Abstract
The autonomic nervous system plays an important role in the modulation of normal cardiac rhythm, but is also implicated in modulating the heart’s susceptibility to re-entrant ventricular and atrial arrhythmias. The mechanisms by which the autonomic nervous system is pro-arrhythmic or anti-arrhythmic is multifaceted and varies for different types of arrhythmia and their cardiac substrates. Despite decades of research in this area, fundamental questions related to how neuron density and spatial organization modulate cardiac wave dynamics remain unanswered. These questions may be ill-posed in intact tissues where the activity of individual cells is often experimentally inaccessible. Development of simplified biological models that would allow us to better understand the influence of neural activation on cardiac activity can be beneficial. This Symposium Review summarizes the development of in vitro cardiomyocyte cell culture models of re-entrant activity, as well as challenges associated with extending these models to include the effects of neural activation.
Introduction
Re-entrant arrhythmias – where an excitatory wave travels in a circuit, repeatedly reactivating its cardiac substrate – are a leading cause of death in the developed world. The transition from normal sinus rhythm, where periodic wavefronts spread from a central pacemaking site to trigger cardiac contraction, to re-entry has been the subject of numerous studies over the last century. Re-entrant tachycardias are often self-terminating, spontaneously converting back to a healthy sinus rhythm. However, termination of the arrhythmia is not guaranteed – and when they occur in ventricular tissue, sustained tachycardias can quickly evolve to a highly disorganized rhythm called fibrillation, which is fatal unless treated within minutes. The dynamics of these arrhythmias is difficult to predict, as they may partly depend on the activity of the autonomic nervous system.
The heart is richly innervated by sympathetic and parasympathetic neurons, which have a well understood role in modulating rate and inotropy in the healthy heart, but also effect arrhythmogenticity in diseased tissue. For example, hyperactivity of the sympathetic nervous system, which often occurs in diseases such as hypertension (Julius, 1998; Esler, 2000) is frequently associated with increased risk of re-entrant arrhythmias, especially in the context of pre-existing conditions such as long QT syndrome and ischaemia (Shen & Zipes, 2014). Sympathetic overflow during seizures may also contribute to sudden unexplained death in epilepsy (SUDEP; Devinsky, 2004). In contrast, vagal stimulation increases the fibrillation threshold in ventricular tissue (Brack et al. 2013), but supports tachyarrhythmia and fibrillatory activity in atria (Chen et al. 2014).
The different effects of sympathetic and parasympathetic nerves on arrhythmogenesis can be partially understood by their heterogeneous distribution and influence on cardiac currents (Fig.1). Sympathetic activity releases noradrenaline (norepinephrine), which acts on cardiac β-adrenergic receptors leading to action potential duration (APD) shortening and an increase in cytoplasmic calcium, which in turn can trigger early and delayed afterdepolarizations (EADs and DADs) in susceptible tissues (Rubart & Zipes, 2005). Sympathetic innervation is also spatially heterogeneous, which can lead to an increase in dispersion of refractoriness when stimulated (Liu et al. 2003; Mantravadi et al. 2007). Ischaemic or scarred myocardium is especially vulnerable to sympathetic drive as diseased tissue can give rise to localized nerve sprouting (Shen & Zipes, 2014), which in turn further increases the cardiac substrate’s electrophysiological heterogeneity. Parasympathetic activity releases acetylcholine, which triggers cardiac muscarinic M2 receptors leading to reduced cytoplasmic calcium. Parasympathetic activity also reduces atrial APD, which can be pro-arrhythmic as it enables atrial tachycardias, but increases APD and flattens the APD restitution curve in the ventricles, which is cardioprotective. Parasympathetic modulation of ventricular APD may be driven by a nitric oxide-dependent pathway (Brack et al. 2013), or might be a consequence of the relatively low concentration of M2 receptors ventricles compared to the atria (Brodde et al. 2001; though see Coote, 2013 for an alternate view) and the ability of vagal stimulation to directly inhibit tonic sympathetic activity (Levy, 1984; Paton et al. 2002). Vagal inhibition of sympathetic activity could potentially increase APD (via reduced slow delayed rectifier K+ current (IKs) current) in the ventricles but not the atria, where greater parasympathetic innervation would result in an increase in muscarinic potassium channel activity, shortening APD.
Figure 1.
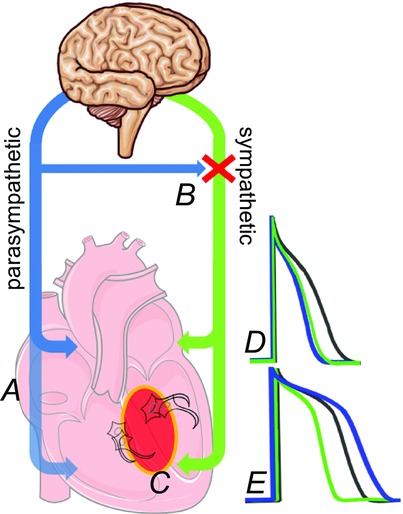
Autonomic nerves and arrhythmogenesis
Parasympathetic (blue) and sympathetic (green) innervate the atria and ventricles in a heterogeneous fashion, with (A) less prominent parasympathetic innervation in the ventricles. Parasympathetic activity can also inhibit sympathetic activity directly (B). Diseases such as myocardial infarction promote sympathetic nerve sprouting (C) which further increases heterogeneity. Activity in either autonomic branch decreases atrial APD (D), which promotes tachyarrhythmia. Sympathetic activity decreases APD in the ventricle (E), while parasympathetic activity both increases ventricular APD and flattens the APD restitution curve, which is cardioprotective.
The arrhythmogenic effects of parasympathetic and sympathetic activity are influenced by a number of parameters that are difficult to address in intact tissue. For example, the spatial organization of neurons on the tissue undoubtedly plays a role in increasing the cardiac substrates heterogeneity, but the distribution of neurons is not under experimental control. Similarly, experiments on isolated cells or cardiac/neuron cell pairs provide valuable mechanistic insights, but cannot address fundamental questions related to the stability of macroscopic propagating waves in myocardium. A biological model system that allows cell-level access while providing insights into tissue level activity is required.
Cardiac monolayers as models of arrhythmogenesis
Biological models with varying degrees of complexity have been developed to shed light on re-entrant arrhythmias. Perhaps the simplest model is the cardiac cell monolayer, a thin layer of tissue grown in culture dishes from embryonic or neonatal cardiac cells. Cardiac cells from very young animals have the capacity to form gap junctional connections with neighbouring cells in culture. After a few days in culture, embryonic cardiac cells are capable of supporting propagating waves of excitation over long distances. Cardiac monolayers were popular 30 years ago as model systems of two-dimensional conduction. More recently, the availability of potential mapping techniques have renewed interest in cultured monolayers, as they allow controlled environments for studying conduction on microscopic (Rohr et al. 1997) and macroscopic (Bub et al. 1998; Entcheva et al. 2000) scales. Monolayer cultures are capable of supporting re-entrant activity in the form of spiral waves, which allow them to be used as simple models of arrhythmogenesis.
Spiral waves in cardiac tissue
Re-entrant waves of excitation in spatially extended systems such as the heart ventricle either travel around an unexcitable obstacle such as a scar or, in homogeneous substrates, form a characteristic spatiotemporal pattern called a spiral wave. Spiral waves (also called vortices and rotors) form naturally when a wavefront travels around a pivot point repeatedly re-exciting the substrate. Spiral waves are a general property of excitable media, and have been observed in the Belousov–Zhabotinsky (BZ) reaction (Keener & Tyson, 1986), intact (Davidenko et al. 1992) and cultured cardiac tissue (Bub et al. 1998), as well as retinal and neural (Shibata & Bures, 1972; Gorelova & Bures, 1983) preparations. Initiating a spiral wave involves generation of a wavefront with a free end, which can curve inward to re-excite tissue forming a wave with a spiral geometry. Once initiated, a spiral can either persist, break up into multiple wavelets, or die out. Spiral wave breakup is a complex phenomenon that has been dealt with in detail by several researchers (see Fenton et al. 2002 for an in-depth review), and is associated with ventricular and atrial fibrillation. Spiral wave termination occurs when the spiral wave tip travels and collides with an unexcitable boundary (Pertsov & Ermakova, 1988; Fast & Efimov, 1991). Spiral waves have been observed in atria (Jalife, 2003), and ventricles, where they typically manifest as a three-dimensional correlate of spirals called scroll waves (Efimov et al. 1999).
Remarkably, cardiac monolayers can generate a range of rhythms similar to those observed in the clinic (Fig.2). Cardiac monolayers display periodic target waves, where activity initiates at a central pacemaking site resulting in an unbroken wavefront propagating throughout the tissue. Target waves are analogous to a regular sinus rhythm in the healthy heart. Cardiac monolayers also support re-entrant activity, in the form of spiral waves, which allows them to be used as models for tachyarrhythmias in the intact heart. Under certain conditions, monolayers also display multiple wavelet re-entry, which could act as a model for fibrillation, or bursting activity driven by the spontaneous onset and offset of re-entrant waves, which generates rhythms similar to paroxysmal tachycardias. These surprising functional similarities exist despite major differences between cell cultures and whole tissue. Cardiac monolayers are derived from neonatal or embryonic tissues, which have a very different phenotype (lower upstroke velocities and increased pacemaking currents) than adult myocardium. Monolayers also lack any three-dimensional structure, which precludes them from displaying the more complex re-entrant phenomena seen in thick tissues, such as scroll waves. Finally, cardiac monolayers lack neural (and other in vivo) inputs, which limits their usefulness in modelling arrhythmias with systemic components such as those associated with ischemia and hypertension.
Figure 2.
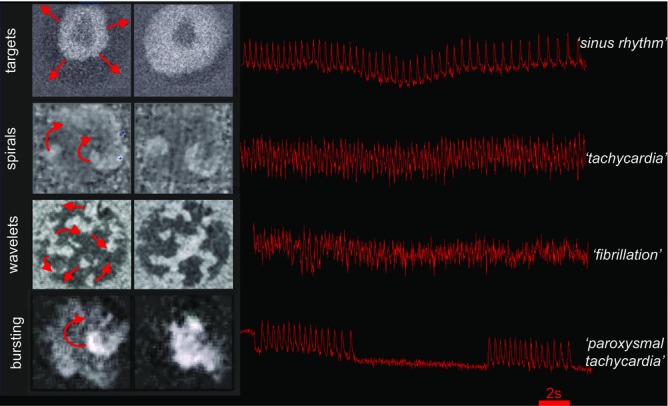
Wave dynamics in cardiac monolayers
Monolayers can display a wide range of rhythms which are similar to those seen in intact hearts. See (Bub et al. 1998, 2002) for experimental details.
Imaging methods
Imaging wave activity at macroscopic scales is typically accomplished by loading the monolayer with an exogenous probe, and recording wave propagation with a fast, sensitive camera. The first spiral waves in cardiac monolayers were recorded using calcium-sensitive dyes, a macroscope constructed using two 35 mm camera lenses, and a sensitive binned CCD (charge-coupled device) running at video frame rates (Bub et al. 1998). Another early system utilized a novel contact fluorescence imaging approach which combined photodiodes and fibre optics placed in a packed hexagonal pattern under the monolayer to measure voltage transients in neonatal rat cultures (Entcheva et al. 2000).Calcium fluorescence was also measured using a standard confocal system to assess calcium dynamics in reperfusion injury in cultured cell networks with a geometrically defined ischaemic zone (Arutunyan et al. 2001). Later, a novel macroscopic phase contrast method which takes advantage of local motion transients to track wave propagation was developed (Hwang et al. 2005), which advantageously does not require dyes or particularly sensitive detectors.
Camera technology has developed significantly over the last decade, allowing for improvements in both imaging speed and resolution. The first high resolution (>1 megapixel) maps of calcium and voltage transients were captured using an intensified CMOS (complementary metal–oxide–semiconductor) system running at 200 frames s–1 (Entcheva & Bien, 2006), which allowed close to cellular resolution while maintaining a large field of view. Our group currently uses a 5.5 megapixel, 100 frames s–1 sCMOS camera (Andor Neo 5.5) to record calcium and motion transients in monolayer preparations. The camera’s high spatial resolution allows us to achieve 10 µm pixel–1 resolution while still capturing spiral waves that propagate over a 2 cm2 area.
Measuring the effects of structure
The simplest culturing technique involves plating dissociated neonatal cardiac cells in a plastic, or glass-bottom culture dish coated with a substrate that is conducive to cell growth (poly-d-lysine, collagen, or fibronectin). Cells organize themselves in an isotropic fashion and do not display a preferred conduction direction as is seen in intact tissue. Several groups have used photolithographic techniques to impose directionality on cell orientation. This technique was pioneered by researchers investigating cardiac conduction by optically mapping cultured cells at microscopic scales (Rohr et al. 1991), and has more recently been used to generate macroscopic patterns in 2D that can mimic the structure of the intact ventricle (Badie & Bursac, 2009). Variations on this approach involve using microlithographically generated stamps (Camelliti et al. 2006), and a relatively simple method where a collagen substrate is mechanically brushed to impose directionality (Bursac et al. 2002). More recently, 3D printing technologies have been adapted to precisely place cardiac cells in 2D and 3D patterns (Jakab et al. 2010). A very promising alternate approach has recently been developed which used an optically activated compound (AzoTAB) to suppress excitability. This method can impose patterns of block in a dynamic fashion on otherwise homogeneous tissue (Magome & Agladze, 2010).
Observing neurons and myocytes in co-cultures
Co-cultures of cardiac and neural cells have been a popular biological model for the last 30 years, partly due to the observation that cardiac cells promote the survival and growth of nerves in culture (Furshpan et al. 1976; Baccaglini & Cooper, 1982). Hearts, like many organ systems, release nerve growth factor (NGF). NGF has been shown to acutely modulate synaptic transmission between sympathetic neurons and cardiac myocytes (Lockhart et al. 1997). Cardiac tissue also increase the release of NGF in response to injury (Zhou et al. 2004), which may contribute to the observed increase in frequency of tachyarrhythmias in damaged tissue due to increased innervation.
Myocyte–neuron co-cultures are typically investigated at microscopic scales to investigate local interactions of small clusters of cardiac cells and neurons. Elegant ultra-structural and immunohistochemistry studies in co-culture systems clarified the structure of the cardiac–neuron junction. Neurons form specialized junctions with cardiac cells, in a process that is mediated by NGF (Lockhart et al. 2000). These specialized junctions are enriched with β1 receptors which drive an increase in myocyte beat rate when the attached neurons are stimulated by nicotine (Shcherbakova et al. 2007).
Our group has recently started investigating the role of neural activation on the generation of abnormal cardiac rhythms in culture. We predominantly image activity at macroscopic scales as we are interested in the effects of abnormal neural activity from neurons from hypertensive animals on propagating wavefront stability. These experiments are challenging as it is difficult to simultaneously measure neural activity and cardiac wave propagation. Neurons are sparsely plated on dense cardiac monolayers, and, due to their low concentration and relatively small calcium transients, fluorescent signals from dye-loaded neurons are difficult to distinguish from cardiac-derived transients. We are currently exploring a variety of different techniques pioneered by other research groups, ranging from maintaining neurons and myocytes in separate but connected compartments (Takeuchi et al. 2011), to virally transfecting neurons with calcium-sensitive markers (Looger & Griesbeck, 2012) and imaging cardiac motion with a second camera using a macroscopic phase contrast approach (Hwang et al. 2005).
Macroscopic myocyte neuron co-cultures can potentially address fundamental questions that are ill-posed in intact tissues or in vivo systems (Fig.3). For example, we can vary the number and spatial organization of neurons on a monolayer to determine if there is critical density of neurons needed to generate macroscopic changes in wave propagation (Fig.3A), or whether neurons are more effective modulators of cardiac activity if they are organized in clusters or spatially dispersed (Fig.3B). We can also investigate how diseased myocytes and neurons from spontaneously hypertensive animals (e.g. the SHR rat) compare to wild-type cultures (Fig.3C), and whether the observed cardiac phenotype is determined by diseased neurons (Fig.3D) or diseased myocytes (Fig.3E) by cross-plating healthy and diseased tissue. Finally, as is the case in myocyte monocultures, each cell in the dish is experimentally accessible, which will allow direct correlation between regional neural activity and the generation of macroscopic spiral waves.
Figure 3.
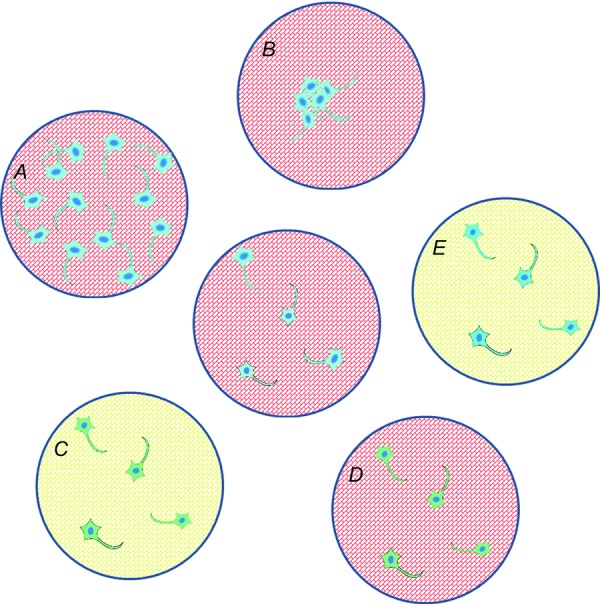
Proposed cardiac–neuron co-culture experiments
The centre monolayer is compared to dishes A–E. A and B, testing the effects of different neuron density and distribution; C–E, cross-plating experiments can isolate the effects of different tissues on macroscopic wave dynamics (blue: wild-type neurons, green: diseased neurons; red: wild-type myocytes; yellow: diseased myocytes).
Looking ahead
A key experimental challenge in this system will be to develop ways of determining how events at the neuron–cardiac junction affect cardiac wavefront stability at macroscopic scales, and relating these to in vivo behaviours. One bottleneck is the lack of ultra-high speed, high resolution cameras capable of resolving microscopic and macroscopic events simultaneously, although we anticipate that the rapid pace of new sensor development (Brady et al. 2012; Bub et al. 2013) will inevitably overcome this limitation. Capturing events at different spatial resolutions is also challenging for current commercially available optical systems, as the numerical aperture of objectives capable of imaging a large field of view limits their resolving power at microscopic scales. Here, we anticipate that the development of novel optical modalities, in the form of specialized objectives with high numerical aperture and wide field of view, or contact fluorescence imaging using a CMOS sensor (Greenbaum et al. 2012; Saini, 2012), perhaps in combination with super-resolution techniques (Gustafsson, 2005), will allow true multiscale imaging at biologically relevant space scales. Finally, we stress that any experimental finding from the monolayer co-culture preparation should be validated in intact tissue. In addition to having a simplified geometry, monolayer preparations are generated from cells harvested from neonatal animals, which will have a different phenotype from the adult. Several groups are currently exploring new methods to measure cellular dynamics in the intact heart and vasculature (Botcherby et al. 2013; Aguirre et al. 2014; Freeman et al. 2014), which we intend to apply to the study of neural cardiac interactions in vivo.
Glossary
- APD
action potential duration
- DAD
delayed afterdepolarization
- EAD
early afterdepolarization
- NGF
nerve growth factor
Biographies
Dr Gil Bub investigates the interplay of activity between neuron and cardiac cells in cell culture, as well as new technologies for imaging excitable cells. Dr Bub did his graduate work at McGill University, Canada on the dynamics of cardiac monolayers, and is currently is University Research Lecturer in Oxford’s Department of Physiology Anatomy and Genetics.
Dr Rebecca Burton obtained a DPhil in Physiology from the University of Oxford. She is currently a Paul Nurse Junior Research Fellow in Biomedical Sciences (Linacre College). Her research interests include understanding the mechanisms of arrhythmias using cardiac monolayers as well as developing novel therapies for the treatment of heart failure by modulating the funny current ‘If’.
Additional information
Competing interests
None declared.
Funding
G.B.’s research is supported by the UK Medical Research Council and the British Heart Foundation Centre of Research Excellence, Oxford (RE/08/004). R.A.B.B. is an EP Abraham Cephalosporin JRF at Linacre College, Oxford.
References
- Aguirre AD, Vinegoni C, Sebas M. Weissleder R. Intravital imaging of cardiac function at the single-cell level. Proc Natl Acad Sci USA. 2014;111:11257–11262. doi: 10.1073/pnas.1401316111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arutunyan A, Webster DR, Swift LM. Sarvazyan N. Localized injury in cardiomyocyte network: a new experimental model of ischemia-reperfusion arrhythmias. Am J Physiol Heart Circ Physiol. 2001;280:H1905–H1915. doi: 10.1152/ajpheart.2001.280.4.H1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccaglini PI. Cooper E. Influences on the expression of acetylcholine receptors on rat nodose neurones in cell culture. J Physiol. 1982;324:441–451. doi: 10.1113/jphysiol.1982.sp014123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badie N. Bursac N. Novel micropatterned cardiac cell cultures with realistic ventricular microstructure. Biophys J. 2009;96:3873–3885. doi: 10.1016/j.bpj.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botcherby EJ, Corbett A, Burton RAB, Smith CW, Bollensdorff C, Booth MJ, Kohl P, Wilson T. Bub G. Fast measurement of sarcomere length and cell orientation in Langendorff-perfused hearts using remote focusing microscopy. Circ Res. 2013;113:863–870. doi: 10.1161/CIRCRESAHA.113.301704. [DOI] [PubMed] [Google Scholar]
- Brack KE, Winter J. Ng GA. Mechanisms underlying the autonomic modulation of ventricular fibrillation initiation – tentative prophylactic properties of vagus nerve stimulation on malignant arrhythmias in heart failure. Heart Fail Rev. 2013;18:389–408. doi: 10.1007/s10741-012-9314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady DJ, Gehm ME, Stack RA, Marks DL, Kittle DS, Golish DR, Vera EM. Feller SD. Multiscale gigapixel photography. Nature. 2012;486:386–389. doi: 10.1038/nature11150. [DOI] [PubMed] [Google Scholar]
- Brodde OE, Bruck H, Leineweber K. Seyfarth T. Presence, distribution and physiological function of adrenergic and muscarinic receptor subtypes in the human heart. Basic Res Cardiol. 2001;96:528–538. doi: 10.1007/s003950170003. [DOI] [PubMed] [Google Scholar]
- Bub G, Glass L, Publicover NG. Shrier A. Bursting calcium rotors in cultured cardiac myocyte monolayers. Proc Natl Acad Sci USA. 1998;95:10283–10287. doi: 10.1073/pnas.95.17.10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bub G, Nebeker N. Light R. Novel Advances in Microsystems Technologies and Their Applications. CRC Press (Taylor and Francis Group); 2013. New approaches for high-speed, high-resolution imaging; pp. 149–167. eds. Francis LA & Iniewski K., Boca Raton, FL. [Google Scholar]
- Bub G, Shrier A. Glass L. Spiral wave generation in heterogeneous excitable media. Phys Rev Lett. 2002;88:058101. doi: 10.1103/PhysRevLett.88.058101. [DOI] [PubMed] [Google Scholar]
- Bursac N, Parker KK, Iravanian S. Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy: a model for functional electrophysiological studies of cardiac muscle. Circ Res. 2002;91:e45–e54. doi: 10.1161/01.res.0000047530.88338.eb. [DOI] [PubMed] [Google Scholar]
- Camelliti P, Gallagher JO, Kohl P. McCulloch AD. Micropatterned cell cultures on elastic membranes as an in vitro model of myocardium. Nat Protoc. 2006;1:1379–1391. doi: 10.1038/nprot.2006.203. [DOI] [PubMed] [Google Scholar]
- Chen P-S, Chen LS, Fishbein MC, Lin S-F. Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114:1500–1515. doi: 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH. Myths and realities of the cardiac vagus. J Physiol. 2013;591:4073–4085. doi: 10.1113/jphysiol.2013.257758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidenko JM, Pertsov AV, Salomonsz R, Baxter W. Jalife J. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature. 1992;355:349–351. doi: 10.1038/355349a0. [DOI] [PubMed] [Google Scholar]
- Devinsky O. Effects of seizures on autonomic and cardiovascular function. Epilepsy Curr. 2004;4:43–46. doi: 10.1111/j.1535-7597.2004.42001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimov IR, Sidorov V, Cheng Y. Wollenzier B. Evidence of three-dimensional scroll waves with ribbon-shaped filament as a mechanism of ventricular tachycardia in the isolated rabbit heart. J Cardiovasc Electrophysiol. 1999;10:1452–1462. doi: 10.1111/j.1540-8167.1999.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Entcheva E. Bien H. Macroscopic optical mapping of excitation in cardiac cell networks with ultra-high spatiotemporal resolution. Prog Biophys Mol Biol. 2006;92:232–257. doi: 10.1016/j.pbiomolbio.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Entcheva E, Lu S, Troppman R, Sharma V. Tung L. Contact fluorescence imaging of reentry in monolayers of cultured neonatal rat ventricular myocytes. J Cardiovasc Electrophysiol. 2000;11:665–676. doi: 10.1111/j.1540-8167.2000.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:S99–S105. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- Fast VG. Efimov IR. Stability of vortex rotation in an excitable cellular medium. Phys D Nonlinear Phenom. 1991;49:75–81. [Google Scholar]
- Fenton FH, Cherry EM, Hastings HM. Evans SJ. Multiple mechanisms of spiral wave breakup in a model of cardiac electrical activity. Chaos. 2002;12:852–892. doi: 10.1063/1.1504242. [DOI] [PubMed] [Google Scholar]
- Freeman K, Tao W, Sun H, Soonpaa MH. Rubart M. In situ three-dimensional reconstruction of mouse heart sympathetic innervation by two-photon excitation fluorescence imaging. J Neurosci Methods. 2014;221:48–61. doi: 10.1016/j.jneumeth.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furshpan EJ, MacLeish PR, O’Lague PH. Potter DD. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci USA. 1976;73:4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova NA. Bures J. Spiral waves of spreading depression in the isolated chicken retina. J Neurobiol. 1983;14:353–363. doi: 10.1002/neu.480140503. [DOI] [PubMed] [Google Scholar]
- Greenbaum A, Luo W, Su T-W, Göröcs Z, Xue L, Isikman SO, Coskun AF, Mudanyali O. Ozcan A. Imaging without lenses: achievements and remaining challenges of wide-field on-chip microscopy. Nat Methods. 2012;9:889–895. doi: 10.1038/nmeth.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson MGL. Nonlinear structured-illumination microscopy: wide-field fluorescence imaging with theoretically unlimited resolution. Proc Natl Acad Sci USA. 2005;102:13081–13086. doi: 10.1073/pnas.0406877102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S-M, Kim TY. Lee KJ. Complex-periodic spiral waves in confluent cardiac cell cultures induced by localized inhomogeneities. Proc Natl Acad Sci USA. 2005;102:10363–10368. doi: 10.1073/pnas.0501539102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G. Forgacs G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010;2:022001. doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalife J. Rotors and spiral waves in atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14:776–780. doi: 10.1046/j.1540-8167.2003.03136.x. [DOI] [PubMed] [Google Scholar]
- Julius S. Effect of sympathetic overactivity on cardiovascular prognosis in hypertension. Eur Heart J. 1998;19(Suppl. F):F14–F18. [PubMed] [Google Scholar]
- Keener JP. Tyson JJ. Spiral waves in the Belousov-Zhabotinskii reaction. Phys D Nonlinear Phenom. 1986;21:307–324. [Google Scholar]
- Levy MN. Cardiac sympathetic-parasympathetic interactions. Fed Proc. 1984;43:2598–2602. [PubMed] [Google Scholar]
- Liu Y-B, Wu C-C, Lu L-S, Su M-J, Lin C-W, Lin S-F, Chen LS, Fishbein MC, Chen P-S. Lee Y-T. Sympathetic nerve sprouting, electrical remodeling, and increased vulnerability to ventricular fibrillation in hypercholesterolemic rabbits. Circ Res. 2003;92:1145–1152. doi: 10.1161/01.RES.0000072999.51484.92. [DOI] [PubMed] [Google Scholar]
- Lockhart S, Mead J, Pisano J, Slonimsky J. Birren S. Nerve growth factor collaborates with myocyte-derived factors to promote development of presynaptic sites in cultured sympathetic neurons. J Neurobiol. 2000;42:460–476. [PubMed] [Google Scholar]
- Lockhart S, Turrigiano G. Birren S. Nerve growth factor modulates synaptic transmission between sympathetic neurons and cardiac myocytes. J Neurosci. 1997;17:9573–9582. doi: 10.1523/JNEUROSCI.17-24-09573.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looger LL. Griesbeck O. Genetically encoded neural activity indicators. Curr Opin Neurobiol. 2012;22:18–23. doi: 10.1016/j.conb.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Magome N. Agladze K. Patterning and excitability control in cardiomyocyte tissue culture. Phys D Nonlinear Phenom. 2010;239:1560–1566. [Google Scholar]
- Mantravadi R, Gabris B, Liu T, Choi B-R, de Groat WC, Ng GA. Salama G. Autonomic nerve stimulation reverses ventricular repolarization sequence in rabbit hearts. Circ Res. 2007;100:e72–80. doi: 10.1161/01.RES.0000264101.06417.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JFR, Kasparov S. Paterson DJ. Nitric oxide and autonomic control of heart rate: a question of specificity. Trends Neurosci. 2002;25:626–631. doi: 10.1016/s0166-2236(02)02261-0. [DOI] [PubMed] [Google Scholar]
- Pertsov A. Ermakova E. Mechanism of the drift of a spiral wave in an inhomogeneous medium. Biofizika. 1988;33:338–342. [Google Scholar]
- Rohr S, Kucera J, Fast V. Kleber A. Paradoxical improvement of impulse conduction in cardiac tissue by partial cellular uncoupling. Science. 1997;275:841–844. doi: 10.1126/science.275.5301.841. [DOI] [PubMed] [Google Scholar]
- Rohr S, Scholly DM. Kleber AG. Patterned growth of neonatal rat heart cells in culture. Morphological and electrophysiological characterization. Circ Res. 1991;68:114–130. doi: 10.1161/01.res.68.1.114. [DOI] [PubMed] [Google Scholar]
- Rubart M. Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest. 2005;115:2305–2315. doi: 10.1172/JCI26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini A. Microscopy. New lens offers scientist a brighter outlook. Science. 2012;335:1562–1563. doi: 10.1126/science.335.6076.1562. [DOI] [PubMed] [Google Scholar]
- Shcherbakova OG, Hurt CM, Xiang Y, Dell’Acqua ML, Zhang Q, Tsien RW. Kobilka BK. Organization of β-adrenoceptor signaling compartments by sympathetic innervation of cardiac myocytes. J Cell Biol. 2007;176:521–533. doi: 10.1083/jcb.200604167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MJ. Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–1021. doi: 10.1161/CIRCRESAHA.113.302549. [DOI] [PubMed] [Google Scholar]
- Shibata M. Bures J. Reverberation of cortical spreading depression along closed-loop pathways in rat cerebral cortex. J Neurophysiol. 1972;35:381–388. doi: 10.1152/jn.1972.35.3.381. [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Nakafutami S, Tani H, Mori M, Takayama Y, Moriguchi H, Kotani K, Miwa K, Lee J, Noshiro M. Jimbo Y. Device for co-culture of sympathetic neurons and cardiomyocytes using microfabrication. Lab Chip. 2011;11:2268–2275. doi: 10.1039/c0lc00327a. [DOI] [PubMed] [Google Scholar]
- Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fishbein MC, Sharifi B. Chen P-S. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res. 2004;95:76–83. doi: 10.1161/01.RES.0000133678.22968.e3. [DOI] [PubMed] [Google Scholar]


