Abstract
In the infarcted myocardium, necrotic cardiomyocytes release danger signals activating an intense inflammatory reaction that serves to clear the wound from dead cells and matrix debris, but may also extend injury. A growing body of evidence suggests an important role for members of the Interleukin (IL)-1 family in injury, repair and remodeling of the infarcted heart. This review manuscript discusses the pathophysiologic functions of IL-1 in the infarcted and remodeling myocardium and its potential role as a therapeutic target in patients with myocardial infarction. Dead cardiomyocytes release IL-1a that may function as a crucial alarmin triggering the post-infarction inflammatory reaction. IL-1b is markedly upregulated in the infarcted myocardium; activation of the inflammasome in both cardiomyocytes and interstitial cells results in release of bioactive IL-1b in the infarcted area. Binding of IL-1 to the type 1 receptor triggers an inflammatory cascade, inducing recruitment of pro-inflammatory leukocytes and stimulating a matrix-degrading program in fibroblasts, while delaying myofibroblast conversion. IL-1 mediates dilative remodeling following infarction and may play a role in the pathogenesis of post-infarction heart failure. As the wound is cleared from dead cells and matrix debris, endogenous inhibitory signals suppress the IL-1 response resulting in repression of inflammation and resolution of the inflammatory infiltrate. Other members of the IL-1 family (such as IL-18 and IL-33) are also implicated in regulation of the inflammatory and reparative response following myocardial infarction. IL-18 may participate in pro-inflammatory signaling, whereas IL-33 may exert cytoprotective effects. Early clinical trials suggest that IL-1 blockade may be a promising therapeutic strategy for patients with myocardial infarction.
Keywords: Myocardial infarction, inflammation, cytokine, cardiac remodeling
1. Introducion
Myocardial infarction triggers an intense inflammatory reaction that serves to clear the wound from dead cells and matrix debris and sets the stage for cardiac repair1. Necrotic cardiomyocytes release danger signals that activate innate immune pathways2, inducing secretion of pro-inflammatory cytokines and chemokines and stimulating adhesion molecule expression by endothelial cells3,4. Activation of adhesive interactions between leukocytes and endothelial cells results in intense infiltration of the infarct with neutrophils and mononuclear cells, which are predominantly localized in the infarct border zone. 20-30 years ago, a large body of experimental evidence, primarily derived from studies in large animal models of reperfused myocardial infarction suggested that inflammatory leukocytes may extend ischemic injury following myocardial infarction5. Broad anti-inflammatory strategies (such as corticosteroids) were tested; however, their wide range of effects on both inflammatory and reparative cells resulted (in some studies) in catastrophic consequences6. More selective strategies inhibiting specific adhesion molecules (such as leukocyte integrins or endothelial selectins) showed great promise in experimental models, markedly reducing the size of the infarct7. Unfortunately, despite the promising findings of the experimental investigations, small clinical trials targeting leukocyte integrins had disappointing results7. These failures greatly diminished enthusiasm regarding inflammatory targets in myocardial infarction. This is unfortunate, considering the important role of inflammatory mediators in adverse remodeling of the infarct heart and in the pathogenesis of heart failure that may not result solely from effects on cardiomyocyte survival. Thus, the quest for new inflammatory targets in myocardial infarction continues.
As the prototypical pro-inflammatory cytokine, interleukin (IL)-1 is involved in the pathogenesis of a wide range of inflammatory diseases8,9. IL-1 blockade is the standard of care for treatment of “autoinflammatory diseases”, a family of conditions characterized by dysfunction of monocytes/macrophages and recurrent bouts of debilitating inflammation10. Moreover, IL-1 neutralization therapy was beneficial in patients with rheumatoid arthritis, reducing symptoms and delaying the progression of joint destruction11. Emerging evidence suggests an important role for members of the IL-1 family in the pathogenesis of post-infarction cardiac remodeling and heart failure. This review manuscript discusses the role of IL-1 signaling in injury, repair and remodeling of the infarcted heart and its potential role as a therapeutic target.
2. The IL-1 family of cytokines
The IL-1 family is comprised of 7 agonist molecules (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36b and IL-36g), 3 receptor antagonists (Ra; IL-1Ra, IL-36Ra and IL-38) and the anti-inflammatory cytokine IL-3712. IL-1 family agonist molecules exhibit profound differences in their expression and regulation. Some members of the family, (such as IL-1α) are constitutively present as precursor proteins in most healthy cells and are released following injury, serving as typical alarmins. Other members (such as IL-1β and IL-18) are generally not expressed by healthy cells, but are synthesized as inactive precursors following stimulation and are processed to generate the active molecules. Generation of active IL-1β is primarily mediated through processing of the precursor protein by the intracellular cysteine protease caspase-1; however, caspase-1-independent mechanisms of IL-1β activation have also been described13. Activation of caspase-1 requires formation of a multi-component platform termed the “inflammasome”; one of the components of the inflammasome, Nucleotide-binding oligomerization domain-Like Receptor with a Pyrin domain 3 (NLRP3), plays an important role in generation of active IL-1β14.
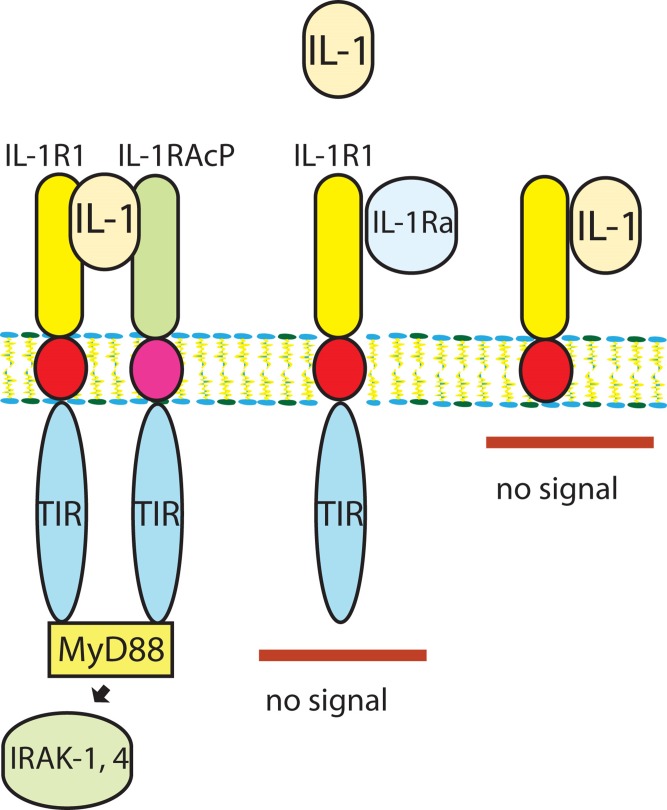
Both IL-1α and IL-1β signal by binding to the type 1 IL-1 receptor (IL-1R1). The family of IL-1 receptors also includes decoy receptors15 (such as IL-1R2) that do not trigger signaling, but serve as molecular sinks for the cytokine, terminating the IL-1-driven response. Regulation of IL-1 signaling is also modulated by endogenous natural inhibitors, such as IL-1 receptor antagonist (IL-1Ra). IL-1Ra is an endogenous natural inhibitor that binds to IL-1R1 but does not activate signaling, preventing recruitment of the IL-1 receptor accessory protein (IL-1RAcP), an essential component of the IL-1 receptor signaling system (Figure 1)16.
Figure 1. Figure 1. Regulation of IL-1 signaling by native inhibitors and decoy receptors.
IL-1α, or IL-1β binds to IL-1R1 and forms a complex with IL-1RAcP, recruiting myeloid differentiation protein 88 (MyD88) and triggering the signaling cascade that involves IRAK-1 and IRAK-4 activation (A). The endogenous inhibitor IL-1Ra also binds to IL-1R1, but does not signal because it fails to form a complex with IL-1RAcP (B). IL-1 binding to the decoy receptor IL-1R2 does not result in stimulation of a signaling cascade, as this receptor lacks a cytoplasmic segment (C).
3. IL-1 upregulation in myocardial infarction
IL-1α release and IL-1β induction are consistently noted in experimental models of myocardial infarction17,18. Necrotic cardiomyocytes release IL-1α18, whereas pro-inflammatory monocyte subsets may be a major source of IL-1β in the infarcted heart19. Increased levels of IL-1 in human patients with myocardial infarction have been less consistently documented. In patients with ST elevation myocardial infarction (STEMI), circulating IL-1β levels were associated with systolic dysfunction and adverse remodeling20. However, other studies did not demonstrate elevated IL-1β levels in patients with myocardial infarction21. Such findings may reflect difficulties in detecting plasma IL-1β, due to binding of the cytokine to large proteins such as a2 macroglobulins, complement, and soluble receptors22,23. Activation of the inflammasome has been documented in many cell types involved in cardiac repair and is critically involved in generation of bioactive IL-1. Activation of the inflammasome in cardiac fibroblasts and in cardiomyocytes has been extensively documented in experimental models of myocardial infarction and may contribute to the post-infarction inflammatory reaction extending injury24-26.
Several other members of the IL-1 family are overexpressed in the infarcted heart. IL-18 is upregulated in the infarcted myocardium27,28; circulating IL-18 levels are increased in patients with acute coronary syndromes29. IL-33 is a biomechanically induced protein30 that is primarily expressed in fibroblasts and may be also upregulated following myocardial infarction31. IL-33 signals through binding to the orphan receptor ST2, increased levels of soluble ST2 have been associated with adverse outcome in patients with STEMI32. The endogenous receptor antagonist IL-1Ra is also upregulated in experimental models of myocardial infarction and is localized in the infarct border zone33. In human patients with myocardial infarction, serum IL-1Ra levels are increased34, preceding the release of markers of necrosis35. Associative studies suggested that plasma IL-1Ra levels correlate with the extent of cardiomyocyte death36 and with the severity of hemodynamic compromise in patients with acute myocardial infarction37.
4. The role of IL-1 in regulation of cardiac injury, repair and remodeling following myocardial infarction
A large body of evidence, derived from genetic loss-of-function studies and antibody neutralization experiments suggests that members of the IL-1 family play crucial roles in injury, repair and remodeling of the infarcted heart38-40. The effects of IL-1 family members may involve several distinct actions on various cell types in the infarcted and remodeling heart.
4.1 Does IL-1 extend ischemic injury following myocardial infarction?
A recent investigation demonstrated that IL-1α, released from necrotic cardiomyocytes, serves a crucial danger signal, implicated in activation of the post-infarction inflammatory response18. It has been suggested that release of constitutive IL-1α and induction of IL-1β may extend ischemic injury, increasing apoptosis of cardiomyocytes. In vitro experiments have demonstrated that IL-1β stimulation activates apoptotic pathways in neonatal rat cardiomyocytes41. Moreover, incubation of rat cardiomyocytes with recombinant human IL-1Ra (anakinra) reduced apoptosis in a simulated ischemia/reperfusion protocol. In vivo, overexpression of human IL-1Ra through gene transfection in heterotopically transplanted rat hearts undergoing ischemia and reperfusion significantly attenuated infarct size, reducing the number of apoptotic cardiomyocytes42. Pro-apoptotic effects of IL-1 were further supported by studies in rodent models of infarction showing that administration of recombinant human IL-1Ra decreased cardiomyocyte apoptosis and prevented cardiac dilation43. It should be noted that not all investigations suggested effects of IL-1 on the size of the infarct. IL-1R1 loss had no effect on the size of the infarct in a model of myocardial ischemia/reperfusion despite a marked attenuation in the inflammatory response44.
4.2 IL-1 signaling is critically involved in activation of the post-infarction inflammatory response
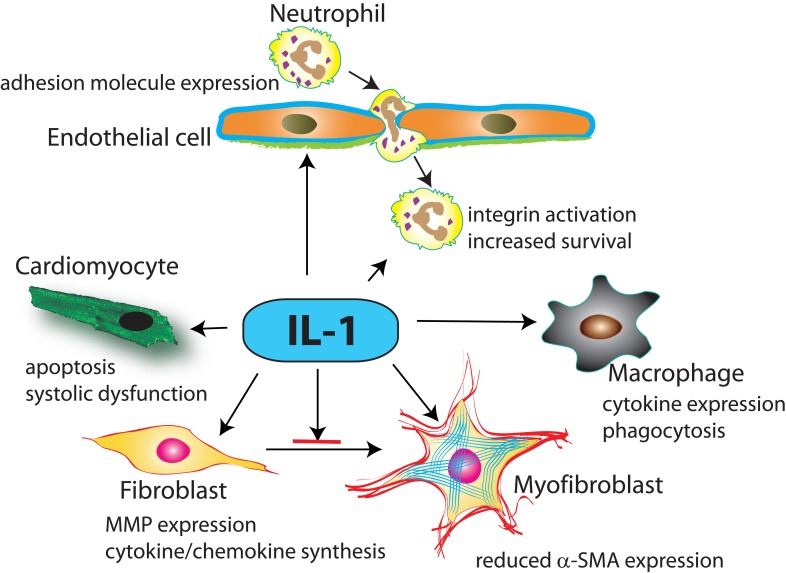
The role of IL-1 in activation of the post-infarction inflammatory response is supported by extensive in vivo and in vitro experimentation. IL-1 activates a pro-inflammatory program in all cells involved in cardiac injury and repair (Figure 2). In endothelial cells, IL-1 induces chemokine and adhesion molecule synthesis, enhancing adhesive interactions implicated in recruitment of leukocytes in injured tissues45. IL-1 also upregulates chemokine synthesis in mononuclear cells and prolongs the lifespan of neutrophils46. In vivo, IL-1Ra overexpression significantly reduced infiltration of the ischemic heart with neutrophils42 and IL-1R1 loss was associated with a marked reduction of peak cytokine and chemokine mRNA expression in the infarcted heart and with attenuated infiltration of the infarct with neutrophils and pro-inflammatory monocytes19,44. Attenuated inflammation in the absence of IL-1 does not result from a reduction in the size of the infarct, but primarily reflects direct IL-1-mediated pro-inflammatory actions19,44.
Figure 2. Figure 2. The cellular targets of IL-1 in myocardial infarction.
IL-1α (released by necrotic cardiomyocytes) and IL-1β (newly synthesized and secreted by resident myocardial cells and infiltrating leukocytes) signal by activating IL-1R1. IL-1 induces cardiomyocyte apoptosis and suppresses cardiomyocyte function, stimulates a matrix-degrading pro-inflammatory program in cardiac fibroblasts and delays fibroblast to myofibroblast transdifferentiation, induces cytokine expression in macrophages, mediates leukocyte recruitment by inducing adhesion molecule expression by endothelial cells and prolongs neutrophil survival.
4.3 Effects of IL-1 on fibroblast activation and on extracellular matrix metabolism
During the inflammatory phase of cardiac repair, resident cardiac fibroblasts undergo pro-inflammatory activation47 and may serve as an important source of cytokines and chemokines. Release of Il-1α, induction of IL-1β and downstream activation of IL-1R1 signaling stimulate an inflammatory program in cardiac fibroblasts18,19,48. In addition to its pro-inflammatory actions, IL-1 also promotes a matrix-degrading phenotype in cardiac fibroblasts, markedly upregulating synthesis of matrix metalloproteinases (MMPs)49,50. Moreover, activation of IL-1 signaling delays myofibroblast transdifferentiation reducing expression of a-smooth muscle actin in cardiac fibroblasts19. Thus, IL-1 signaling may prevent premature conversion of cardiac fibroblasts into matrix-synthetic myofibroblasts, until the wound is cleared from dead cells and matrix debris.
4.4 IL-1 promotes adverse dilative remodeling of the infarcted heart
IL-1Ra overexpression studies and loss-of-function experiments targeting the IL-1 signaling cascade demonstrated that disruption of IL-1 attenuates dilative remodeling following myocardial infarction44,51. The beneficial actions of IL-1 disruption in post-infarction remodeling may be mediated through attenuation of pro-inflammatory signaling, or through loss of direct IL-1-mediated actions on matrix metabolism and on function of cardiac fibroblasts. Excessive matrix degradation reduces the tensile strength of the wound and may deprive surviving cardiomyocytes in the border zone from key pro-survival signals52,53.
4.5 Termination of IL-1 signaling
Repair of the infarcted heart is dependent on timely repression of the inflammatory reaction and subsequent resolution of the inflammatory infiltrate1. Suppression of the inflammatory reaction is not a passive process, but requires activation of STOP signals that inhibit pro-inflammatory signaling. Considering the intense pro-inflammatory actions of IL-1, suppression and termination of IL-1 signaling is crucial for the transition from inflammation to repair. Several molecular signals may participate in suppression of the IL-1 response. First, induction of secreted anti-inflammatory mediators, such as IL-1054 and Transforming Growth Factor (TGF)-β55, may deactivate mononuclear cells, reducing IL-1 transcription. Although this is a plausible hypothesis, it should be noted that IL-10 null animals and wildtype controls had comparable myocardial IL-1β mRNA levels 24 h after reperfused infarction56. Second, mediators inhibiting the inflammasome may limit generation of bioactive IL-1β57. Third, activation of intracellular negative regulators, such as Interleukin receptor-associated kinase (IRAK)-M in macrophages and fibroblasts suppresses pro-inflammatory IL-1/Toll Like Receptor (TLR) signaling58. Fourth, upregulation of decoy receptors (such as IL-1R2) in modulated M2 macrophages of the infarct may serve as a molecular sink that terminates the IL-1 response19. Recruitment of monocyte and lymphocyte subsets with inhibitory properties may play an important role in suppressing the IL-1 response59-61.
4.6 Does IL-1 exert protective actions on the infarcted heart?
Cytokines are highly pleiotropic mediators, exerting a wide range of actions on all cell types implicated in cardiac repair. The recent experience with interventions targeting the Tumor Necrosis Factor (TNF)-a system in patients with heart failure highlighted the unpredictable consequences of interfering with cytokine signaling62. TNF-a exerts both protective and injurious actions on the failing heart63,64; as a result, anti-TNF therapy did not prove beneficial in heart failure patients. Although the bulk of experimental evidence indicates that activation of IL-1 signaling exerts deleterious effects on the infarcted and remodeling heart, could IL-1 activation regulate pathways with a critical role in cardioprotection, or in repair of the injured myocardium?
Hard evidence suggesting protective effects of IL-1β on ischemic cardiomyocytes is lacking. However, a study using antibody neutralization to neutralize IL-1β suggested important effects of IL-1 in cardiac repair. Administration of a single intraperitoneal injection of a neutralizing anti-IL-1β antibody immediately after coronary ligation in a model of non-reperfused infarction significantly increased the incidence of cardiac rupture, reducing collagen accumulation in the infarcted area. Surviving animals exhibited accentuated chamber dilation65. The findings of this study are in conflict with several other investigations that showed attenuated adverse remodeling in animals with defective IL-1 signaling44 and in animals treated with IL-1 antagonists42,43,51. The basis for the contradictory findings is unclear. The differences in outcome may be related, at least in part, to the use of non-reperfused vs. reperfused models of myocardial infarction. Reperfused infarction is associated with more intense and early activation of pro-inflammatory signaling cascades. Moreover, in the presence of a permanently occluded coronary, early administration of an anti-IL-1 antibody will likely exclusively target the perfused non-infarcted area and may not significantly affect the reparative response in the infarct. Effectiveness of IL-1 inhibition may also depend on the dose and method used for IL-1 neutralization. Timing of the therapeutic intervention may also play a critical role in determining outcome.
5. IL-1 as a therapeutic target in human patients with acute myocardial infarction
A growing body of experimental evidence suggests an important role for IL-1-driven inflammation in the pathogenesis of atherothrombotic disease. The availability of anakinra, a nonglycosylated recombinant human IL-1Ra that binds to IL-1R1 competitively inhibiting IL-1 signaling, provides an interesting and safe tool for therapeutic intervention. Anakinra has been approved for treatment of patients with rheumatoid arthritis with poor responses to disease modifying agents. Clinical trials examining the effects of IL-1β antibody inhibition on the incidence of cardiovascular events in high-risk patients are currently underway66. Considering the evidence suggesting a role for IL-1 in post-infarction remodeling, acute myocardial infarction represents a promising opportunity for the therapeutic use of IL-1 antagonists. The effectiveness of IL-1 blockade in patients with myocardial infarction was studied in small clinical investigations. Pilot studies suggested that in patients with STEMI, a 2-week course of anakinra is safe, may attenuate adverse remodeling67 and may reduce the incidence of post-infarction heart failure68,69. Large clinical studies are needed to test the effectiveness of IL-1 inhibition in STEMI patients. It should be emphasized that anti-IL-1 approaches may be particularly beneficial in patient subpopulations with overactive and prolonged post-infarction inflammatory responses70. These patients could be identified through the use of carefully selected biomarkers or imaging strategies71.
6. Other members of the IL-1 family: the role of IL-18 and IL-33
Although IL-1α and IL-1β remain the best-studied members of the family, emerging evidence implicates IL-18 and IL-33 in regulation of the post-infarction inflammatory reaction. IL-18 inhibition studies have added IL-18 to the long list of pro-inflammatory mediators that may extend ischemic injury in mouse models72. Moreover, therapy with mesenchymal stem cells expressing IL-18 binding protein, a natural IL-18 inhibitor, protected the ischemic heart73. A role for IL-18 in mediating IL-1-dependent pro-inflammatory actions has been suggested28.
IL-33, on the other hand, exerts a wide range of pro-inflammatory actions, activating dendritic cells and enhancing LPS-dependent cytokine synthesis by macrophages12. In the healing infarct, IL-33 has protective actions attenuating cardiomyocyte apoptosis through binding to ST231. The mechanisms responsible for the cardioprotective effects of the IL-33/ST2 axis remain poorly understood.
7. Conclusions
Despite intensive efforts, implementation of strategies targeting the inflammatory cascade in patients with myocardial infarction has been unsuccessful. The pleiotropic effects of inflammatory mediators that exert both beneficial and detrimental actions on many different cell types pose major challenges in designing an effective treatment approach. Redundant actions of various inflammatory mediators further complicate translational efforts.
Considering the strong evidence suggesting a key role for IL-1 in myocardial injury and remodeling, and the availability of safe and effective agents for IL-1 inhibition, IL-1 blockade should be considered a highly promising therapeutic approach in myocardial infarction. Studies in rodent models suggest a crucial role for IL-1 in activation of the post-infarction inflammatory reaction. IL-1 signaling also regulates fibroblast phenotype inducing a matrix-degrading program and promoting dilative remodeling. Early clinical studies testing the effectiveness of anti-IL-1 approaches in patients with acute myocardial infarction have produced promising results. Future studies need to expand our understanding of the role of IL-1 family members in the pathophysiology of myocardial infarction, while aggressively pursuing clinical translation. The significance of specific cellular actions of IL-1 on cardiomyocytes, immune cells, fibroblasts and vascular cells needs to be dissected in vivo. The mechanisms of IL-1-mediated injury need to be studied; the possibility for cytoprotective actions should be carefully considered. The molecular signals involved in negative regulation of the IL-1 response in the healing infarct need to be understood. Pathophysiologic functions of the newer members of the IL-1 family need to be explored. Understanding the biology of the IL-1 system in myocardial infarction is essential in order to design therapies for attenuation of post-infarction remodeling and for protection from the development of heart failure.
KEY POINTS
◊ Several members of the Interleukin (IL)-1 family, including IL-1a, IL-1b, IL-18, IL-33 and the endogenous inhibitor IL-1Ra are released in the infarcted myocardium.
◊ IL-1α is released by necrotic cells, whereas IL-1β is synthesized and activated through a molecular platform called the “inflammasone”.
◊ Both IL-1α and IL-1β signal through the type 1 IL-1 receptor (IL-1R1). IL-1R1 plays a critical role in activation of post-infarction inflammation, promotes a matrix-degrading phenotype in fibroblasts, delays myofibroblast transdifferentiation and may induce cardiomyocyte apoptosis.
◊ IL-1 mediates adverse remodeling and dysfunction following myocardial infarction.
◊Pilot clinical studies suggest that IL-1 neutralization may attenuate adverse remodeling and heart failure in patients with acute myocardial infarction.
OPEN Question
◊ Which cellular effects of IL-1 are critical in the pathogenesis of post-infarction remodeling?
Acknowledgments
Dr. Frangogiannis’ laboratory is supported by NIH grants R01 HL76246 and R01 HL85440.
Footnotes
Conflict of interests: The authors declare no conflict of interest.
Interleukin (IL); nucleotide-binding oligomerization domain-Like Receptor with a Pyrin domain 3 (NLRP3); interleukin-1 receptor (IL-1R); interkeukin-1 receptor antagonist (IL-1Ra); interleukin-1 receptor accessory protein (IL-1RAcP); ST elevation myocardial infarction (STEMI); matrix metalloproteinase (MMP); transforming growth factor-b (TGF- b interleukin receptor-associated kinase (IRAK); toll-like receptor (TLR), tumor necrosis factor-a (TNF); myeloid differentiation protein 88 (MyD88)
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.Regulation of the inflammatory response in cardiac repair. Frangogiannis Nikolaos G. Circulation research. 2012;110(1):159–73. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Innate immune signaling in cardiac ischemia. Arslan Fatih, de Kleijn Dominique P, Pasterkamp Gerard. Nature reviews. Cardiology. 2011;8(5):292–300. doi: 10.1038/nrcardio.2011.38. [DOI] [PubMed] [Google Scholar]
- 3.Repair after myocardial infarction, between fantasy and reality: the role of chemokines. Liehn Elisa A, Postea Otilia, Curaj Adelina, Marx Nikolaus. Journal of the American College of Cardiology. 2011;58(23):2357–62. doi: 10.1016/j.jacc.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Chemokines in ischemia and reperfusion. Frangogiannis Nikolaos G. Thrombosis and haemostasis. 2007;97(5):738–47. [PubMed] [Google Scholar]
- 5.Inflammation in the course of early myocardial ischemia. Entman M L, Michael L, Rossen R D, Dreyer W J, Anderson D C, Taylor A A, Smith C W. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1991;5(11):2529–37. doi: 10.1096/fasebj.5.11.1868978. [DOI] [PubMed] [Google Scholar]
- 6.Deleterious effects of methylprednisolone in patients with myocardial infarction. Roberts R, DeMello V, Sobel B E. Circulation. 1976;53(3 Suppl):I204–6. [PubMed] [Google Scholar]
- 7.Targeting inflammatory pathways in myocardial infarction. Christia Panagiota, Frangogiannis Nikolaos G. European journal of clinical investigation. 2013;43(9):986–95. doi: 10.1111/eci.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A clinical perspective of IL-1β as the gatekeeper of inflammation. Dinarello Charles A. European journal of immunology. 2011;41(5):1203–17. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 9.Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Dinarello Charles A. Blood. 2011;117(14):3720–32. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*). Masters Seth L, Simon Anna, Aksentijevich Ivona, Kastner Daniel L. Annual review of immunology. 2009;27:621–68. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA. Nam J L, Winthrop K L, van Vollenhoven R F, Pavelka K, Valesini G, Hensor E M A, Worthy G, Landewé R, Smolen J S, Emery P, Buch M H. Annals of the rheumatic diseases. 2010;69(6):976–86. doi: 10.1136/ard.2009.126573. [DOI] [PubMed] [Google Scholar]
- 12.The interleukin-1 family: back to the future. Garlanda Cecilia, Dinarello Charles A, Mantovani Alberto. Immunity. 2013;39(6):1003–18. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inflammasome-independent regulation of IL-1-family cytokines. Netea Mihai G, van de Veerdonk Frank L, van der Meer Jos W M, Dinarello Charles A, Joosten Leo A B. Annual review of immunology. 2015;33:49–77. doi: 10.1146/annurev-immunol-032414-112306. [DOI] [PubMed] [Google Scholar]
- 14.NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Agostini Laetitia, Martinon Fabio, Burns Kimberly, McDermott Michael F, Hawkins Philip N, Tschopp Jürg. Immunity. 2004;20(3):319–25. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 15.Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, Giri J G, Dower S K, Sims J E, Mantovani A. Science (New York, N.Y.) 1993;261(5120):472–5. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- 16.IL-1 receptor accessory protein is an essential component of the IL-1 receptor. Cullinan E B, Kwee L, Nunes P, Shuster D J, Ju G, McIntyre K W, Chizzonite R A, Labow M A. Journal of immunology (Baltimore, Md. : 1950) 1998;161(10):5614–20. [PubMed] [Google Scholar]
- 17.Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Dewald Oliver, Ren Guofeng, Duerr Georg D, Zoerlein Martin, Klemm Christina, Gersch Christine, Tincey Sophia, Michael Lloyd H, Entman Mark L, Frangogiannis Nikolaos G. The American journal of pathology. 2004;164(2):665–77. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutting edge: IL-1α is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. Lugrin Jérôme, Parapanov Roumen, Rosenblatt-Velin Nathalie, Rignault-Clerc Stéphanie, Feihl François, Waeber Bernard, Müller Olivier, Vergely Catherine, Zeller Marianne, Tardivel Aubry, Schneider Pascal, Pacher Pal, Liaudet Lucas. Journal of immunology (Baltimore, Md. : 1950) 2015;194(2):499–503. doi: 10.4049/jimmunol.1401948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. Saxena Amit, Chen Wei, Su Ya, Rai Vikrant, Uche Olisambu U, Li Na, Frangogiannis Nikolaos G. Journal of immunology (Baltimore, Md. : 1950) 2013;191(9):4838–48. doi: 10.4049/jimmunol.1300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Increased interleukin-1β levels are associated with left ventricular hypertrophy and remodelling following acute ST segment elevation myocardial infarction treated by primary percutaneous coronary intervention. Ørn S, Ueland T, Manhenke C, Sandanger Ø, Godang K, Yndestad A. Journal of Internal Medicine. 2012;272(3):267–276. doi: 10.1111/j.1365-2796.2012.02517.x. [DOI] [PubMed] [Google Scholar]
- 21.Interleukin-1 and tumor necrosis factor-alpha in plasma of patients with acute ischemic heart disease who undergo thrombolytic therapy: a randomized, placebo-controlled study. Munkvad S, Gram J, Jespersen J. Lymphokine and cytokine research. 1991;10(4):325–7. [PubMed] [Google Scholar]
- 22.Biologic basis for interleukin-1 in disease. Dinarello C A. Blood. 1996;87(6):2095–147. [PubMed] [Google Scholar]
- 23.Elevated levels of shed type II IL-1 receptor in sepsis. Potential role for type II receptor in regulation of IL-1 responses. Giri J G, Wells J, Dower S K, McCall C E, Guzman R N, Slack J, Bird T A, Shanebeck K, Grabstein K H, Sims J E. Journal of immunology (Baltimore, Md. : 1950) 1994;153(12):5802–9. [PubMed] [Google Scholar]
- 24.The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Sandanger Øystein, Ranheim Trine, Vinge Leif Erik, Bliksøen Marte, Alfsnes Katrine, Finsen Alexandra V, Dahl Christen P, Askevold Erik T, Florholmen Geir, Christensen Geir, Fitzgerald Katherine A, Lien Egil, Valen Guro, Espevik Terje, Aukrust Pål, Yndestad Arne. Cardiovascular research. 2013;99(1):164–74. doi: 10.1093/cvr/cvt091. [DOI] [PubMed] [Google Scholar]
- 25.The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Mezzaroma Eleonora, Toldo Stefano, Farkas Daniela, Seropian Ignacio M, Van Tassell Benjamin W, Salloum Fadi N, Kannan Harsha R, Menna Angela C, Voelkel Norbert F, Abbate Antonio. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(49):19725–30. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Kawaguchi Masanori, Takahashi Masafumi, Hata Takeki, Kashima Yuichiro, Usui Fumitake, Morimoto Hajime, Izawa Atsushi, Takahashi Yasuko, Masumoto Junya, Koyama Jun, Hongo Minoru, Noda Tetsuo, Nakayama Jun, Sagara Junji, Taniguchi Shun'ichiro, Ikeda Uichi. Circulation. 2011;123(6):594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 27.Increased cardiac IL-18 mRNA, pro-IL-18 and plasma IL-18 after myocardial infarction in the mouse; a potential role in cardiac dysfunction. Woldbaek Per Reidar, Tønnessen Theis, Henriksen Unni Lie, Florholmen Geir, Lunde Per Kristian, Lyberg Torstein, Christensen Geir. Cardiovascular research. 2003;59(1):122–31. doi: 10.1016/s0008-6363(03)00339-0. [DOI] [PubMed] [Google Scholar]
- 28.Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Toldo Stefano, Mezzaroma Eleonora, O'Brien Laura, Marchetti Carlo, Seropian Ignacio M, Voelkel Norbert F, Van Tassell Benjamin W, Dinarello Charles A, Abbate Antonio. American journal of physiology. Heart and circulatory physiology. 2014;306(7):H1025–31. doi: 10.1152/ajpheart.00795.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Increased plasma concentrations of interleukin-18 in acute coronary syndromes. Mallat Z, Henry P, Fressonnet R, Alouani S, Scoazec A, Beaufils P, Chvatchko Y, Tedgui A. Heart (British Cardiac Society) 2002;88(5):467–9. doi: 10.1136/heart.88.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. Sanada Shoji, Hakuno Daihiko, Higgins Luke J, Schreiter Eric R, McKenzie Andrew N J, Lee Richard T. The Journal of clinical investigation. 2007;117(6):1538–49. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Seki Kenjiro, Sanada Shoji, Kudinova Anastacia Y, Steinhauser Matthew L, Handa Vandna, Gannon Joseph, Lee Richard T. Circulation. Heart failure. 2009;2(6):684–91. doi: 10.1161/CIRCHEARTFAILURE.109.873240. [DOI] [PubMed] [Google Scholar]
- 32.Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Sabatine Marc S, Morrow David A, Higgins Luke J, MacGillivray Catherine, Guo Wei, Bode Christophe, Rifai Nader, Cannon Christopher P, Gerszten Robert E, Lee Richard T. Circulation. 2008;117(15):1936–44. doi: 10.1161/CIRCULATIONAHA.107.728022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Immunolocalization of interleukin-1 receptor antagonist in healthy and infarcted myocardium. Bonetti A, Marchini M, Ortolani F. Histology and histopathology. 2008;23(9):1093–102. doi: 10.14670/HH-23.1093. [DOI] [PubMed] [Google Scholar]
- 34.Cytokines in acute myocardial infarction: selective increase in circulating tumor necrosis factor, its soluble receptor, and interleukin-1 receptor antagonist. Latini R, Bianchi M, Correale E, Dinarello C A, Fantuzzi G, Fresco C, Maggioni A P, Mengozzi M, Romano S, Shapiro L. Journal of cardiovascular pharmacology. 1994;23(1):1–6. [PubMed] [Google Scholar]
- 35.Early interleukin-1 receptor antagonist elevation in patients with acute myocardial infarction. Patti Giuseppe, D'Ambrosio Andrea, Mega Simona, Giorgi Gabriele, Zardi Enrico Maria, Zardi Domenico Maria, Dicuonzo Giordano, Dobrina Aldo, Di Sciascio Germano. Journal of the American College of Cardiology. 2004;43(1):35–8. doi: 10.1016/j.jacc.2003.07.032. [DOI] [PubMed] [Google Scholar]
- 36.Interleukin-1 receptor antagonist levels correlate with extent of myocardial loss in patients with acute myocardial infarction. Patti Giuseppe, Mega Simona, Pasceri Vincenzo, Nusca Annunziata, Giorgi Gabriele, Zardi Enrico Maria, D'Ambrosio Andrea, Dobrina Aldo, Di Sciascio Germano. Clinical cardiology. 2005;28(4):193–6. doi: 10.1002/clc.4960280409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elevated plasma levels of interleukin-1 receptor antagonist and interleukin-10 in patients with acute myocardial infarction. Shibata M, Endo S, Inada K, Kuriki S, Harada M, Takino T, Sato N, Arakawa N, Suzuki T, Aoki H, Suzuki T, Hiramori K. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 1997;17(3):145–50. doi: 10.1089/jir.1997.17.145. [DOI] [PubMed] [Google Scholar]
- 38.The role of IL-1 in the pathogenesis of heart disease. Bujak Marcin, Frangogiannis Nikolaos G. Archivum immunologiae et therapiae experimentalis. 2009;57(3):165–76. doi: 10.1007/s00005-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The immune system and the remodeling infarcted heart: cell biological insights and therapeutic opportunities. Frangogiannis Nikolaos G. Journal of cardiovascular pharmacology. 2014;63(3):185–95. doi: 10.1097/FJC.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Interleukin-18 as a therapeutic target in acute myocardial infarction and heart failure. O'Brien Laura C, Mezzaroma Eleonora, Van Tassell Benjamin W, Marchetti Carlo, Carbone Salvatore, Abbate Antonio, Toldo Stefano. Molecular medicine (Cambridge, Mass.) 2014;20:221–9. doi: 10.2119/molmed.2014.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modulation of cytokine-induced cardiac myocyte apoptosis by nitric oxide, Bak, and Bcl-x. Ing D J, Zang J, Dzau V J, Webster K A, Bishopric N H. Circulation research. 1999;84(1):21–33. doi: 10.1161/01.res.84.1.21. [DOI] [PubMed] [Google Scholar]
- 42.Overexpression of interleukin-1 receptor antagonist provides cardioprotection against ischemia-reperfusion injury associated with reduction in apoptosis. Suzuki K, Murtuza B, Smolenski R T, Sammut I A, Suzuki N, Kaneda Y, Yacoub M H. Circulation. 2001;104(12 Suppl 1):I308–I3. doi: 10.1161/hc37t1.094871. [DOI] [PubMed] [Google Scholar]
- 43.Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Abbate Antonio, Salloum Fadi N, Vecile Elena, Das Anindita, Hoke Nicholas N, Straino Stefania, Biondi-Zoccai Giuseppe G L, Houser Jon-Erik, Qureshi Ian Z, Ownby Evan D, Gustini Edoardo, Biasucci Luigi M, Severino Anna, Capogrossi Maurizio C, Vetrovec George W, Crea Filippo, Baldi Alfonso, Kukreja Rakesh C, Dobrina Aldo. Circulation. 2008;117(20):2670–83. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 44.Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Bujak Marcin, Dobaczewski Marcin, Chatila Khaled, Mendoza Leonardo H, Li Na, Reddy Anilkumar, Frangogiannis Nikolaos G. The American journal of pathology. 2008;173(1):57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. Colotta F, Borré A, Wang J M, Tattanelli M, Maddalena F, Polentarutti N, Peri G, Mantovani A. Journal of immunology (Baltimore, Md. : 1950) 1992;148(3):760–5. [PubMed] [Google Scholar]
- 46.Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Blood. 1992;80(8):2012–20. [PubMed] [Google Scholar]
- 47.Fibroblasts in myocardial infarction: a role in inflammation and repair. Shinde Arti V, Frangogiannis Nikolaos G. Journal of molecular and cellular cardiology. 2014;70:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Interleukin-1alpha stimulates proinflammatory cytokine expression in human cardiac myofibroblasts. Turner Neil A, Das Anupam, Warburton Philip, O'Regan David J, Ball Stephen G, Porter Karen E. American journal of physiology. Heart and circulatory physiology. 2009;297(3):H1117–27. doi: 10.1152/ajpheart.00372.2009. [DOI] [PubMed] [Google Scholar]
- 49.Modulatory effect of interleukin-1α on expression of structural matrix proteins, MMPs and TIMPs in human cardiac myofibroblasts: role of p38 MAP kinase. Turner Neil A, Warburton Philip, O'Regan David J, Ball Stephen G, Porter Karen E. Matrix biology : journal of the International Society for Matrix Biology. 2010;29(7):613–20. doi: 10.1016/j.matbio.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Interleukin-1 and Tumor Necrosis Factor- Decrease Collagen Synthesis and Increase Matrix Metalloproteinase Activity in Cardiac Fibroblasts In Vitro. Siwik D. A., Chang D. L.-F., Colucci W. S. Circulation Research. 2000;86(12):1259-1265. doi: 10.1161/01.res.86.12.1259. [DOI] [PubMed] [Google Scholar]
- 51.Transplantation of skeletal myoblasts secreting an IL-1 inhibitor modulates adverse remodeling in infarcted murine myocardium. Murtuza B., Suzuki K., Bou-Gharios G., Beauchamp J. R., Smolenski R. T., Partridge T. A., Yacoub M. H. Proceedings of the National Academy of Sciences. 2004;101(12):4216-4221. doi: 10.1073/pnas.0306205101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.ECM remodeling in hypertensive heart disease. Berk Bradford C., Fujiwara Keigi, Lehoux Stephanie. Journal of Clinical Investigation. 2007;117(3):568-575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.The pathogenesis of cardiac fibrosis. Kong Ping, Christia Panagiota, Frangogiannis Nikolaos G. Cellular and Molecular Life Sciences. 2013;71(4):549-574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.IL-10 Is Induced in the Reperfused Myocardium and May Modulate the Reaction to Injury. Frangogiannis N. G., Mendoza L. H., Lindsey M. L., Ballantyne C. M., Michael L. H., Smith C. W., Entman M. L. The Journal of Immunology. 2000;165(5):2798-2808. doi: 10.4049/jimmunol.165.5.2798. [DOI] [PubMed] [Google Scholar]
- 55.Transforming growth factor (TGF)-β signaling in cardiac remodeling. Dobaczewski Marcin, Chen Wei, Frangogiannis Nikolaos G. Journal of Molecular and Cellular Cardiology. 2011;51(4):600-606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Interleukin-10 is not a critical regulator of infarct healing and left ventricular remodeling. ZYMEK P, NAH D, BUJAK M, REN G, KOERTING A, LEUCKER T, HUEBENER P, TAFFET G, ENTMAN M, FRANGOGIANNIS N. Cardiovascular Research. 2007;74(2):313-322. doi: 10.1016/j.cardiores.2006.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MFGE8 inhibits inflammasome-induced IL-1β production and limits postischemic cerebral injury. Deroide Nicolas, Li Xuan, Lerouet Dominique, Van Vré Emily, Baker Lauren, Harrison James, Poittevin Marine, Masters Leanne, Nih Lina, Margaill Isabelle, Iwakura Yoichiro, Ryffel Bernhard, Pocard Marc, Tedgui Alain, Kubis Nathalie, Mallat Ziad. The Journal of clinical investigation. 2013;123(3):1176–81. doi: 10.1172/JCI65167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Endogenous IRAK-M attenuates postinfarction remodeling through effects on macrophages and fibroblasts. Chen Wei, Saxena Amit, Li Na, Sun Jinyu, Gupta Amit, Lee Dong-Wook, Tian Qi, Dobaczewski Marcin, Frangogiannis Nikolaos G. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(11):2598–608. doi: 10.1161/ATVBAHA.112.300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. Nahrendorf Matthias, Swirski Filip K, Aikawa Elena, Stangenberg Lars, Wurdinger Thomas, Figueiredo Jose-Luiz, Libby Peter, Weissleder Ralph, Pittet Mikael J. The Journal of experimental medicine. 2007;204(12):3037–47. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Weirather Johannes, Hofmann Ulrich D W, Beyersdorf Niklas, Ramos Gustavo C, Vogel Benjamin, Frey Anna, Ertl Georg, Kerkau Thomas, Frantz Stefan. Circulation research. 2014;115(1):55–67. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 61.Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Saxena Amit, Dobaczewski Marcin, Rai Vikrant, Haque Zaffar, Chen Wei, Li Na, Frangogiannis Nikolaos G. American journal of physiology. Heart and circulatory physiology. 2014;307(8):H1233–42. doi: 10.1152/ajpheart.00328.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Targeted anticytokine therapy and the failing heart. Mann Douglas L. The American journal of cardiology. 2005;95(11A):9C–16C; discussion 38C. doi: 10.1016/j.amjcard.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 63.The cytoprotective effects of tumor necrosis factor are conveyed through tumor necrosis factor receptor-associated factor 2 in the heart. Burchfield Jana S, Dong Jian-Wen, Sakata Yasushi, Gao Feng, Tzeng Huei-Ping, Topkara Veli K, Entman Mark L, Sivasubramanian Natarajan, Mann Douglas L. Circulation. Heart failure. 2010;3(1):157–64. doi: 10.1161/CIRCHEARTFAILURE.109.899732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. Haudek Sandra B, Taffet George E, Schneider Michael D, Mann Douglas L. The Journal of clinical investigation. 2007;117(9):2692–701. doi: 10.1172/JCI29134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neutralization of interleukin-1beta in the acute phase of myocardial infarction promotes the progression of left ventricular remodeling. Hwang M W, Matsumori A, Furukawa Y, Ono K, Okada M, Iwasaki A, Hara M, Miyamoto T, Touma M, Sasayama S. Journal of the American College of Cardiology. 2001;38(5):1546–53. doi: 10.1016/s0735-1097(01)01591-1. [DOI] [PubMed] [Google Scholar]
- 66.Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Ridker Paul M, Thuren Tom, Zalewski Andrew, Libby Peter. American heart journal. 2011;162(4):597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 67.Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Abbate Antonio, Kontos Michael C, Grizzard John D, Biondi-Zoccai Giuseppe G L, Van Tassell Benjamin W, Robati Roshanak, Roach Lenore M, Arena Ross A, Roberts Charlotte S, Varma Amit, Gelwix Christopher C, Salloum Fadi N, Hastillo Andrea, Dinarello Charles A, Vetrovec George W. The American journal of cardiology. 2010;105(10):1371–1377.e1. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 68.Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Abbate Antonio, Van Tassell Benjamin Wallace, Biondi-Zoccai Giuseppe, Kontos Michael Christopher, Grizzard John Dallas, Spillman Debra Whittaker, Oddi Claudia, Roberts Charlotte Susan, Melchior Ryan David, Mueller George Herman, Abouzaki Nayef Antar, Rengel Lenore Rosemary, Varma Amit, Gambill Michael Lucas, Falcao Raquel Appa, Voelkel Norbert Felix, Dinarello Charles Anthony, Vetrovec George Wayne. The American journal of cardiology. 2013;111(10):1394–400. doi: 10.1016/j.amjcard.2013.01.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Abbate Antonio, Kontos Michael Christopher, Abouzaki Nayef Antar, Melchior Ryan David, Thomas Christopher, Van Tassell Benjamin Wallace, Oddi Claudia, Carbone Salvatore, Trankle Cory Ross, Roberts Charlotte Susan, Mueller George Herman, Gambill Michael Lucas, Christopher Sanah, Markley Roshanak, Vetrovec George Wayne, Dinarello Charles Anthony, Biondi-Zoccai Giuseppe. The American journal of cardiology. 2015;115(3):288–92. doi: 10.1016/j.amjcard.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 70.The prognostic value of monocyte chemoattractant protein-1/CCL2 in acute coronary syndromes. Frangogiannis Nikolaos G. Journal of the American College of Cardiology. 2007;50(22):2125–7. doi: 10.1016/j.jacc.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 71.Biomarkers: hopes and challenges in the path from discovery to clinical practice. Frangogiannis Nikolaos G. Translational research : the journal of laboratory and clinical medicine. 2012;159(4):197–204. doi: 10.1016/j.trsl.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury. Venkatachalam Kaliyamurthi, Prabhu Sumanth D, Reddy Venkatapuram Seenu, Boylston William H, Valente Anthony J, Chandrasekar Bysani. The Journal of biological chemistry. 2009;284(12):7853–65. doi: 10.1074/jbc.M808824200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.IL-18 binding protein-expressing mesenchymal stem cells improve myocardial protection after ischemia or infarction. Wang Meijing, Tan Jiangning, Wang Yue, Meldrum Kirstan K, Dinarello Charles A, Meldrum Daniel R. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(41):17499–504. doi: 10.1073/pnas.0908924106. [DOI] [PMC free article] [PubMed] [Google Scholar]