Abstract
Rett syndrome, a prototypical neurological disorder caused by loss of function of the transcriptional regulator methyl-CpG-binding protein 2 (MeCP2) gene, is associated with a severely disordered breathing pattern and reduced ventilatory CO2 sensitivity. In a mouse model of Rett syndrome (MeCP2 knockout), re-introduction of the MeCP2 gene selectively in astrocytes rescues normal respiratory phenotype. In the present study we determined whether the metabolic and/or signalling functions of astrocytes are affected by testing the hypotheses that in conditions of MeCP2 deficiency, medullary astrocytes are unable to produce/release appropriate amounts of lactate or detect changes in 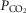 /[H+], or both. No differences in tonic or hypoxia-induced release of lactate from the ventral surface of the medulla oblongata or cerebral cortex in brain slices of MeCP2-knockout and wild-type mice were found. In brainstem slices of wild-type mice, respiratory acidosis triggered robust elevations in [Ca2+]i in astrocytes residing near the ventral surface of the medulla oblongata. The magnitude of CO2-induced [Ca2+]i responses in medullary astrocytes was markedly reduced in conditions of MeCP2 deficiency, whereas [Ca2+]i responses to ATP were unaffected. These data suggest that (i) metabolic function of astrocytes in releasing lactate into the extracellular space is not affected by MeCP2 deficiency, and (ii) MeCP2 deficiency impairs the ability of medullary astrocytes to sense changes in
/[H+], or both. No differences in tonic or hypoxia-induced release of lactate from the ventral surface of the medulla oblongata or cerebral cortex in brain slices of MeCP2-knockout and wild-type mice were found. In brainstem slices of wild-type mice, respiratory acidosis triggered robust elevations in [Ca2+]i in astrocytes residing near the ventral surface of the medulla oblongata. The magnitude of CO2-induced [Ca2+]i responses in medullary astrocytes was markedly reduced in conditions of MeCP2 deficiency, whereas [Ca2+]i responses to ATP were unaffected. These data suggest that (i) metabolic function of astrocytes in releasing lactate into the extracellular space is not affected by MeCP2 deficiency, and (ii) MeCP2 deficiency impairs the ability of medullary astrocytes to sense changes in 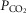 /[H+]. Taken together with the evidence of severely blunted ventilatory sensitivity to CO2 in mice with conditional MeCP2 deletion in astroglia, these data support the hypothesis of an important role played by astrocytes in central respiratory CO2/pH chemosensitivity.
/[H+]. Taken together with the evidence of severely blunted ventilatory sensitivity to CO2 in mice with conditional MeCP2 deletion in astroglia, these data support the hypothesis of an important role played by astrocytes in central respiratory CO2/pH chemosensitivity.
Key points
Rett syndrome is a prototypical neurological disorder characterised by abnormal breathing pattern and reduced ventilatory CO2 sensitivity. Medullary astrocytes are a crucial component of central CO2/pH chemosensitivity.
This study tested the hypotheses that methyl-CpG-binding protein 2 (MeCP2) deficient medullary astrocytes are (i) unable to produce/release appropriate amounts of lactate, and/or (ii) unable to sense changes in
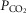 /[H+].
/[H+].
We found no differences in tonic or hypoxia-induced release of lactate from the ventral surface of the medulla oblongata or cerebral cortex between MeCP2-knockout and wild-type mice.
Respiratory acidosis triggered robust [Ca2+]i responses in wild-type astrocytes residing near the ventral surface of the medulla oblongata. CO2-induced [Ca2+]i responses in astrocytes were dramatically reduced in conditions of MeCP2 deficiency.
These data suggest that (i) ‘metabolic’ function of astrocytes in releasing lactate into the extracellular space is not affected by MeCP2 deficiency, and (ii) MeCP2 deficiency impairs the ability of medullary astrocytes to sense changes in
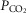 /[H+].
/[H+].
Introduction
Astrocytes provide neurones with structural and metabolic support. They are well positioned to respond rapidly to changes in chemical composition of the blood supplying the brain as well as to sense metabolic demands of the neuronal networks acting as an essential cellular component of the neurovascular interface. The astrocyte–neurone lactate shuttle hypothesis proposes that astrocytes support neuronal activity by supplying neuronal networks with metabolic substrate in the form of lactate (Magistretti et al. 1993, 1994; Pellerin & Magistretti, 1994). In low 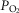 conditions (hypoxia), lactate production by astrocytes increases and appears to be essential for the recovery of synaptic function during re-oxygenation (Schurr et al. 1997a,b,c). Recent evidence suggests that astrocytes play an important role in the central nervous mechanisms controlling breathing. Astrocytes residing at and near the ventral surface of the medulla oblongata are intrinsically sensitive to changes in parenchymal
conditions (hypoxia), lactate production by astrocytes increases and appears to be essential for the recovery of synaptic function during re-oxygenation (Schurr et al. 1997a,b,c). Recent evidence suggests that astrocytes play an important role in the central nervous mechanisms controlling breathing. Astrocytes residing at and near the ventral surface of the medulla oblongata are intrinsically sensitive to changes in parenchymal  /[H+] and during hypercapnia contribute to the adaptive increases in breathing (Gourine et al. 2010).
/[H+] and during hypercapnia contribute to the adaptive increases in breathing (Gourine et al. 2010).
Mutations in the human transcriptional regulator methyl-CpG-binding protein 2 (MeCP2) gene leading to loss or reduction of protein function cause a neurodevelopmental disorder called Rett syndrome (Amir et al. 1999). Severely arrhythmic breathing which includes frequent apnoeas, hypopneas and periods of hyperventilation is a hallmark of this prototypical neurological disorder (Southall et al. 1988; Viemari et al. 2005; Ramirez et al. 2013). The respiratory dysrhythmia often results in hypoxaemia and hypocapnia (De Felice et al. 2014). In animal (mouse) models, MeCP2 deficiency is associated with significantly depressed ventilatory sensitivity to CO2, especially in response to mild and moderate levels of hypercapnia (Zhang et al. 2011; Bissonnette et al. 2014). MeCP2 deficiency also significantly increases the CO2 apnoeic threshold (Toward et al. 2013). Conversely, ventilatory responses to hypoxia appear to be enhanced (Bissonnette & Knopp, 2006; Voituron et al. 2009; Bissonnette et al. 2014), while a higher level of inspired oxygen worsens the occurrence of periodic breathing in mice with reduced MeCP2 function (Bissonnette & Knopp, 2008). Interestingly, in the affected individuals and animal models, regularity of breathing is improved by supplemental CO2 provided in the inspiratory gas mixture (Smeets et al. 2006; Bissonnette & Knopp, 2008).
MeCP2 is expressed in astrocytes (Yasui et al. 2013; Forbes-Lorman et al. 2014) and loss of MeCP2 function causes critical astrocytic defects (Okabe et al. 2012). There are in vitro data showing that the function of wild-type neurones is compromised when they are co-cultured with MeCP2-deficient astrocytes (Ballas et al. 2009; Maezawa et al. 2009; Maezawa & Jin, 2010). Earlier reports highlighted the importance of astroglial dysfunction in the development of Rett syndrome and demonstrated that re-expression of MeCP2 selectively in astrocytes rescues normal respiratory pattern in MeCP2 deficient mice (Lioy et al. 2011). Since astrocytes located at and near the ventral surface of the medulla oblongata contribute in a significant manner to the mechanisms of central respiratory CO2 chemosensitivity, we proposed that impaired astroglial CO2 sensitivity could underlie the respiratory phenotype observed in Rett syndrome.
In this study we first determined whether the ‘metabolic’ function of astrocytes is compromised by MeCP2 deficiency by measuring the amount of lactate released by the brain slices of MeCP2-null and wild-type male mice. Then we determined whether Ca2+ signalling and CO2/pH sensitivity of medullary astrocytes are affected in the absence of MeCP2 function.
Methods
All the experiments were performed in accordance with the European Commission Directive 2010/63/EU (European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes) and the UK Animals (Scientific Procedures) Act (1986).
MeCP2-null mice
In total 28 MeCP2-null and 26 littermate wild-type male mice (65–80 days old) were used for the experiments. MeCP2 is an X-linked gene subject to random X inactivation (Braunschweig et al. 2004) and MeCP2 mutant heterozygous females show mosaicism. Since phenotype depends on both cell-autonomous and non-cell-autonomous mechanisms (Braunschweig et al. 2004), only male mice were used in the current study. We used two MeCP2-null strains: B6.129P2(C)-MeCP2tm1.1Bird/J (Bird, n = 26) as a model of a complete MeCP2 knockout (Guy et al. 2001), and MeCP2tm1.1Jtc/SchvJ (Coyle, n = 2), which carries one of the most common mutations that affect humans where a non-functional truncated MeCP2 protein transcript is present (Lawson-Yuen et al. 2007). The former was maintained on a C57BL/6J background and the latter on a mixed background ∼87–93% C57BL/6J and ∼13–6% 129S6/SvEvTac. Null males were obtained from breeding heterozygous females to wild-type males. Heterozygous MeCP2tm1.1Bird females were obtained from The Jackson Laboratory (Bar Harbor, Maine, USA). Their offspring were genotyped using standard PCR, and primer sequences were as follows: common (AAA TTG GGT TAC ACC GCT GA), mutant reverse (CCA CCT AGC CTG CCT GTA CT) and wild-type reverse (CTG TAT CCT TGG GTC AAG CTG). Heterozygous MeCP2tm1.1Jtc were donated by Dr Laura R. Schaevitz (Tufts University). Their offspring were genotyped using Sanger sequencing, and primer sequences were as follows: MeCP2 forward (AGG TGG GAG ACA CCT CCT TG) and MeCP2 reverse (GAC CTG GGC AGA TGT GGT AG).
Slice preparation
Mice were terminally anaesthetised with a halothane inhalation overdose. The brains were removed and placed in ice cold artificial cerebrospinal fluid (aCSF) containing 124 mm NaCl, 26 mm NaHCO3, 3 mm KCl, 2 mm CaCl2, 1.25 mm NaH2PO4, 1 mm MgSO4, 10 mm glucose, saturated with 95% O2–5% CO2 (pH 7.4) with an addition of 9 mm Mg2+. Horizontal brainstem slices containing the ventral surface of the medulla oblongata (thickness ∼300 μm; the pia mater was kept intact and undisturbed during slicing) or coronal brainstem and cortical slices (thickness 250 μm) were cut using a vibrating-blade microtome and then incubated at room temperature for 1 h in a standard aCSF saturated with 95% O2–5% CO2.
Biosensor recordings
Lactate release was measured using amperometric lactate biosensors (Sarissa Biomedical, Coventry, UK) placed in direct contact with the surface of the slice. A lactate biosensor uses lactate oxidase that in the presence of oxygen converts lactate to pyruvate and H2O2 (which is detected electrochemically). A dual recording configuration of a null sensor (lacking lactate oxidase) placed in an equivalent position along the lactate biosensor was used (Fig.1A). The null sensor serves as a control to determine whether any non-specific electroactive interferents were released and confounded the measurements. Null sensor currents were subtracted from the lactate biosensor currents. Biosensors were calibrated with 100 μm lactate before and after the recordings (Fig.1A). To convert changes in sensor current to changes in lactate concentration, the means of the initial and final calibrations were used. Recordings were made from the slice placed on an elevated grid in a flow chamber at 35°C. Hypoxic conditions were induced for 2 min by replacement of oxygen in the medium with nitrogen (perfusion of the chamber with aCSF saturated with 95% N2 and 5% CO2). Since the lactate biosensor requires oxygen to operate, decreases in O2 concentration reduce biosensor currents (Fig.1A). Therefore, peak lactate release induced by hypoxia was determined upon re-oxygenation (Fig.1A).
Figure 1.
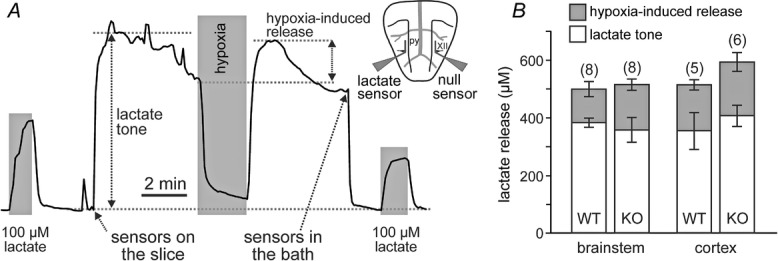
Tonic and hypoxia-induced release of lactate by the brainstem and cerebral cortex are not affected in conditions of MeCP2 deficiency
A, representative example of changes in lactate biosensor current during calibration by application of l-lactate (100 μm), after biosensor placement in direct contact with the surface of the brainstem slice (revealing lactate tone), and in response to a hypoxic challenge (perfusion with aCSF saturated with 95% N2–5% CO2). Peak hypoxia-induced lactate release is measured upon re-oxygenation. Inset: schematic drawing of a horizontal brainstem slice illustrating dual recording configuration of lactate and null (control) biosensors placed on the ventral medullary surface. Difference in current between lactate and null biosensors was used to determine the amount of lactate release. py, pyramidal tract; XII hypoglossal rootlets; B, summary data showing tonic and hypoxia-induced release of lactate measured at the surface of the horizontal brainstem and coronal cortical slices of MeCP2-knockout (KO) and wild-type (WT) mice. Numbers in parentheses indicate sample sizes.
Calcium imaging
Optical measurements of changes in [Ca2+]i in brainstem astrocytes were performed using an upright Olympus FV1000 microscope (Olympus, Japan), equipped with 25× and 40× water immersion objectives. Regions for imaging were selected based on the distance from the pyramidal tracts, the boundary of the pons and the edge of the slice, targeting cells residing near the surface of the brainstem in locations corresponding to the chemosensitive retrotrapezoid nucleus of the rostroventrolateral medulla oblongata. [Ca2+]i responses were visualised using a conventional Ca2+ indicator, Oregon Green-488 BAPTA-1, AM (OGB-1). Slices were incubated for 60 min at room temperature in 95 O2–5% CO2-saturated aCSF containing OGB-1 (10 μm) followed by 15 min incubation with sulforhodamine 101 (SR101, 30 μm) to aid identification of astroglial cells. Then, slices were washed for 15 min at room temperature in aCSF (saturated with 95% O2–5% CO2) for complete esterification of OGB-1. Imaging of Ca2+ responses was performed from the slice placed on an elevated grid in a flow chamber (volume 2 ml) at 35°C. The rate of perfusion was 4 ml min−1. The 488 nm argon laser line was used to excite OGB-1 fluorescence, which was measured using a bandpass filter of 505–550 nm. Illumination intensity was kept to a minimum (at 0.5–0.7% of laser output). SR101 fluorescence was excited at 514 nm, and emitted fluorescence at 641–716 nm was recorded. Hypercapnic conditions (respiratory acidosis) were induced by perfusion of the chamber with aCSF saturated with 90% O2–10% CO2. Fluorescence intensities in the regions of interest (ROIs) corresponding to astroglial somas were measured and background level was subtracted. On average responses of ∼10 labelled cells per slice were captured and analysed.
Data analysis
Lactate biosensor measurements were processed using a 1401 interface and analysed using Spike2 software (Cambridge Electronic Design Ltd, Cambridge, UK). Built-in Olympus analysis software was used to analyse the results of the imaging experiments. Data are reported as means ± SEM. Datasets were compared using Wilcoxon’s signed rank test or Student’s t test for paired or unpaired data, as appropriate. Differences between groups with P values of less than 0.05 were considered to be significant.
Results
Astrocytes are the only type of brain cell that store glycogen, and according to the evidence supporting the ‘lactate shuttle hypothesis’ produce and release lactate into the extracellular space to be taken up by neighbouring neurones (Pellerin & Magistretti, 1994). We first thought to determine whether the ‘metabolic’ function of astrocytes is compromised in conditions of MeCP2 deficiency by measuring the amount of lactate released by the brainstem slices of MeCP2-null and wild-type mice. For comparison, lactate release by cortical slices was also evaluated in order to determine whether MeCP2 deficiency is associated with global inability of astrocytes to release an appropriate amount of lactate. Tonic lactate release (lactate tone) was detected when lactate biosensors were placed in direct contact with the uncut ventral surface of the brainstem (Fig.1A) or coronal cortical slices of the MeCP2-null mice and their wild-type counterparts. No differences in the amount of lactate released by the brainstem slices were observed between preparations of the MeCP2-knockout (301 ± 27 μm, n = 19) and wild-type (310 ± 18 μm; n = 21) mice. Facilitated release of lactate in response to hypoxia was assessed in eight brainstem slice preparations of MeCP2-knockout and eight brainstem slice preparations of wild-type mice as well as in six cortical slice preparations of MeCP2-knockout and five cortical slice preparations of wild-type mice. No significant differences in the amount of lactate released in response to the hypoxic challenge were observed between brainstem and cortical slices of MeCP2-knockout and wild-type mice (Fig.1B).
Astrocytes which reside near the ventral surface of the medulla oblongata display [Ca2+]i responses to chemosensory challenges and release gliotransmitters (predominantly ATP) which excite neurones of the brainstem respiratory network contributing to the adaptive increases in central respiratory drive (Gourine et al. 2010). We next determined whether CO2/pH sensitivity of medullary astrocytes is affected by MeCP2 deficiency.
Efficacy of SR101 labelling of brainstem astroglia was previously reported to be low (Schnell et al. 2012). Therefore, chemosensitive astrocytes of the ventral medulla oblongata were identified using several criteria: (i) labelling with SR101 (Fig.2A); (ii) Ca2+ response to hypercapnia (Fig.2B); (iii) lack of Ca2+ response to application of KCl (Fig.2B); and (iv) Ca2+ response to application of ATP (Fig.2B). In wild-type mice, respiratory acidosis (perfusion of the recording chamber with aCSF saturated with 90% O2–10% CO2) triggered robust elevations in [Ca2+]i in astrocytes residing near the ventral surface of the medulla oblongata (Figs 3A and 4A). The magnitude of CO2-induced [Ca2+]i responses in ventral medullary astrocytes was markedly reduced in conditions of MeCP2 deficiency (Figs 3B and 4A). CO2-induced [Ca2+]i responses were equally suppressed in medullary astrocytes of both strains of MeCP2-null mice (Bird and Coyle, Fig.3B). Ca2+ responses triggered by application of exogenous ATP were not affected (Fig.3B, left panel).
Figure 2.
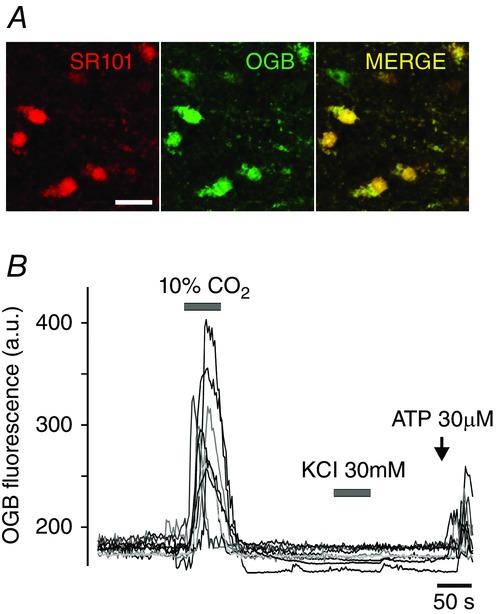
Chemosensitive astrocytes of the ventral medulla oblongata in mice
A, putative astrocytes residing near the ventral surface of the medulla oblongata loaded with Ca2+ indicator Oregon Green-488 BAPTA-1, AM (OGB) and identified by labelling with sulforhodamine 101 (SR101). Scale bar, 25 μm; B, CO2/pH-sensitive astrocytes of the ventral medulla oblongata of a wild-type mouse identified by a [Ca2+]i response to the respiratory acidosis (perfusion of the chamber with aCSF saturated with 90% O2–10% CO2), lack of Ca2+ response to application of KCl, and Ca2+ response to application of ATP.
Figure 3.
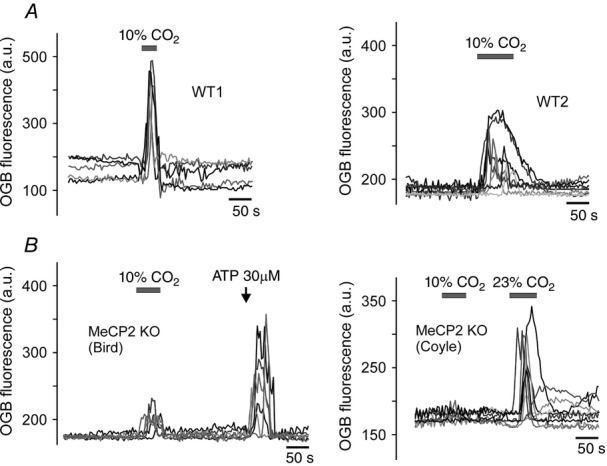
CO2-chemosensitivity of medullary astrocytes is compromised in conditions of MeCP2 deficiency
A, representative examples of CO2-evoked [Ca2+]i responses in astrocytes recorded in brainstem slice preparations of two individual wild-type (WT) mice; B, representative examples of CO2-evoked [Ca2+]i responses in astrocytes recorded in brainstem slice preparations of Bird (left) and Coyle (right) strains of MeCP2-knockout (KO) mice.
Figure 4.
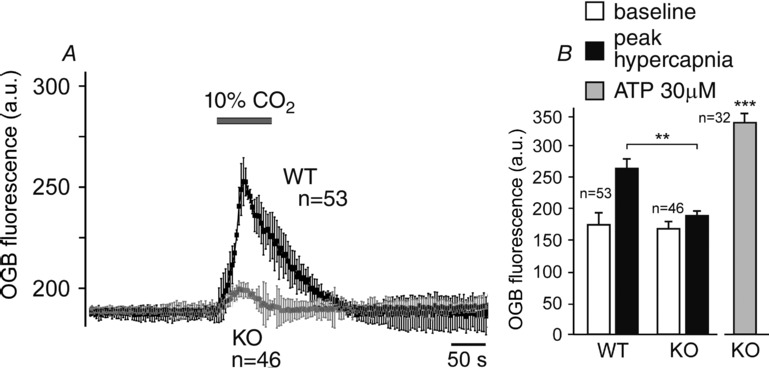
Summary data illustrating the effect of MeCP2 deficiency on CO2-induced Ca2+ responses of ventral medullary astrocytes
A, time course of [Ca2+]i changes induced by respiratory acidosis (perfusion of the chamber with aCSF saturated with 90% O2–10% CO2) in astrocytes recorded in brainstem slices of MeCP2-knockout (KO) mice and their wild-type (WT) counterparts; B, summary data of basal OGB fluorescence and peak CO2-induced increases in OGB fluorescence in astrocytes recorded in brainstem slices of MeCP2-knockout and wild-type mice. Values of n indicate the numbers of CO2-sensitive astrocytes recorded in 8 knockout and 8 wild-type brainstem slice preparations. **Significant difference in peak CO2-evoked response between wild-type and MeCP2-knockout astrocytes (P < 0.01). ***Significant difference in OGB fluorescence at the peak of the ATP-induced response compared to the baseline (P < 0.001).
In astrocytes recorded in slice preparations of MeCP2-knockout animals which failed to respond to a standard chemosensory challenge (10% CO2), [Ca2+]i responses were triggered when severe hypercapnia/acidosis was applied (Fig.3B, right panel). These data demonstrate that MeCP2 deficiency reduces the ability of medullary astrocytes to sense changes in 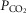 /[H+]. Preserved Ca2+ responses to activation of ATP receptors suggests that the mechanisms underlying Ca2+ recruitment from the intracellular stores and/or Ca2+ entry are not affected by MeCP2 deficiency.
/[H+]. Preserved Ca2+ responses to activation of ATP receptors suggests that the mechanisms underlying Ca2+ recruitment from the intracellular stores and/or Ca2+ entry are not affected by MeCP2 deficiency.
Discussion
Rett syndrome – a prototypical neurological disorder caused by MeCP2 deficiency – is associated with a severely disordered breathing pattern (Southall et al. 1988; Julu et al. 2001; Weese-Mayer et al. 2006; De Felice et al. 2014) and reduced ventilatory CO2 sensitivity (Zhang et al. 2011; Bissonnette et al. 2014). In a mouse model of Rett syndrome (MeCP2 gene knockout), re-introduction of the MeCP2 gene selectively in astrocytes rescues normal respiratory phenotype (Lioy et al. 2011), suggesting that astroglial dysfunction underlies the development of the disease. Moreover, in mice selective and inducible deletion of MeCP2 in astrocytes leads to a dramatically reduced ventilatory sensitivity to CO2 (Bissonnette et al. 2012), indicating that the respiratory neural circuits, including putative central chemosensitive neurones, are unable to generate appropriate CO2-sensitive respiratory drive when astrocytes are devoid of MeCP2. These data support the hypothesis of a key role played by astrocytes in the mechanisms of central CO2 chemosensitivity (Gourine et al. 2010). However, the mechanisms underlying reduced ventilatory CO2 sensitivity in conditions of MeCP2 deficiency are not known. Since astrocytes provide neural circuits with structural and metabolic support (metabolic function) and also have a signalling role, it remains unclear which of these two key astroglial functions is affected.
In the present study we found no differences in tonic and hypoxia-induced release of lactate measured from the ventral surface of the medulla oblongata or cerebral cortex in brain slices of MeCP2-knockout and wild-type mice. However, Ca2+ responses of ventral medullary astrocytes of MeCP2-knockout animals evoked by standard experimental chemosensory challenge (10% CO2) were dramatically reduced, while Ca2+ elevations induced by application of the exogenous ATP were preserved. In conditions of MeCP2 deficiency, robust Ca2+ responses in medullary astrocytes were only elicited when severe chemosensory challenge (>20% CO2) was applied.
Preserved lactate tone and unaltered release of lactate in low oxygen conditions in preparations of MeCP2-null mice suggest that metabolic function of astrocytes in providing neurones with the energy substrate in the form of lactate is not affected in the absence of MeCP2 function. This is somewhat surprising considering the variety of astroglial gene pathways controlled by this nuclear protein (Yasui et al. 2013; Forbes-Lorman et al. 2014). The ability of MeCP2 deficient brain tissue to release an appropriate amount of lactate might be an important factor in preventing or delaying widespread neuronal death and allowing recovery of synaptic function after acute tissue hypoxia (Schurr et al. 1997a,c,b), despite significant oxidative stress associated with recurrent episodes of intermittent hypoxia (De Felice et al. 2012, 2014). Indeed, it is not uncommon for Rett syndrome patients to experience episodes of severe oxygen desaturation (S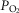 ≤ 80%) (Andaku et al. 2005; Hagebeuk et al. 2012; Carotenuto et al. 2013), yet post-mortem reports revealed no evidence of widespread neuronal degeneration (Cornford et al. 1994; Armstrong, 1995; Bauman et al. 1995). This is also supported by the evidence demonstrating that acute re-expression of MeCP2 in adult knockout mice rescues the overt neurological phenotype, indicating that MeCP2 deficiency is associated with limited neurodegeneration (Guy et al. 2007). Interestingly, in patients with Rett syndrome, elevated cerebrospinal fluid (CSF) level of lactate positively correlates with respiratory dysfunction but not with other neurological features (Matsuishi et al. 1992, 1994; Lappalainen & Riikonen, 1994). The data reported here suggest that the high levels of lactate in the CSF of patients with Rett syndrome may reflect enhanced astroglial release in response to recurrent episodes of hypoxia that inevitably accompany the disordered breathing pattern associated with this condition (the significance of high CSF lactate in Rett patients in the context of the findings obtained in the present study is discussed below).
≤ 80%) (Andaku et al. 2005; Hagebeuk et al. 2012; Carotenuto et al. 2013), yet post-mortem reports revealed no evidence of widespread neuronal degeneration (Cornford et al. 1994; Armstrong, 1995; Bauman et al. 1995). This is also supported by the evidence demonstrating that acute re-expression of MeCP2 in adult knockout mice rescues the overt neurological phenotype, indicating that MeCP2 deficiency is associated with limited neurodegeneration (Guy et al. 2007). Interestingly, in patients with Rett syndrome, elevated cerebrospinal fluid (CSF) level of lactate positively correlates with respiratory dysfunction but not with other neurological features (Matsuishi et al. 1992, 1994; Lappalainen & Riikonen, 1994). The data reported here suggest that the high levels of lactate in the CSF of patients with Rett syndrome may reflect enhanced astroglial release in response to recurrent episodes of hypoxia that inevitably accompany the disordered breathing pattern associated with this condition (the significance of high CSF lactate in Rett patients in the context of the findings obtained in the present study is discussed below).
The results of the present study also show that MeCP2 deficiency dramatically reduced the ability of brainstem astrocytes to sense changes in 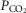 /[H+]. Astrocytes are not electrically excitable and their signalling function is governed by changes in intracellular [Ca2+]. We previously demonstrated high sensitivity of medullary astrocytes to physiological changes in pH (Gourine et al. 2010). Astrocytes respond to hypercapnia/acidosis with robust elevations in [Ca2+]i, leading to the release of ATP, which propagates astrocytic activation and excites neurones of the brainstem respiratory network, contributing to the adaptive increases in central respiratory drive (Gourine et al. 2005, 2010; Kasymov et al. 2013). Astroglial CO2/pH sensitivity has been incorporated in the current models of brain respiratory chemosensitivity, centred on a group of highly pH sensitive neurones of the medullary retrotrapezoid nucleus (RTN), which are proposed to play the most significant role, with neighbouring astrocytes providing an additional 20–30% of the chemosensory drive to breathe (Guyenet, 2014).
/[H+]. Astrocytes are not electrically excitable and their signalling function is governed by changes in intracellular [Ca2+]. We previously demonstrated high sensitivity of medullary astrocytes to physiological changes in pH (Gourine et al. 2010). Astrocytes respond to hypercapnia/acidosis with robust elevations in [Ca2+]i, leading to the release of ATP, which propagates astrocytic activation and excites neurones of the brainstem respiratory network, contributing to the adaptive increases in central respiratory drive (Gourine et al. 2005, 2010; Kasymov et al. 2013). Astroglial CO2/pH sensitivity has been incorporated in the current models of brain respiratory chemosensitivity, centred on a group of highly pH sensitive neurones of the medullary retrotrapezoid nucleus (RTN), which are proposed to play the most significant role, with neighbouring astrocytes providing an additional 20–30% of the chemosensory drive to breathe (Guyenet, 2014).
This study contributes to the body of evidence suggesting that the role of astrocytes in the central nervous mechanisms linking changes in brain parenchymal 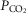 /[H+] and central respiratory drive is greater than was previously appreciated. First, sensitivity of RTN neurones to changes in pH (as assessed in the organotypic brainstem slice preparation) is, to a large extent, mediated by prior release of gliotransmitter(s), primarily ATP (Gourine et al. 2010). Other experiments conducted in acute slices estimated that ATP actions contribute some 30% to the overall response of RTN neurones to respiratory acidosis (Wenker et al. 2012). Second, specific genetic targeting and silencing of RTN neurones reduces phrenic nerve response to systemic hypercapnia in vivo by ∼30% (Marina et al. 2010) – the effect similar in magnitude to that observed following blockade of ATP receptors within the region (Gourine et al. 2005; Wenker et al. 2012). Third, in mice astrocyte-specific inducible deletion of MeCP2 results in a dramatically reduced ventilatory sensitivity to CO2 (Bissonnette et al. 2012), the effect being similar to that observed in animals with global MeCP2 deficiency (Zhang et al. 2011; Bissonnette et al. 2014). If astrocytes contribute only a small fraction of the overall respiratory response to systemic hypercapnia, then intact functional chemosensitive RTN neurones would be expected to mount an appropriate ventilatory response regardless of astroglial involvement (especially when we consider that the metabolic function of astrocytes does not appear to be affected by MeCP2 deficiency). Finally, the data reported here demonstrate that astroglial sensitivity to changes in
/[H+] and central respiratory drive is greater than was previously appreciated. First, sensitivity of RTN neurones to changes in pH (as assessed in the organotypic brainstem slice preparation) is, to a large extent, mediated by prior release of gliotransmitter(s), primarily ATP (Gourine et al. 2010). Other experiments conducted in acute slices estimated that ATP actions contribute some 30% to the overall response of RTN neurones to respiratory acidosis (Wenker et al. 2012). Second, specific genetic targeting and silencing of RTN neurones reduces phrenic nerve response to systemic hypercapnia in vivo by ∼30% (Marina et al. 2010) – the effect similar in magnitude to that observed following blockade of ATP receptors within the region (Gourine et al. 2005; Wenker et al. 2012). Third, in mice astrocyte-specific inducible deletion of MeCP2 results in a dramatically reduced ventilatory sensitivity to CO2 (Bissonnette et al. 2012), the effect being similar to that observed in animals with global MeCP2 deficiency (Zhang et al. 2011; Bissonnette et al. 2014). If astrocytes contribute only a small fraction of the overall respiratory response to systemic hypercapnia, then intact functional chemosensitive RTN neurones would be expected to mount an appropriate ventilatory response regardless of astroglial involvement (especially when we consider that the metabolic function of astrocytes does not appear to be affected by MeCP2 deficiency). Finally, the data reported here demonstrate that astroglial sensitivity to changes in 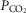 /[H+] is dramatically reduced in the absence of MeCP2, the effect being consistent with associated respiratory phenotype and the hypothesis of a prominent role played by astroglia in the mechanisms of central respiratory CO2 chemosensitivity. It was possible to trigger [Ca2+]i responses in MeCP2-deficient astrocytes by application of a severe hypercapnic stimulus (>20% CO2) suggesting a shift in sensitivity, although we did not investigate this systematically. Interestingly, shift in ventilatory CO2 sensitivity has also been demonstrated in MeCP2-null mice (Zhang et al. 2011; Toward et al. 2013; Bissonnette et al. 2014).
/[H+] is dramatically reduced in the absence of MeCP2, the effect being consistent with associated respiratory phenotype and the hypothesis of a prominent role played by astroglia in the mechanisms of central respiratory CO2 chemosensitivity. It was possible to trigger [Ca2+]i responses in MeCP2-deficient astrocytes by application of a severe hypercapnic stimulus (>20% CO2) suggesting a shift in sensitivity, although we did not investigate this systematically. Interestingly, shift in ventilatory CO2 sensitivity has also been demonstrated in MeCP2-null mice (Zhang et al. 2011; Toward et al. 2013; Bissonnette et al. 2014).
The mechanisms of intracellular Ca2+ recruitment evoked by activation of purinoceptors do not appear to be affected, as the responses to the exogenously applied ATP were preserved in astrocytes of MeCP2-null mice. These data suggest that reduced CO2 sensitivity in conditions of MeCP2 deficiency is upstream from astroglial release of ATP (which under normal conditions propagates Ca2+ excitation among ventral medullary astrocytes; Gourine et al. 2010). However, the mechanisms of CO2/pH sensitivity of astrocytes remain unknown. A number of putative mechanisms have been proposed including involvement of (i) certain ion channels (e.g. pH sensitive inward rectifier K+ channels – Kirs) (Mulkey & Wenker, 2011), (ii) a molecular CO2 sensor (e.g. connexin 26) (Huckstepp et al. 2010a,b), and (iii) an electrogenic Na+/HCO3− cotransporter (Mulkey & Wenker, 2011).
Of these mechanisms MeCP2 deficiency has been shown to alter expression of the pH sensitive Kir4.1 channels in the locus coeruleus (Zhang et al. 2011) – one of the functional respiratory CO2 chemosensitive brainstem areas. It was suggested that overexpressed Kir4.1 subunit may favour formation of homomeric Kir4.1 channels, which are less sensitive to changes in pH than heteromeric Kir4.1–Kir5.1 (Zhang et al. 2011). Kir4.1–Kir5.1 heteromeric channels are expressed by astrocytes and have been proposed by some studies to underlie astrocytic CO2/pH sensitivity (Wenker et al. 2010; Mulkey & Wenker, 2011). Signalling molecules released by activated astrocytes (ATP and lactate) have a significant impact on the activity of locus coeruleus neurones (Harms et al. 1992; Tang et al. 2014), suggesting that reduced chemosensory responses of these neurones in conditions of MeCP2 deficiency (Zhang et al. 2011) may also be secondary to impaired CO2 sensitivity of local astrocytes. Indeed, in astrocytes Kir4.1 expression is regulated by DNA methylation throughout development, and therefore MeCP2 deficiency may lead to Kir4.1 overexpression (Nwaobi et al. 2014). However, more recent studies demonstrated that Kir4.1 expression is reduced in cortical astrocytes of heterozygous MeCP2 deficient female mice (Cuddapah et al. 2013) and the earlier evidence argues against any significant role of Kir4.1–Kir5.1 heteromeric channels in the mechanisms of central respiratory chemosensitivity (Trapp et al. 2011).
One potential mechanism which may underlie reduced CO2 sensitivity of MeCP2 deficient astrocytes may be related to the disruption of brain-derived neurotrophic factor (BDNF)-mediated signalling (Amaral et al. 2007). MeCP2 is an important regulator of BDNF transcription (Chen et al. 2003; Martinowich et al. 2003). BDNF protein levels are reduced in conditions of MeCP2 deficiency (Chang et al. 2006; Wang et al. 2006), while stimulation of BDNF receptors improves respiratory pattern in MePC2 null mice (Schmid et al. 2012; Kron et al. 2014). Part of the BDNF signalling pathway involves activation of certain transient receptor potential channels (TRPCs) (Amaral et al. 2007). Noteworthy is a recently described TRPA1 channel that plays an important role in generation of Ca2+ transients in astrocytes (Shigetomi et al. 2013). Together, these pieces of evidence suggest that disruption of BDNF- and TRPC-mediated Ca2+ homeostasis and signalling in astrocytes may underlie reduced Ca2+ responses to CO2 in medullary astrocytes of MeCP2-null mice.
The combination of preserved tonic and hypoxia-induced lactate release and markedly reduced CO2 sensitivity of medullary astrocytes could underlie severely disordered breathing observed in Rett syndrome. Cheyne–Stokes-like breathing pattern characterised by recurrent central apnoeas followed by periods of hyperventilation is frequent in Rett syndrome patients (Julu et al. 2001) and is also observed in mouse models (Bissonnette & Knopp, 2008; Abdala et al. 2010). Recurrent apnoeas followed by periods of hyperventilation are associated with chronic hypoxaemia and hypocapnia, as O2 saturation does not fully recover from apnoeas, and hyperventilation washes out CO2 (De Felice et al. 2014). Our data suggest that the acute bouts of severe hypoxaemia and hyperventilation would lead to the increased levels of brain lactate, as was demonstrated by measurements of high CSF lactate in Rett syndrome patients (Matsuishi et al. 1992, 1994; Lappalainen & Riikonen, 1994). This, in turn, would contribute to brain parenchymal acidification, which would facilitate hyperventilation, washing out more CO2. Increased CO2 apnoeic threshold and reduced CO2 sensitivity would favour prolonged apnoeas.
In summary, the data obtained in the present study suggest that MeCP2 deficiency is associated with a dramatically reduced sensitivity of medullary astrocytes to changes in 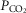 /[H+]. Metabolic function of astrocytes in providing neurones with readily available metabolic substrate in the form of lactate does not appear to be affected. Taken together with the evidence of a severely blunted ventilatory response to CO2 in conditions of inducible deletion of MeCP2 specifically in astrocytes (Bissonnette et al. 2012), these data support the hypothesis of an important role played by astroglia in the mechanisms of central respiratory CO2/pH chemosensitivity.
/[H+]. Metabolic function of astrocytes in providing neurones with readily available metabolic substrate in the form of lactate does not appear to be affected. Taken together with the evidence of a severely blunted ventilatory response to CO2 in conditions of inducible deletion of MeCP2 specifically in astrocytes (Bissonnette et al. 2012), these data support the hypothesis of an important role played by astroglia in the mechanisms of central respiratory CO2/pH chemosensitivity.
Acknowledgments
We are grateful to The Wellcome Trust, the International Rett Syndrome Foundation (IRSF-ANGEL#3101) and the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH-NINDS, R01 NS069220) for financial support. A.V.G. is a Wellcome Trust Senior Research Fellow (ref. 095064). We thank Prof. John Bissonnette for helpful discussions and critical review of the manuscript.
Glossary
- aCSF
artificial cerebrospinal fluid
- BDNF
brain-derived neurotrophic factor
- CSF
cerebrospinal fluid
- KO
knockout
- MeCP2
methyl-CpG-binding protein 2
- OGB
Oregon Green-488 BAPTA-1 AM
- SR101
sulforhodamine 101
- TRPC
transient receptor potential channels
- WT
wild-type
Additional information
Competing interests
The authors have no competing interests to disclose.
Author contributions
A.V.G. conceived and designed the experiments. A.P.A. bred and genotyped the MeCP2-knockout mice. E.T. and A.K. collected, assembled, analysed and interpreted the data. A.V.G. and A.P.A. wrote the article. All authors revised the article critically for important intellectual content.
References
- Abdala AP, Dutschmann M, Bissonnette JM. Paton JF. Correction of respiratory disorders in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2010;107:18208–18213. doi: 10.1073/pnas.1012104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral MD, Chapleau CA. Pozzo-Miller L. Transient receptor potential channels as novel effectors of brain-derived neurotrophic factor signaling: potential implications for Rett syndrome. Pharmacol Ther. 2007;113:394–409. doi: 10.1016/j.pharmthera.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U. Zoghbi HY. Rett syndrome is caused by mutations in X-linked MeCP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Andaku DK, Mercadante MT. Schwartzman JS. Buspirone in Rett syndrome respiratory dysfunction. Brain Dev. 2005;27:437–438. doi: 10.1016/j.braindev.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Armstrong DD. The neuropathology of Rett syndrome– overview 1994. Neuropediatrics. 1995;26:100–104. doi: 10.1055/s-2007-979736. [DOI] [PubMed] [Google Scholar]
- Ballas N, Lioy DT, Grunseich C. Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Arin DM. Pervasive neuroanatomic abnormalities of the brain in three cases of Rett’s syndrome. Neurology. 1995;45:1581–1586. doi: 10.1212/wnl.45.8.1581. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM, Garg SK, Lioy DT, Knopp SJ. Mandel G. 2012 Neuroscience Meeting Planner. Washington, DC Society for Neuroscience, 2012. Online. 2012. p. Program No. 897.19. Depletion of methyl-CpG-binding protein 2 (MeCP2) in astrocytes depresses chemosensitivity.
- Bissonnette JM. Knopp SJ. Separate respiratory phenotypes in methyl-CpG-binding protein 2 (Mecp2) deficient mice. Pediatr Res. 2006;59(4):513–518. doi: 10.1203/01.pdr.0000203157.31924.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette JM. Knopp SJ. Effect of inspired oxygen on periodic breathing in methy-CpG-binding protein 2 (Mecp2) deficient mice. J Appl Physiol. 2008;104:198–204. doi: 10.1152/japplphysiol.00843.2007. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM, Schaevitz LR, Knopp SJ. Zhou Z. Respiratory phenotypes are distinctly affected in mice with common Rett syndrome mutations MeCP2 T158A and R168X. Neuroscience. 2014;267:166–176. doi: 10.1016/j.neuroscience.2014.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Simcox T, Samaco RC. LaSalle JM. X-Chromosome inactivation ratios affect wild-type MeCP2 expression within mosaic Rett syndrome and Mecp2−/+ mouse brain. Hum Mol Genet. 2004;13:1275–1286. doi: 10.1093/hmg/ddh142. [DOI] [PubMed] [Google Scholar]
- Carotenuto M, Esposito M, D’Aniello A, Rippa CD, Precenzano F, Pascotto A, Bravaccio C. Elia M. Polysomnographic findings in Rett syndrome: a case-control study. Sleep Breath. 2013;17:93–98. doi: 10.1007/s11325-012-0654-x. [DOI] [PubMed] [Google Scholar]
- Chang Q, Khare G, Dani V, Nelson S. Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–348. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R. Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Cornford ME, Philippart M, Jacobs B, Scheibel AB. Vinters HV. Neuropathology of Rett syndrome: case report with neuronal and mitochondrial abnormalities in the brain. J Child Neurol. 1994;9:424–431. doi: 10.1177/088307389400900419. [DOI] [PubMed] [Google Scholar]
- Cuddapah VA, Nwaobi S, Thompson C. Olsen M. Methyl-CpG-binding protein 2 (MeCP2) deficiency leads to altered astrocyte function. 2013 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2013. Online. 2013. p. Program No. 718.09.
- De Felice C, Della Ragione F, Signorini C, Leoncini S, Pecorelli A, Ciccoli L, Scalabrì F, Marracino F, Madonna M, Belmonte G, Ricceri L, De Filippis B, Laviola G, Valacchi G, Durand T, Galano JM, Oger C, Guy A, Bultel-Poncé V, Guy J, Filosa S, Hayek J. D’Esposito M. Oxidative brain damage in Mecp2-mutant murine models of Rett syndrome. Neurobiol Dis. 2014;68:66–77. doi: 10.1016/j.nbd.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice C, Rossi M, Leoncini S, Chisci G, Signorini C, Lonetti G, Vannuccini L, Spina D, Ginori A, Iacona I, Cortelazzo A, Pecorelli A, Valacchi G, Ciccoli L, Pizzorusso T. Hayek J. Inflammatory lung disease in Rett syndrome. Mediators Inflamm. 2014;2014:560120. doi: 10.1155/2014/560120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice C, Signorini C, Leoncini S, Pecorelli A, Durand T, Valacchi G, Ciccoli L. Hayek J. The role of oxidative stress in Rett syndrome: an overview. Ann N Y Acad Sci. 2012;1259:121–135. doi: 10.1111/j.1749-6632.2012.06611.x. [DOI] [PubMed] [Google Scholar]
- Forbes-Lorman RM, Kurian JR. Auger AP. MeCP2 regulates GFAP expression within the developing brain. Brain Res. 2014;1543:151–158. doi: 10.1016/j.brainres.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K. Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N. Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S. Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE. Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Guyenet PG( Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol. 2014;4:1511–1562. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagebeuk EE, Bijlmer RP, Koelman JH. Poll-The BT. Respiratory disturbances in Rett syndrome: don’t forget to evaluate upper airway obstruction. J Child Neurol. 2012;27:888–892. doi: 10.1177/0883073811429859. [DOI] [PubMed] [Google Scholar]
- Harms L, Finta M, Tschöpl M. Illes P. Depolarization of rat locus coeruleus neurons by adenosine 5′-triphosphate. Neuroscience. 1992;48:941–952. doi: 10.1016/0306-4522(92)90282-7. [DOI] [PubMed] [Google Scholar]
- Huckstepp RT, Eason R, Sachdev A. Dale N. CO2-dependent opening of connexin 26 and related beta connexins. J Physiol. 2010a;588:3921–3931. doi: 10.1113/jphysiol.2010.192096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV. Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol. 2010b;588:3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julu PO, Kerr AM, Apartopoulos F, Al-Rawas S, Engerstrom IW, Engerstrom L, Jamal GA. Hansen S. Characterisation of breathing and associated central autonomic dysfunction in the Rett disorder. Arch Dis Child. 2001;85:29–37. doi: 10.1136/adc.85.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasymov V, Larina O, Castaldo C, Marina N, Patrushev M, Kasparov S. Gourine AV. Differential sensitivity of brainstem versus cortical astrocytes to changes in pH reveals functional regional specialization of astroglia. J Neurosci. 2013;33:435–441. doi: 10.1523/JNEUROSCI.2813-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron M, Lang M, Adams IT, Sceniak M, Longo F. Katz DM. A BDNF loop-domain mimetic acutely reverses spontaneous apneas and respiratory abnormalities during behavioral arousal in a mouse model of Rett syndrome. Dis Model Mech. 2014;7:1047–1055. doi: 10.1242/dmm.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen R. Riikonen RS. Elevated CSF lactate in the Rett syndrome: cause or consequence? Brain Dev. 1994;16:399–401. doi: 10.1016/0387-7604(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Lawson-Yuen A, Liu D, Han L, Jiang ZI, Tsai GE, Basu AC, Picker J, Feng J. Coyle JT. Ube3a mRNA and protein expression are not decreased in Mecp2R168X mutant mice. Brain Res. 2007;1180:1–6. doi: 10.1016/j.brainres.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N. Mandel G. A role for glia in the progression of Rett’s syndrome. Nature. 2011;475:497–500. doi: 10.1038/nature10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I. Jin LW. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J Neurosci. 2010;30:5346–5356. doi: 10.1523/JNEUROSCI.5966-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Swanberg S, Harvey D, LaSalle JM. Jin LW. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci. 2009;29:5051–5061. doi: 10.1523/JNEUROSCI.0324-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Sorg O, Naichen Y, Pellerin L, de Rham S. Martin JL. Regulation of astrocyte energy metabolism by neurotransmitters. Ren Physiol Biochem. 1994;17:168–171. doi: 10.1159/000173810. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Sorg O, Yu N, Martin JL. Pellerin L. Neurotransmitters regulate energy metabolism in astrocytes: implications for the metabolic trafficking between neural cells. Dev Neurosci. 1993;15:306–312. doi: 10.1159/000111349. [DOI] [PubMed] [Google Scholar]
- Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF. Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci. 2010;30:12466–12473. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G. Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Matsuishi T, Urabe F, Komori H, Yamashita Y, Naito E, Kuroda Y, Horikawa M. Ohtaki E. The Rett syndrome and CSF lactic acid patterns. Brain Dev. 1992;14:68–70. doi: 10.1016/s0387-7604(12)80283-x. [DOI] [PubMed] [Google Scholar]
- Matsuishi T, Urabe F, Percy AK, Komori H, Yamashita Y, Schultz RS, Ohtani Y, Kuriya N. Kato H. Abnormal carbohydrate metabolism in cerebrospinal fluid in Rett syndrome. J Child Neurol. 1994;9:26–30. doi: 10.1177/088307389400900105. [DOI] [PubMed] [Google Scholar]
- Mulkey DK. Wenker IC. Astrocyte chemoreceptors: mechanisms of H+ sensing by astrocytes in the retrotrapezoid nucleus and their possible contribution to respiratory drive. Exp Physiol. 2011;96:400–406. doi: 10.1113/expphysiol.2010.053140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwaobi SE, Lin E, Peramsetty SR. Olsen ML. DNA methylation functions as a critical regulator of Kir4.1 expression during CNS development. Glia. 2014;62:411–427. doi: 10.1002/glia.22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y, Takahashi T, Mitsumasu C, Kosai K, Tanaka E. Matsuishi T. Alterations of gene expression and glutamate clearance in astrocytes derived from an MeCP2-null mouse model of Rett syndrome. PLoS One. 2012;7:e35354. doi: 10.1371/journal.pone.0035354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L. Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Ward CS. Neul JL. Breathing challenges in Rett syndrome: lessons learned from humans and animal models. Respir Physiol Neurobiol. 2013;189:280–287. doi: 10.1016/j.resp.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid DA, Yang T, Ogier M, Adams I, Mirakhur Y, Wang Q, Massa SM, Longo FM. Katz DM. A TrkB small molecule partial agonist rescues TrkB phosphorylation deficits and improves respiratory function in a mouse model of Rett syndrome. J Neurosci. 2012;32:1803–1810. doi: 10.1523/JNEUROSCI.0865-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr A, Payne RS, Miller JJ. Rigor BM. Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: further in vitro validation. J Neurochem. 1997a;69:423–426. doi: 10.1046/j.1471-4159.1997.69010423.x. [DOI] [PubMed] [Google Scholar]
- Schurr A, Payne RS, Miller JJ. Rigor BM. Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: an in vitro study. Brain Res. 1997b;744:105–111. doi: 10.1016/s0006-8993(96)01106-7. [DOI] [PubMed] [Google Scholar]
- Schurr A, Payne RS, Miller JJ. Rigor BM. Glia are the main source of lactate utilized by neurons for recovery of function posthypoxia. Brain Res. 1997c;774:221–224. doi: 10.1016/s0006-8993(97)81708-8. [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Jackson-Weaver O, Huckstepp RT, O’Dell TJ. Khakh BS. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J Neurosci. 2013;33:10143–10153. doi: 10.1523/JNEUROSCI.5779-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets EE, Julu PO, van Waardenburg D, Engerstrom IW, Hansen S, Apartopoulos F, Curfs LM. Schrander-Stumpel CT. Management of a severe forceful breather with Rett syndrome using carbogen. Brain Dev. 2006;28:625–632. doi: 10.1016/j.braindev.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Schnell C, Hagos Y. Hülsmann S. Active sulforhodamine 101 uptake into hippocampal astrocytes. PLoS One. 2012;7:e49398. doi: 10.1371/journal.pone.0049398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southall DP, Kerr AM, Tirosh E, Amos P, Lang MH. Stephenson JB. Hyperventilation in the awake state: potentially treatable component of Rett syndrome. Arch Dis Child. 1988;63:1039–1048. doi: 10.1136/adc.63.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Lane S, Korsak A, Paton JF, Gourine AV, Kasparov S. Teschemacher AG. Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat Commun. 2014;5:3284. doi: 10.1038/ncomms4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toward MA, Abdala AP, Knopp SJ, Paton JF. Bissonnette JM. Increasing brain serotonin corrects CO2 chemosensitivity in methyl-CpG-binding protein 2 (Mecp2)-deficient mice. Exp Physiol. 2013;98:842–849. doi: 10.1113/expphysiol.2012.069872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Tucker SJ. Gourine AV. Respiratory responses to hypercapnia and hypoxia in mice with genetic ablation of Kir5.1 (Kcnj16) Exp Physiol. 2011;96:451–459. doi: 10.1113/expphysiol.2010.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Peña F, Zanella S, Bévengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L. Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voituron N, Zanella S, Menuet C, Dutschmann M. Hilaire G. Early breathing defects after moderate hypoxia or hypercapnia in a mouse model of Rett syndrome. Respir Physiol Neurobiol. 2009;168:109–118. doi: 10.1016/j.resp.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Wang H, Chan SA, Ogier M, Hellard D, Wang Q, Smith C. Katz DM. Dysregulation of brain-derived neurotrophic factor expression and neurosecretory function in Mecp2 null mice. J Neurosci. 2006;26:10911–10915. doi: 10.1523/JNEUROSCI.1810-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Silvestri JM. Ramirez JM. Autonomic nervous system dysregulation: breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr Res. 2006;60:443–449. doi: 10.1203/01.pdr.0000238302.84552.d0. [DOI] [PubMed] [Google Scholar]
- Wenker IC, Kreneisz O, Nishiyama A. Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1–Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol. 2010;104:3042–3052. doi: 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Sobrinho CR, Takakura AC, Moreira TS. Mulkey DK. Regulation of ventral surface CO2/H+-sensitive neurons by purinergic signalling. J Physiol. 2012;590:2137–2150. doi: 10.1113/jphysiol.2012.229666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui DH, Xu H, Dunaway KW, Lasalle JM, Jin LW. Maezawa I. MeCP2 modulates gene expression pathways in astrocytes. Mol Autism. 2013;4:3. doi: 10.1186/2040-2392-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Su J, Cui N, Gai H, Wu Z. Jiang C. The disruption of central CO2 chemosensitivity in a mouse model of Rett syndrome. Am J Physiol Cell Physiol. 2011;301:C729–738. doi: 10.1152/ajpcell.00334.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


