Abstract
Introduction
Prevention of mother-to-child HIV transmission (PMTCT) strategies include combined short-course antiretrovirals during pregnancy (Option A), triple-drug antiretroviral treament (ART) during pregnancy and breastfeeding (Option B), or lifelong ART (Option B+). The WHO also recommends ART for HIV treatment and prevention of sexual transmission of HIV. The impact of PMTCT strategies on prevention of sexual HIV transmission of HIV is not known. We estimated the population-level impact of PMTCT interventions on heterosexual HIV transmission in southwestern Uganda and KwaZulu-Natal, South Africa, two regions with different HIV prevalence and fertility rates.
Materials and Methods
We constructed and validated dynamic, stochastic, network-based HIV transmission models for each region. PMTCT Options A, B, and B+ were simulated over ten years under three scenarios: 1) current ART and PMTCT coverage, 2) current ART and high PMTCT coverage, and 3) high ART and PMTCT coverage. We compared adult HIV incidence after ten years of each intervention to Option A (and current ART) at current coverage.
Results
At current coverage, Options B and B+ reduced heterosexual HIV incidence by about 5% and 15%, respectively, in both countries. With current ART and high PMTCT coverage, Option B+ reduced HIV incidence by 35% in Uganda and 19% in South Africa, while Option B had smaller, but meaningful, reductions. The greatest reductions in HIV incidence were achieved with high ART and PMTCT coverage. In this scenario, all PMTCT strategies yielded similar results.
Discussion
Implementation of Options B/B+ reduces adult HIV incidence, with greater effect (relative to Option A at current levels) in Uganda than South Africa. These results are likely driven by Uganda’s higher fertility rates.
Introduction
The 2013 World Health Organization (WHO) guidelines to prevent mother-to-child transmission (PMTCT) of HIV recommend triple-drug antiretroviral treatment (ART) for all pregnant women for the duration of pregnancy and breastfeeding (Option B) or for life (Option B+) [1]. Previous guidelines recommended Option B or Option A, a short-course regimen of zidovudine (AZT) during pregnancy, single dose nevaripine (NVP) during labor, AZT plus lamivudine for seven days postpartum, and infant NVP during breastfeeding [2]. In all cases, pregnant women eligible for combination antiretroviral therapy (ART) by national treatment guidelines should receive lifelong ART for their own health [1,2].
Option B provides significant clinical and programmatic advantages over Option A because it utilizes a single regimen for both treatment and PMTCT [1]. Option B+ has the potential to simplify Option B by keeping pregnant women on treatment for life, rather than requiring repeated initiation and cessation of treatment for multiple pregnancies [3]. Although Option B+ may be more effective than Option B for PMTCT, the extent to which this is so will depend on factors such as fertility rates, breastfeeding duration, and retention on treatment, and current evidence remains inconclusive [1]. ART reduces the risk of sexual HIV transmission through viral suppression [4], hence, Options B and B+ also have the potential to decrease HIV incidence due to sexual transmission.
Prior work has shown that pregnancy is associated with increased genital HIV shedding [5–8], and an increased risk of sexual HIV transmission [aHR = 2.47; 95% CI 1.26,4.85] within HIV serodiscordant partnerships [9]. PMTCT interventions target women during this period of increased transmissibility, and hence may have a disproportionate impact on HIV incidence. Additionally, pregnant women are a target group that is already involved in care, and there is room to scale up coverage of PMTCT to expand the possible benefits of the treatment-as-prevention potential of PMTCT. However, little is known about the population-level effects of Options B or B+; a full assessment requires a detailed analysis of impact on sexual transmission of HIV. Two unanswered questions are: How do Options B and B+ impact adult HIV incidence, at different levels of coverage? Is there a substantial reduction in adult HIV incidence under Option B+, given that it may be simpler but costlier to implement? Previously, an ethnographic study in Tanzania showed that women preferred Option B due to its shorter treatment duration [10], and a clinical trial studying benefits of Option B+ is in progress in Malawi [11]. Two modeling studies on the benefits of WHO PMTCT guidelines focused exclusively on outcomes related to mother-to-child transmission [12,13], and one other considered sexual transmissions in addition to MTCT [14]. Option B+ was found to have the largest improvement for maternal and infant life expectancy in Zimbabwe [13], and was also the most cost-effective in a comparative analysis of Kenya, Zimbabwe, South Africa and Vietnam [14]. However, none of the prior modeling studies explicitly compared different PMTCT regimens at different coverage levels, or used partnership-level data and the sexual networks through which HIV infections transmit. While Gopalappa et al. [14] did consider adult HIV infections, they relied on an estimate of the number of infections averted per person-year on ART rather than modeling sexual transmission of HIV directly.
Thus, while some work on the effects of mother-to-child transmission has been undertaken, very little is known about the impact of PMTCT on adult HIV incidence, in particular the amount by which different PMTCT regimens may reduce horizontal transmission, and how these reductions vary with PMTCT coverage. We analyze the impact of PMTCT regimens exclusively on adult HIV incidence, and consider the impact on incidence at different coverage levels, while focusing explicitly on fertility rates as a determinant of this impact.
We hypothesized that Option B+ substantially decreases HIV incidence in adults due to sexual transmission compared to Option B, which decreases HIV incidence more than Option A, and that the magnitude of this decrease correlates with fertility rates. While it seems likely that Options B and B+ will each have a greater effect on HIV incidence, we have little intuition on how much greater the effect will be, especially when coverage levels and fertility rates are incorporated in the model. These hypotheses were tested in two sub-Saharan African regions: southwestern (SW) Uganda, with moderate HIV prevalence (about 10% [15]) and a high fertility rate (total fertility rate of 5.9 per woman [16]), and KwaZulu-Natal (KZN), South Africa, with a high HIV prevalence (about 25% [17]) and lower fertility rate (total fertility rate of 2.2 per woman [16]). Mathematical models were used to estimate the impact of Options A, B, and B+ on HIV incidence due to heterosexual transmission at different coverage levels in each settings. In scenario analyses, we assessed the impact of fertility rates on our results, and their sensitivity to ART initiation at a higher CD4 count.
Materials and Methods
Dynamic, stochastic, network-based models simulated HIV transmission among persons aged 18–55 years in SW Uganda and KZN, and were parameterized using demographic, biological, behavioral, and treatment data from those settings. Methods are summarized here and provided in full detail in the Supplementary Appendix. The models were derived from the exponential-family random graph modeling (ERGM) framework, and programmed using the Statnet [18] packages in the R programming language. In all models, one simulated timestep was defined to be 14 days.
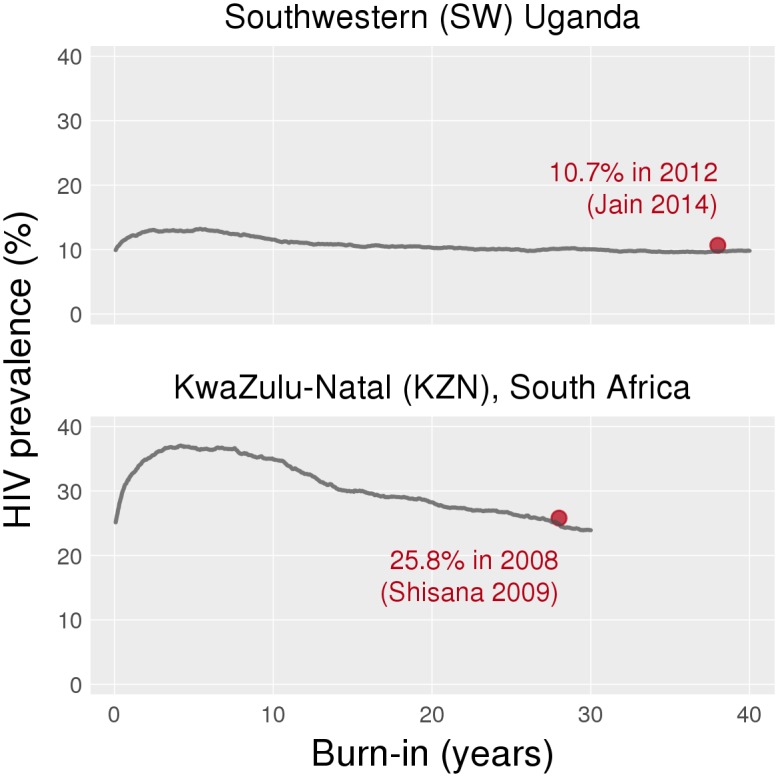
Baseline HIV epidemics were simulated to capture existing epidemic features in the two countries, including ART coverage levels and Option A for PMTCT. (For the rest of this paper, the term “ART” denotes combination antiretroviral treatment that is initiated not as part of a PMTCT regimen.) The baseline simulation ran for 40 years in SW Uganda and 30 years in KZN to achieve approximately stable HIV incidence and prevalence (Fig 1). These baseline models were created to capture the late-stage HIV epidemics in Uganda [19] and South Africa [20], and the current rates of new infections in both countries. While both countries show signs of declining incidence, it is important to remember that both ART and PMTCT have been scaled up in the recent past; calibration of our baseline models did not include ART or PMTCT scale-up, and we did not aim to model these trends. Our goal was to obtain reasonable epidemic outcomes (incidence [21,22] and prevalence [15,17]) that are consistent with estimates from other published studies, to serve as the starting point for modeling interventions. The baseline models should not be interpreted as a reconstruction of the historical trajectory of the epidemic.
Fig 1. Prevalence plots to produce baseline epidemics in Uganda and South Africa.
After the baseline phase, PMTCT interventions Options A, B, and B+ were simulated over ten years. Our baseline and intervention simulations only included heterosexual transmission; we did not include the impact of treatment on mother-to-child transmission (MTCT) in our models. Our primary question was to consider changes in heterosexual incidence rates over 10 years, and our modeled population included individuals between 18 and 55 years of age. Because the interventions were only modeled over 10 years, any children born during this time would not age enough to join the sexually active model population. Therefore, including transmission to newborn infants would not directly impact outcomes. However, we did include HIV prevalence at entry consistent with data for infection rates among 18-year old men and women (on average, about 3% for both Uganda [23] and South Africa [17]).
In Option A, HIV-infected pregnant women with CD4>350 cells/μl received AZT from the first antenatal visit until delivery [2]. In Option B, HIV-infected pregnant women with CD4>350 cells/μl received triple-drug PMTCT from the first antenatal visit until the conclusion of breast-feeding at 12 months postpartum [2]. In Options A and B, pregnant women with CD4≤350 cells/μl initiated lifelong ART. In Option B+, all HIV-infected pregnant women initiated lifelong ART regardless of CD4 count [1].
Each PMTCT intervention was simulated at three population coverage levels: “Current ART and PMTCT”, “High PMTCT” (with ART at Current levels), and “High ART and PMTCT” (Table 1). Current coverage included initiation at 22 weeks gestation, with 57% [23] of HIV-infected pregnant women accessing either PMTCT or ART (“uptake”) in Uganda and 89% [24] in South Africa. We assumed 75% adherence to PMTCT in this population [25]. Coverage was calculated as the product of uptake and adherence: 43% in SW Uganda and 67% in KZN. This method makes the conservative assumption that non-adherent individuals receive no benefit of treatment and have the same outcomes as untreated individuals.
Table 1. Coverage levels for the three scenarios that we modeled.
Parameter estimates for Southwestersn Uganda (SW Uganda) and KwaZulu-Natal (KZN) are separated by commas.
| Coverage Level | |||
|---|---|---|---|
| Current | High PMTCT | High ART and PMTCT | |
| PMTCT | |||
| PMTCT initiation (weeks) | 22, 22 | 14 [62], 14 [62] | Same as High PMTCT |
| PMTCT uptake (%) | 57 [23], 53 [24] | 90, 90 [assumption] | Same as High PMTCT |
| ART | |||
| CD4 count at ART initiation (cells/μl) | 131 [26], 100 [63] | 131 [26], 100 [26] | 350, 350 [assumption] |
| ART uptake (%) | 48 [64], 53 [64] | 48 [64], 53 [64] | 90, 90 [assumption] |
| Proportion of those on ART who are virally suppressed (%) | 88 [64], 85 [64] | 88 [64], 85 [64] | Same as Current |
Current coverage assumed ART initiation for males and non-pregnant females at a CD4 count of 131 cells/μl [26] at 48% uptake in Uganda, and 100 cells/μl [27] at 53% uptake in South Africa. Adherence was estimated by the proportion of individuals on treatment who were virally suppressed: 88% in SW Uganda and 85% in KZN [28]. As with PMTCT, ART coverage was the product of uptake and adherence: 43% in Uganda and 45% in South Africa. These coverage levels determine the proportion of HIV-infected individuals who will ever access ART in the baseline model.
We defined High PMTCT coverage as PMTCT initiation at 14 weeks gestation [1], with 90% uptake in both regions. Adherence remained at 75%. In KZN, the main change in this scenario was earlier initiation of PMTCT, because uptake was already close to 90%. In Uganda, the High PMTCT scenario included substantive changes to uptake and timing of PMTCT initiation.
Under High ART and PMTCT coverage, the High PMTCT settings were retained and ART uptake for men and non-pregnant women was set to 90% in both regions, with initiation at CD4≤350 cells/μl). ART adherence levels did not change [28]. All interventions are rolled out in the first year of the intervention at the specified levels.
Data
Our primary data source was a prospective study of home HIV testing and counseling (HTC) to improve testing and linkage to care [28]. Home HTC was offered to consenting adults (≥18 years) in defined geographic regions in the Mbarara district of SW Uganda, and Vulindlela district of KZN, South Africa. A total of 2,121 individuals in Uganda and 1,272 individuals in South Africa were tested. The study also included a pre-intervention community survey (n = 232 in SW Uganda and n = 268 in KZN) [28]. The study provided model parameters for sexual network characteristics, including the momentary (cross-sectional) distribution of the number of partnerships, start and end dates of the last three partnerships, mixing by age, and ART uptake and adherence. The sexual network data from the empirical study represented longer-term primary and casual partnerships. Due to limitations of this data, we did not model short-term or once-off sexual partnerships. Published sources were used to parameterize additional components of our models, as described in the Supplementary Appendix.
Components of Simulation
Each country was populated with 5,000 individuals at the start of the simulations. At each timestep, our simulations contained the following steps, with input parameters described in Table 2:
Table 2. Input parameters for our models.
| SW Uganda | KZN | References | |
|---|---|---|---|
| Behavior | |||
| Mean partnership duration (years) | 11.8 | 8.8* | [28] |
| Mean number of partnerships (per person) | 0.8 | 1.0 | [28] |
| Frequency of unprotected sex (per partnership per week) | 2.4 | 2.4 | [49] |
| Distribution of number of partnerships | Men: 0 (35%), 1 (52)%, 2 (10%), 3 (3); Women: 0 (22%), 1 (76%), 2 (2%) | Men: 0 (14%), 1 (66%), 2 (11%), 3 (5%), 4 (2.5%), 5 (2.5%); Women: 0 (24%), 1 (73%), 2 (2%), | [28] |
| Age mixing | Mixing matrix in appendix | Mixing matrix in appendix | [28] |
| Biology | |||
| Duration of: Acute stage Chronic stage Late stage | 135 days | [65] | |
| 1742 days | [66] | ||
| 1424 days | [67] | ||
| Level of: Peak viremia Viral set point Max. late stage viremia | 6.17 log | [68] | |
| 4.2 log | [68] | ||
| 5.05 log | [68] | ||
| Lifespan of untreated individuals | 3301 days | [67] | |
| Infection Transmission | |||
| Per act (chronic stage) | Specific to viral load (details in appendix) | [48] | |
| Mulitipliers for: | |||
| Acute stage | 4.98 | [49] | |
| Late stage | 3.49 | [49] | |
| HSV infection in either partner | 2.14*population prevalence (68%) | [48] | |
| Pregnancy of HIV-infected | 1.7 | [69] | |
| Pregnancy of HIV-uninfected | 2.5 | [69] | |
| Circumcision of uninfected man | 0.53 | [48] | |
| Combination ART | |||
| Change in CD4 | CD4 count recovers by 15 cells/μl every month until pre-infection level [40] or for 3 years [38], whichever is first | Inline | |
| Changes in viral load | Declines to 50 copies/ml in 4 months | [46] | |
| PMTCT | |||
| CD4 count during treatment | Option A: Increases 50 cells/μl from initiation to delivery; Option B: Increases 15 cells/μl every month until pre-infection level; Option B+: Increases 15 cells/μl every month until pre-infection level or 3 years, whichever is first | [40] | |
| CD4 count upon cessation of treatment | Option A: Declines to pre-treatment levels in 1 month [40]; Option B: Declines to pre-treatment levels in 2 months; Option B+[45]: NA | Inline | |
| Viral load during treatment | Option A: Declines 1.1 log from initiation to delivery [40]; Option B: Declines to 50 copies/ml in 4 months [45]; Option B+: Same as Option B [45] | Inline | |
| Viral load upon cessation of treatment | Option A: Increases to pre-treatment levels in 1 month [41]; Option B: Increases to pre-treatment levels in 2 months; Option B+: NA | Inline | |
| Pregnancy | |||
| Age-specific fertility rates (per 1000 person years)*** | See Table S4 in S1 Text | [35] | |
| Eligibility for pregnancy | Age between 15 and 49 years ≥15 months since onset of last pregnancy | ||
| HIV infection and pregnancy | HIV-infected women have a 47% lower age-specificfertility rate | [36] | |
* We increased the mean partnership duration by approximately 3 years to match incidence data.
** We decreased the proportion of women with 0 partners and increased the proportion of women with 1 partner by 20% to balance the total number of partnerships between men and women.
*** Annual fertility rates in UN Data increased by 15% to match empirical data on proportion of 18–49 year old pregnant women at any cross-section
Mortality: The model included age-specific mortality for uninfected individuals [29,30] and CD4-dependent mortality [31,32] for infected individuals. Additionally, individuals exited the model at age 55 when they were no longer a part of the population of interest.
Entry into population: Individuals entered the population at age 18, with entry rates selected to achieve a net national population growth rates of 3–4% per year in Uganda [33] and 1–2% per year in South Africa [34].
Formation and dissolution of partnerships: Only heterosexual partnerships were modeled. The process of partnership formation depended on the partners’ ages and the number of existing partnerships for each individual. The mean number of partnerships and momentary distribution of partnership numbers were parameterized from the Home HTC study [28]. All partnerships had an equal probability of dissolution per time step (i.e. lengths of partnerships were geometrically distributed), estimated using the mean duration of extant (ongoing) partnerships (Table 2).
Pregnancy: Women aged 18–49 years whose last pregnancy started ≥15 months ago were eligible to become pregnant. Pregnancy onset was modeled as a Bernoulli event with probabilities estimated from country- and age-specific fertility rates [35]. HIV-infected women were 47% less likely to become pregnant than HIV-uninfected women [36].
Update of CD4 count: All adults had a sex-specific uniform CD4 count until seroconversion (518 cells/μl in men and 570 cells/μl in women [37]). After seroconversion, CD4 counts of untreated individuals declined as a function of age and sex [37]. For individuals initiating ART or Option B+, CD4 counts increased for three years after initiation [38], or until they returned to pre-infection levels [39], whichever occurred first. Under Options A or B, CD4 counts increased for the duration of treatment [40], and declined to pre-treatment levels in one [41] or two [42] months after cessation of treatment, respectively.
Update of viral load: Viral load trajectories were modeled as curves [43,44] defined by: time to peak viremia; duration of acute, chronic, and late-stage infection; magnitude of peak viremia; set-point viral load; and maximum late-stage viral load. We assumed each part of this curve to be linear. For women receiving Option A, viral load declined by 1.1 log (base 10) copies/mL between treatment initiation and delivery [40]. For individuals receiving ART, or Options B or B+, viral load declined to 50 copies/mL over 4 months [45,46]. Viral load returned to pre-treatment levels in one and two months upon cessation of Options A [41] and B [47], respectively.
HIV transmission: The probability of HIV transmission per sex act in serodiscordant couples was a function of viral load of the infected partner [48], circumcision status of the uninfected male partner, and pregnancy status of the female partner, and was adjusted for population prevalence of herpes simplex virus type 2 (HSV-2). The probabilities of transmission per timestep were calculated using the binomial formula, assuming a constant frequency of unprotected heterosexual intercourse [49].
To obtain reasonable HIV prevalence and incidence as outputs in the model, in South Africa, we increased the mean partnership duration by approximately 3 years to match incidence data. In Uganda, to balance the number of partners reported by men and women, we decreased the proportion of women with 0 partners and increased the proportion of women with 1 partner by 20%. In both countries, annual fertility rates in UN Data were increased by 15% to match empirical data on proportion of 18–49 year old pregnant women at any cross-section.
Outcomes
The primary outcome was adult HIV incidence in the tenth year of implementation (not cumulative over the ten years), averaged over 10 model simulations. We computed 95% confidence intervals using a theoretical t-distribution, defined as , where X is the outcome of interest, n is the number of simulation runs, and σ is the standard deviation. These confidence intervals only capture variation across simulations, not variation due to changing parameter values.
A secondary outcome was the proportion of all HIV-infected individuals (including treated and untreated individuals in the denominator) who were virally suppressed (viral load ≤100 copies/mL) for all interventions and coverage levels (see Supplementary Appendix). To explore the relationship between PMTCT coverage, viral suppression, and HIV incidence, we calculated incidence rates and the proportion virally suppressed after ten years for Option B+ at Current ART coverage and PMTCT coverage of 20%, 40%, 60%, and 80%, with initation at 14 weeks gestation.
Scenario Analyses
To understand the relative impact of age-specific fertility rates (ASFRs) as a determinant of the impact of PMTCT on HIV incidence rates, the two countries’ ASFRs were switched and the change in incidence between Options A at Current coverage and Option B+ at High PMTCT (and Current ART) coverage was estimated.
In January 2014, Ugandan HIV treatment guidelines extended ART to all HIV-infected individuals with CD4≤500 cells/μl [50]. South Africa started to implement the same policy in January 2015 [51]. We estimated the effect of PMTCT interventions with this revised ART eligibility criterion for each country. We revised the mean CD4 count at initiation to 174 cells/μl in SW Uganda, and 168 cells/μl in KZN. ART coverage for SW Uganda did not change, and was revised to 51% of all HIV-infected individuals for KZN (see Supplementary Appendix for details). We estimated incidence rates for each region (with their original ASFRs) under Option A at Current coverage, and Option B+ at Current ART and High PMTCT coverage.
Results
For SW Uganda, the baseline model resulted in 10% HIV prevalence and an incidence of 1.00 per 100 person years (py) [95% CI: 0.94, 1.05]; these estimates compared well with observed data (Fig 1) [15,21]. Similarly, for KZN, model output for current HIV prevalence (25%) and incidence (2.52 per 100 py; [95% CI: 2.31, 2.73]) were consistent with observed estimates [17,22]. We compared incidence rates under different PMTCT interventions at different coverage levels to baseline estimates, where baseline reflects a continuation of Option A at Current coverage levels for 10 years. All mean incidence rates apply to the tenth year of intervention in units of new infections per 100 py, with the 95% CI in brackets.
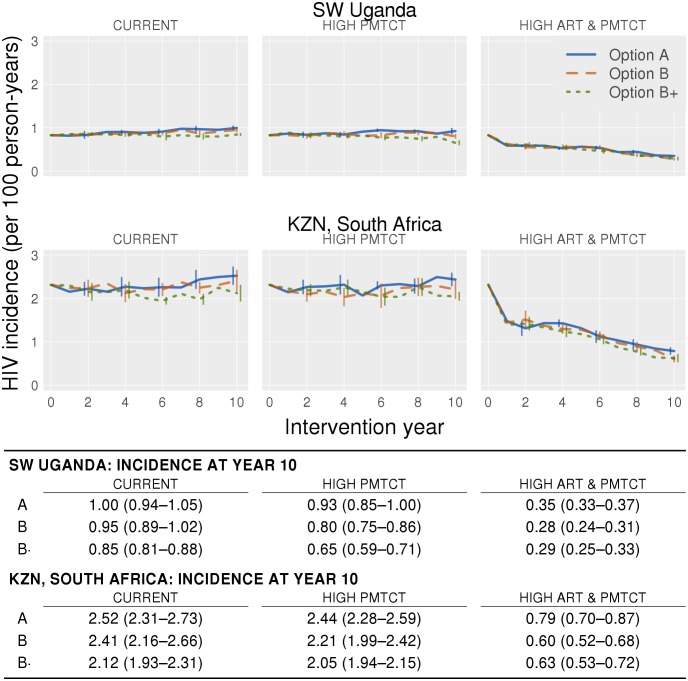
For SW Uganda, Options B and B+ at Current coverage produced mean incidence rates of 0.95 [0.89, 1.02] and 0.85 [0.81, 0.88] (Fig 2), corresponding to declines of 5% and 15% from baseline, respectively. With Options A, B, and B+, the High PMTCT intervention produced mean incidence rates of 0.93 [0.85, 1.00], 0.80 [0.75, 0.86] and 0.65 [0.59, 0.71], respectively, corresponding to declines of 7%, 20% and 35% relative to baseline. With the High ART and PMTCT intervention, Option A produced a mean incidence rate of 0.35 [0.33, 0.37] (mean decline of 65% relative to baseline), while Options B and B+ produced similar incidence rates of 0.28 [0.24, 0.31] and 0.29 [0.25, 0.33] (mean declines of 72% and 71%), respectively.
Fig 2. Annual incidence rates averaged over ten simulations for the ten-year intervention period in Uganda (top row) and South Africa (bottom row).
Each graph shows all three PMTCT interventions; the first, second and third columns represent Current, High PMTCT and High ART and PMTCT coverage respectively. The error bars show 95% confidence intervals. The table below shows the mean incidence rate in the tenth year of the intervention, 95% confidence intervals are in the parentheses.
In KZN, at Current coverage, Options B and B+ produced mean incidence rates of 2.41 [2.16, 2.66] and 2.12 [1.93, 2.31], corresponding to relative declines of 4% and 16% from baseline, respectively (Fig 2). At High PMTCT coverage, Options A, B, and B+ produced mean incidence rates of 2.44 [2.28, 2.59], 2.21 [1.99, 2.42], and 2.05 [1.94, 2.15]–declines of 3%, 12% and 19% respectively. At High ART and PMTCT coverage, Options A, B, and B+ produced mean incidence rates of 0.79 [0.70, 0.87], 0.60 [0.52, 0.68] and 0.63 [0.53, 0.72], corresponding to mean declines of 69%, 76%, and 75%, respectively.
As coverage for Option B+ increased from 20% to 80% with ART coverage held at Current levels, the proportion of all HIV-infected individuals who were virally suppressed increased from 31% to 47% in SW Uganda, and from 31% to 38% in KZN (Fig 3). For the same coverage levels, mean incidence in SW Uganda declined from 0.89 [0.85, 0.93] to 0.63 [0.59, 0.68] (mean decline of 29%), and in KZN from 2.45 [2.28, 2.62] to 1.95 [1.79, 2.12] (mean decline of 20%).
Fig 3. Mean HIV incidence rate in tenth year of Option B+ with High PMTCT coverage set at 20%, 40%, 60% and 80%.
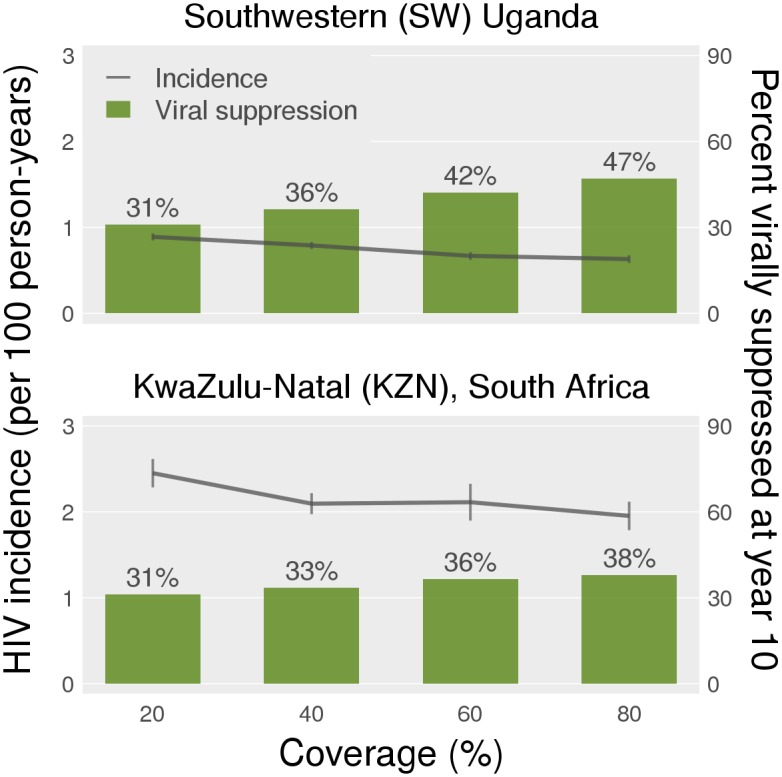
ART coverage is held constant at Current levels. On the right axis, we see the mean proportion of virally suppressed individuals (viral load < 100 counts/ml), at the end of the tenth year of intervention.
Scenario Analyses
In SW Uganda with South African ASFRs, mean HIV incidence was 0.99 [0.90, 1.08] in the tenth year under Option A at Current coverage, and 0.83 [0.73, 0.92] under Option B+ at High PMTCT coverage, a decline of 16%. In KZN with Ugandan ASFRs, mean incidence was 2.25 [2.06, 2.45] with Option A at Current coverage, and declined by 27% to 1.64 [1.45, 1.83] under Option B+ at High PMTCT coverage.
In SW Uganda, under revised ART eligibility criterion of CD4≤500 cells/μl, mean HIV incidence rates were 0.86 [0.77, 0.94] under Option A at Current coverage, and 0.63 [0.56, 0.69] under Option B+ at High PMTCT coverage, corresponding to a 27% decline. In KZN, under the revised criterion, mean incidence rates were 2.04 [1.89, 2.19] and 1.66 [1.51, 1.81] under the two interventions, respectively, corresponding to a 19% decline.
Discussion
In addition to improving the health of mothers and reducing infant HIV infections, expanded coverage of PMTCT has the potential to reduce population-level adult HIV incidence in both high and low fertility settings in sub-Saharan Africa. At current levels of ART and PMTCT coverage, changing from Option A to Options B or B+ could reduce HIV incidence by similar percentages in SW Uganda and KZN (approximately 5% and 15% for Option B and Option B+, respectively). In models with increased PMTCT coverage at maintained ART coverage (High PMTCT, Current ART model), Option B+ had a substantial impact in Uganda, reducing HIV incidence by 35%, while Option B reduced incidence by 20%. In KZN, where both HIV prevalence and PMTCT coverage are high, the incidence reductions attributable to Options B and B+ were not substantially changed by improving PMTCT coverage. As expected, the largest declines in incidence in both countries were associated with higher overall ART coverage in treatment programs among adults (High PMTCT, High ART model). In this scenario, the incremental benefit of both Options B and B+ were reduced. Our conclusions are consistent with prior modeling work on the population-level benefits of early ART initiation for HIV prevention [52–55].
Our secondary analyses suggest that the variation in the findings between the two regions is attributable to the proportion virally suppressed and to fertility rates. We found a direct relationship between Option B+ coverage, the proportion of virally suppressed HIV-infected individuals, and reduction in HIV incidence. However, as Option B+ coverage increased, there was a greater increase in the proportion of HIV-infected individuals achieving viral suppression in SW Uganda than KZN, likely because the higher fertility rates in SW Uganda allowed PMTCT interventions to cover more women, and/or provide coverage for a longer period. Option B+ had a reduced impact on population HIV incidence in SW Uganda when ASFRs were lowered to South African levels, and an increased impact in KZN when ASFRs were raised to Ugandan levels. The higher mean fertility rates are correlated with a) a greater proportion of of women who are pregnant and b) a lower age at first pregnancy. Both these factors increasing the coverage of Option B+, and thus magnify its effect.
With ART eligibility at CD4≤500 cells/μl the incremental benefits of PMTCT interventions were reduced in SW Uganda. In KZN, at all coverage levels, Options B and B+ had the same impact on adult HIV incidence under the new ART eligibility criteria as under the original criteria of CD4≤350 cells/μl. In Uganda, a moderate HIV prevalence country, higher CD4 at initiation of ART may offset the added benefit of PMTCT. In a high prevalence setting such as South Africa, however, the benefit of PMTCT is retained with expanded ART coverage. Note that if ART is initiated at CD4≤500 cells/μl, Option B+ still has a greater relative impact on HIV incidence in Uganda than in South Africa.
This study has several important limitations. Self-reported partnership data had to be adjusted to balance the partnership numbers reported by men and women, and social desirability may have limited reporting of multiple partners. We only modeled long-term partnerships and did not consider casual sexual contacts because of the available data. Not including subpopulations such as commercial sex workers may have led to an overestimation of the prevention potential of PMTCT. We also assumed that all partnerships were homogeneous and equally likely to dissolve; future empirical and modeling work may consider the typologies of these partnerships, and their dissolution rates. Some model parameters were based on older data; updated parameters could influence model conclusions. The model did not reflect the dependencies between unprotected sex and risk of pregnancy. However, it did capture increased risks of HIV acquisition and transmission during pregnancy. In addition, we did not take into account certain factors that could affect HIV transmission and ART effectiveness, such as migration and mobility patterns of individuals [56], dry sex [57], HIV subtypes [58], and drug resistance [59].
The model did not consider the process of hemodilution [49], in which CD4 counts in HIV-infected pregnant women are lower than in HIV-infected, non-pregnant women, after adjusting for time of infection. Pregnant women may thus become eligible for ART sooner, and the incidence reduction associated with PMTCT may be overestimated here. Also, a high proportion of individuals on ART achieved viral suppression according to our empirical data; the impact of Option B+ on HIV incidence might be lower in other settings. We computed ART coverage as a product of uptake and adherence, equivalent to assuming that individuals who do not adhere to treatment never initiate ART. In reality, partial treatments may have some population-level effects, which implies that the effects of interventions in our study may be underestimated. Conversely, although our parameterization of PMTCT adherence does combine estimates for antepartum and postpartum adherence, we did not model the two periods separately, or account for changes in adherence after cessation of breastfeeding for women on Option B+. Since recent studies report low adherence among women in this group, we may overestimate the true impact of Option B+ [60,61]. Finally, we did not account for program costs, or conduct a full cost-effectiveness analysis. As policymakers assess which strategies are most efficient, an explicit consideration of costs is essential. We hope that our models (which are publicly available) will provide the groundwork for future work in accounting for costs.
When choosing between PMTCT regimens and selecting coverage targets, policy-makers will take into account estimates from many empirical studies and mathematical models on the efficacy and cost-effectiveness of different regimens for preventing vertical transmission. Yet in addition to those considerations, there are biological and epidemiological reasons for strengthening PMTCT programs beyond the benefits to the mother and her infant: pregnant women are at increased risk of acquiring and transmitting HIV, and high coverage of antenatal care provides good opportunities to link them to ART. Due to resource constraints, countries may have to choose between implementing Option B+ or expanding coverage under Option B, and our model results may help inform those decisions. The size of the effect of each regimen depends on PMTCT coverage, fertility rates, and coverage of ART for treatment. The difference between Options B and B+ is minimal when ART coverage is high, and is more pronounced at lower coverage. Overall, increasing Option B coverage or implementing Option B+ at current coverage both have potential to reduce HIV incidence, especially in Uganda, where fertility is high and PMTCT coverage can be improved. In South Africa, larger reductions may be achieved by expanding ART coverage to all HIV-infected persons instead of implementing Option B+.
By considering our findings on the impact of PMTCT regimens on heterosexual transmission, in addition to evidence on vertical transmission, policy makers can thus derive more realistic estimates of each regimen’s impact on overall HIV incidence. We hope that this work will provide motivation to policymakers to consider PMTCT as not just a strategy to reduce new infections in infants, but also as a strategy to reduce adult HIV incidence.
Supporting Information
Blue, orange and green bars show Options A, B, and B+, respectively.
(TIFF)
(DOCX)
Acknowledgments
We thank Justin Brantley for support on figures and tables. Support for STR was through the University of Washington Graduate School and the ARCS Foundation Seattle Chapter Endowment Fund.
Data Availability
All code files are available at: https://github.com/khanna7/Development. Relevant citations for empirical data are provided in the paper.
Funding Statement
This work was supported by the NIH (RC4 AI092552, KL2 TR000421, P30 AI027757, R24 HD042828, R00 HD057533, R01 DA033875) and the HIV Modeling Consortium (RFA 3.2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization (2013) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Available: http://www.who.int/hiv/pub/guidelines/arv2013/en/. [PubMed]
- 2.World Health Organization (2010) Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: towards universal access. Available: http://www.who.int/hiv/pub/mtct/antiretroviral2010/en/. [PubMed]
- 3. Kieffer MP, Mattingly M, Giphart A, van de Ven R, Chouraya C, et al. (2014) Lessons Learned From Early Implementation of Option B+. JAIDS J Acquir Immune Defic Syndr 67: S188–S194. Available: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00126334-201412011-00004. 10.1097/QAI.0000000000000372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. (2011) Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365: 493–505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clemetson DB, Moss GB, Willerford DM, Hensel M, Emonyi W, et al. (1993) Detection of HIV DNA in cervical and vaginal secretions. Prevalence and correlates among women in Nairobi, Kenya. JAMA 269: 2860–2864. [PubMed] [Google Scholar]
- 6. Gardella B, Roccio M, Maccabruni A, Mariani B, Panzeri L, et al. (2011) HIV shedding in cervico-vaginal secretions in pregnant women. Curr HIV Res 9: 313–320. [DOI] [PubMed] [Google Scholar]
- 7. García-Bujalance S, Ruiz G, De Guevara CL, Peña JM, Bates I, et al. (2004) Quantitation of human immunodeficiency virus type 1 RNA loads in cervicovaginal secretions in pregnant women and relationship between viral loads in the genital tract and blood. Eur J Clin Microbiol Infect Dis 23: 111–115. 10.1007/s10096-003-1058-4 [DOI] [PubMed] [Google Scholar]
- 8. John GC, Nduati RW, Mbori-Ngacha DA, Richardson BA, Panteleeff D, et al. (2001) Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis 183: 206–212. 10.1086/317918 [DOI] [PubMed] [Google Scholar]
- 9. Mugo NR, Heffron R, Donnell D, Wald A, Were EO, et al. (2011) Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1 serodiscordant couples. AIDS 25: 1887–1895. 10.1097/QAD.0b013e32834a9338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ngarina M, Tarimo EAM, Naburi H, Kilewo C, Mwanyika-Sando M, et al. (2014) Women’s preferences regarding infant or maternal antiretroviral prophylaxis for prevention of mother-to-child transmission of HIV during breastfeeding and their views on Option B+ in Dar es Salaam, Tanzania. PLoS One 9: e85310 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3899007&tool=pmcentrez&rendertype=abstract. Accessed 19 December 2014. 10.1371/journal.pone.0085310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenberg NE, van Lettow M, Tweya H, Kapito-Tembo A, Bourdon CM, et al. (2014) Improving PMTCT uptake and retention services through novel approaches in peer-based family-supported care in the clinic and community: a 3-arm cluster randomized trial (PURE Malawi). J Acquir Immune Defic Syndr 67 Suppl 2: S114–S119. Available: http://www.ncbi.nlm.nih.gov/pubmed/25310116. Accessed 30 December 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ciaranello AL, Perez F, Maruva M, Chu J, Engelsmann B, et al. (2011) WHO 2010 guidelines for prevention of mother-to-child HIV transmission in Zimbabwe: modeling clinical outcomes in infants and mothers. PLoS One 6: e20224 10.1371/journal.pone.0020224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ciaranello AL, Perez F, Engelsmann B, Walensky RP, Mushavi A, et al. (2013) Cost-effectiveness of World Health Organization 2010 guidelines for prevention of mother-to-child HIV transmission in Zimbabwe. Clin Infect Dis 56: 430–446. 10.1093/cid/cis858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gopalappa C, Stover J, Shaffer N, Mahy M (2014) The costs and benefits of Option B+ for the prevention of mother-to-child transmission of HIV. AIDS 28 Suppl 1: 5–14. [DOI] [PubMed] [Google Scholar]
- 15. Jain V, Byonanebye DM, Liegler T, Kwarisiima D, Chamie G, et al. (2014) Changes in population HIV RNA levels in Mbarara, Uganda, during scale-up of HIV antiretroviral therapy access. J Acquir Immune Defic Syndr 65: 327–332. 10.1097/QAI.0000000000000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bureau USC (n.d.) International Database.
- 17. Shisana O., Rehle T, Simbayi LC, Z K., et al. (2008) South African national HIV prevalence, incidence, behaviour and communication survey 2008: A turning tide among teenager. Cape Town, South Africa: HSRC Press. [Google Scholar]
- 18.Handcock MS, Hunter DR, Butts CT, Goodreau SM, Morris M (2003) statnet: Software tools for the Statistical Modeling of Network Data. Available: http://statnetproject.org. [DOI] [PMC free article] [PubMed]
- 19.UNAIDS (n.d.) Uganda. Available: http://www.unaids.org/sites/default/files/epidocuments/UGA.pdf.
- 20.UNAIDS (n.d.) South Africa. Available: http://www.unaids.org/sites/default/files/epidocuments/ZAF.pdf.
- 21. Ruzagira E, Wandiembe S, Abaasa A, Levin J, Bwanika A, et al. (2011) Prevalence and incidence of HIV in a rural community-based HIV vaccine preparedness cohort in Masaka, Uganda. PLoS One 6: e20684 10.1371/journal.pone.0020684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML (2013) High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science (80-) 339: 966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uganda Ministry of Health and ICF International (n.d.) 2011 Uganda AIDS Indicator Survey: Key Findings.
- 24.Goga A, Dinh T, Jackson D, and for the SAPMTCTE study Group (2012) Evaluation of the effectiveness of the national prevention of mother-to-child transmission (PMTCT) programme on infant HIV measured at six weeks postpartum in South Africa. Available: http://www.doh.gov.za/docs/reports/2012/pmtcteffectiveness.pdf.
- 25. Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, et al. (2012) Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS 26: 2039–2052. 10.1097/QAD.0b013e328359590f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geng EH, Bwana MB, Muyindike W, Glidden D V, Bangsberg DR, et al. (2013) Failure to initiate antiretroviral therapy, loss to follow-up and mortality among HIV-infected patients during the pre-ART period in Uganda. J Acquir Immune Defic Syndr 63: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bassett I V, Giddy J, Wang B, Lu Z, Losina E, et al. (2008) Routine, voluntary HIV testing in Durban, South Africa: correlates of HIV infection. HIV Med 9: 863–867. 10.1111/j.1468-1293.2008.00635.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barnabas R V, van Rooyen H, Tumwesigye E, Murnane PM, Baeten JM, et al. (2014) Initiation of antiretroviral therapy and viral suppression after home HIV testing and counselling in KwaZulu-Natal, South Africa, and Mbarara district, Uganda: a prospective, observational intervention study. Lancet HIV 1: e68–e76. Available: http://linkinghub.elsevier.com/retrieve/pii/S2352301814700244. Accessed 3 January 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uganda Bureau of Statistics and ICF International (n.d.) Uganda Demographic and Health Survey 2011. Available: http://www.ubos.org/onlinefiles/uploads/ubos/UDHS/UDHS2011.pdf.
- 30.Anderson B, Phillips H (n.d.) The Changing Pattern of Adult Mortality in South Africa, 1997–2005: HIV and Other Sources. Available: http://www.psc.isr.umich.edu/pubs/pdf/rr08-649.pdf.
- 31. Fielding K, Koba A, Grant AD, Charalambous S, Day J, et al. (2011) Cytomegalovirus viremia as a risk factor for mortality prior to antiretroviral therapy among HIV-infected gold miners in South Africa. PLoS One 6: e25571 10.1371/journal.pone.0025571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pantazis N, Morrison C, Amornkul PN, Lewden C, Salata RA, et al. (2012) Differences in HIV natural history among African and non-African seroconverters in Europe and seroconverters in sub-Saharan Africa. PLoS One 7: e32369 10.1371/journal.pone.0032369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.United States Census Bureau (n.d.) International Database. Available: http://www.census.gov/population/international/data/idb/informationGateway.php.
- 34.Statistics South Africa (n.d.). Available: http://beta2.statssa.gov.za/.
- 35.United Nations Statistics Division (n.d.) UN Data. A World of Information. Available: http://data.un.org/.
- 36. Ross A, der Paal L, Lubega R, Mayanja BN, Shafer LA, et al. (2004) HIV-1 disease progression and fertility: the incidence of recognized pregnancy and pregnancy outcome in Uganda. AIDS 18: 799–804. [DOI] [PubMed] [Google Scholar]
- 37. Pantazis N, Morrison C, Amornkul PN, Lewden C, Salata RA, et al. (2012) Differences in HIV natural history among African and non-African seroconverters in Europe and seroconverters in sub-Saharan Africa. PLoS One 7: e32369 10.1371/journal.pone.0032369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bussmann H, Wester CW, Ndwapi N, Grundmann N, Gaolathe T, et al. (2008) Five-year outcomes of initial patients treated in Botswana’s National Antiretroviral Treatment Program. AIDS 22: 2303–2311. 10.1097/QAD.0b013e3283129db0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fairall LR, Bachmann MO, Louwagie GM, van Vuuren C, Chikobvu P, et al. (2008) Effectiveness of antiretroviral treatment in a South African program: a cohort study. Arch Intern Med 168: 86–93. 10.1001/archinternmed.2007.10 [DOI] [PubMed] [Google Scholar]
- 40. Dioulasso B, Faso B, Meda N, Fao P, Ky-Zerbo O, et al. (2012) Maternal HIV-1 disease progression 18–24 months postdelivery according to antiretroviral prophylaxis regimen (triple-antiretroviral prophylaxis during pregnancy and breastfeeding vs zidovudine/single-dose nevirapine prophylaxis): The Kesho Bora randomized. Clin Infect Dis 55: 449–460. 10.1093/cid/cis461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chung MH, Kiarie JN, Richardson BA, Lehman DA, Overbaugh J, et al. (2008) Highly active antiretroviral therapy versus zidovudine/nevirapine effects on early breast milk HIV type-1 Rna: a phase II randomized clinical trial. Antivir Ther (Lond) 13: 799–807. [PMC free article] [PubMed] [Google Scholar]
- 42. Danel C, Moh R, Chaix ML, Gabillard D, Gnokoro J, et al. (2009) Two-months-off, four-months-on antiretroviral regimen increases the risk of resistance, compared with continuous therapy: a randomized trial involving West African adults. J Infect Dis 199: 66–76. 10.1086/595298 [DOI] [PubMed] [Google Scholar]
- 43. Goodreau SM, Carnegie NB, Vittinghoff E, Lama JR, Sanchez J, et al. (2012) What Drives the US and Peruvian HIV Epidemics in Men Who Have Sex with Men (MSM)? PLoS One 7: e50522 10.1371/journal.pone.0050522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khanna AS, Goodreau SM, Gorbach PM, Daar E, Little SJ (2014) Modeling the Impact of Post-Diagnosis Behavior Change on HIV Prevalence in Southern California Men Who Have Sex with Men (MSM). AIDS Behav 18: 1523–1531. 10.1007/s10461-013-0646-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rizzardi GP, De Boer RJ, Hoover S, Tambussi G, Chapuis A, et al. (2000) Predicting the duration of antiviral treatment needed to suppress plasma HIV-1 RNA. J Clin Invest 105: 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hammer SM, Eron JJ, Reiss P, Schooley RT, Thompson MA, et al. (2008) Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA 300: 555–570. 10.1001/jama.300.5.555 [DOI] [PubMed] [Google Scholar]
- 47. El-Sadr WM, Grund B, Neuhaus J, Babiker A, Cohen CJ, et al. (2008) Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy: a randomized trial. Ann Intern Med 149: 289–299. [DOI] [PubMed] [Google Scholar]
- 48. Hughes JP, Baeten JM, Lingappa JR, Magaret AS, Wald A, et al. (2012) Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis 205: 358–365. 10.1093/infdis/jir747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li XB, et al. (2005) Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis 191: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 50.Republic of Uganda. Ministry of Health (2013) ADDENDUM TO THE NATIONAL ANTIRETROVIRAL TREATMENT GUIDELINES. Available: http://preventcrypto.org/wp-content/uploads/2012/07/Uganda-National-ART-Guidelines_2014.pdf.
- 51.Republic of South Africa. Department of Health (2014) National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Available: http://www.health.gov.za/docs/Policies/2014/HIV_Guidelines_Jan2015-final_edits-YP.pdf.
- 52. Eaton JW, Menzies NA, Stover J, Cambiano V, Chindelevitch L, et al. (2014) Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: A combined analysis of 12 mathematical models. Lancet Glob Heal 2. [DOI] [PubMed] [Google Scholar]
- 53. Martin NK, Devine A, Eaton JW, Miners A, Hallett TB, et al. (2014) Modeling the impact of early antiretroviral therapy for adults coinfected with HIV and hepatitis B or C in South Africa. AIDS 28 Suppl 1: S35–S46. Available: http://www.ncbi.nlm.nih.gov/pubmed/24468945. 10.1097/QAD.0000000000000084 [DOI] [PubMed] [Google Scholar]
- 54. Mountain E, Pickles M, Mishra S, Vickerman P, Alary M, et al. (2014) The HIV care cascade and antiretroviral therapy in female sex workers: implications for HIV prevention. Expert Rev Anti Infect Ther 12: 1203–1219. Available: 10.1586/14787210.2014.948422 [DOI] [PubMed] [Google Scholar]
- 55. Eaton JW, Johnson LF, Salomon JA, Barnighausen T, Bendavid E, et al. (2012) HIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa. PLoS Med 9: e1001245 10.1371/journal.pmed.1001245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lurie MN, Williams BG (2014) Migration and Health in Southern Africa: 100 years and still circulating. Heal Psychol Behav Med 2: 34–40. Available: http://www.ncbi.nlm.nih.gov/pubmed/24653964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mbikusita-Lewanika M, Stephen H, Thomas J (2009) The prevalence of the use of “dry sex” traditional medicines, among Zambian women, and the profile of the users. Psychol Health Med 14: 227–238. 10.1080/13548500802270364 [DOI] [PubMed] [Google Scholar]
- 58. Walter BL, Armitage AE, Graham SC, de Oliveira T, Skinhøj P, et al. (2009) Functional characteristics of HIV-1 subtype C compatible with increased heterosexual transmissibility. AIDS 23: 1047–1057. 10.1097/QAD.0b013e32832a1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Celum C, Hallett TB, Baeten JM (2013) HIV-1 prevention with ART and PrEP: Mathematical modeling insights into resistance, effectiveness, and public health impact. J Infect Dis 208: 189–191. 10.1093/infdis/jit154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tenthani L, Haas AD, Tweya H, Jahn A, van Oosterhout JJ, et al. (2014) Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+') in Malawi. AIDS 28: 589–598. Available: http://www.ncbi.nlm.nih.gov/pubmed/24468999. 10.1097/QAD.0000000000000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim MH, Ahmed S, Hosseinipour MC, Giordano TP, Chiao EY, et al. (2015) Implementation and Operational Research: the impact of option B+ on the antenatal PMTCT cascade in Lilongwe, Malawi. JAIDS J Acquir Immune Defic Syndr 68: e77–e83. Available: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00126334-201504150-00019. 10.1097/QAI.0000000000000517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.World Health Organization (2012) Programmatic Update: Use of Antiretroviral Drugs in Treating Pregnant Women and Preventing HIV Infection in Infants. [PubMed]
- 63. Bassett I V, Giddy J, Nkera J, Wang B, Losina E, et al. (2007) Routine voluntary HIV testing in Durban, South Africa: the experience from an outpatient department. J Acquir Immune Defic Syndr 46: 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barnabas R, Van Rooyen H, Tumwesigye E, Krows M, Murnane P, et al. (2014) Community HIV testing and linkage to care reduces population viral load in South Africa and Uganda. Conference On Retroviruses And Opportunistic Infections, Boston, USA (Abstract 148).
- 65. Ribeiro RM, Qin L, Chavez LL, Li D, Self SG, et al. (2010) Estimation of the initial viral growth rate and basic reproductive number during acute HIV-1 infection. J Virol 84: 6096–6102. 10.1128/JVI.00127-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Morrison CS, Demers K, Kwok C, Bulime S, Rinaldi A, et al. (2010) Plasma and cervical viral loads among Ugandan and Zimbabwean women during acute and early HIV-1 infection. AIDS 24: 573–582. 10.1097/QAD.0b013e32833433df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Van der Paal L, Shafer LA, Todd J, Mayanja BN, Whitworth JA, et al. (2007) HIV-1 disease progression and mortality before the introduction of highly active antiretroviral therapy in rural Uganda. AIDS 21 Suppl 6: S21–S29. [DOI] [PubMed] [Google Scholar]
- 68. Pilcher CD, Price MA, Hoffman IF, Galvin S, Martinson FEA, et al. (2004) Frequent detection of acute primary HIV infection in men in Malawi. AIDS 18: 517–524. [DOI] [PubMed] [Google Scholar]
- 69. Mugo N, Heffron R, Donnell D (2011) Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1 serodiscordant couples. AIDS 25: 1887–1895. 10.1097/QAD.0b013e32834a9338 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Blue, orange and green bars show Options A, B, and B+, respectively.
(TIFF)
(DOCX)
Data Availability Statement
All code files are available at: https://github.com/khanna7/Development. Relevant citations for empirical data are provided in the paper.