Abstract
Background
A substantial fraction of all American healthcare expenditures are potentially wasted, and practices that are not evidence-based could contribute to such waste. We sought to characterize whether Prothrombin Time (PT) and activated Partial Thromboplastin Time (aPTT) tests of preoperative patients are used in a way unsupported by evidence and potentially wasteful.
Methods and Findings
We evaluated prospectively-collected patient data from 19 major teaching hospitals and 8 hospital-affiliated surgical centers in 7 states (Delaware, Florida, Maryland, Massachusetts, New Jersey, New York, Pennsylvania) and the District of Columbia. A total of 1,053,472 consecutive patients represented every patient admitted for elective surgery from 2009 to 2012 at all 27 settings. A subset of 682,049 patients (64.7%) had one or both tests done and history and physical (H&P) records available for analysis. Unnecessary tests for bleeding risk were defined as: PT tests done on patients with no history of abnormal bleeding, warfarin therapy, vitamin K-dependent clotting factor deficiency, or liver disease; or aPTT tests done on patients with no history of heparin treatment, hemophilia, lupus anticoagulant antibodies, or von Willebrand disease. We assessed the proportion of patients who received PT or aPTT tests who lacked evidence-based reasons for testing.
Conclusions
This study sought to bring the availability of big data together with applied comparative effectiveness research. Among preoperative patients, 26.2% received PT tests, and 94.3% of tests were unnecessary, given the absence of findings on H&P. Similarly, 23.3% of preoperative patients received aPTT tests, of which 99.9% were unnecessary. Among patients with no H&P findings suggestive of bleeding risk, 6.6% of PT tests and 7.1% of aPTT tests were either a false positive or a true positive (i.e. indicative of a previously-undiagnosed potential bleeding risk). Both PT and aPTT, designed as diagnostic tests, are apparently used as screening tests. Use of unnecessary screening tests raises concerns for the costs of such testing and the consequences of false positive results.
Introduction
Estimates suggest that 20% to 30% of total American healthcare expenditures may be unnecessary. [1–4] Over-diagnosis of disease has been described as a modern epidemic in high-income countries.[5] A comprehensive review of 146 medical practices found that 40% of those practices recommended when new were reversed upon more rigorous evaluation; some practices were unhelpful, and some were found to substantially increase patient costs without improving outcomes.[6]
Recently, there has been a focus on using objective evidence to combat over-diagnosis and over-treatment of disease.[7] This strategy is motivated by the need to contain medical costs as mandated by the Affordable Care Act; but, also derives from a sense that there are human as well as economic costs to consider when allocating treatment.[8]
Factors that potentially could contribute to higher medical costs include practices that have persisted in medicine and surgery without objective validation of their efficacy. One such practice may be ordering a panel of pre-operative tests that include a prothrombin time (PT) test and/or an activated partial thromboplastin time (aPTT) test prior to surgery to determine whether bleeding is a potential surgical risk.[9–11]
We hypothesize that if PT and aPTT tests are used correctly as diagnostic tests (rather than as screening tests), then there should be specific findings on the patient’s history and physical (H&P) chart to justify such tests. Further, these indications should be consistent with current guidelines as to when PT and aPTT tests should be ordered. Conversely, if PT and aPTT tests are used for screening, then specific findings in a patient’s H&P will not necessarily be present.[12–14] Therefore, we compared each patient’s PT and/or aPTT results with findings on that patient’s H&P to determine whether the tests had been used as diagnostic tests or as screening tests.
Materials and Methods
We utilized a web-based patient information warehouse and designed a tool for our comparative-effectiveness research. The warehouse is used by hospitals to manage information required for scheduled surgeries that originates with their affiliated surgeons. Our research tool provided us access to de-identified and aggregated patient information through appropriate de-identification provisions (e.g., via hospital service agreements). [15, 16] This research was reviewed and approved by the Rockefeller University Institutional Review Board (MCA-0669) on June 10, 2014.
De-identified pre-surgical patient data (H&Ps and lab reports), generated at 19 hospitals and 8 associated ambulatory surgery centers (Table 1) were aggregated. The time period covered was 48 months (from 2008 through 2012), with the exception of facilities 6, 16, 22, and 27 (data aggregated for 36 months) and facilities 9 and 24 (data aggregated for 46 months).
Table 1. H&P and Lab Data of Patients Who Underwent Surgery.
| Facility | Facility Location | Facility Type | Patients | H&Ps | % | Labs | % | H&Ps & Labs | % | Data Set | % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertiary HospitalsS | 1 | Delaware | Tertiary Hospital | 78,473 | 62,176 | 79% | 47,738 | 61% | 43,483 | 55% | 62,176 | 79% |
| 2 | New Jersey | Tertiary Hospital | 23,846 | 8,397 | 35% | 19,200 | 81% | 7,325 | 31% | 8,397 | 35% | |
| 3 | New Jersey | Tertiary Hospital | 40,439 | 27,780 | 69% | 20,792 | 51% | 18,579 | 46% | 27,780 | 69% | |
| 4 | Maryland | Tertiary Hospital | 62,300 | 21,639 | 35% | 22,360 | 36% | 13,660 | 22% | 21,639 | 35% | |
| 5 | New York | Tertiary Hospital | 46,562 | 37,201 | 80% | 36,680 | 79% | 33,444 | 72% | 37,201 | 80% | |
| 6 | Florida | Tertiary Hospital | 14,554 | 11,199 | 77% | 11,248 | 77% | 9,726 | 67% | 11,199 | 77% | |
| 7 | New Jersey | Tertiary Hospital | 41,733 | 30,161 | 72% | 20,570 | 49% | 17,163 | 41% | 30,161 | 72% | |
| 8 | New Jersey | Tertiary Hospital | 62,234 | 46,570 | 75% | 38,040 | 61% | 35,028 | 56% | 46,570 | 75% | |
| 9 | New York | Tertiary Hospital | 55,670 | 21,455 | 39% | 15,108 | 27% | 11,326 | 20% | 21,455 | 39% | |
| 10 | New Jersey | Tertiary Hospital | 29,355 | 19,960 | 68% | 19,840 | 68% | 15,752 | 54% | 19,960 | 68% | |
| 11 | Pennsylvania | Tertiary Hospital | 28,079 | 19,023 | 68% | 14,768 | 53% | 13,790 | 49% | 19,023 | 68% | |
| 12 | D. of Columbia | Tertiary Hospital | 57,865 | 35,278 | 61% | 16,877 | 29% | 15,080 | 26% | 35,278 | 61% | |
| 13 | Florida | Tertiary Hospital | 25,518 | 19,590 | 77% | 16,200 | 63% | 15,260 | 60% | 19,590 | 77% | |
| 14 | New York | Tertiary Hospital | 31,438 | 19,596 | 62% | 11,908 | 38% | 11,169 | 36% | 19,596 | 62% | |
| 15 | Delaware | Tertiary Hospital | 22,859 | 17,672 | 77% | 11,230 | 49% | 10,114 | 44% | 17,672 | 77% | |
| 16 | New York | Tertiary Hospital | 16,804 | 12,192 | 73% | 5,129 | 31% | 4,236 | 25% | 12,192 | 73% | |
| 17 | New York | Orthopedic Hospital | 113,646 | 72,806 | 64% | 30,833 | 27% | 24,531 | 22% | 72,806 | 64% | |
| 18 | New York | Eye and Ear Hospital | 42,311 | 33,359 | 79% | 27,955 | 66% | 27,229 | 64% | 33,359 | 79% | |
| 19 | Massachusetts | Eye and Ear Hospital | 59,407 | 53,525 | 90% | 31,094 | 52% | 30,857 | 52% | 53,525 | 90% | |
| Subtotal | 853,079 | 569,579 | 67% | 417,570 | 49% | 357,752 | 42% | 569,579 | 67% | |||
| AmbSurg Centers | 20 | Delaware | Hospital 1 Surgery Center | 37,414 | 28,732 | 77% | 15,497 | 41% | 14,302 | 38% | 28,732 | 77% |
| 21 | Maryland | Hospital 4 Surgery Center | 26,411 | 11,385 | 43% | 9,065 | 34% | 5,968 | 23% | 11,385 | 43% | |
| 22 | Florida | Hospital 6 Surgery Center | 9,737 | 2,539 | 26% | 2,549 | 26% | 1,850 | 19% | 2,539 | 26% | |
| 23 | New Jersey | Hospital 8 Surgery Center | 27,392 | 19,148 | 70% | 9,652 | 35% | 8,509 | 31% | 19,148 | 70% | |
| 24 | New York | Hospital 9 Surgery Center | 29,469 | 12,215 | 41% | 7,286 | 25% | 5,902 | 20% | 12,215 | 41% | |
| 25 | New Jersey | Hospital 10 Surgery Center | 18,247 | 10,123 | 55% | 7,719 | 42% | 6,324 | 35% | 10,123 | 55% | |
| 26 | Delaware | Hospital 15 Surgery Center | 21,001 | 16,377 | 78% | 8,870 | 42% | 8,337 | 40% | 16,377 | 78% | |
| 27 | New York | Hospital 16 Surgery Center | 30,708 | 11,951 | 39% | 3,777 | 12% | 3,054 | 10% | 11,951 | 39% | |
| Subtotal | 200,379 | 112,470 | 56% | 64,415 | 32% | 54,246 | 27% | 112,470 | 56% | |||
| Total | 1,053,472 | 682,049 | 65% | 481,985 | 46% | 411,998 | 39% | 682,049 | 65% | |||
The research tool accessed scheduling systems for elective surgery at each hospital. In-patient and emergency room patients who underwent surgeries were not included; such patient information resides on in-house hospital systems, not accessible to the tool. This approach yielded patient information on a consecutive sample of 1,053,472 patients, representing every patient admitted for elective surgery between 2009 and 2012 at all 27 settings, provided that these patients were scheduled, confirmed, and actually underwent surgery (Table A in S1 File).
Joint Commission requirements mandate that all hospitals have a recent H&P in place for every patient scheduled for surgery. Therefore, each hospital has an H&P for each patient either in the data warehouse (from its surgeons) or on their own EHR system[17] Lab tests are not required for surgery and may not be available on the research tool.
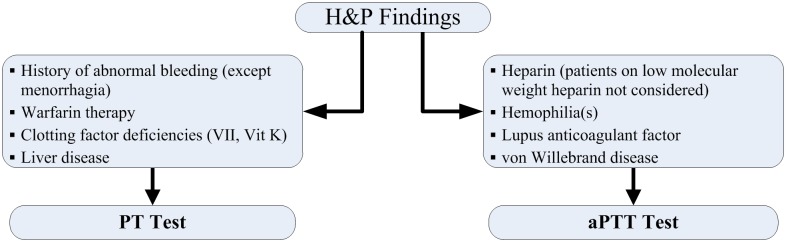
Patient H&P records were evaluated to determine whether there was justification for PT and aPTT testing. Unnecessary tests were defined as PT tests done on patients without a history of: 1) abnormal bleeding, 2) warfarin therapy, 3) vitamin K-dependent clotting factor deficiency, or 4) liver disease; or aPTT tests done on patients without a history of: 1) heparin use, 2) hemophilia, 3) antiphospholipid antibodies (lupus anticoagulant), or 4) von Willebrand disease (Fig 1).
Fig 1. H&P Findings That Prompt PT and aPTT Testing.
Results
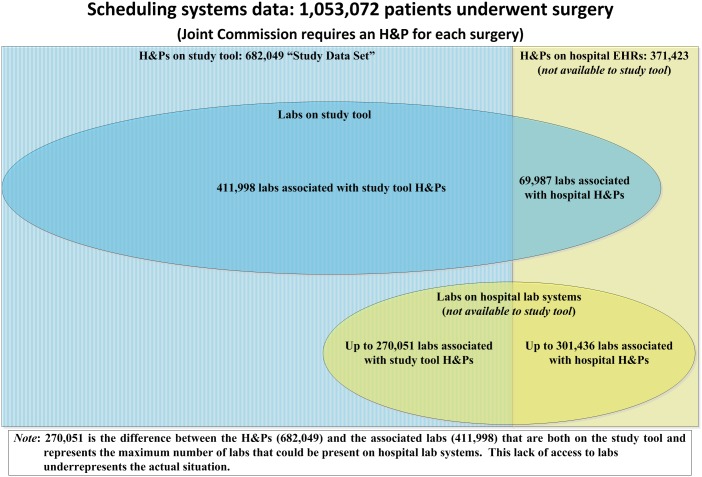
Our research tool included 682,049 H&Ps from the 1,053,472 patient records in surgeon EHRs. Thus 65% of patients had H&Ps in our data set (Table 1). The remaining 371,423 H&Ps were in-hospital EHRs and therefore not available for analysis.
Among the 682,049 H&Ps, we found 411,998 associated with PT and aPTT tests (60.4%) (Fig 2). Some of the remaining 270,051 surgeries may have had associated labs on hospital lab systems that were not accessible to us; therefore, this analysis under-represents the actual ratio of labs to H&Ps. Roughly 39.1% of all potential records (411,998/1,053,472) were evaluated in this study. We cannot assess how many patients received PT and aPTT testing whose records are not available to us.
Fig 2. Origin of Study Data.
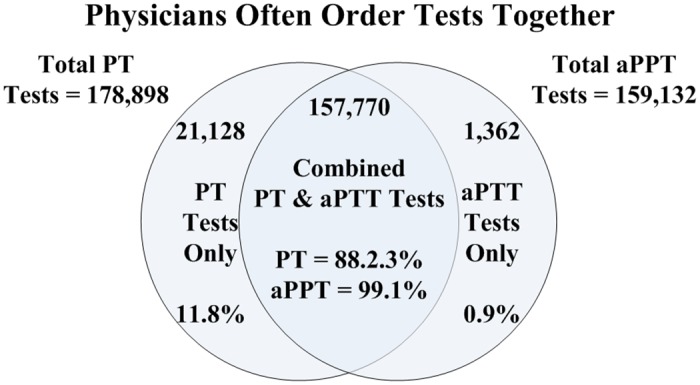
Roughly 26.2% of all pre-surgical patients accessible in the database received PT tests, of which 94.3% of tests were deemed unnecessary, given the absence of findings on the H&P (Table 2); this means that at least 158,378 unnecessary PT tests were done. Similarly, 23.3% of all pre-surgical patients received aPTT tests, of which 99.9% were deemed unnecessary given an absence of H&P findings (Table 2); this is equivalent to at least 149,484 unnecessary aPTT tests. In most cases, PT and aPTT tests were ordered together (Fig 3). The PT test was ordered 178,898 times, and the aPTT test was ordered 159,132 times. The tests were ordered together in 157,770 instances. This represents 88.2% of all PT tests ordered, and 99.1% of all aPTT tests ordered. The aPTT test was ordered on its own in only 1362 cases.
Table 2. Unnecessary Testing—Where H&Ps Show No Findings.
| Facility | Data Set | PT Tests Ordered and % of Data Set | H&Ps Showing no PT Findings (Unnecessary Tests) | aPTT Tests Ordered and % of Data Set | H&Ps Showing no aPTT Findings (Unnecessary Tests) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tertiary Hospitals | 1 | 62,176 | 9,881 | 15.9% | 8,811 | 89.2% | 4,240 | 6.8% | 4,238 | 100.0% |
| 2 | 8,397 | 4,833 | 57.6% | 4,549 | 94.1% | 4,753 | 56.6% | 4,751 | 100.0% | |
| 3 | 27,780 | 9,913 | 35.7% | 9,255 | 93.4% | 9,173 | 33.0% | 9,170 | 100.0% | |
| 4 | 21,639 | 10,077 | 46.6% | 9,591 | 95.2% | 8,235 | 38.1% | 8,231 | 100.0% | |
| 5 | 37,201 | 29,005 | 78.0% | 28,005 | 96.6% | 27,376 | 73.6% | 27,366 | 100.0% | |
| 6 | 11,199 | 2,707 | 24.2% | 2,459 | 90.8% | 2,391 | 21.4% | 2,388 | 99.9% | |
| 7 | 30,161 | 8,114 | 26.9% | 7,698 | 94.9% | 7,939 | 26.3% | 7,927 | 99.8% | |
| 8 | 46,570 | 11,892 | 25.5% | 10,843 | 91.2% | 10,441 | 22.4% | 10,433 | 99.9% | |
| 9 | 21,455 | 2,283 | 10.6% | 2,020 | 88.5% | 2,117 | 9.9% | 2,109 | 99.6% | |
| 10 | 19,960 | 10,281 | 51.5% | 9,787 | 95.2% | 9,979 | 50.0% | 9,974 | 99.9% | |
| 11 | 19,023 | 3,886 | 20.4% | 3,278 | 84.4% | 3,790 | 19.9% | 3,787 | 99.9% | |
| 12 | 35,278 | 4,983 | 14.1% | 4,701 | 94.3% | 4,426 | 12.5% | 4,423 | 99.9% | |
| 13 | 19,590 | 9,000 | 45.9% | 8,704 | 96.7% | 8,397 | 42.9% | 8,396 | 100.0% | |
| 14 | 19,596 | 7,798 | 39.8% | 7,449 | 95.5% | 7,384 | 37.7% | 7,380 | 99.9% | |
| 15 | 17,672 | 2,096 | 11.9% | 2,008 | 95.8% | 1,235 | 7.0% | 1,234 | 99.9% | |
| 16 | 12,192 | 2,446 | 20.1% | 2,314 | 94.6% | 2,403 | 19.7% | 2,400 | 99.9% | |
| 17 | 72,806 | 12,456 | 17.1% | 12,029 | 96.6% | 11,983 | 16.5% | 11,958 | 99.8% | |
| 18 | 33,359 | 21,285 | 63.8% | 20,729 | 97.4% | 19,770 | 59.3% | 19,768 | 100.0% | |
| 19 | 53,525 | 5,050 | 9.4% | 4,148 | 82.1% | 3,555 | 6.6% | 3,551 | 99.9% | |
| 569,579 | 167,986 | 29.5% | 158,378 | 94.3% | 149,587 | 26.3% | 149,484 | 99.9% | ||
| AmbSurg Centers | 20 | 28,732 | 563 | 2.0% | 510 | 90.6% | 383 | 1.3% | 383 | 100.0% |
| 21 | 11,385 | 3,654 | 32.1% | 3,460 | 94.7% | 2,999 | 26.3% | 2,997 | 99.9% | |
| 22 | 2,539 | 243 | 9.6% | 223 | 91.8% | 208 | 8.2% | 208 | 100.0% | |
| 23 | 19,148 | 1,325 | 6.9% | 1,267 | 95.6% | 1,136 | 5.9% | 1,136 | 100.0% | |
| 24 | 12,215 | 853 | 7.0% | 787 | 92.3% | 792 | 6.5% | 788 | 99.5% | |
| 25 | 10,123 | 2,919 | 28.8% | 2,776 | 95.1% | 2,764 | 27.3% | 2,762 | 99.9% | |
| 26 | 16,377 | 520 | 3.2% | 491 | 94.4% | 458 | 2.8% | 458 | 100.0% | |
| 27 | 11,951 | 835 | 7.0% | 811 | 97.1% | 805 | 6.7% | 805 | 100.0% | |
| Subtotal | 112,470 | 10,912 | 9.7% | 10,325 | 94.6% | 9,545 | 8.5% | 9,537 | 99.9% | |
| Total | 682,049 | 178,898 | 26.2% | 168,703 | 94.3% | 159,132 | 23.3% | 159,021 | 99.9% | |
Fig 3. Distribution of PT Only, aPTT Only, and Combined PT/aPTT Tests.
There is a wide range between facilities in the frequency with which PT and aPTT tests are ordered. For example, facilities 18 and 19 are both eye and ear specialty hospitals; hospital 18 ordered PT tests for 63.8% of patients, while hospital 19 ordered the same tests for 9.4% of patients.
Across all hospitals and centers, the proportion of unnecessary PT tests ranged from 82.1% to 97.4% and the proportion of unnecessary aPTT tests ranged from 99% to 100% (Table 2). Extrapolating the lowest of these proportions to all patients, some of whom had no H&P records available, enables us to calculate that 90.0% of all patients may have received unnecessary PT tests, and 99.6% of all patients may have received unnecessary aPTT tests (Table 2).
The number and proportion of unnecessary PT and aPTT tests that nevertheless produced abnormal findings is shown (Fig 4). The rate of abnormal test results was significantly higher in patients with relevant findings on their H&P than in patients with no relevant findings. There were a substantial number of patients for whom unnecessary tests were positive (6.6% and 7.1%). We lack sufficient information to tell whether these results are unanticipated true positives or false positives.
Fig 4. Abnormal Findings for PT and aPTT Tests.
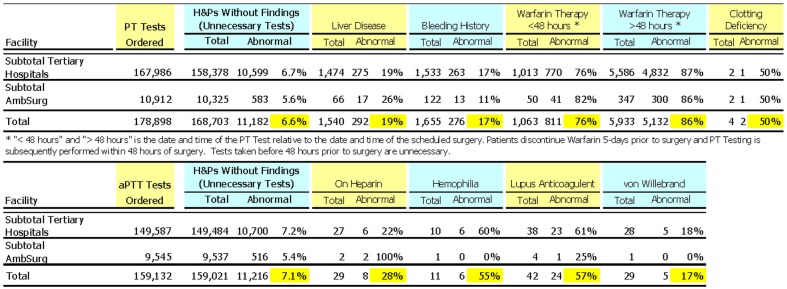
The rate of abnormal test results declined with patient age (Table 3). Abnormal PT and aPTT tests were nearly 3-fold more prevalent in patients younger than 30 years than in patients older than 50 years.
Table 3. Age Distribution of Abnormal PT and aPTT Test Rates.
| PT | Tests—Age <30 | Tests—Age 30–50 | Tests—Age >50 | |||||||
| Facility | Tests | Total | Abnormal | Total | Abnormal | Total | Abnormal | |||
| Subtotal Tertiary Hospitals | 167,986 | 13,225 | 1,267 | 9.6% | 39,426 | 2,195 | 5.6% | 115,327 | 13,278 | 3.7% |
| Subtotal AmbSurg | 10,912 | 1,149 | 76 | 6.6% | 2,545 | 145 | 5.7% | 7,218 | 734 | 1.4% |
| Total | 178,898 | 14,374 | 1,343 | 9.3% | 41,971 | 2,340 | 5.6% | 122,545 | 14,012 | 3.4% |
| aPTT | Tests—Age <30 | Tests—Age 30–50 | Tests—Age >50 | |||||||
| Facility | Tests | Total | Abnormal | Total | Abnormal | Total | Abnormal | |||
| Subtotal Tertiary Hospitals | 149,587 | 12,611 | 792 | 6.3% | 36,603 | 1,832 | 5.0% | 100,369 | 8,116 | 2.3% |
| Subtotal AmbSurg | 9,545 | 1,122 | 62 | 5.5% | 2,331 | 87 | 3.7% | 6,092 | 370 | 0.7% |
| Total | 159,132 | 13,733 | 854 | 6.2% | 38,934 | 1,919 | 4.9% | 106,461 | 8,486 | 2.1% |
Statistical Analysis
We tested a hypothesis that the percentage of abnormal results on both the PT and aPTT tests is the same (Fig 5). This hypothesis was rejected, the p-value being practically 0. The 95% confidence intervals (PT: 6.48–6.72) and (aPTT: 6.97–7.23) were non-overlapping, indicating that the proportion of false positives on PT and aPTT tests are independent of one another. This suggests that patients who get a false positive on one test are not more likely to get an abnormal result on the other test.
Fig 5. Statistical Overview.
Similarly, a χ2 test was used to test the hypothesis that the PT and aPTT tests were used independently. The fourfold contingency table (see Table 4 and Fig 2) for the two tests shows that this hypothesis can be rejected (again the p-value being practically 0), suggesting that physicians tend to order both tests together. This is also evident from the large number of patients who were given both tests or neither test (Table 4).
Table 4. Fourfold Contingency Table.
| PT Test Ordered | PT Test Not Ordered | Total | |
|---|---|---|---|
| aPTT Test Ordered | 157,770 | 1,362 | 159,132 |
| aPTT Test Not Ordered | 21,128 | 501,789 | 522,917 |
| Total | 178,898 | 503,151 | 682,049 |
Discussion
We have demonstrated that 94.3% of all PT tests and 99.9% of all aPTT tests in our data set were ordered without documented justification in patient H&Ps. Our results clearly show that both PT and aPTT tests are routinely used as screening tests (Table 2), although no rationale exists to conclude that these tests are anything other than diagnostic.
Unnecessary PT tests may actually comprise 97.6% rather than 94.3% (Fig 4) if the data is adjusted to eliminate patients on warfarin whose tests were ordered too early (typically patients should be off warfarin therapy five days prior to surgery allowing PT levels to normalize and tested within 24–48 h of surgery to confirm the patient has stopped warfarin therapy). [18]
Though it is well known that these tests are often ordered with no clinical justification, we have shown that this practice of ordering these tests is widespread, at least in the surgical environment.[19] If extrapolated on a national and international level, the scope of unnecessary testing could be significant as well as the direct and indirect healthcare costs and burdens. For instance, the CDC estimates that in the US, there are over 50 million surgical patients operated on annually. [20, 21]
Given the extent to which surgeons order PT and aPTT tests, they must believe that results are important in predicting bleeding complications. The following two questions provide a better perspective regarding these tests.
How useful are the tests in predicting bleeding complications?
Does an abnormal test result represent a false positives or a true positive?
How useful are the tests in predicting bleeding complications?[22] The PT test was introduced in 1935 for the management of warfarin therapy[23], while the aPTT test was introduced in 1953 and became the test of choice for the management of heparin therapy.[24] Both tests are useful when employed for their intended purposes. However, under most circumstances, even if there is an H&P finding that suggests a need for testing, it is still unlikely that the patient will have a prolonged PT or aPTT as well as a meaningful bleeding complication, since most of the findings on an H&P (disseminated intravascular coagulation, liver disease[25], vitamin K deficiency, congenital factor VII deficiency[26], dysfibrinogenemia[27], factors XII, XI, IX, and VII, lupus anticoagulant, and von Willebrand disease[28–30]) must be quite advanced, and these patients would already have been identified. Accordingly, in contrast with the eight H&P findings commonly used by physicians, the literature only supports the use of these tests where the patient is utilizing warfarin or heparin. Further, near-unanimous results from peer reviewed publications have demonstrated that abnormalities on coagulation tests are not predictive of bleeding events.[31–39]
Does an abnormal test result represent a false positives or a true positive? Abnormal test results in the absence of H&P findings were observed at rates of 6.6% for PT tests and 7.1% for aPTT tests (Fig 4). These findings may be false positives or true positives (i.e. potential bleeding complication in a patient about to undergo surgery). It has been shown that the prevalence of asymptomatic coagulopathies is so low that false-positive test results greatly outnumber true-positive results.[40] Accordingly, an abnormal test result is most likely a false positive for one or more of the following reasons[41]:
Inadequate determination of reference standards (reagents and instrumentation)[42];
High patient hematocrit resulting in an artifactual prolongation of the clotting time[43];
Variations in citrate anticoagulant (3.2% or 3.8%), which is known to affect results[44];
Fasting state of the patient (plasma turbidity can interfere with optical systems in non-fasting lipemic, hemolyzed, or icteric specimens)[45];
PT tests should be done within 24 h of collection, and aPTT should be determined within 4 h, especially if the sample is heparinized.[46]
Some weaknesses of the present study may limit the scope of our conclusions. First, our study tool could not access all H&P and lab information from the various sites. Patient information was received from some hospital surgeons who did not utilize the hospital’s in-house EMR and/or lab systems; these patients may constitute a different patient population than other patients in the hospitals studied here. Second, the Joint Commission mandates that an H&P exist for each surgery undertaken. Therefore, the study tool should have had access to an H&P for each of the 1,053,472 surgeries. In contrast, we had access to only 65% of these H&Ps, because all other H&Ps were on hospital systems that were not accessible to our tool. The research tool also had access to only some labs, which underrepresents the actual ratio of H&Ps to labs (Fig 1). Third, on a geographic basis, our sample reflects surgeon ordering practices compiled from data from seven states and the District of Columbia, which may or may not be representative of surgeon ordering practices in the unevaluated portion of the United States. Fourth, the study did not include pregnancy morbidity as an indicator, which is relevant to diagnosing antiphospholipid syndrome (APS) and a potential factor prompting PT and aPTT testing and unexplained thrombosis.
Since a meaningful percentage of surgeons use these tests as screening tests (88.2% of PT tests and 99.1% of aPTT tests) it would appear these tests are being ordered by surgeons as part of their routine pre-surgical process. Accordingly, given the previously mentioned cost burdens, consideration by the relevant industry constituencies could be given to exploring the use of change agents to evolve these apparent pre-surgical processes to conform with evidence-based practices. Some hospitals appear to be proactive in reducing unnecessary testing (e.g., Table 2: facility 19 vs. facility 18, both specialty eye and ear hospitals). Our data reflect substantially lower levels of unnecessary testing at some hospitals. For example, one hospital performed roughly 15% as many tests as a comparable hospital with similar surgery cases. The burdens and costs of unnecessary testing may not only refer to the test per se, but also extend to the follow-on professional obligations placed on health-care professionals.
Conclusions
We report what we believe to be the largest prospective sample of surgical patients ever assembled. Our sample includes 1,053,472 consecutive patients from 27 medical facilities enrolled from 2009 to 2012, and we were able to gather complete data for a subset of 65% of those patients (N = 682,049). Our results show that both PT and aPTT are used as screening tests, though no rationale exists to conclude that these tests are anything other than diagnostic. Overall, 26.2% of patients received PT testing, and 94.3% of those tests were not necessary, given the absence of findings on the patient H&P. Similarly, 23.3% of preoperative patients received aPTT testing, of which 99.9% of tests were unnecessary. For patients with no H&P findings suggestive of bleeding risk, 6.6% of PT tests and 7.1% of aPTT tests were positive, indicating either a false positive or an unanticipated true positive finding. Given that bleeding conditions are likely to be diagnosed symptomatically prior to surgery, most positive findings are likely to be false positives.
We therefore document routine pre-surgical practices for which there is no clinical justification and which can put patients at risk of false-positive findings. Useless tests are clinically inappropriate because they consume resources, yet bring no benefit to patients or clinicians. Empty testing is also ethically wrong, because it puts patients at risk to no purpose. If our study set is representative of national practices in the United States, then modification of current testing practices could substantially reduce the number of unnecessary PT and aPTT tests, thereby saving hospitals, the Centers for Medicare and Medicaid Services, and insurance companies the costs of unnecessary testing. Our tool offers an unprecedented window into unnecessary testing in the United States.
Supporting Information
(XLSX)
Acknowledgments
We would like to acknowledge the advice and sharing of expertise from: Donna Brassil, Barry Coller M.D., Emil Gotschlich, M.D. of Rockefeller University; Jayant Deshpande, Ph.D., Department of Statistics, Michigan State University; Walter H. Dzik, MD, of Massachusetts General Hospital; Malgorzata B. Trela and Ashwini Valimbe of MMF Systems who contributed significantly to the data collection process; and the encouragement of Paul Verkuil, J.S.D. of the Administrative Council of the United States.
Data Availability
Data in support of the paper are provided in the Supporting Information. The ultimate data owners of the underlying dataset are the participating hospitals who allowed MMF Systems to deidentify and aggregate relevant patient information. As system access does not prevent access to personal health information, further access can be arranged by contacting the corresponding author.
Funding Statement
Support was provided by Rockefeller University Center for Clinical and Translational Science (grant # UL1 TR000043 from NCATS, NIH, CTSA Program) and application development resources were contributed by MMF Systems, Inc. MMF Systems, of which FA, JCB, MNC, and RGS are employees or consultants, provided access to its proprietary data on de-identified hospital patients.
References
- 1. Brook RH. The role of physicians in controlling medical care costs and reducing waste. Jama. 2011;306(6):650–1. 10.1001/jama.2011.1136 . [DOI] [PubMed] [Google Scholar]
- 2. Emanuel EJ. Where are the health care cost savings? Jama. 2012;307(1):39–40. 10.1001/jama.2011.1927 . [DOI] [PubMed] [Google Scholar]
- 3. McMahon LF Jr., Chopra V. Health care cost and value: the way forward. Jama. 2012;307(7):671–2. 10.1001/jama.2012.136 . [DOI] [PubMed] [Google Scholar]
- 4. Berwick DM, Hackbarth AD. Eliminating waste in US health care. Jama. 2012;307(14):1513–6. 10.1001/jama.2012.362 . [DOI] [PubMed] [Google Scholar]
- 5. Hoffman JR, Cooper RJ. Overdiagnosis of disease: a modern epidemic. Archives of internal medicine. 2012;172(15):1123–4. 10.1001/archinternmed.2012.3319 . [DOI] [PubMed] [Google Scholar]
- 6. Prasad V, Vandross A, Toomey C, Cheung M, Rho J, Quinn S, et al. A decade of reversal: an analysis of 146 contradicted medical practices. Mayo Clinic proceedings. 2013;88(8):790–8. 10.1016/j.mayocp.2013.05.012 . [DOI] [PubMed] [Google Scholar]
- 7. Moynihan R, Henry D, Moons KG. Using evidence to combat overdiagnosis and overtreatment: evaluating treatments, tests, and disease definitions in the time of too much. PLoS medicine. 2014;11(7):e1001655 10.1371/journal.pmed.1001655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glasziou P, Moynihan R, Richards T, Godlee F. Too much medicine; too little care. Bmj. 2013;347:f4247 10.1136/bmj.f4247 . [DOI] [PubMed] [Google Scholar]
- 9. Mancuso CA. Impact of new guidelines on physicians' ordering of preoperative tests. Journal of general internal medicine. 1999;14(3):166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pilsczek FH, Rifkin WD, Walerstein S. Overuse of prothrombin and partial thromboplastin coagulation tests in medical inpatients. Heart & lung: the journal of critical care. 2005;34(6):402–5. 10.1016/j.hrtlng.2005.07.004 . [DOI] [PubMed] [Google Scholar]
- 11. Jaffer AK. Perioperative management of warfarin and antiplatelet therapy. Cleveland Clinic journal of medicine. 2009;76 Suppl 4:S37–44. 10.3949/ccjm.76.s4.07 . [DOI] [PubMed] [Google Scholar]
- 12.Dugdale, DC. Prothrombin time (PT). Medline Plus. US National Library of Medicine, National Institutes of Health. 2013. Available: http://www.nlm.nih.gov/medlineplus/ency/article/003652.htm.
- 13.Dugdale, DC. Partial thromboplastin time (PTT), Medline Plus. US National Library of Medicine, National Institutes of Health. 2013. Available: http://www.nlm.nih.gov/medlineplus/ency/article/003653.htm.
- 14. Eckman MH, Erban JK, Singh SK, Kao GS. Screening for the risk for bleeding or thrombosis. Annals of internal medicine. 2003;138(3):W15–24. . [DOI] [PubMed] [Google Scholar]
- 15. VanLare JM, Conway PH, Sox HC. Five next steps for a new national program for comparative-effectiveness research. The New England journal of medicine. 2010;362(11):970–3. 10.1056/NEJMp1000096 . [DOI] [PubMed] [Google Scholar]
- 16.Hirsch R, Deixler H. HIPAA Business Associates and Health-Care Big Data: Big Promise, Little Guidance. Bureau of National Affairs. 17 February 2014. Available: http://www.bna.com/hipaa-business-associates-and-health-care-big-data-big-promise-little-guidance/. Accessed 15 August 2014.
- 17. Joint Commission on Accreditation of Healthcare Organizations. 2015 Hospital Accreditation Standards. Oakbrook Terrace: Joint Commission on Accreditation of Healthcare Organizations; 2014. [Google Scholar]
- 18. Committee on S, Practice P, Apfelbaum JL, Connis RT, Nickinovich DG, American Society of Anesthesiologists Task Force on Preanesthesia E, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116(3):522–38. 10.1097/ALN.0b013e31823c1067 . [DOI] [PubMed] [Google Scholar]
- 19. amal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clinic proceedings. 2007;82(7):864–73. 10.4065/82.7.864 . [DOI] [PubMed] [Google Scholar]
- 20. Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. National health statistics reports. 2010;(29):1–20, 4 . [PubMed] [Google Scholar]
- 21. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. National health statistics reports. 2009;(11):1–25. . [PubMed] [Google Scholar]
- 22. Newman MF, Fleisher LA, Fink MP. Perioperative medicine: managing for outcome. Philadelphia: Saunders Elsevier; 2008. xix, 723 p. p. [Google Scholar]
- 23. Quick AJ. The prothrombin time in hemophilia and in obstructive jaundice. J Biol Chem 1935;109:73–4. [Google Scholar]
- 24. Owren PA. The coagulation of blood. Investigations on a new clotting factor. Acta Med Scand 1947;194:521–49. [Google Scholar]
- 25. Cobas M. Preoperative assessment of coagulation disorders. International anesthesiology clinics. 2001;39(1):1–15. . [DOI] [PubMed] [Google Scholar]
- 26. Acharya SS, Coughlin A, Dimichele DM, North American Rare Bleeding Disorder Study G. Rare Bleeding Disorder Registry: deficiencies of factors II, V, VII, X, XIII, fibrinogen and dysfibrinogenemias. Journal of thrombosis and haemostasis: JTH. 2004;2(2):248–56. . [DOI] [PubMed] [Google Scholar]
- 27. Cunningham MT, Brandt JT, Laposata M, Olson JD. Laboratory diagnosis of dysfibrinogenemia. Archives of pathology & laboratory medicine. 2002;126(4):499–505. . [DOI] [PubMed] [Google Scholar]
- 28. Fujikawa K. Historical perspective of factor XI. Thrombosis research. 2005;115(6):441–50. 10.1016/j.thromres.2004.10.013 . [DOI] [PubMed] [Google Scholar]
- 29. Seligsohn U. Factor XI deficiency. Thrombosis and haemostasis. 1993;70(1):68–71. . [PubMed] [Google Scholar]
- 30. Mannucci PM. Treatment of von Willebrand's Disease. The New England journal of medicine. 2004;351(7):683–94. 10.1056/NEJMra040403 . [DOI] [PubMed] [Google Scholar]
- 31. Gabriel P, Mazoit X, Ecoffey C. Relationship between clinical history, coagulation tests, and perioperative bleeding during tonsillectomies in pediatrics. Journal of clinical anesthesia. 2000;12(4):288–91. . [DOI] [PubMed] [Google Scholar]
- 32. Scheckenbach K, Bier H, Hoffmann TK, Windfuhr JP, Bas M, Laws HJ, et al. [Risk of hemorrhage after adenoidectomy and tonsillectomy. Value of the preoperative determination of partial thromboplastin time, prothrombin time and platelet count]. Hno. 2008;56(3):312–20. . [DOI] [PubMed] [Google Scholar]
- 33. Dzik WH. Predicting hemorrhage using preoperative coagulation screening assays. Current hematology reports. 2004;3(5):324–30. . [PubMed] [Google Scholar]
- 34. Schwaab M, Hansen S, Gurr A, Dazert S. [Significance of blood tests prior to adenoidectomy]. Laryngo- rhino- otologie. 2008;87(2):100–6. . [DOI] [PubMed] [Google Scholar]
- 35. Asaf T, Reuveni H, Yermiahu T, Leiberman A, Gurman G, Porat A, et al. The need for routine pre-operative coagulation screening tests (prothrombin time PT/partial thromboplastin time PTT) for healthy children undergoing elective tonsillectomy and/or adenoidectomy. International journal of pediatric otorhinolaryngology. 2001;61(3):217–22. . [DOI] [PubMed] [Google Scholar]
- 36. Chee YL, Greaves M. Role of coagulation testing in predicting bleeding risk. The hematology journal: the official journal of the European Haematology Association / EHA. 2003;4(6):373–8. 10.1038/sj.thj.6200306 . [DOI] [PubMed] [Google Scholar]
- 37. Garcia Callejo FJ, Pardo Mateu L, Velert Vila MM, Orts Alborch M, Monzo Gandia R, Marco Algarra J. [Usefulness of preoperative coagulation tests in the prevention of post-tonsillectomy hemorrhage in children]. Acta otorrinolaringologica espanola. 1997;48(6):473–8. . [PubMed] [Google Scholar]
- 38. Peterson P, Hayes TE, Arkin CF, Bovill EG, Fairweather RB, Rock WA Jr., et al. The preoperative bleeding time test lacks clinical benefit: College of American Pathologists' and American Society of Clinical Pathologists' position article. Archives of surgery. 1998;133(2):134–9. . [DOI] [PubMed] [Google Scholar]
- 39. Rohrer MJ, Michelotti MC, Nahrwold DL. A prospective evaluation of the efficacy of preoperative coagulation testing. Annals of surgery. 1988;208(5):554–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suchman AL, Griner PF. Diagnostic uses of the activated partial thromboplastin time and prothrombin time. Annals of internal medicine. 1986;104(6):810–6. . [DOI] [PubMed] [Google Scholar]
- 41. Eisenberg JM, Clarke JR, Sussman SA. Prothrombin and partial thromboplastin times as preoperative screening tests. Archives of surgery. 1982;117(1):48–51. . [DOI] [PubMed] [Google Scholar]
- 42. Andrew M, Vegh P, Johnston M, Bowker J, Ofosu F, Mitchell L. Maturation of the hemostatic system during childhood. Blood. 1992;80(8):1998–2005. . [PubMed] [Google Scholar]
- 43. Adcock DM, Kressin DC, Marlar RA. Minimum specimen volume requirements for routine coagulation testing: dependence on citrate concentration. American journal of clinical pathology. 1998;109(5):595–9. . [DOI] [PubMed] [Google Scholar]
- 44. Adcock DM, Kressin DC, Marlar RA. Effect of 3.2% vs 3.8% sodium citrate concentration on routine coagulation testing. American journal of clinical pathology. 1997;107(1):105–10. . [DOI] [PubMed] [Google Scholar]
- 45. Mann KG. Biochemistry and physiology of blood coagulation. Thrombosis and haemostasis. 1999;82(2):165–74. . [PubMed] [Google Scholar]
- 46. Adcock D, Kressin D, Marlar RA. The effect of time and temperature variables on routine coagulation tests. Blood coagulation & fibrinolysis: an international journal in haemostasis and thrombosis. 1998;9(6):463–70. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
Data in support of the paper are provided in the Supporting Information. The ultimate data owners of the underlying dataset are the participating hospitals who allowed MMF Systems to deidentify and aggregate relevant patient information. As system access does not prevent access to personal health information, further access can be arranged by contacting the corresponding author.