Abstract
Why sympathetic activity rises in neurogenic hypertension remains unknown. It has been postulated that changes in the electrical excitability of medullary pre-sympathetic neurones are the main causal mechanism for the development of sympathetic overactivity in experimental hypertension. Here we review recent data suggesting that enhanced sympathetic activity in neurogenic hypertension is, at least in part, dependent on alterations in the electrical excitability of medullary respiratory neurones and their central modulation of sympatho-excitatory networks. We also present results showing a critical role for carotid body tonicity in the aetiology of enhanced central respiratory modulation of sympathetic activity in neurogenic hypertension. We propose a novel hypothesis of respiratory neurone channelopathy induced by carotid body overactivity in neurogenic hypertension that may contribute to sympathetic excess. Moreover, our data support the notion of targeting the carotid body as a potential novel therapeutic approach for reducing sympathetic vasomotor tone in neurogenic hypertension.
Introduction
The mechanisms underlying the increased arterial pressure in neurogenic hypertension are not fully understood. The role of sympathetic outflow in the pathogenesis of hypertension has been an issue of continuous interest for several years. It has been described that sympathetic overactivity is present in hypertensive patients and populations at risk in developing hypertension (Grassi, 1998; Esler, 2000; Schlaich et al. 2004; Fisher & Paton, 2012). In a rat model of neurogenic hypertension, the spontaneously hypertensive (SH) rat, there is a significant increase in sympathetic activity and vascular resistance even in neonatal and juvenile rats (Simms et al. 2009, 2010). In addition, sympathectomy in young SH rats prevents the increase in arterial pressure and vascular hypertrophy (Korner et al. 1993; Zicha & Kunes, 1999). In this regard, it is established that the rostral ventrolateral medulla (RVLM) pre-sympathetic neurones are a main source of excitatory drive to sympathetic pre-ganglionic neurones (Guertzenstein & Silver, 1974; Ross et al. 1984; Dampney et al. 2003; Guyenet, 2006). Despite a lack of direct evidence, it has been postulated that changes in the electrical excitability of RVLM pre-sympathetic neurones are the main causal mechanism for the development of sympathetic overactivity in experimental hypertension (Bergamaschi et al. 1995; Ito et al. 2000, 2001; Stocker et al. 2007).
We have addressed this issue using the ventral approach of the in situ perfused preparation of rat (Paton, 1996), which has mechanical stability allowing long-term intracellular recording of brainstem (Fig.1; Dutschmann & Paton, 2003; Paton & St-John, 2005). We used whole cell patch clamp to record RVLM barosensitive bulbospinal pre-sympathetic neurones in pre-hypertensive SH and Wistar rats at 3–4 weeks old (Moraes et al. 2014d). In SH rats, there was increased vascular resistance and sympathetic activity in both lumbar and cervical nerves and increased firing frequency in two different populations of RVLM pre-sympathetic neurones: one which expressed enzymes required for the production of adrenaline (Fig.2Ai and ii, and Ci–iii), and thus part of the C1 cell group, and the other which was found to be non-catecholaminergic (Fig.3Ai and ii, and Ci–iii; Moraes et al. 2013a).
Figure 1.
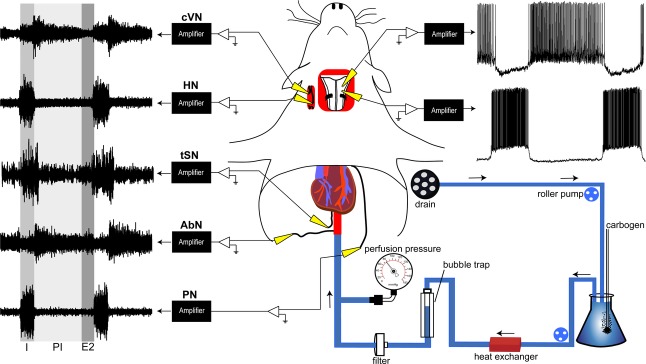
Schematic representation of ventral approach of in situ perfused preparation of rats
The brainstem was adequately oxygenated by perfusing carbogenated Ringer solution at 31°C via descending aorta. Perfusion pressure was recorded via the catheter inside the aorta. A eupnoeic respiratory motor pattern was recorded from phrenic (PN), abdominal (AbN), hypoglossal (HN) and cervical vagus (cVN) nerves, which showed three respiratory phases (inspiration (I), post-inspiration (PI) and second half of expiration (E2). The sympathetic outflow was recorded from thoracic sympathetic nerve (tSN). Note the respiratory modulation of sympathetic activity starting during I with the peak at PI. The trachea, oesophagus, all muscles and connective tissues covering the basilar surface of occipital bone were removed. The basilar portion of the atlantooccipital membrane was also cut and the bone carefully removed to expose the ventral surface of the medulla in the anteroposterior extension from the vertebral arteries to the pontine nuclei. Blind whole cell patch clamp recordings were made from pre-sympathetic and respiratory neurones located within ventrolateral medulla (Moraes et al. 2013a). Recordings of ventral medullary expiratory (upper) and inspiratory (lower) neurones are shown in the upper right corner.
Figure 2.
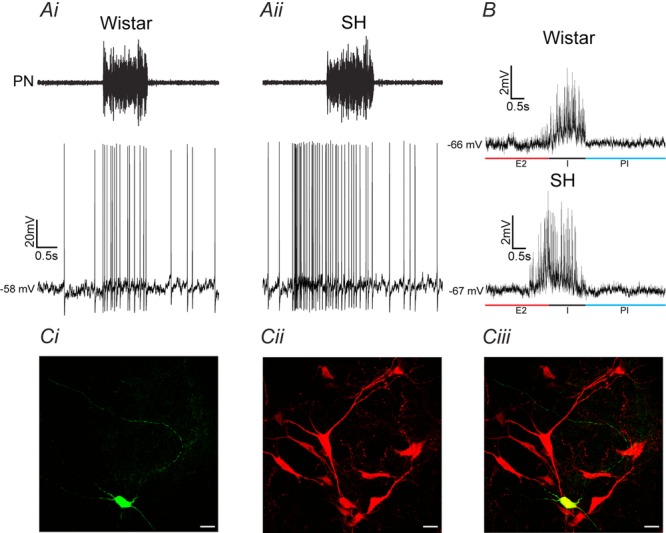
Inspiratory-modulated RVLM pre-sympathetic neurones from Wistar and SH rats
Raw records of phrenic nerve (PN) and whole cell patch clamp of RVLM pre-sympathetic neurones with inspiratory modulation in Wistar (Ai) and SH (Aii) rats. All RVLM pre-sympathetic neurones were barosensitive and spinal stimulation (T8–T12) evoked constant latency antidromic action potentials. SH rats showed higher firing frequency because of the additional action potentials during the pre-inspiratory phase (Moraes et al. 2014c). B, reduced spike discharge frequency with a small continuous hyperpolarizing current revealed spontaneous excitatory postsynaptic potentials (sEPSPs) during inspiration (I) in RVLM pre-sympathetic neurones from Wistar and SH rats. Additional sEPSPs were also observed at the end of second half of expiration (E2) or pre-inspiratory phase only in RVLM pre-sympathetic neurones from SH rat. C, photomicrographs of one representative RVLM pre-sympathetic neurone with inspiratory modulation labelled with biocytin (i), tyrosine hydroxylase (TH) immunohistochemical staining (ii) and its co-localization (iii). Note that this RVLM pre-sympathetic neurone is a positive TH cell as shown in a previous study (Moraes et al. 2013a). PI: post-inspiratory phase. Scale bar, 20 μm.
Figure 3.
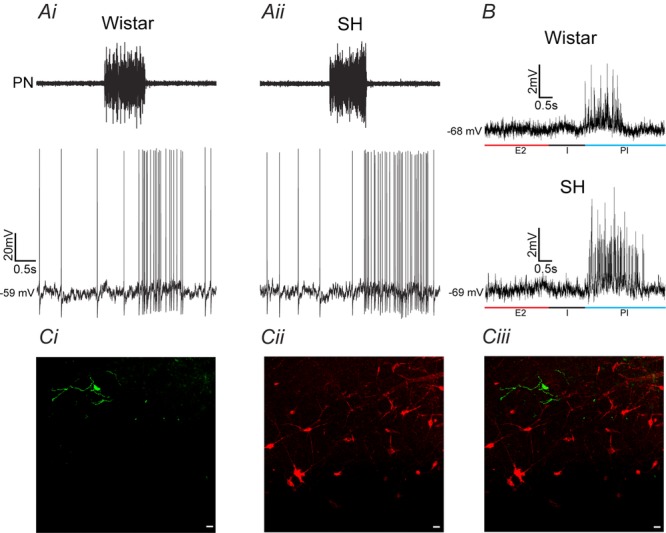
Post-inspiratory-modulated RVLM pre-sympathetic neurones from Wistar and SH rats
Raw records of phrenic nerve (PN) and whole cell patch clamp of RVLM pre-sympathetic neurones with post-inspiratory modulation in Wistar (Ai) and SH (Aii) rats. All RVLM pre-sympathetic neurones were barosensitive and spinal stimulation (T8–T12) evoked constant latency antidromic action potentials. SH rats showed increased firing frequency because of the additional action potentials during the post-inspiratory phase (Moraes et al. 2014c). B, reduced spike discharge frequency with a small continuous hyperpolarizing current revealed sEPSPs during post-inspiration (PI) in RVLM pre-sympathetic neurones from Wistar and SH rats. Increases in frequency and amplitude of sEPSPs were also observed during PI in RVLM pre-sympathetic neurones from SH rat. C, photomicrographs of one representative RVLM pre-sympathetic neurone with post-inspiratory modulation labelled with biocytin (i), tyrosine hydroxylase (TH) immunohistochemical staining (ii) and its overlay (iii). Note that this RVLM pre-sympathetic neurone is a negative TH cell as shown in a previous study (Moraes et al. 2013a). I: inspiration; E2: second half of expiration. Scale bar, 20 μm.
We next evaluated whether sympathetic overactivity and increased vascular resistance in SH rats is determined by an increase in the intrinsic excitability of these RVLM pre-sympathetic neurones. By comparing RVLM pre-sympathetic neurones from SH and Wistar rats after blockade of fast excitatory and inhibitory synaptic transmission, their intrinsic pacemaker firing frequency and intrinsic electrophysiological properties were found to be similar (Moraes et al. 2014d). Therefore, these data do not support the current dogma that sympathetic overactivity in neurogenic hypertension is due to an elevated intrinsic excitability of RVLM pre-sympathetic neurones (Matsuura et al. 2002).
Our recordings of RVLM pre-sympathetic neurones in in situ preparations of rats showed the expected respiratory-related activity (Fig.2Ai and ii; Fig.3Ai and ii; Haselton & Guyenet, 1989; Moraes et al. 2013a, 2014b,d). Notably, there was a dramatic enhancement of the respiratory-related excitatory modulation of their firing frequency during the post-inspiratory period in SH rats compared to Wistar rats (Fig.3Ai and ii). Additional action potentials were also observed during the pre-inspiratory phase (before inspiratory phrenic nerve activity) in inspiratory-modulated RVLM pre-sympathetic neurones from SH but not Wistar rats (Fig.2Ai and ii). Considering the absence of changes in the intrinsic electrophysiological properties of RVLM pre-sympathetic neurones in SH rats (Moraes et al. 2014d), we next evaluated the respiratory-related spontaneous excitatory postsynaptic potentials. By hyperpolarizing the neurones (∼ −70 mV) to eliminate the action potentials, our results showed that the increased firing frequency of these different RVLM pre-sympathetic neurones in SH rats is due to increased synaptic drive from respiratory neurones during the pre-inspiratory phase in C1 neurones (Fig.2B) and during the post-inspiratory phase in non-C1 neurones (Fig.3B).
Medullary respiratory neurones: are they new players in generating sympathetic overactivity in neurogenic hypertension?
Altered respiratory modulation of sympathetic activity in neurogenic hypertension was first described in experiments using adult SH rats, in which changes in the pattern of the respiratory-modulated peak of sympathetic activity was observed (Czyzyk-Krzeska & Trzebski, 1990). These changes are not a consequence of the hypertensive state, since it was described subsequently in pre-hypertensive neonatal and juvenile SH rats (Simms et al. 2009, 2010). Rats submitted to chronic intermittent hypoxia (CIH), as a rodent model of obstructive sleep apnoea (OSA), and rats submitted to sustained hypoxia, also exhibited hypertension associated with an enhancement of sympathetic activity in the expiratory phase of the respiratory cycle. This also translated to increased expiratory activity of abdominal nerves and muscles triggering forced exhalation (Zoccal et al. 2008; Moraes et al. 2013a, 2014b). Thus, we can state that under various conditions of hypertension the respiratory pattern changes and animals no longer expire passively, but actively, at rest: a pathological behaviour. Alterations in the relationship between control of respiration and sympathetic neural drive have also been proposed to underlie the increased sympathetic outflow in chronic heart failure rats and humans (Goso et al. 2001; Marcus et al. 2013). It should be noted that changes in respiratory modulation of sympathetic activity were also described in hypertensive OSA patients. Fatouleh et al. (2014) examined the temporal profile of respiratory modulation of muscle sympathetic nerve activity and demonstrated that respiratory–sympathetic coupling was stronger in the OSA patients than in the control subjects. Although there are distinctions in the temporal patterning and phase of respiratory modulation of sympathetic activity between different models of hypertension, we propose that changes in the respiratory pattern and/or strength of its central modulation of sympatho-excitatory networks contribute to its overactive state in the hypertensive phenotype.
How then does respiration produce sympathetic overactivity since there is no evidence of any augmentation in inspiratory activity either in terms of amplitude or frequency in rodent models of hypertension (Zoccal et al. 2008; Simms et al. 2009; Moraes et al. 2014b) or even in hypertensive patients (Fatouleh et al. 2014)? It should be pointed out that the frequent measurement of respiratory output is typically central inspiratory drive only; there is often no measure of centrally generated expiratory drive, either post-inspiration or late-expiratory activity (or pre-inspiration), which is better reflected in recordings of respiratory motor outputs to the upper airway and abdominal muscles, respectively (Smith et al. 2007, 2013). To evaluate enhanced expiratory modulation of sympathetic activity as a potential mechanism for neurogenic hypertension, we sought to determine differences in respiratory pattern generation within SH rats (Moraes et al. 2014d). Our recent data showed that the respiratory network of SH rat is reconfigured to a pattern dominated by heightened respiratory motor activity and control of upper airway resistance during pre-inspiration and post-inspiration, without changes in inspiration. This was caused by increasing the intrinsic excitability of both ventral medullary pre-inspiratory neurones located in the pre-Bötzinger complex (pre-BötC) and post-inspiratory neurones located in the Bötzinger complex (BötC) (Smith et al. 2007, 2013). Therefore, we suggest that these respiratory neurones provide the additional synaptic-mediated excitatory drive to RVLM pre-sympathetic neurones that explains the enhanced phase-related respiratory modulation of sympathetic nerves in SH rats.
In these different medullary respiratory neurones distinct potassium channels appear responsible for generating the enhanced intrinsic neuronal excitability (Moraes et al. 2014d). The increased excitability of BötC post-inspiratory neurones was due to reductions in potassium currents mediated by the large conductance calcium-activated potassium channel (BKCa) producing less spike frequency adaptation. Blocking BKCa increased post-inspiratory neurone excitability in normotensive rats, but was ineffective in SH rats, suggesting its loss in this rat strain. Importantly, blocking BKCa within the BötC of normotensive rats also enhanced the respiratory modulation of sympathetic activity (similar to the SH rat), but was ineffective in SH rats. Additionally, the increased excitability of pre-BötC pre-inspiratory neurones in SH rats was due to a reduction in a pH-sensitive potassium-dominated leak conductance (Koizumi et al. 2010; Moraes et al. 2014d). We rescued the hyperexcitability of pre-inspiratory neurones from SH rats by inhibiting the leak conductance with 3% CO2. Altogether, these data provide strong evidence suggesting the intriguing idea that sympathetic overactivity in a neurogenic model of hypertension results, at least in part, from changes in potassium channels in selective respiratory neurone types, as we found in other models of hypertension (Moraes et al. 2013b, 2014b; Almado et al. 2014).
Although the above experiments suggest a possible respiratory neurone channelopathy for neurogenic hypertension, major questions still remain: are these respiratory neurones unique in having changes in their intrinsic membrane properties, making them more electrically excitable in the hypertensive phenotype; are there other neural pathways enhancing respiratory modulation of sympathetic activity and contributing to increased vascular resistance and hypertension in rats. In angiotensin II–salt hypertension, RVLM pre-sympathetic neurones also show enhanced post-inspiratory-related activity (Toney et al. 2010), suggesting that the proposed central respiratory modulation of sympathetic activity also exists in an environmentally induced hypertensive phenotype. On the other hand, rats submitted to chronic hypoxia (intermittent or sustained) presented increased firing frequency in RVLM pre-sympathetic neurones modulated at the end of expiration (or pre-inspiration), but not during post-inspiration (Moraes et al. 2013a, 2014b). Thus, it seems that there are multiple mechanisms that can trigger enhanced phasic excitatory synaptic drives to sympatho-excitatory networks to enhance the respiratory modulation of sympathetic activity in hypertensive animals. A recent schematic representation for the central generation of respiratory and sympathetic activities has been proposed that depicts intersecting networks involved in the generation of respiratory and sympathetic rhythms and patterns (Baekey et al. 2010; Molkov et al. 2011). The specific interaction between medullary respiratory neurones and RVLM pre-sympathetic neurones has been difficult to establish using only electrophysiology and pharmacological approaches, but recent experiments targeting respiratory neurone populations optogenetically (Abdala et al. 2014) is now allowing temporal control of respiratory neural activity that is needed to study its impact on sympatho-excitatory networks (Moraes et al. 2014a). Additionally, optogenetic control of respiratory neurones allows either reduced or increased control of excitability of medullary inspiratory and expiratory neurones. Such an approach will be crucial to better understand the mechanisms of respiratory–sympathetic coupling and, with selective genetic targeting of specific respiratory neuronal phenotypes, provide the proof of principle demonstrating that altering medullary respiratory neurone excitability can directly drive sympathetic overactivity in neurogenic hypertension. This could then lead to novel approaches for controlling sympathetic activity generation chronically, which would have clinical implications.
What induces channelopathy in respiratory neurones and increases sympatho-excitatory network activity in SH rats?
Accumulating evidence suggests a critical role for the carotid body (CB) peripheral chemoreceptors in cardiovascular disease states, including neurogenic hypertension (Abdala et al. 2012; Paton et al. 2013; Prabhakar, 2013). Peripheral chemoreceptor reflex sensitivity has been shown to be significantly enhanced in both patients with neurogenic hypertension and SH rats (Tafil-Klawe et al. 1985; Somers et al. 1988a, b1988; Tan et al. 2010; Sinski et al. 2012). This is consistent with the increased minute ventilation (Honig et al. 1981), larger carotid bodies (Fukuda et al. 1987) and enhanced carotid sinus nerve discharge during hypoxia in SH rats (Weil et al. 1998). Repeated CB activation in both rats and humans produces enhanced respiratory modulation of sympathetic activity and increased muscle vasoconstrictor activity, which may contribute to the development of hypertension (Narkiewicz & Somers, 2003; Zoccal et al. 2008; Simms et al. 2009). In this regard, carotid sinus nerve denervation has been shown not only to prevent the development of hypertension, but also reduce established hypertension in the SH rat (Abdala et al. 2012; McBryde et al. 2013). With direct inputs to the caudal aspects of nucleus tractus solitarii, CB chemoreceptors generate sympathetic activity reflexly by activating RVLM pre-sympathetic neurones (Haselton & Guyenet, 1989; Sun & Reis, 1994a, b), and indirectly by exciting expiratory neurones that in turn send projections to these sympathetic neurones (Moraes et al. 2012). Interestingly, CB stimulation increases both pre-inspiratory and post-inspiratory neuronal activities (Paton, 1997), which may contribute to the higher sympatho-excitation response in SH rats (Tan et al. 2010) and their modulation (Moraes et al. 2014c). Importantly, carotid chemoreceptors are essential for inducing chronic potentiation of sympathetic activity and hypertension seen after chronic intermittent hypoxia (Fletcher et al. 1992; Peng et al. 2014; Moraes & Machado, 2015) or heart failure (Marcus et al. 2013) in rats. Thus, we hypothesize that chronic activation of CB chemoreceptors can cause long-term synaptic plasticity within brainstem sympatho-excitatory networks, by increasing the respiratory synaptic inputs to RVLM pre-sympathetic neurones.
To address this hypothesis, we evaluated the contribution of CB afferent inputs to the increased intrinsic excitability of medullary post-inspiratory neurones in SH rats (Moraes et al. 2014c). Whole cell patch recordings of BötC post-inspiratory neurones were performed in in situ preparations of Wistar, SH and CB-denervated (CBD) SH rats at 3–4 weeks old (CB denervation 5 days before the experiments; McBryde et al. 2013; Moraes & Machado, 2015). In these experiments, post-inspiratory neurones from SH rats presented increased firing frequency and intrinsic excitability due to a reduction in their spike frequency adaptation in relation to post-inspiratory neurones from Wistar rats, as we showed before (Moraes et al. 2014d). CBD was able to normalize the intrinsic excitability and firing frequency of post-inspiratory neurones from SH rats by enhancing their spike frequency adaptation (Moraes et al. 2014c). Considering that BKCa is expressed in post-inspiratory neurones and it is also involved in their spike frequency adaptation (Pierrefiche et al. 1995), we next evaluated whether the effects of CBD on intrinsic excitability of post-inspiratory neurones of SH rats is due to an increase of potassium currents mediated by BKCa (Moraes et al. 2014c). Using voltage-clamp configuration all tested neurones were initially held at −40 mV to avoid activation of transient outward potassium currents, followed by 2 s of voltage test pulses from −80 to 60 mV, with 10 mV of increments. Iberiotoxin (IBTX)-sensitive outward currents were determined as the indicator of BKCa activity. The total IBTX-sensitive outward current was significantly reduced in post-inspiratory neurones from SH rats in relation to neurones from Wistar rats, without changes in BKCa activation properties. Five days after CBD, post-inspiratory neurones from SH rats exhibited similar levels of the IBTX-sensitive outward current relative to neurones from Wistar rats (Moraes et al. 2014c). These data suggest that CB afferent inputs to the brainstem are capable of modulating specifically the expression of BKCa in medullary post-inspiratory neurones.
In addition to the effect on BKCa and intrinsic properties of post-inspiratory neurones, CBD was effective in normalizing the higher baseline perfusion pressure and increased post-inspiratory-related bursts of lumbar sympathetic nerve (lSN) activity of SH rats (Moraes et al. 2014c). We used phrenic cycle-triggered averaging to analyse the temporal relationship between respiratory cycle, lSN activity and the respiratory change in perfusion pressure (Traube–Hering waves) in in situ preparations of rats (Simms et al. 2009). Across preparations the average level of perfusion pressure, Traube–Hering waves and post-inspiratory-related lSN activity was significantly greater in SH in relation to Wistar rats. CBD in SH rats eliminated the higher baseline perfusion pressure, Traube–Hering waves and post-inspiratory-related bursts of lSN activity (Moraes et al. 2014c). These findings indicate that the augmentation of medullary neuronal post-inspiratory activity and its modulation on lSN activity and perfusion pressure seen in SH rats is the product of heightened activity of CB chemoreceptors in this rat strain.
How might CB overactivity produce respiratory neurone channelopathy?
Neuronal network plasticity requires communication between electrical events in cell membrane surface, intracellular calcium signalling and nuclear gene expression (Ma et al. 2014). Therefore, basic questions arise about the mechanisms that link CB-induced respiratory neuronal overactivity to transcriptional events (of BKCa, for example) and future studies are now needed to evaluate: (i) whether neuronal depolarization or synaptic activity per se determine the changes in respiratory neurone excitability, and in particular BKCa, and (ii) whether there is a link between specific pattern of synaptic inputs from CB (intermittent or tonic activation) to a restricted set of transcriptional events (e.g. specific ionic channels) in respiratory neurones or even whether this is restricted to functionally specific types of respiratory neurone. We propose that a single cell transcriptomic analysis of specific medullary respiratory neurone types in different models of hypertension with and without intact CB will help determine the mechanisms of respiratory neurone channelopathy, hence, a mechanism of neurogenic hypertension.
Clinical perspectives
There is now overwhelming evidence of elevations in CB activity in terms of both hyper-reflexia and tonicity affecting ventilatory and sympathetic systems in hypertension. We have described here that this is due to alterations in the respiratory pattern and a change from passive to active expiration in SH rats that increases the generation of sympathetic output. Future clinical studies are now needed to determine the role of the CB in human hypertension and whether this also involves changing expiratory motor behaviour. Moreover, our data support the notion of targeting the CB as a potential novel therapy for hypertension. A key objective will be to reduce the sensitivity and tonicity of this organ without removing chemoreflex function in animal models and human hypertensive patients; this remains a critical challenge for the future.
Acknowledgments
We would like to thank Melina Pires da Silva for help with Fig.1 construction.
Glossary
- BKCa
large conductance calcium-activated potassium channel
- BötC
Bötzinger complex
- CB
carotid body
- CBD
carotid body denervated
- RVLM
rostral ventrolateral medulla
- SH
spontaneously hypertensive
Biographies
Davi J. A.Moraes received a PhD in Physiology from the University of São Paulo, Brazil (2011). He was postdoctoral fellow in the University of São Paulo and University of Bristol (2012-2014). He is now an Assistant Professor at the Department of Physiology, School of Medicine of Ribeirão Preto, University of São Paulo, exploring the maturation of brainstem circuitry, at cellular and molecular levels, involved in generation of respiratory rhythm and pattern during postnatal development.
Benedito H. Machado is Professor at the Department of Physiology, School of Medicine of Ribeirão Preto, University of São Paulo, Brazil, and the focus of his cardiovascular, respiratory and autonomic studies are the synaptic transmission of the cardiovascular reflexes in the NTS; the central mechanisms underlying the sympathetic overactivity observed in models of neurogenic hypertension and the changes in the coupling of sympathetic and respiratory neural activities in rats submitted to experimental models of hypoxia.
Additional information
Competing interests
The authors have no competing interests to disclose.
Funding
J.F.R.P. is funded by the Biotechnology and Biological Science Research Council, the British Heart Foundation and the National Institutes of Health (R01 NS069220). B.H.M. is funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (Thematic Project 2013/06077-5). D.J.A.M. is funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (Young Investigator Project 2013/10484-5).
References
- Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV. Paton JF. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol. 2012;590:4269–4277. doi: 10.1113/jphysiol.2012.237800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala AP, Paton JF. Smith JC. Defining inhibitory neurone function in respiratory circuits: opportunities with optogenetics? J Physiol. 2014 doi: 10.1113/jphysiol.2014.280610. (in press; DOI: 10.1113/jphysiol.2014.280610 ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almado CE, Leao RM. Machado BH. Intrinsic properties of rostral ventrolateral medulla presympathetic and bulbospinal respiratory neurons of juvenile rats are not affected by chronic intermittent hypoxia. Exp Physiol. 2014;99:937–950. doi: 10.1113/expphysiol.2013.077800. [DOI] [PubMed] [Google Scholar]
- Baekey DM, Molkov YI, Paton JF, Rybak IA. Dick TE. Effect of baroreceptor stimulation on the respiratory pattern: insights into respiratory–sympathetic interactions. Respir Physiol Neurobiol. 2010;174:135–145. doi: 10.1016/j.resp.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi C, Campos RR, Schor N. Lopes OU. Role of the rostral ventrolateral medulla in maintenance of blood pressure in rats with Goldblatt hypertension. Hypertension. 1995;26:1117–1120. doi: 10.1161/01.hyp.26.6.1117. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF. Trzebski A. Respiratory-related discharge pattern of sympathetic nerve activity in the spontaneously hypertensive rat. J Physiol. 1990;426:355–368. doi: 10.1113/jphysiol.1990.sp018142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dampney RA, Horiuchi J, Tagawa T, Fontes MA, Potts PD. Polson JW. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand. 2003;177:209–218. doi: 10.1046/j.1365-201X.2003.01070.x. [DOI] [PubMed] [Google Scholar]
- Dutschmann M. Paton JF. Whole cell recordings from respiratory neurones in an arterially perfused in situ neonatal rat preparation. Exp Physiol. 2003;88:725–732. doi: 10.1113/eph8802639. [DOI] [PubMed] [Google Scholar]
- Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13:99S–105S. doi: 10.1016/s0895-7061(00)00225-9. [DOI] [PubMed] [Google Scholar]
- Fatouleh R, McKenzie DK. Macefield VG. Respiratory modulation of muscle sympathetic nerve activity in obstructive sleep apnoea. Exp Physiol. 2014;99:1288–1298. doi: 10.1113/expphysiol.2013.077511. [DOI] [PubMed] [Google Scholar]
- Fisher JP. Paton JF. The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens. 2012;26:463–475. doi: 10.1038/jhh.2011.66. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Behm R, Miller CC, 3rd, Stauss H. Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol (1985) 1992;72:1978–1984. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Sato A. Trzebski A. Carotid chemoreceptor discharge responses to hypoxia and hypercapnia in normotensive and spontaneously hypertensive rats. J Auton Nerv Syst. 1987;19:1–11. doi: 10.1016/0165-1838(87)90139-1. [DOI] [PubMed] [Google Scholar]
- Goso Y, Asanoi H, Ishise H, Kameyama T, Hirai T, Nozawa T, Takashima S, Umeno K. Inoue H. Respiratory modulation of muscle sympathetic nerve activity in patients with chronic heart failure. Circulation. 2001;104:418–423. doi: 10.1161/hc2901.093111. [DOI] [PubMed] [Google Scholar]
- Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens. 1998;16:1979–1987. doi: 10.1097/00004872-199816121-00019. [DOI] [PubMed] [Google Scholar]
- Guertzenstein PG. Silver A. Fall in blood pressure produced from discrete regions of the ventral surface of the medulla by glycine and lesions. J Physiol. 1974;242:489–503. doi: 10.1113/jphysiol.1974.sp010719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Haselton JR. Guyenet PG. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol. 1989;256:R739–R750. doi: 10.1152/ajpregu.1989.256.3.R739. [DOI] [PubMed] [Google Scholar]
- Honig A, Habeck JO, Pfeiffer C, Schmidt M, Huckstorf C, Rotter H. Eckermann P. The carotid bodies of spontaneously hypertensive rats (SHR): a functional and morphologic study. Acta Biol Med Ger. 1981;40:1021–1030. [PubMed] [Google Scholar]
- Ito S, Komatsu K, Tsukamoto K. Sved AF. Excitatory amino acids in the rostral ventrolateral medulla support blood pressure in spontaneously hypertensive rats. Hypertension. 2000;35:413–417. doi: 10.1161/01.hyp.35.1.413. [DOI] [PubMed] [Google Scholar]
- Ito S, Komatsu K, Tsukamoto K. Sved AF. Tonic excitatory input to the rostral ventrolateral medulla in Dahl salt-sensitive rats. Hypertension. 2001;37:687–691. [PubMed] [Google Scholar]
- Koizumi H, Smerin SE, Yamanishi T, Moorjani BR, Zhang R. Smith JC. TASK channels contribute to the K+-dominated leak current regulating respiratory rhythm generation in vitro. J Neurosci. 2010;30:4273–4284. doi: 10.1523/JNEUROSCI.4017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner P, Bobik A, Oddie C. Friberg P. Sympathoadrenal system is critical for structural changes in genetic hypertension. Hypertension. 1993;22:243–252. doi: 10.1161/01.hyp.22.2.243. [DOI] [PubMed] [Google Scholar]
- Ma H, Groth RD, Cohen SM, Emery JF, Li B, Hoedt E, Zhang G, Neubert TA. Tsien RW. γCaMKII shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell. 2014;159:281–294. doi: 10.1016/j.cell.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJ, Sobotka PA. Paton JF. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Commun. 2013;4:2395. doi: 10.1038/ncomms3395. [DOI] [PubMed] [Google Scholar]
- Marcus NJ, Del Rio R, Schultz EP, Xia XH. Schultz HD. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol. 2013;592:391–408. doi: 10.1113/jphysiol.2013.266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura T, Kumagai H, Kawai A, Onimaru H, Imai M, Oshima N, Sakata K. Saruta T. Rostral ventrolateral medulla neurons of neonatal Wistar-Kyoto and spontaneously hypertensive rats. Hypertension. 2002;40:560–565. doi: 10.1161/01.hyp.0000032043.64223.87. [DOI] [PubMed] [Google Scholar]
- Molkov YI, Zoccal DB, Moraes DJ, Paton JF, Machado BH. Rybak IA. Intermittent hypoxia-induced sensitization of central chemoreceptors contributes to sympathetic nerve activity during late expiration in rats. J Neurophysiol. 2011;105:3080–3091. doi: 10.1152/jn.00070.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes DJ, Abdala AP, Machado BH. Paton JF. Functional connectivity between Bötzinger complex glycinergic neurons and parafacial late-expiratory neurons for expiratory and sympathetic control. FASEB J. 2014;28(1 Suppl):712.17. [Google Scholar]
- Moraes DJ, Bonagamba LG, Costa KM, Costa-Silva JH, Zoccal DB. Machado BH. Short-term sustained hypoxia induces changes in the coupling of sympathetic and respiratory activities in rats. J Physiol. 2014;592:2013–2033. doi: 10.1113/jphysiol.2013.262212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes DJ, da Silva MP, Bonagamba LG, Mecawi AS, Zoccal DB, Antunes-Rodrigues J, Varanda WA. Machado BH. Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats. J Neurosci. 2013;33:19223–19237. doi: 10.1523/JNEUROSCI.3041-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes DJ, da Silva MP, Zoccal DB, Varanda WA. Machado BH. Changes in ionic currents of respiratory neurons produce sympathetic overactivity in chronic intermittent hypoxic rats. FASEB J. 2013;27(Suppl):1135.6. [Google Scholar]
- Moraes DJ. Machado BH. Electrophysiological properties of laryngeal motoneurones in rats submitted to chronic intermittent hypoxia. J Physiol. 2015;593:619–634. doi: 10.1113/jphysiol.2014.283085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes DJ, Machado BH. Paton JF. Carotid body induced post-inspiratory neuron channelopathy for neurogenic hypertension. FASEB J. 2014;28(1 Suppl):872.9. [Google Scholar]
- Moraes DJ, Machado BH. Paton JF. Specific respiratory neuron types have increased excitability that drive presympathetic neurones in neurogenic hypertension. Hypertension. 2014;63:1309–1318. doi: 10.1161/HYPERTENSIONAHA.113.02283. [DOI] [PubMed] [Google Scholar]
- Moraes DJ, Zoccal DB. Machado BH. Sympathoexcitation during chemoreflex active expiration is mediated by L-glutamate in the RVLM/Bötzinger complex of rats. J Neurophysiol. 2012;108:610–623. doi: 10.1152/jn.00057.2012. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K. Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177:385–390. doi: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Paton JF. Rhythmic bursting of pre- and post-inspiratory neurones during central apnoea in mature mice. J Physiol. 1997;502:623–639. doi: 10.1111/j.1469-7793.1997.623bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF, Ratcliffe L, Hering D, Wolf J, Sobotka PA. Narkiewicz K. Revelations about carotid body function through its pathological role in resistant hypertension. Curr Hypertens Rep. 2013;15:273–280. doi: 10.1007/s11906-013-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF. St-John WM. Long-term intracellular recordings of respiratory neuronal activities in situ during eupnea, gasping and blockade of synaptic transmission. J Neurosci Methods. 2005;147:138–145. doi: 10.1016/j.jneumeth.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Khan S, Nanduri J, Makarenko VV, Reddy VD, Vasavda C, Kumar GK, Semenza GL. Prabhakar NR. Regulation of hypoxia-inducible factor-αisoforms and redox state by carotid body neural activity in rats. J Physiol. 2014;592:3841–3858. doi: 10.1113/jphysiol.2014.273789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrefiche O, Champagnat J. Richter DW. Calcium-dependent conductances control neurones involved in termination of inspiration in cats. Neurosci Lett. 1995;184:101–104. doi: 10.1016/0304-3940(94)11179-m. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Sensing hypoxia: physiology, genetics and epigenetics. J Physiol. 2013;591:2245–2257. doi: 10.1113/jphysiol.2012.247759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Joh TH, Park DH. Reis DJ. Rostral ventrolateral medulla: selective projections to the thoracic autonomic cell column from the region containing C1 adrenaline neurons. J Comp Neurol. 1984;228:168–185. doi: 10.1002/cne.902280204. [DOI] [PubMed] [Google Scholar]
- Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A. Esler MD. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Aangiotensin neuromodulation. Hypertension. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- Simms AE, Paton JF, Allen AM. Pickering AE. Is augmented central respiratory-sympathetic coupling involved in the generation of hypertension? Respir Physiol Neurobiol. 2010;174:89–97. doi: 10.1016/j.resp.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Simms AE, Paton JF, Pickering AE. Allen AM. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension? J Physiol. 2009;587:597–610. doi: 10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinski M, Lewandowski J, Przybylski J, Bidiuk J, Abramczyk P, Ciarka A. Gaciong Z. Tonic activity of carotid body chemoreceptors contributes to the increased sympathetic drive in essential hypertension. Hypertens Res. 2012;35:487–491. doi: 10.1038/hr.2011.209. [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Borgmann A, Rybak IA. Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 2013;36:152–162. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA. Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, Mark AL. Abboud FM. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension. 1988;11:608–612. doi: 10.1161/01.hyp.11.6.608. [DOI] [PubMed] [Google Scholar]
- Somers VK, Mark AL. Abboud FM. Sympathetic activation by hypoxia and hypercapnia–implications for sleep apnea. Clin Exp Hypertens A. 1988;10(Suppl. 1):413–422. doi: 10.3109/10641968809075998. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Meador R. Adams JM. Neurons of the rostral ventrolateral medulla contribute to obesity-induced hypertension in rats. Hypertension. 2007;49:640–646. doi: 10.1161/01.HYP.0000254828.71253.dc. [DOI] [PubMed] [Google Scholar]
- Sun MK. Reis DJ. Central neural mechanisms mediating excitation of sympathetic neurons by hypoxia. Prog Neurobiol. 1994;44:197–219. doi: 10.1016/0301-0082(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Sun MK. Reis DJ. Hypoxia selectively excites vasomotor neurons of rostral ventrolateral medulla in rats. Am J Physiol. 1994;266:R245–R256. doi: 10.1152/ajpregu.1994.266.1.R245. [DOI] [PubMed] [Google Scholar]
- Tafil-Klawe M, Trzebski A, Klawe J. Palko T. Augmented chemoreceptor reflex tonic drive in early human hypertension and in normotensive subjects with family background of hypertension. Acta Physiol Pol. 1985;36:51–58. [PubMed] [Google Scholar]
- Tan ZY, Lu Y, Whiteis CA, Simms AE, Paton JF, Chapleau MW. Abboud FM. Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circ Res. 2010;106:536–545. doi: 10.1161/CIRCRESAHA.109.206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toney GM, Pedrino GR, Fink GD. Osborn JW. Does enhanced respiratory–sympathetic coupling contribute to peripheral neural mechanisms of angiotensin II–salt hypertension? Exp Physiol. 2010;95:587–594. doi: 10.1113/expphysiol.2009.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil JV, Stevens T, Pickett CK, Tatsumi K, Dickinson MG, Jacoby CR. Rodman DM. Strain-associated differences in hypoxic chemosensitivity of the carotid body in rats. Am J Physiol. 1998;274:L767–L774. doi: 10.1152/ajplung.1998.274.5.L767. [DOI] [PubMed] [Google Scholar]
- Zicha J. Kunes J. Ontogenetic aspects of hypertension development: analysis in the rat. Physiol Rev. 1999;79:1227–1282. doi: 10.1152/physrev.1999.79.4.1227. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF. Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]


