Introduction
Fear of falling (FoF) is an autonomic, cognitive and behavioural response to a real or imminent threat of a fall (Hadjistavropoulos et al. 2011). Given its multidimensional nature, the assessment of fear usually relies on self-report and converging evidence from a number of independent but related measures, such as anxiety (Davis et al. 2010), confidence (self-efficacy) and arousal (Hadjistavropoulos et al. 2011). Recent studies, in which young healthy adults have been exposed to a postural threat (e.g. standing on an elevated platform where the consequences of a fall are severe), have shown that fear and related factors directly affect control of quiet standing and dynamic balance responses (Brown & Frank, 1997; Carpenter et al. 2001, 2004; Sibley et al. 2010); however, the mechanisms underlying these fear-related postural changes are yet to be understood fully. Of recent debate is whether FoF-related changes in vestibular function, as evidenced by modulation (or lack thereof) of vestibular-evoked balance responses, might contribute to threat-related adjustments in balance behaviours (Osler et al. 2013; Horslen et al. 2014). Our position is that FoF does influence vestibular-evoked balance responses.
Evidence in support of our position
We would expect to observe changes in vestibular-evoked balance responses with FoF because of the strong excitatory reciprocal projections between all vestibular nuclei and neural regions responsible for fear-related processes, including the amygdala, the parabrachial nuclei (Balaban, 2002) and the histaminergic system (de Waele et al. 1992). These fear-related networks have been implicated in the relationship between anxiety and vestibular or dizziness disorders (Furman & Jacob, 2001; Staab et al. 2013). These networks are also thought to be engaged transiently to limit body movement with threat (Balaban, 2002), as part of the ‘freezing’ response to threatening stimuli (Lang et al. 2000). As such, larger vestibular-evoked balance responses may be a result of excitation of the central vestibular system, which might normally serve to limit movement, in the presence of a postural threat (Horslen et al. 2014).
Vestibular-evoked balance responses can be probed with percutaneous electrical stimulation over the mastoid processes bilaterally to modulate vestibular afferent firing rates (Goldberg et al. 1984). This activation of vestibular afferents leads to a virtual head perturbation (Fitzpatrick & Day, 2004). Electrical vestibular stimulation (EVS) evokes patterned activity in axial and appendicular muscles which, when added vectorially, exert a net force onto the ground causing whole-body movement (Britton et al. 1993; Fitzpatrick & Day, 2004; Forbes et al. 2015). The early responses are most likely to reflect the body’s compensation to an isolated vestibular perturbation (Fitzpatrick & Day, 2004). If the stimulation persists, then feedback from non-vestibular sources can be used to counteract the evoked balance response (Day & Guerraz, 2007). Continuously varying stochastic electrical vestibular stimulation (SVS; Fitzpatrick et al. 1996; Dakin et al. 2007) evokes muscle and balance responses similar to those elicited with EVS (Dakin et al. 2007). Cross-correlations (between SVS and physiological recordings) can resolve the short- (SL) and medium-latency (ML) responses typically examined in response to EVS (Dakin et al. 2007, 2010; Reynolds, 2010). Likewise, frequency-based analyses can be used to assess the strength of input–output coupling and gain of the relationship (Dakin et al. 2010).
In recent experiments, we showed increased vestibular-evoked balance responses to SVS when subjects stood with their toes at the edge of a platform 3.2 m high, compared with standing at ground level (Horslen et al. 2014). Specifically, height-induced threat significantly increased vestibular-evoked SL and ML peak force amplitudes (Fig. 1A), as well as gain and coherence between SVS and ground reaction forces. Vestibular-evoked balance responses were also increased in postural muscles when subjects stood under the threat of unpredictable lateral support surface tilt perturbations (Lim, 2014). Both SL and ML peak muscle responses were larger (Fig. 1B and C), and gain and coherence were increased when the threat of perturbation was present, compared with no-threat conditions. Taken together, these findings indicate that the threat of increased consequence or likelihood of a fall increases vestibular gain, as measured by vestibular-evoked balance responses. Osler et al. (2013), in contrast, used square-wave EVS to evoke balance responses in subjects who stood with feet in a tandem orientation on an elevated beam and found that trunk kinematic responses were only affected (reduced) in later phases of the response (>800 ms). They concluded that FoF has no effect on early ‘feedforward vestibular-evoked balance responses’, but ‘strongly attenuates the feedback’ response (Osler et al. 2013). While these results may seem contradictory to the observations of Horslen et al. (2014) and Lim (2014), methodological considerations may account for the reported differences. In particular, the high-frequency threat-related changes observed in ground reaction forces (Horslen et al. 2014) and muscle activity (Lim, 2014) would be less evident in trunk kinematics because of natural low-pass filtering in conversion from muscle activity or force to sway (Dakin et al. 2010; Forbes et al. 2015). Likewise, differences in the level of stability due to foot position (tandem vs. side by side), threat location/type (both sides vs. front vs. support surface tilt) and/or EVS characteristics (square-wave vs. zero-mean stochastic) may offer additional explanations for the incongruent observations of threat-related changes in early vestibular-evoked balance responses between studies (Osler et al. 2013; Horslen et al. 2014; Lim, 2014).
Figure 1.
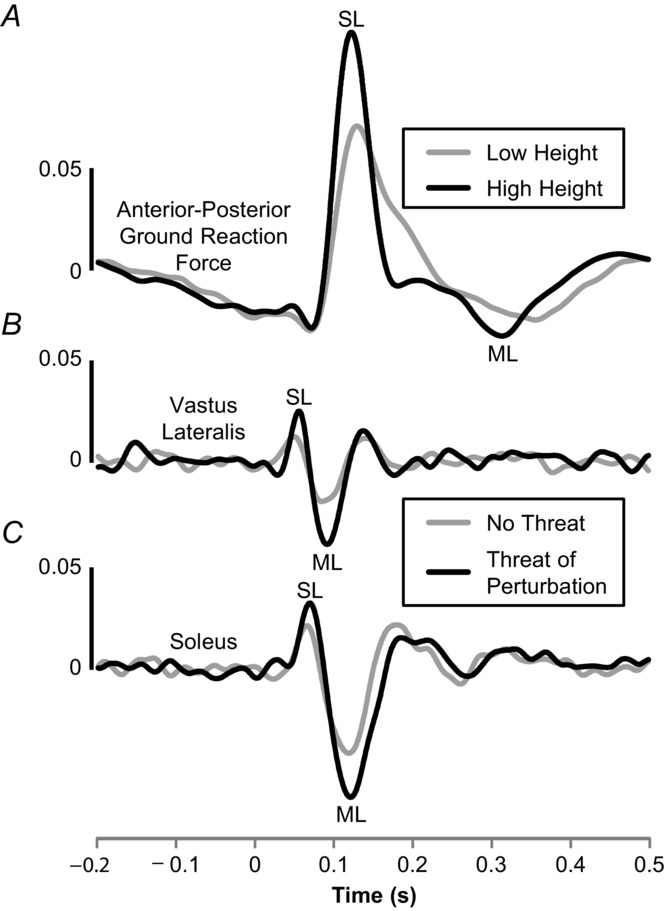
Short- (SL) and medium-latency (ML) vestibular-evoked balance responses with threat
Amplitude-normalized cumulant density plots representing cross-correlation between stochastic electrical vestibular stimulation (SVS) and anterior–posterior ground reaction forces acting on the body (A), vastus lateralis (B) and soleus muscle activity (C). In all cases, positive peaks reflect a positive correlation between SVS and the respective measure [e.g. a positive current caused a forward-directed force (A) or increase in muscle activity (B and C)]. The ground reaction force trace (A) is reproduced from Horslen et al. (2014; their Fig. 2C); SVS (2–25 Hz bandwidth) evoked balance responses while subjects (n = 10) stood at low and high surface heights with feet at the edge and head turned 90 deg to the right. The muscle activity traces (B and C) were reproduced with permission from Lim (2014; her Fig. 3.12); SVS (0–25 Hz) evoked responses while subjects (n = 13) stood with the head facing forward with and without the threat of laterally directed support surface perturbations.
Further evidence supporting fear-related influences on vestibular-evoked responses can be drawn from studies that have used alternative methods to probe vestibular function. Vestibular-evoked myogenic potentials (VEMPs) use loud auditory tones or clicks to activate the vestibular receptors directly and evoke short-latency reflexes in tonically engaged muscles (Rosengren et al. 2010). Naranjo et al. (2015) observed significant increases in VEMP amplitudes in neck and leg muscles actively involved in stabilizing the body and head when subjects stood at the edge of a high compared with a low surface. Furthermore, changes in VEMP amplitude were positively correlated with changes in both FoF and anxiety. These results are consistent with prior evidence of increased vestibulo-ocular reflex gain in conditions of increased anxiety (Yardley et al. 1995) or vigilance (Collins, 1988) that would normally accompany a fear response. The SVS, VEMP and vestibulo-ocular reflex studies all demonstrate anxiety- or fear-related excitation of vestibular responses. Combined, this evidence implicates the vestibular nuclei as a likely site for modulation, because vestibular-evoked reflexes in the leg, neck and eye muscles all relay through the vestibular nuclei.
Concluding remarks
Based on the evidence reviewed here, we conclude that FoF increases the amplitude of vestibular-evoked balance responses. One question that remains is how (and if) changes in vestibular-evoked balance res-ponses with FoF contribute to the incre-ases in balance-correcting responses to whole-body perturbation with threat (Brown & Frank, 1997; Carpenter et al. 2004; Sibley et al. 2010). Vestibular-evoked balance responses are thought to reflect reactions to virtual head perturbation and are distinct from balance-correcting responses to whole-body support surface perturbations (Wardman et al. 2003). However, support surface perturbations induce early head accelerations (15–40 ms; Carpenter et al. 1999), and balance-correcting responses are known to be attenuated with vestibular deficits (Horlings et al. 2009). As such, it is possible that the networks responsible for vestibular-evoked responses can contribute, at least in part, to fear-related changes in balance-correcting responses.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief (250 word) comment. Comments may be submitted up to 6 weeks after publication of the article, at which point the discussion will close and the CrossTalk authors will be invited to submit a ‘Last Word’. Please email your comment to jphysiol@physoc.org.
Biographies
Brian Horslen is a Natural Sciences and Engineering Research Council of Canada-funded PhD candidate supervised by Mark Carpenter in the Neural Control of Posture and Movement Laboratory of the School of Kinesiology at the University of British Columbia. He earned his MSc at the University of British Columbia and BSc at the University of Waterloo. Brian Horslen’s research focuses on the effects of fear, anxiety and arousal on sensorimotor function,particularly related to the control of standing balance.
MarkCarpenter is an Associate Professor in the School of Kinesiology at the University of British Columbia. He completed his PhD at the University of Waterloo and postdoctoral training at the University Hospital Basel and the Karolinska Institute. Mark Carpenter’s research is focused on understanding the physiological and psychological factors that contribute to the control of standing balance in healthy individuals and those with balance deficits associated with age, Parkinson’s disease, vestibular loss and spinal cord injury.
Additional information
Competing interests
None declared.
Funding
Natural Sciences and Engineering Research Council of Canada (NSERC) to M.G.C. (application #326910).
References
- Balaban CD. Neural substrates linking balance control and anxiety. Physiol Behav. 2002;77:469–475. doi: 10.1016/s0031-9384(02)00935-6. [DOI] [PubMed] [Google Scholar]
- Britton TC, Day BL, Brown P, Rothwell JC, Thompson PD. Marsden CD. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- Brown LA. Frank JS. Postural com-pensations to the potential consequences of instability: kinematics. Gait Posture. 1997;6:89–97. [Google Scholar]
- Carpenter MG, Allum JH. Honegger F. Directional sensitivity of stretch reflexes and balance corrections for normal subjects in the roll and pitch planes. Exp Brain Res. 1999;129:93–113. doi: 10.1007/s002210050940. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Adkin AL, Paton A. Allum JHJ. Influence of postural anxiety on postural reactions to multi-directional surface rotations. J Neurophysiol. 2004;92:3255–3265. doi: 10.1152/jn.01139.2003. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Silcher CP. Peysar GW. The influence of postural threat on the control of upright stance. Exp Brain Res. 2001;138:210–218. doi: 10.1007/s002210100681. [DOI] [PubMed] [Google Scholar]
- Collins WE. Some effects of sleep loss on vestibular responses. Aviat Space Environ Med. 1988;59:523–529. [PubMed] [Google Scholar]
- Dakin CJ, Lee Son GM, Inglis JT. Blouin JS. Frequency response of human vestibular reflexes characterized by stochastic stimuli. J Physiol. 2007;583:1117–1127. doi: 10.1113/jphysiol.2007.133264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin CJ, Luu BL, van den Doel K, Inglis JT. Blouin JS. Frequency-specific modulation of vestibular-evoked sway responses in humans. J Neurophysiol. 2010;103:1048–1056. doi: 10.1152/jn.00881.2009. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L. Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL. Guerraz M. Feedforward versus feedback modulation of human vestibular-evoked balance responses by visual self-motion information. J Physiol. 2007;582:153–161. doi: 10.1113/jphysiol.2007.132092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waele C, Serafin M, Khateb A, Vibert N, Yabe T, Arrang JM, Mulhethaler M. Vidal PP. An in vivo and in vitro study of the vestibular nuclei histaminergic receptors in the guinea pig. Ann N Y Acad Sci. 1992;656:550–565. doi: 10.1111/j.1749-6632.1992.tb25235.x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D. Gandevia SC. Loop gain of reflexes controlling human standing measured with the use of postural and vestibular disturbances. J Neurophysiol. 1996;76:3994–4008. doi: 10.1152/jn.1996.76.6.3994. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC. Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol. 2004;96:2301–2316. doi: 10.1152/japplphysiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- Forbes PA, Siegmund GP, Schouten AC. Blouin JS. Task, muscle and frequency dependent vestibular control of posture. Front Integr Neurosci. 2015;8:94. doi: 10.3389/fnint.2014.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman JM. Jacob RG. A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J Anxiety Disord. 2001;15:9–26. doi: 10.1016/s0887-6185(00)00040-2. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE. Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984;51:1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- Hadjistavropoulos T, Delbaere K. Fitzgerald TD. Reconceptualizing the role of fear of falling and balance confidence in fall risk. J Aging Health. 2011;23:3–23. doi: 10.1177/0898264310378039. [DOI] [PubMed] [Google Scholar]
- Horlings CG, Carpenter MG, Honegger F. Allum JH. Vestibular and proprioceptive contributions to human balance corrections: aiding these with prosthetic feedback. Ann N Y Acad Sci. 2009;1164:1–12. doi: 10.1111/j.1749-6632.2009.03872.x. [DOI] [PubMed] [Google Scholar]
- Horslen BC, Dakin CJ, Inglis JT, Blouin JS. Carpenter MG. Modulation of human vestibular reflexes with increased postural threat. J Physiol. 2014;592:3671–3685. doi: 10.1113/jphysiol.2014.270744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Davis M. Öhman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Lim SB. 2014. Vancouver, Canada University of British Columbia Graduate and Postdoctoral Studies Modulation of stochastic vestibular stimulation-induced reflexes within a dynamic balance paradigm: the effect of response phase and emotional state, PhD Thesis,
- Naranjo EN, Allum JH, Inglis JT. Carpenter MG. Increased gain of vestibulospinal potentials evoked in neck and leg muscles when standing under height-induced postural threat. Neuroscience. 2015;293:45–54. doi: 10.1016/j.neuroscience.2015.02.026. [DOI] [PubMed] [Google Scholar]
- Osler CJ, Tersteeg MC, Reynolds RF. Loram ID. Postural threat differentially affects the feedforward and feedback components of the vestibular-evoked balance response. Eur J Neurosci. 2013;38:3239–3247. doi: 10.1111/ejn.12336. [DOI] [PubMed] [Google Scholar]
- Reynolds RF. The effect of voluntary sway control on the early and late components of the vestibular-evoked postural response. Exp Brain Res. 2010;201:133–139. doi: 10.1007/s00221-009-2017-9. [DOI] [PubMed] [Google Scholar]
- Rosengren SM, Welgampola MS. Colebatch JG. Vestibular evoked myogenic potentials: past, present and future. Clin Neurophysiol. 2010;121:636–651. doi: 10.1016/j.clinph.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Sibley KM, Mochizuki G, Frank JS. McIlroy WE. The relationship between physiological arousal and cortical and autonomic responses to postural instability. Exp Brain Res. 2010;203:533–540. doi: 10.1007/s00221-010-2257-8. [DOI] [PubMed] [Google Scholar]
- Staab JP, Balaban CD. Furman JM. Threat assessment and locomotion: clinical applications of an integrated model of anxiety and postural control. Semin Neurol. 2013;33:297–306. doi: 10.1055/s-0033-1356462. [DOI] [PubMed] [Google Scholar]
- Wardman DL, Taylor JL. Fitzpatrick RC. Effects of galvanic vestibular stimulation on human posture and perception while standing. J Physiol. 2003;551:1033–1042. doi: 10.1113/jphysiol.2003.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley L, Watson S, Britton J, Lear S. Bird J. Effects of anxiety arousal and mental stress on the vestibulo-ocular reflex. Acta Otolaryngol. 1995;115:597–602. doi: 10.3109/00016489509139373. [DOI] [PubMed] [Google Scholar]


