Abstract
To better understand the underlying molecular basis of polycythemia vera (PV), we performed whole-exome sequencing and DNA copy-number analysis of 31 JAK2V617F-positive patients and further investigated the evolution of somatic mutations using longitudinal samples. In addition to JAK2V617F and 9pUPD, we identified frequent recurrent somatic mutation in ASXL1, TET2, DNMT3A, SF3B1 and NF1. Forty two percent of patients had a somatic mutation in at least one epigenetic modifier gene. In 4 of 31 patients, variant allele abundance suggested mutation of JAK2V617F was preceded by other somatic mutations including ASXL1, DNMT3A and SF3B1. Strikingly, in 7 patients, apparent germline variants were detected at COSMIC codons in one or more PV-related genes in which we had also discovered somatic mutations across the cohort, suggesting that some pre-JAK2V617F mutations contribute to substantial T-lymphocyte progeny. This study contributes to novel understanding of the complexity of PV pathogenesis.
Polycythemia vera (PV) is a myeloproliferative neoplasm (MPN) characterized by an elevated red cell mass caused by excessive myelopoiesis with propensity to transformation to myelofibrosis and acute leukemia1. JAK2V617F mutation2 and acquired uniparental disomy on chromosome 9p (9pUPD)3, 4 are the most frequent somatic alterations. JAK2 is mutated in over 90% of PV patients2; however, in spite of its prevalence it is regarded as necessary by not sufficient to cause the disease5. Yet, systematic mutation analysis of exome coding sequence has to be described. We here analyzed 31 JAK2V617F-positive patients by whole-exome sequencing (WES) and SNP array and further investigated the mutational evolution using longitudinal samples collected from 7 patients (Supplementary Methods).
WES targeting ~43 Mb of coding sequence yielded an average of 125-fold coverage (Supplementary Figure 1), with 95% of targeted bases covered to a depth of 20x (Supplementary Figure 2). In this study, we use the exomes of T cells as a reference genome and we defined sequence variants present in granulocytes but not in the T-cells from the same patient as somatic mutations. DNA copy-number analysis revealed multiple recurrent chromosomal arm-length changes including 9p UPD, trisomy of 9p and 1q, LOH at 18p, and single patients with 2p and 9q LOH, trisomy 2q and UPD at 22q. Through serial sampling, we observed a focal hemizygous deletion in PV28 (Supplementary Figure 3) at 9q22.31-32. No known tumor suppressors or oncogenes were found in this region and its functional importance is unclear at this time.
We validated 87 somatic non-silent mutations and a subset of important germline variants (Supplementary Table 1). The average non-silent somatic mutation rate was 0.08 per megabase (Mb), which was much lower than other adult cancers6. G->T transversion was the most frequent base change, followed by C->T transition (Supplementary Figure 4). The recurrent G1849T mutation leading to JAK2V617F accounted for the majority of G->T transversions since all patients harbored JAK2V617F. The most frequently mutated class of genes (Figure 1) was that involved in epigenetic modification. Forty-two percent of patients had at least one somatic mutation in an epigenetic modifier, indicating epigenetic regulation plays a key role in PV pathogenesis, as it does in other myeloid neoplasms. Many other known cancer-related genes were mutated in single patients as shown in Figure 1.
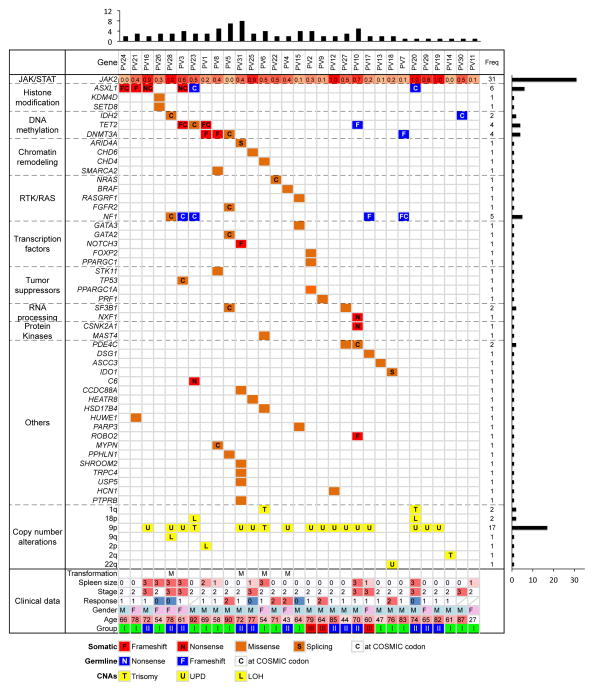
Figure 1. Summary of somatic mutations, germline variants and somatic DNA copy-number alterations identified in 31 PV cases.
Somatic mutations were shown in orange and red based on their mutation type and germline variants were shown in blue. For germline variants, only those frame-shift variants, nonsense variants or those variants reported in COSMIC database (http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/) were shown and only those genes with somatic mutations identified in the cohort were listed. The total number of somatic mutations identified per patient and gene was showed at the top and right, respectively. The allelic fraction of JAK2V617F was shown at the top row and color scaled. The group classification was based on the relationship of JAK2V617F and 9p aUPD. Subgroup I, patients harbored JAK2V617F in a heterozygous state without detectable 9p aUPD; Subgroup II, patients had JAK2V617F with an allelic fraction in direct proportion to the level of 9p aUPD; Subgroup III, patients harbored 9p aUPD at approximately twice the level of the JAK2V617F allelic burden. The clinical data were summarized at the bottom. CNAs, somatic DNA copy-number alterations: T, trisomy; L, hemizygous deletion; U, uniparental disomy. Transformation: M, myelofibrosis. Spleen size: 0, the size of spleen is not palpable; 1, < 3cm; 2, < 6cm; 3, > 6cm. Stage: 1, low; 2, intermediate; 3, high. Response to therapy: 0, no response; 1, partial response; 2, complete response. Gender: M, male; F, female.
Recurrent somatic mutations were observed in: ASXL1, DNMT3A, TET2, SF3B1 and PDE4C (Figure 1). ASXL1 belongs to the Enhancer of trithorax and Polycomb gene families, which are responsible for maintaining activation and silencing of PcG and trxG proteins7. Disruption of ASXL1, through inactivating mutations in exon 12 is prevalent in post-PV myelofibrosis, but is identified only rarely in cases of essential thrombocytosis or PV8. Stein and coworkers sequenced exon 12 of ASXL1 in 42 PV cases but found only one patient (2%) with a nonsense mutation8. We identified 4 inactivating somatic mutations in ASXL1 (12.9%), 2 frame-shift and 2 nonsense. All 4 loss-of-function mutations were identified in exon 12. This was a 6-fold higher mutation rate than previously reported10 and is similar to other MPN.
Somatic DNMT3A mutations were reported at low frequency in PV (2.7%)9. The reported mutations were identified in the terminal exon, at position M880 and R882. In this study, we identified 3 somatic DNMT3A mutations (9.7%), one was identified at the known hotspot, R882, and the other two were novel frame-shift mutations at codon K456. SF3B1 encodes subunit 1 of the splicing factor 3b, which is important for anchoring the spliceosome to precursor mRNA. Mutation of SF3B1 is frequent in most MPN, having been reported in myelodysplasia with ring sideroblasts (65%)10, myelodysplastic syndrome (20%)10, primary myelofibrosis (7%)11 and essential thombocythaemia (3%), but it has not been reported in PV10. In this study, we identified 3 SF3B1 mutations in 2 patients (9.7%), patient PV5 carried two mutations and both were reported by COSMIC (http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/). Interestingly, phosphodiesterase 4C, PDE4C, is mutated in two patients. PDE4C hydrolyzes the second messenger cAMP and therefore mediates a variety of responses to extracellular signals. Although mutation in this gene is rarely observed in cancer, one of the mutations we discovered was reported in COSMIC, suggesting it may be functionally relevant.
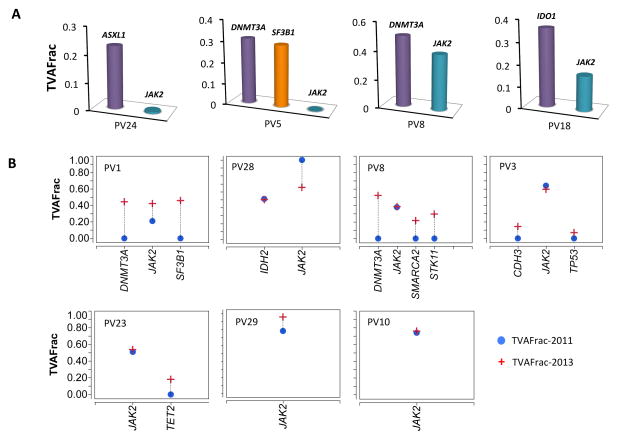
The fraction of reads with a given mutation, the variant allele fraction, is proportional to the number of nuclei in the tumor sample harboring the mutation. Since the granulocytes in PV patients are clonal, by X-inactivation in females12, the variant allele fraction of the mutations reported in Figure 1, should correspond to the order in which they appeared in the patient. Three patients, PV5, PV8 and PV24, exhibited tumor variant allele fraction in the key epigenetic modifier genes, ASXL1, DNMT3A, or SF3B1 that were higher than JAK2V617F (Figure 2A). Interestingly mutation in a gene associated with immunosuppression in solid tumors, IDO1, is also found at higher variant allele burden than that of JAK2V617F. These results suggest that mutation of these genes might have preceded the acquisition of JAK2V617F.
Figure 2. The pre-JAK2 mutations and signatures of mutational evolution.
(A) Patients with pre-JAK2 somatic mutations. (B) Changes of somatic mutations during PV progression. The longitudinal samples were collected from 7 PV patients during a 2-year period from 2011 to 2013. TVAFrac, the variant allelic fraction in granulocytes.
In 7 patients we could determine the order of appearance of mutations directly by longitudinal sampling (Figure 2B). Patients PV1, PV3, PV8, PV10, PV23 and PV29, harbored only JAK2V617F mutation in 2011. Upon follow-up in 2013, four of them had acquired additional mutations, particularly in key epigenetic modifier genes DNMT3A, SMARCA2 and TET2, but also in SF3B1, STK11, CDH3 and TP53. One patient harbored IDH2 and JAK2 in 2011, but did not acquire any additional mutations. Results from a parallel study of these patients showed that 10% of them acquired 9p UPD prior to JAK2V617F mutations (Figure 1, Group III patients), whereas 42% had acquired JAK2V617F mutation first (Figure I, Group II, AMBER13-LEU-1191).
Novel sequence variants found in both granulocytes and T-cells from the same patients are putative germline mutations. However, in 7 patients, these putative germline variants were in genes that were somatically mutated in other patients in the cohort (Figure 1, blue tiles). Moreover, a high proportion of these so-called germline mutations were likely to be functionally relevant either because they were truncating frameshift or nonsense mutations or the same mutations could be found in COSMIC. For example, the tumor suppressor NF1 was mutated in 4 patients’ T-cells and granulocytes. Among them, 3 variants are presented in COSMIC. Germline mutation of NF1 is linked to neurofibromatosis type 1, a debilitating dominant genetic disorder characterized by a higher risk for juvenile myelomonocytic leukemia with a potential progression to acute myeloid leukemia (AML)13. Symptoms of neurofibromatosis type 1 were not observed in our PV patients, thus it is highly unlikely these patients have true germline mutation in this gene. Similar variants were also found in two patients in ASXL1, and one patient each in TET2, DNMT3A and IDH2. The IDH2 variant R140Q is a hotspot for somatic mutation in AML and other cancers. Based on these results, NF1 emerges as a frequently mutated gene (16%) in PV; ASXL1 mutated in 19% of patients, 5-fold higher than previously reported (P = 0.02, Fisher’s exact test); and DNMT3A and TET2 each mutated in 13% of our cohort.
These mutations could not be explained by contamination of the T-cells by granulocytes because the T-cells harbored little or no JAK2V617F, whereas these mutations are present in similar variant allele fractions in the T-cells and granulocytes (Supplementary Figure 5). Therefore we conclude these were somatic mutations in long-lived T cells. For the mutations to have populated both the T-cell and granulocyte lineages in equal proportions, they must have occurred in pluripotent stem cells early in the lives of these patients, well before the JAK2V617F mutation that favors myeloid differentiation.
The involvement of T cells by some of the somatic mutations was unexpected. Studies based on the distribution of X-chromosome allelic usage have been used to assess the relative contribution of pluripotent stem cells to B and T cell lymphopoiesis versus myelopoiesis. Indeed, in normal healthy females the X-chromosome allelic usage of myeloid and lymphoid progeny was identical suggesting that these are all derived from relative small number of hematopoietic stem cells present in female embryo at the time of X-chromosome inactivation14. However, the majority of T cells, unlike myeloid progeny in PV are polyclonal15 suggesting that the mutations were observed were derived from stem cells prior to the clonal event necessary for discernible PV phenotype. However, a small proportion of T cells may be part of the PV clone3, and JAK2V617F mutant cells have preferentially myeloid potential but not exclusively as some JAK2V617F lymphoid progeny are also formed16. In this report we present a novel observation that some pre- JAK2V617F somatic mutations contribute to substantial T-lymphocyte progeny.
In summary, we sequenced 31 PV pairs of granulocytes and T cells presumed to have germline genome and identified multiple novel driver genes and pre-JAK2 mutations and described the signatures of clonal evolution during PV progression in some patients. This study contributes to our understanding of the pathogenesis of PV and underscores the complexity of genetic lesions in the typical PV genome.
Supplementary Material
Supplementary Table 1. The somatic mutations and germline ariants identified in 31 PV patients.
Acknowledgments
This study was supported by research funding from the National Human Genome Research Institute (NHGRI, grant number: 5U54HG003273) to DW and from the National Institutes of Health (NIH, grant number: NIH-P01CA108671) to JP. We thank Christian Buhay, Donna Morton, Huyen Dinh, Ritika Raj, Lora Lewis, Christie Kovar, Sandra Lee, Michelle Bellair and Zhu Yiming for their excellent technical support.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary information is available at Nature Leukemia’s website.
References
- 1.Tefferi A, Spivak JL. Polycythemia vera: scientific advances and current practice. Seminars in hematology. 2005;42(4):206–20. doi: 10.1053/j.seminhematol.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 2.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, Guan Y, Prchal JT. Acquired uniparental disomy of chromosome 9p is a frequent stem cell defect in polycythemia vera. Experimental hematology. 2002;30(3):229–36. doi: 10.1016/s0301-472x(01)00789-5. [DOI] [PubMed] [Google Scholar]
- 4.Wang K, Swierczek S, Hickman K, Hakonarson H, Prchal JT. Convergent mechanisms of somatic mutations in polycythemia vera. Discovery medicine. 2011;12(62):25–32. [PMC free article] [PubMed] [Google Scholar]
- 5.Nussenzveig RH, Swierczek SI, Jelinek J, Gaikwad A, Liu E, Verstovsek S, et al. Polycythemia vera is not initiated by JAK2V617F mutation. Experimental hematology. 2007;35(1):32–8. doi: 10.1016/j.exphem.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler DA, Wang L. From human genome to cancer genome: The first decade. Genome Res. 2013;23(7):1054–62. doi: 10.1101/gr.157602.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher CL, Pineault N, Brookes C, Helgason CD, Ohta H, Bodner C, et al. Loss-of-function Additional sex combs like 1 mutations disrupt hematopoiesis but do not cause severe myelodysplasia or leukemia. Blood. 2010;115(1):38–46. doi: 10.1182/blood-2009-07-230698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein BL, Williams DM, O’Keefe C, Rogers O, Ingersoll RG, Spivak JL, et al. Disruption of the ASXL1 gene is frequent in primary, post-essential thrombocytosis and post-polycythemia vera myelofibrosis, but not essential thrombocytosis or polycythemia vera: analysis of molecular genetics and clinical phenotypes. Haematologica. 2011;96(10):1462–9. doi: 10.3324/haematol.2011.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao N, Butcher CM, Lewis ID, Ross DM, Melo JV, Scott HS, et al. Clonal and lineage analysis of somatic DNMT3A and JAK2 mutations in a chronic phase polycythemia vera patient. British journal of haematology. 2012;156(2):268–70. doi: 10.1111/j.1365-2141.2011.08837.x. [DOI] [PubMed] [Google Scholar]
- 10.Papaemmanuil E, Cazzola M, Boultwood J, Malcovati L, Vyas P, Bowen D, et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. The New England journal of medicine. 2011;365(15):1384–95. doi: 10.1056/NEJMoa1103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lasho TL, Finke CM, Hanson CA, Jimma T, Knudson RA, Ketterling RP, et al. SF3B1 mutations in primary myelofibrosis: clinical, histopathology and genetic correlates among 155 patients. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2012;26(5):1135–7. doi: 10.1038/leu.2011.320. [DOI] [PubMed] [Google Scholar]
- 12.Swierczek SI, Agarwal N, Nussenzveig RH, Rothstein G, Wilson A, Artz A, et al. Hematopoiesis is not clonal in healthy elderly women. Blood. 2008;112(8):3186–93. doi: 10.1182/blood-2008-03-143925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Side L, Taylor B, Cayouette M, Conner E, Thompson P, Luce M, et al. Homozygous inactivation of the NF1 gene in bone marrow cells from children with neurofibromatosis type 1 and malignant myeloid disorders. The New England journal of medicine. 1997;336(24):1713–20. doi: 10.1056/NEJM199706123362404. [DOI] [PubMed] [Google Scholar]
- 14.Prchal JT, Prchal JF, Belickova M, Chen S, Guan Y, Gartland GL, et al. Clonal stability of blood cell lineages indicated by X-chromosomal transcriptional polymorphism. The Journal of experimental medicine. 1996;183(2):561–7. doi: 10.1084/jem.183.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prchal JT, Guan YL, Prchal JF, Barany F. Transcriptional analysis of the active X-chromosome in normal and clonal hematopoiesis. Blood. 1993;81(1):269–71. [PubMed] [Google Scholar]
- 16.Jamieson CH, Gotlib J, Durocher JA, Chao MP, Mariappan MR, Lay M, et al. The JAK2 V617F mutation occurs in hematopoietic stem cells in polycythemia vera and predisposes toward erythroid differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(16):6224–9. doi: 10.1073/pnas.0601462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. The somatic mutations and germline ariants identified in 31 PV patients.