Abstract
Tibetans living at high altitude have adapted genetically such that many display a low erythropoietic response, resulting in near sea-level haemoglobin (Hb) concentration. We hypothesized that absence of the erythropoietic response would be associated with greater exercise capacity compared to those with high [Hb] as a result of beneficial changes in oxygen transport. We measured, in 21 Tibetan males with [Hb] ranging from 15.2 g dl−1 to 22.9 g dl−1 (9.4 mmol l−1 to 14.2 mmol l−1), [Hb], ventilation, volumes of O2 and CO2 utilized at peak exercise ( and
and  ), heart rate, cardiac output and arterial blood gas variables at peak exercise on a cycle ergometer at ∼4200 m. Lung and muscle O2 diffusional conductances were computed from these measurements. [Hb] was related (negatively) to
), heart rate, cardiac output and arterial blood gas variables at peak exercise on a cycle ergometer at ∼4200 m. Lung and muscle O2 diffusional conductances were computed from these measurements. [Hb] was related (negatively) to  kg–1 (r = −0.45, P< 0.05), cardiac output kg–1 (QT kg–1, r = −0.54, P < 0.02), and O2 diffusion capacity in muscle (DM kg–1, r = −0.44, P<0.05), but was unrelated to ventilation, arterial partial pressure of O2 (
kg–1 (r = −0.45, P< 0.05), cardiac output kg–1 (QT kg–1, r = −0.54, P < 0.02), and O2 diffusion capacity in muscle (DM kg–1, r = −0.44, P<0.05), but was unrelated to ventilation, arterial partial pressure of O2 ( ) or pulmonary diffusing capacity. Using multiple linear regression, variance in peak
) or pulmonary diffusing capacity. Using multiple linear regression, variance in peak  kg–1 was primarily attributed to QT, DM, and
kg–1 was primarily attributed to QT, DM, and  (R2 = 0.88). However, variance in pulmonary gas exchange played essentially no role in determining peak
(R2 = 0.88). However, variance in pulmonary gas exchange played essentially no role in determining peak 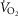 . These results (1) show higher exercise capacity in Tibetans without the erythropoietic response, supported mostly by cardiac and muscle O2 transport capacity and ventilation rather than pulmonary adaptations, and (2) support the emerging hypothesis that the polycythaemia of altitude, normally a beneficial response to low cellular
. These results (1) show higher exercise capacity in Tibetans without the erythropoietic response, supported mostly by cardiac and muscle O2 transport capacity and ventilation rather than pulmonary adaptations, and (2) support the emerging hypothesis that the polycythaemia of altitude, normally a beneficial response to low cellular  , may become maladaptive if excessively elevated under chronic hypoxia. The cause and effect relationships among [Hb], QT, DM, and
, may become maladaptive if excessively elevated under chronic hypoxia. The cause and effect relationships among [Hb], QT, DM, and 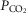 remain to be elucidated.
remain to be elucidated.
Key points
We hypothesized that sea-level range haemoglobin concentration ([Hb]) at altitude, previously linked with adaptive genetic factors in Tibetans, would be associated with greater exercise capacity, explained by changes in steps of the oxygen transport system in this population.
In 21 Tibetan and 9 Han Chinese males resident at 4200–4300 m, we measured [Hb], ventilation, volumes of O2 and CO2 utilized at peak exercise (
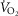 and
and 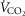 ), heart rate, cardiac output and arterial blood gas variables at peak exercise on a cycle ergometer and determined oxygen (O2) diffusion capacity in lung and muscle.
), heart rate, cardiac output and arterial blood gas variables at peak exercise on a cycle ergometer and determined oxygen (O2) diffusion capacity in lung and muscle.
Tibetans with low [Hb] exhibit greater peak
 kg–1, which was explained mostly by variation in cardiac output, ventilation and O2 diffusional conductances in muscle.
kg–1, which was explained mostly by variation in cardiac output, ventilation and O2 diffusional conductances in muscle.
These results suggest that polycythaemia may be an excessive response to low
 at altitude.
at altitude.
Introduction
Humans have occupied the Tibetan highlands for hundreds of generations despite the unavoidable stress of high-altitude hypoxia and exhibit a set of unique physiological traits distinct from native highland counterparts and lowlanders at altitude (Moore, 2001; Beall, 2007; Petousi & Robbins, 2014). One such difference is that many Tibetans exhibit haemoglobin concentrations ([Hb]) within the range expected at sea level, also common among Amhara Ethiopians (Beall et al. 2002, 2006; Alkorta-Aranburu et al. 2012), while healthy sojourners and Andeans at high altitude exhibit polycythaemia (Winslow et al. 1989; Beall et al. 1998; Beall, 2000, 2007; Moore, 2001; Wu et al. 2005). Polycythaemia is thought to enhance oxygenation to tissues by carrying more O2 per millilitre of blood and thus might be expected to enhance O2 transport and support exercise capacity. However, acute experimental studies dispute this hypothesis (Calbet et al. 2002), and excessive [Hb] is associated with severe complications and disease such as chronic mountain sickness (CMS) (Monge, 1976; Monge et al. 1992; Vargas & Spielvogel, 2006b) suggesting excessive [Hb] at high altitude in response to high-altitude hypoxia may become maladaptive.
The physiological significance of a markedly low in many Tibetans has not been established, particularly with respect to exercise capacity and steps of the O2 transport system necessary to support exercise. Previous studies show that Tibetan natives, compared to other high-altitude residents, exhibit greater exercise capacity at high altitude (Sun et al. 1990; Ge et al. 1994; Ge, 1995; Niu et al. 1995; Chen et al. 1997, Moore et al. 1992), although this is not observed in all studies (Kayser et al. 1994). If and how these characteristics relate to [Hb] and/or genetic variation in Tibetans is unknown.
Progress in genomics suggests that several genes underlie Tibetan adaptation to high altitude (Simonson et al. 2012; Petousi & Robbins, 2014). Low, sea-level range, [Hb] among Tibetans at altitude is associated with adaptive changes in hypoxia-related genes (Beall et al. 2010; Simonson et al. 2010), suggesting that this reduction, at least in part, has a genetic basis. Whether [Hb] is secondary to other physiological changes at one or more steps of the O2 transport cascade or rather a direct target of adaptation (e.g. [Hb] is specifically targeted for reduction to ameliorate negative effects associated with high blood viscosity) is unknown (Moore, 2001; Beall et al. 2004; Leon-Velarde et al. 2005; Julian et al. 2007; Storz, 2010; Storz et al. 2010).
Considering that adaptive genetic factors in Tibetans are associated with non-elevated [Hb] at altitude, we challenged traditional thoughts that polycythaemia at altitude improves O2 availability and thus exercise capacity. By making use of the natural variation in their [Hb], we tested the hypothesis that this relatively low [Hb] in Tibetans is associated with higher exercise capacity at altitude, which, if found to be true, would imply that the usual polycythaemic response to altitude seen in most populations may be a consequence of the erythropoeitic system’s usual, and generally beneficial, response to cellular hypoxia, such as after blood loss. If the hypothesis is correct, then one or more steps of the O2 transport pathway must be of greater capacity in Tibetans with lower [Hb], which directed the focus of this study. Such differences in the O2 transport pathway may reflect a suite of sequential adaptive events in this population, some which are related to [Hb].
Methods
Subjects
Twenty-one healthy, non-athletic, but active, non-smoking Tibetan male subjects aged 18–40 who were permanent residents at elevations >4000 m and nine Han Chinese males who had been resident above 4000 m for more than 2 years were recruited for this study and another study (Table2; additional information is reported in Table2 of Simonson et al. 2014) and screened for exercise studies as previously described (Simonson et al. 2014). No subjects exhibited illness, including chronic mountain sickness, as only screened healthy subjects were eligible to participate. Subjects provided written informed consent, obtained by Chinese members of the project team who spoke the local dialect (health assessment and consent led by an established Chinese academic physician Ri-Li Ge), and the University of California San Diego and University of Qinghai Medical College review boards approved all studies, which conformed to the standards set by the Declarationof Helsinki.
Table 2.
Oxygen transport data collected from 21 Tibetan and 9 Han Chinese males at >4200 m during maximal exercise
| Ethnicity | Age | Height (cm) | Weight (cm) |
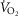 kg–1 kg–1
|
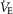 kg–1 kg–1
|
DL/VC | A-a 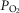 difference (mmHg) difference (mmHg) |
Base deficit | QT kg–1 | DM kg–1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Tibetan | 23 ± 5 | 168 ± 7 | 61 ± 12 | 37.5 ± 7.0 | 1.92 ± 0.40 | 18.4 ± 3.3 | 16.3 ± 4.0 | 13 ± 3 | 0.26 ± 0.05 | 1.3 ± 0.4 |
| Han Chinese | 27 ± 10 | 173 ± 4 | 60 ± 9 | 36.8 ± 5.2 | 1.95 ± 0.38 | 15.5 ± 2.5 | 18.5 ± 2.8 | 13 ± 2 | 0.22 ± 0.05 | 1.3 ± 0.3 |
Values are means ± SD.
See Simonson et al. 2014 for additional published variables including [Hb],  , P50, arterial
, P50, arterial  ,
,  , and pH;
, and pH;
individual values available upon request.)
Study design
Studies were performed in a hospital outpatient room in either of two neighbouring villages from which the subjects were recruited at an altitude between 4200 and 4300 m. After detailed explanations of the protocol and measurement procedures, spirometry was performed to determine vital capacity (VC), forced vital capacity (FVC), forced expiratory volume in one second (FEV1), maximum mid-expiratory flow (FEF25-75), and peak expiratory flow (PEF). Individuals exhibiting signs of significant respiratory disease based on spirometry data were excluded from the study. None of the 30 subjects displayed any spirometric abnormalities.
Following spirometry, a forehead oximeter (Nellcor N-395 Louisville, KY, USA) probe was taped to the skin, and a 20-gauge radial arterial catheter was then placed percutaneously under local anaesthetic using sterile technique. Six electrodes were taped onto cleansed skin in customary regions (two each on the back, neck and chest) for impedance cardiography measurements (PhysioFlow Enduro, Paris, France). A tight-fitting facemask covering both nose and mouth was secured and connected to a Carefusion ‘Oxycon’  /
/ measurement system.
measurement system.
Given the lack of familiarity of the subjects with research studies, a straightforward, simplified, incremental exercise protocol to exhaustion was adopted (Simonson et al. 2014). Subjects were seated on a cycle ergometer, resting until heart rate and respiratory rate were stable, at which point resting data were collected. Subjects were then asked to pedal at light exercise breathing room air as a warm-up, the load being varied according to each individual’s capacity. After 2 min, subjects were asked to pedal at a moderate workload intended to target about 70–90% of maximal power, as judged by both frequent questioning of each subject, as he pedalled, and heart rate. After 2 min at this work rate, further data collection was performed, and when completed, subjects then pedalled at maximal effort, the load again being varied to achieve exhaustion in 2–3 min. Towards the end of this period, data were again collected.
After removal of the arterial line, securing of haemostasis, and at least a 30 min rest, subjects repeated the exercise as above, but now breathing 100% O2, which was administered once they reached their previous submaximal level. Subjects exercised again to exhaustion to determine whether maximal power output would increase after elimination of ambient hypoxia, as it does in acclimatized lowlanders (Calbet et al. 2002). While 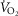 could not be measured while breathing 100% O2, the maximal power output and heart rate were recorded and compared to maximal watts and heart rate achieved without supplemental oxygen.
could not be measured while breathing 100% O2, the maximal power output and heart rate were recorded and compared to maximal watts and heart rate achieved without supplemental oxygen.
Variables measured and data collection
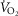 ,
,  , expiratory ventilation (
, expiratory ventilation ( ), cardiac output (QT) and heart rate (HR) were measured continuously from rest until exhaustion, using 10 s averaging, under ambient conditions (barometric pressure (PB) = 454–459 mmHg, Davis Perception II). At rest, and during the final 2 min of both submaximal and maximal exercise, arterial blood samples (2 ml) were taken anaerobically into sterile heparinized syringes that were kept in an ice slurry before and after sample collection. Blood gas variables were measured immediately or within a few minutes of collection. All of the data collected, equipment, and methods used in this study are summarized in Table1. [Hb] and haematocrit values were measured from arterial blood samples and analysed using a Mindray Hematology Analyser (BC-2300, Shenzhen, People’s Republic of China).
), cardiac output (QT) and heart rate (HR) were measured continuously from rest until exhaustion, using 10 s averaging, under ambient conditions (barometric pressure (PB) = 454–459 mmHg, Davis Perception II). At rest, and during the final 2 min of both submaximal and maximal exercise, arterial blood samples (2 ml) were taken anaerobically into sterile heparinized syringes that were kept in an ice slurry before and after sample collection. Blood gas variables were measured immediately or within a few minutes of collection. All of the data collected, equipment, and methods used in this study are summarized in Table1. [Hb] and haematocrit values were measured from arterial blood samples and analysed using a Mindray Hematology Analyser (BC-2300, Shenzhen, People’s Republic of China).  ,
, 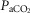 and pH were measured using an iSTAT hand-held electrode system at 37ºC. Core temperature could not be measured, and was estimated at 37.0ºC for all subjects as the short duration and limited power output during peak exercise (<2 min) would not be expected to affect core temperature beyond an average of 0.6ºC (data from Wagner et al. 1986, 1987). Peak power averaged only 151 W, due to hypoxia, which further acted to limit core temperature changes. Since no lactate analyser was available, base excess was calculated from pH and
and pH were measured using an iSTAT hand-held electrode system at 37ºC. Core temperature could not be measured, and was estimated at 37.0ºC for all subjects as the short duration and limited power output during peak exercise (<2 min) would not be expected to affect core temperature beyond an average of 0.6ºC (data from Wagner et al. 1986, 1987). Peak power averaged only 151 W, due to hypoxia, which further acted to limit core temperature changes. Since no lactate analyser was available, base excess was calculated from pH and 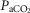 using the Siggaard-Andersen nomogram to quantify metabolic acidosis generated during exercise. Mixed venous [O2] and
using the Siggaard-Andersen nomogram to quantify metabolic acidosis generated during exercise. Mixed venous [O2] and 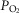 (and [CO2] and
(and [CO2] and 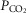 ) were calculated by mass conservation using the Fick principle and measured values of
) were calculated by mass conservation using the Fick principle and measured values of 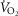 , QT, and arterial O2 concentration. The partial pressure of O2 in the blood at which Hb is 50% saturated (P50) was computed from the
, QT, and arterial O2 concentration. The partial pressure of O2 in the blood at which Hb is 50% saturated (P50) was computed from the 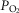 –saturation relationship (Simonson et al. 2014). Previously established Fortran programs were used to determine diffusing capacity in lung (DL) and muscle (DM) using a forward integration algorithm (Wagner & West, 1972) that is based on the Kelman subroutines describing the O2 and CO2 dissociation curves (Kelman, 1966, 1967). These algorithms find the values of diffusing capacity in each tissue that match measured arterial
–saturation relationship (Simonson et al. 2014). Previously established Fortran programs were used to determine diffusing capacity in lung (DL) and muscle (DM) using a forward integration algorithm (Wagner & West, 1972) that is based on the Kelman subroutines describing the O2 and CO2 dissociation curves (Kelman, 1966, 1967). These algorithms find the values of diffusing capacity in each tissue that match measured arterial 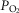 in the case of lung and measured venous
in the case of lung and measured venous 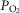 in the case of muscle, assuming a homogeneous organ in each case.
in the case of muscle, assuming a homogeneous organ in each case.
Table 1.
Measurements collected and data analysed at rest and during graded exercise to determine values for analyses of each step of the oxygen transport chain
 , , 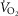 , , 
|
Expired gas analysis (Oxycon, Carefusion); measured |
Arterial  , ,  , pH, base excess (BE) , pH, base excess (BE) |
Arterial sample (i-STAT, Abbott); measured |
| Cardiac output (QT) [Hb], haematocrit | Impedance cardiography (Physioflow); measured Arterial blood sample; measured |
| Oxygen saturation, [O2] | Forehead oximetry (Nelcor); measured |
| Standard P50 | Computed from 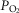 /saturation relationship; calculated /saturation relationship; calculated |
Venous [O2], 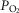
|
Calculated by mass conservation; calculated |
| Lung diffusing capacity (DL) | Bohr Integration using above data; calculated |
| Muscle diffusing capacity (DM) |
Analysis
Individual O2 transport variables at maximal exercise were first plotted as a function of [Hb] using linear regression in MatLab 2013v. Variables examined included 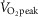 kg–1, minute ventilation (
kg–1, minute ventilation (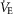 kg–1),
kg–1),  ,
, 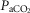 , base deficit, alveolar–arterial
, base deficit, alveolar–arterial  (A-a
(A-a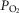 ) difference, diffusion capacity in lung (normalized to vital capacity, VC), i.e. DL/VC, cardiac output (QT kg–1), and diffusion capacity in muscle (DM kg–1), some of which are published in Simonson et al. 2014. Next,
) difference, diffusion capacity in lung (normalized to vital capacity, VC), i.e. DL/VC, cardiac output (QT kg–1), and diffusion capacity in muscle (DM kg–1), some of which are published in Simonson et al. 2014. Next,  kg–1 was plotted as a function of individual O2 transport variables, including
kg–1 was plotted as a function of individual O2 transport variables, including 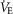 kg–1,
kg–1, 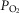 ,
, 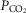 , A-a
, A-a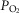 difference, base deficit, DL/VC, QT kg–1, and DM kg–1. Multiple linear regression (MatLab 2013v) was used to assess the significance of standardized predictors, including [Hb],
difference, base deficit, DL/VC, QT kg–1, and DM kg–1. Multiple linear regression (MatLab 2013v) was used to assess the significance of standardized predictors, including [Hb], 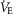 ,
, 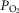 ,
,  , A-a
, A-a difference, base deficit, DL/VC, QT kg–1, DM kg–1 and age. Interaction effects were further assessed for significant predictors. P values < 0.05 were used to establish statistical significance.
difference, base deficit, DL/VC, QT kg–1, DM kg–1 and age. Interaction effects were further assessed for significant predictors. P values < 0.05 were used to establish statistical significance.
Results
Pertinent O2 transport data in the 21 Tibetan and nine Han Chinese subjects at maximal exercise breathing air at 4200–4300 m are provided in Table2 (and Simonson et al. 2014). The wide range of [Hb] in Tibetans provided variation to test the hypothesis that exercise capacity would relate negatively to [Hb]. All subjects included in this study fulfilled at least two of the following criteria for peak 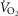 : (1) maximal heart rate at exhaustion as previous reported in individuals of Tibetan ethnicity (176 beats min–1 3 days after descending from an altitude of 4400 m, within 100 m of that of our studies, to 3685 m; Curran et al. 1998); (2) respiratory exchange ratio > 1.10; (3) no further increase (or a decrease) in
: (1) maximal heart rate at exhaustion as previous reported in individuals of Tibetan ethnicity (176 beats min–1 3 days after descending from an altitude of 4400 m, within 100 m of that of our studies, to 3685 m; Curran et al. 1998); (2) respiratory exchange ratio > 1.10; (3) no further increase (or a decrease) in 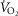 with increasing workload; (4) no further increase in heart rate despite an increase in workload; (5) rest to peak exercise increase in base deficit of 6 mmol l−1 or more. Overall, at exhaustion, average heart rate was 172 beats min–1; RER was 1.19; and base deficit was 13 mmol l−1 (rising from 4 mmol l−1 at rest), similarly in Tibetans and Han Chinese. There was no [Hb] dependence of these
with increasing workload; (4) no further increase in heart rate despite an increase in workload; (5) rest to peak exercise increase in base deficit of 6 mmol l−1 or more. Overall, at exhaustion, average heart rate was 172 beats min–1; RER was 1.19; and base deficit was 13 mmol l−1 (rising from 4 mmol l−1 at rest), similarly in Tibetans and Han Chinese. There was no [Hb] dependence of these 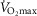 criteria.
criteria.
Oxygen transport and exercise capacity
Tibetans with lower [Hb] reached a higher  kg–1 compared to Tibetans with higher [Hb] (r = −0.45; P < 0.05; Fig.1). In addition, data were collected from Han Chinese subjects at altitude, although this population is genetically distinct and, as a historically lowland population, has not been exposed to hundreds of generations of selective pressure (Simonson et al. 2012; Wuren et al. 2014). The Han Chinese subjects, whose [Hb] falls in a higher range than more than half of the Tibetan subjects examined (from 18.1 to 21.1 g/dl −1 (11.2 – 13.1 mmol−1) in nine Han Chinese males), exhibit a similar relationship to exercise capacity (r = −0.83; y = −3.2x + 99).
kg–1 compared to Tibetans with higher [Hb] (r = −0.45; P < 0.05; Fig.1). In addition, data were collected from Han Chinese subjects at altitude, although this population is genetically distinct and, as a historically lowland population, has not been exposed to hundreds of generations of selective pressure (Simonson et al. 2012; Wuren et al. 2014). The Han Chinese subjects, whose [Hb] falls in a higher range than more than half of the Tibetan subjects examined (from 18.1 to 21.1 g/dl −1 (11.2 – 13.1 mmol−1) in nine Han Chinese males), exhibit a similar relationship to exercise capacity (r = −0.83; y = −3.2x + 99).
Figure 1.
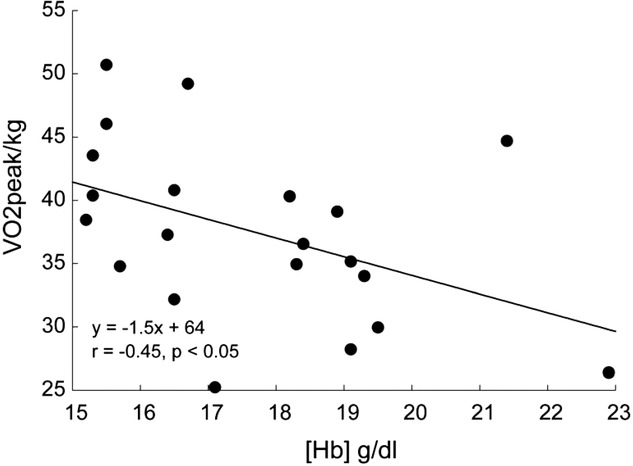
Haemoglobin concentration ([Hb]) versus peak 
Relationships between haemoglobin concentration ([Hb]) and peak 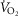 (
(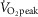 kg–1) in 21 male Tibetan subjects at 4200 m.
kg–1) in 21 male Tibetan subjects at 4200 m.
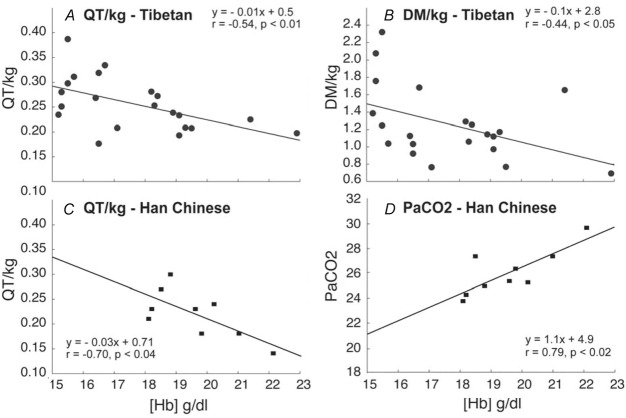
Figure 2 shows the relationships between [Hb] and significant variables in Tibetans and, in separate panels, Han Chinese. Relationships between [Hb] and QT kg–1 (r = –0.54; P < 0.01; Fig.2A) and between [Hb] and DM kg–1 (r = –0.44; P < 0.05; Fig.2B) indicate that both cardiac output and muscle diffusing capacity were higher at lower [Hb]. In contrast, 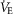 ,
, 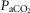 (ventilation), and diffusion in the lung (as reflected by both the A-a
(ventilation), and diffusion in the lung (as reflected by both the A-a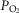 difference and DL/VC) were not associated with [Hb] and are therefore not shown. The nine Han Chinese who exhibit elevated [Hb] also exhibit significant relationships between [Hb] and and QT kg–1 (r = −0.70; y = −0.03x + 0.7) and
difference and DL/VC) were not associated with [Hb] and are therefore not shown. The nine Han Chinese who exhibit elevated [Hb] also exhibit significant relationships between [Hb] and and QT kg–1 (r = −0.70; y = −0.03x + 0.7) and  (r = 0.79; y = 1.1x + 4.9) (Fig.2C and D), and there is no statistical difference between Tibetan and Han Chinese in each of the relationships examined.
(r = 0.79; y = 1.1x + 4.9) (Fig.2C and D), and there is no statistical difference between Tibetan and Han Chinese in each of the relationships examined.
Figure 2.
Haemoglobin concentration ([Hb]) compared to components of oxygen transport at 4200 m
Components of the oxygen transport cascade compared to haemoglobin concentration ([Hb]) in Tibetan and Han Chinese males. Cardiac output (QT kg–1; A) and diffusing capacity in muscle (DM kg–1; B) are significantly related to [Hb]; arterial 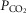 (
( , ventilation) and lung-related (alveolar–arterial difference) components are not associated with [Hb] in Tibetans. Relationships between [Hb] and QT kg–1 (P < 0.04; C) and
, ventilation) and lung-related (alveolar–arterial difference) components are not associated with [Hb] in Tibetans. Relationships between [Hb] and QT kg–1 (P < 0.04; C) and 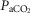 (P < 0.02; D) are also shown for 9 Han Chinese.
(P < 0.02; D) are also shown for 9 Han Chinese.
The above O2 transport-related variables were next individually examined with respect to 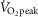 kg–1, again using only the Tibetan data for the regression line construction. In addition to its significant relationship with [Hb] (Fig.1),
kg–1, again using only the Tibetan data for the regression line construction. In addition to its significant relationship with [Hb] (Fig.1), 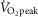 kg–1 was found to be positively and strongly associated with QT kg–1 (r = 0.71; P < 0.001; Fig.3B), diffusion capacity in muscle (r = 0.84; P < 0.001; Fig.3C), and negatively associated with
kg–1 was found to be positively and strongly associated with QT kg–1 (r = 0.71; P < 0.001; Fig.3B), diffusion capacity in muscle (r = 0.84; P < 0.001; Fig.3C), and negatively associated with 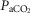 (r = –0.54; P < 0.02; Fig.3A). In nine Han Chinese subjects, peak
(r = –0.54; P < 0.02; Fig.3A). In nine Han Chinese subjects, peak 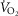 kg–1 tracked with
kg–1 tracked with 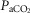 (r = –0.75; y = −2.1x + 92; Fig.3D) and DM kg–1 (r = 0.72; y = 12x + 22; Fig.3E), and no statistical difference was observed between Tibetan and Han Chinese data sets. In a multifactorial system such as O2 transport, such pairwise linear regressions are limited in their information content, which led us to conduct multiple linear regression analysis for the 21 Tibetan subjects. Each of the significant univariate predictors was tested and included in the model (QT kg–1 and DM kg–1 at P < 0.001;
(r = –0.75; y = −2.1x + 92; Fig.3D) and DM kg–1 (r = 0.72; y = 12x + 22; Fig.3E), and no statistical difference was observed between Tibetan and Han Chinese data sets. In a multifactorial system such as O2 transport, such pairwise linear regressions are limited in their information content, which led us to conduct multiple linear regression analysis for the 21 Tibetan subjects. Each of the significant univariate predictors was tested and included in the model (QT kg–1 and DM kg–1 at P < 0.001;  at P < 0.05), and explains the majority of variation in
at P < 0.05), and explains the majority of variation in 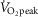 kg–1 (R2 = 0.88; y = −0.19
kg–1 (R2 = 0.88; y = −0.19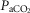 + 0.38QT kg–1 + 0.63DM kg–1; Fig.4).
+ 0.38QT kg–1 + 0.63DM kg–1; Fig.4).
Figure 3.

Components of oxygen transport associated with exercise capacity
Relationships observed between ventilation (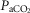 , P <0.01; A), QT kg–1 (P < 0.001l; B), and DM kg–1 (P < 0.001; C), with
, P <0.01; A), QT kg–1 (P < 0.001l; B), and DM kg–1 (P < 0.001; C), with  kg–1 in Tibetans;
kg–1 in Tibetans;  and A-a
and A-a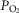 difference did not exhibit a relationship with
difference did not exhibit a relationship with 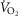 peak kg–1. Relationships between
peak kg–1. Relationships between 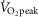 kg–1 and
kg–1 and 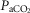 (P < 0.02; D), and DM kg–1 (P < 0.03; E) are also shown for 9 Han Chinese.
(P < 0.02; D), and DM kg–1 (P < 0.03; E) are also shown for 9 Han Chinese.
Figure 4.
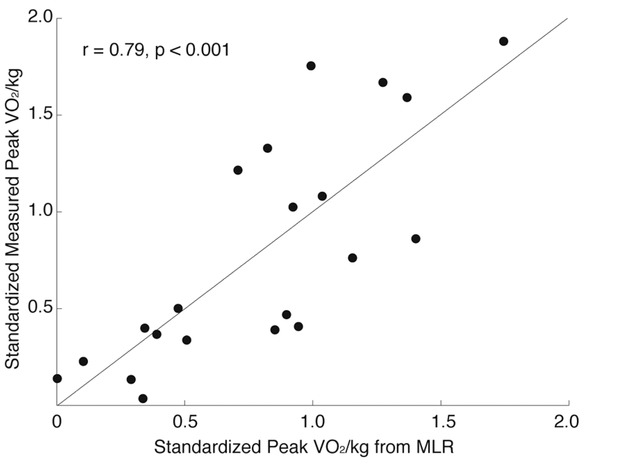
Measured versus predicted peak VO2 in 21 Tibetan males at 4200 m. Highly significant relationship (r = 0.95; p < 0.001) between measured VO2peak/kg (y axis) compared to that predicted by linear regression; standardized predictors include PaCO2 (b = −0.18), cardiac output (QT)/kg (b = 0.38), diffusion capacity in muscle (DM)/kg (b = 0.63).
Subjects breathing 100% O2 exhibited an average increase in peak power of 16 W (from 152 to 168 W) (P < 0.0001), which was consistent among Tibetan (n = 21) and Han Chinese (n = 8; no data available for one Han Chinese subject) groups. Therefore, the increase in available O2 resulted in only a 10% increase in maximal power output, which is relatively minor compared to the corresponding ∼30% increase observed in individuals of European ancestry acclimatized to altitudes of 4200–4300 m (Reeves et al. 1992; Pronk et al. 2003).
Discussion
Summary of main findings
The principal finding was that Tibetans with sea-level range [Hb] had enhanced exercise capacity compared to those with elevated [Hb]. The second major finding was that this [Hb] dependency of 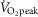 within Tibetans was facilitated by higher cardiac output and greater muscle O2 diffusing capacity, but not by respiratory factors including ventilation and gas exchange. As a group, however, Tibetans exhibit greater DL/VC compared to the nine Han Chinese counterparts. The third major finding was that eliminating hypoxaemia by breathing 100% O2 led to an average increase in peak work rate of only 16 W, or 10% compared to acclimatized lowlanders at this altitude, who would have been able to increase work rate by ∼30% (i.e. back to prior sea level values) upon breathing O2 (Pronk et al. 2003). The fourth major finding was that Han Chinese and Tibetans with the same high [Hb] performed similarly in all respects examined.
within Tibetans was facilitated by higher cardiac output and greater muscle O2 diffusing capacity, but not by respiratory factors including ventilation and gas exchange. As a group, however, Tibetans exhibit greater DL/VC compared to the nine Han Chinese counterparts. The third major finding was that eliminating hypoxaemia by breathing 100% O2 led to an average increase in peak work rate of only 16 W, or 10% compared to acclimatized lowlanders at this altitude, who would have been able to increase work rate by ∼30% (i.e. back to prior sea level values) upon breathing O2 (Pronk et al. 2003). The fourth major finding was that Han Chinese and Tibetans with the same high [Hb] performed similarly in all respects examined.
[Hb] in this group of subjects and their health status
[Hb] among the high-altitude (>4200 m) inhabitants examined (Tibetan = 17.7 ± 2.1 g dl−1 (11.0 ± 1.3 mmol l−1); Han Chinese = 19.0 ± 0.8 g dl−1 (11.8 ± 0.5 mmol l−1); P < 0.02) is consistent with previous reports at comparable altitude (Beall, 2000; Wu et al. 2005; Beall et al. 2010; Simonson et al. 2010). It is therefore reasonable to believe that subjects were representative of a high-altitude Tibetan resident population. While iron stores or binding capacity were not measured, medical history including dietary information gave no reason to suspect a pathological cause of the sea-level [Hb] in those Tibetans with non-elevated [Hb], nor a lifestyle or health-related reason for less exercise capacity in the higher [Hb] group (all subjects reported as non-athletic, many self-described as herders). Andean highlanders exhibiting anaemia (defined as [Hb] < 15.8 g dl−1) at altitude exhibit both a reduced aerobic capacity (Tufts et al. 1985) and a left-shifted Hb-dissociation curve that maintains arterial saturation similar to pre-exercise levels, suggesting different mechanisms for anaemia compensation at high versus low altitude in this population (Beard et al. 1988). The Tibetans studied here, exhibiting [Hb] 15.2–22.9 g dl−1 (9.4–14.2 mmol l−1), also exhibit an increased blood O2 binding affinity, although this is not associated with [Hb], alveolar–arterial 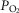 difference, or peak O2 uptake per kilogram (Simonson et al. 2014).
difference, or peak O2 uptake per kilogram (Simonson et al. 2014).
There were no symptoms or signs of chronic mountain sickness (CMS) in the subjects with elevated [Hb] at altitude. If the low [Hb] was due to undiscovered pathology, it is all the more remarkable that these Tibetan subjects had greater exercise capacity. It is, however, recognized that it is not possible to exclude confounding factors in the [Hb]–exercise capacity relationship completely.
Physiological basis for greater exercise capacity in subjects with low [Hb] at altitude relative to those with higher [Hb]
Considering that increased [Hb] leads to more O2 binding sites and thus more O2 carried in blood at a given  , it seems counterintuitive that Tibetans have adapted with a low [Hb] at altitude. This is because a lower [Hb], if the only factor different between low and high [Hb] Tibetans, would only reduce capacity to transport O2 to the muscles. Moreover, since polycythaemia is a universal response to ascent to altitude in healthy lowlanders, having more red cells should be beneficial to exercise at altitude. Similarly, the horse has a huge splenic red cell reservoir that is pumped into the circulation within seconds of galloping which has been shown (by splenectomy) to greatly enhance their exercise capacity (Persson & Bergsten, 1975). Blood volume, a separate benefit of splenic contraction, may be more important than [Hb] as most of the loss in exercise after splenectomy is reversed by infusion of blood with resting [Hb] in horses (Hopper et al. 1991). The literature contains many reports suggesting that a high [Hb] (even short of chronic mountain sickness) does not promote exercise capacity as demonstrated by acute isovolumic dilution in Danish subjects acclimatized to 5260 m (Calbet et al. 2002) nor upon acute [Hb] reduction in a single Peruvian high-altitude native (Winslow et al. 1985). Additional studies suggest optimal [Hb] is rather near sea-level values (Villafuerte et al. 2004; Schuler et al. 2010). The likely explanation for lack of benefit to exercise capacity from an elevated [Hb] at altitude is that the gains in circulatory transport afforded by a higher [Hb] with more O2 binding sites are mostly offset by reductions in the ability to move O2 by diffusion between alveolar gas and capillary blood in the lungs and between muscle microcirculatory vessels and the mitochondria (Wagner, 1996). When [Hb] is elevated, time to diffusive equilibration in these vascular beds is prolonged. Piiper & Scheid (1981) expressed this best when they showed that the compound constant D/(ßQ) determined the degree of diffusion equilibration to be expected in the lungs (where D is lung diffusing capacity) or muscle (where D is muscle diffusing capacity). Here Q is muscle blood flow or cardiac output, and ß is the (average) slope of the O2–Hb dissociation curve. Since ß must increase when total [Hb] increases, D/(ßQ) must fall, other factors being equal, and diffusion equilibration takes longer. As a result,
, it seems counterintuitive that Tibetans have adapted with a low [Hb] at altitude. This is because a lower [Hb], if the only factor different between low and high [Hb] Tibetans, would only reduce capacity to transport O2 to the muscles. Moreover, since polycythaemia is a universal response to ascent to altitude in healthy lowlanders, having more red cells should be beneficial to exercise at altitude. Similarly, the horse has a huge splenic red cell reservoir that is pumped into the circulation within seconds of galloping which has been shown (by splenectomy) to greatly enhance their exercise capacity (Persson & Bergsten, 1975). Blood volume, a separate benefit of splenic contraction, may be more important than [Hb] as most of the loss in exercise after splenectomy is reversed by infusion of blood with resting [Hb] in horses (Hopper et al. 1991). The literature contains many reports suggesting that a high [Hb] (even short of chronic mountain sickness) does not promote exercise capacity as demonstrated by acute isovolumic dilution in Danish subjects acclimatized to 5260 m (Calbet et al. 2002) nor upon acute [Hb] reduction in a single Peruvian high-altitude native (Winslow et al. 1985). Additional studies suggest optimal [Hb] is rather near sea-level values (Villafuerte et al. 2004; Schuler et al. 2010). The likely explanation for lack of benefit to exercise capacity from an elevated [Hb] at altitude is that the gains in circulatory transport afforded by a higher [Hb] with more O2 binding sites are mostly offset by reductions in the ability to move O2 by diffusion between alveolar gas and capillary blood in the lungs and between muscle microcirculatory vessels and the mitochondria (Wagner, 1996). When [Hb] is elevated, time to diffusive equilibration in these vascular beds is prolonged. Piiper & Scheid (1981) expressed this best when they showed that the compound constant D/(ßQ) determined the degree of diffusion equilibration to be expected in the lungs (where D is lung diffusing capacity) or muscle (where D is muscle diffusing capacity). Here Q is muscle blood flow or cardiac output, and ß is the (average) slope of the O2–Hb dissociation curve. Since ß must increase when total [Hb] increases, D/(ßQ) must fall, other factors being equal, and diffusion equilibration takes longer. As a result,  is lower, and muscle venous
is lower, and muscle venous 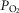 higher (i.e. extraction is less) when [Hb] is higher (again, all other factors being constant), and overall O2 transport is impaired, counterbalancing the positive effect of more circulatory transport capacity.
higher (i.e. extraction is less) when [Hb] is higher (again, all other factors being constant), and overall O2 transport is impaired, counterbalancing the positive effect of more circulatory transport capacity.
This line of reasoning requires that positive changes in other parts of the O2 transport system must explain greater exercise capacity when [Hb] is low, and a major purpose of this study was to identify any such changes should a low [Hb] be found to be associated with greater exercise capacity. The changes associated with [Hb] are cardiac output and in muscle O2 diffusional conductance. Moreover, if we consider that the Tibetans with higher [Hb] reflect the pre-adaptive ‘norm’ – that is, the [Hb] without genetic changes that lead to reduced [Hb] – it is notable that the outcome is not just maintained exercise capacity as [Hb] falls, but greatly increased exercise capacity in Tibetans with lower compared to higher [Hb] (Fig.1).
The positive relationships observed between [Hb] and cardiac output and exercise capacity suggest that changes in cardiac preload, contractility, or afterload (singly or in combination) are involved in increased cardiac output when [Hb] is reduced. Similarly, greater muscle O2 diffusive transport, modelled as described in the Methods section, is observed in Tibetan males with lower [Hb] in addition to a higher 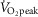 kg–1, suggesting more efficient O2 transfer from red cells to mitochondria of metabolizing cells. Such changes could be attributed to muscle capillarity (i.e. increased number of capillaries per muscle fibre), decreased fibre diameter, or alterations in fibre type. Assessment of these variables (in addition to others such as red cell mass and plasma volume), as well as gene expression and protein levels in respective tissues, will help to determine the structural and functional basis of these correlations, but such data were not obtained in the current study.
kg–1, suggesting more efficient O2 transfer from red cells to mitochondria of metabolizing cells. Such changes could be attributed to muscle capillarity (i.e. increased number of capillaries per muscle fibre), decreased fibre diameter, or alterations in fibre type. Assessment of these variables (in addition to others such as red cell mass and plasma volume), as well as gene expression and protein levels in respective tissues, will help to determine the structural and functional basis of these correlations, but such data were not obtained in the current study.
Although arterial 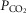 (representing ventilation in relation to metabolic load) is not associated with [Hb] (P > 0.08), lower
(representing ventilation in relation to metabolic load) is not associated with [Hb] (P > 0.08), lower  is associated with greater
is associated with greater 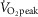 kg–1 in Tibetan males (Fig.3A). Previous reports indicate higher offspring survival in Tibetan women with elevated O2 saturation (Beall et al. 2004), and our previous work indicates an inverse relationship between [Hb] and O2 saturation in females but not males (Simonson et al. 2010). Whether this may be attributed to alterations in female ventilatory responses compared to males, and furthermore attributed to specific genetic changes, has yet to be determined. It will therefore be necessary to determine whether differences in exercise capacity and response vary between sexes.
kg–1 in Tibetan males (Fig.3A). Previous reports indicate higher offspring survival in Tibetan women with elevated O2 saturation (Beall et al. 2004), and our previous work indicates an inverse relationship between [Hb] and O2 saturation in females but not males (Simonson et al. 2010). Whether this may be attributed to alterations in female ventilatory responses compared to males, and furthermore attributed to specific genetic changes, has yet to be determined. It will therefore be necessary to determine whether differences in exercise capacity and response vary between sexes.
High [Hb] Tibetans exhibit similar exercise capacity to Han Chinese
It is important to note the similarity of exercise capacity and response among Tibetans with elevated [Hb] and Han Chinese subjects who exhibit equivalent [Hb]. This indicates that Tibetans with erythrocytosis at altitude exhibit comparable physiological outcomes to native lowland Han Chinese at altitude, at least with respect to exercise capacity and the O2 transport components measured here. No statistical difference was detected between Tibetan and Han Chinese in each of the relationships examined. The variation in the Tibetan sample, however, extends across a much larger range (11 Tibetans have [Hb] lower than the lowest level in the Han), reflecting innate, gene-by-environment, differences in these populations.
It is unclear whether components observed in high [Hb] Tibetans and Han Chinese are a result of the same, non-adaptive genetic backgrounds or if such similarities reflect different physiological mechanisms that converge towards elevated [Hb] and the exercise capacity and responses examined. The nine Han Chinese examined in this study, however, had successfully resided at altitudes above 4000 m for more than 2 years without illness and may not be an accurate representation of the population (as Han exhibit greater incidence of altitude illness compared to Tibetans (MacInnis et al. 2010) and such subjects would not have been included in our study). Additionally, ancestral ‘standing’ beneficial genetic variants may be present in both groups. All this considered, it will be necessary to determine whether the ‘least’ adapted high [Hb] Tibetans and well-acclimatized lowland Han Chinese share genetic variants which relate to elevated [Hb] and exercise capacity and response.
Elimination of hypoxaemia and peak work rate
The difference between exercise capacity in ambient conditions and upon administration of 100% O2 is significant (P < 0.0001) but relatively small (16 W) compared to the corresponding ∼30% increase observed in individuals of European ancestry acclimatized to altitudes of 4200–4300 m (Reeves et al. 1992; Pronk et al. 2003). This raises the possibility that Tibetans have developed fine-tuned mechanisms (possibly a reduction in mitochondrial mass to minimize metabolic machinery/biogenesis costs (Kayser et al. 1996)) to efficiently utilize limited O2 available at high altitude. Similar results have been reported in Andeans (Hochachka, 1998), suggesting such conservation may be utilized in both highland groups.
Primary and secondary targets of adaptive change within Tibetans
A question these findings raise is whether low [Hb] at altitude, comparable to sea-level values, is the primary target of adaptive change, i.e. did low [Hb] come first and QT kg–1 and/or DM kg–1 follow or vice versa. Altitude-induced polycythaemia and the associated increase in blood viscosity is not beneficial, and may underlie the pathogenesis of chronic mountain sickness (CMS) (Vargas & Spielvogel, 2006a). The incidence of CMS is lower among Tibetans (less than a few per cent; Wu et al. 1992) compared to Andeans (15.6%; Monge et al. 1992), who exhibit [Hb] few to several grams per decilitre higher than Tibetan natives. Yet the late onset of CMS (post-reproductive age; Moore et al. 2007) suggests protection from CMS alone would not explain the adaptive genetic signals that have been associated with lower [Hb] compared to other ethnic groups at altitude (Simonson et al. 2012), although maternal [Hb] during pregnancy could be a primary target. In either case, if [Hb] was the primary driver of evolutionary change, alterations at one or more steps of the O2 transport cascade would be necessary to compensate for reduction in O2 transport capacity. While examination within a Tibetan population exhibiting a range of [Hb] is shown here, further consideration of population differences, as observed in Tibetan–Han DL/VC (Table2), may indicate fixed or nearly fixed differences compared to native lowland groups.
An alternative hypothesis based on the data presented within Tibetan subjects examined here is that changes related to cardiac and/or skeletal muscle were the primary targets of adaptation and, consequently, the shift in [Hb] towards sea-level values was secondary to improved cellular O2 availability resulting from enhanced cardiovascular function and/or muscle diffusion capacity. We suggest that this view has merit because most compensatory changes tend to restore function towards baseline rather than resulting in greater function than before. Thus, were the Hb change primary, compensation might have been expected to restore exercise capacity, but to a much lesser degree than shown by Tibetans with lower [Hb]. However, the observation was that exercise capacity was greater in those Tibetans with [Hb] in the sea-level range. In this case, it is plausible that the genetic associations with [Hb] are in fact secondary signals (side-effects), or possibly occurred separately following changes in circulatory and/or O2 muscle diffusion components of the O2 transport cascade.
Considering that male Tibetans examined here with lower [Hb] have increased exercise capacity, cardiac output and O2 diffusion capacity in muscle, it will be important to determine whether associations between each of these O2 transport components and the [Hb]-associated adaptive loci are significant or whether cardiac and muscle changes are related via increased blood flow, which serves to increase O2 conductance. Increased flow may result in gene expression changes in muscle (Drummond et al. 2008) or, alternatively, genetic changes in muscle and heart attributed to the same or independent outcomes. Such gains in efficiency would diminish the stimulus to elevate [Hb], which may be reduced as the result of a separate adaptive event.
Genomic targets of selection at EGLN1, PPARA, and EPAS1 loci are associated with low [Hb] in Tibetans at altitude (Beall et al. 2010; Simonson et al. 2010; Yi et al. 2010). While not examined here, it is plausible that adaptive copies at these loci underlie variation in other components of O2 transport. These genes, in addition to others that have not been associated with [Hb] but reported as selection candidates in multiple genomic studies (Simonson et al. 2012), may also be related to physiological variables highlighted in this study (i.e. heart/skeletal muscle structure and function, ventilation). Aside from EGLN1 (Lorenzo et al. 2014; Song et al. 2014), however, precise functional variants remain unknown. Ultimately, adaptive functional genetic variants, and combinations thereof, should be examined in a physiological context.
Limitations
As our study was conducted in a remote village at 4200 m, various logistical challenges were anticipated and addressed as follows. One concern was altitude and inhospitable conditions would affect equipment performance of the  /
/ system, the device used to measure cardiac output, and the blood gas analyser. We therefore collected
system, the device used to measure cardiac output, and the blood gas analyser. We therefore collected  /
/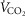 data during an incremental exercise protocol from a single subject at 2200 m and again at simulated 4200 m and from another subject at 2200 m and under ambient conditions at 4200 m in the Tibetan village. Repeated daily measurements in both subjects at different altitudes indicate consistency in the
data during an incremental exercise protocol from a single subject at 2200 m and again at simulated 4200 m and from another subject at 2200 m and under ambient conditions at 4200 m in the Tibetan village. Repeated daily measurements in both subjects at different altitudes indicate consistency in the 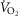 /
/ system, and a slope of the relationship between
system, and a slope of the relationship between 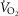 and watts that lay within the normal range of 9–11 ml min−1 W−1 (supplemental material available).
and watts that lay within the normal range of 9–11 ml min−1 W−1 (supplemental material available).
While we were unable to engage subjects in additional tests for repeated measurement of blood gas variables (requiring an additional arterial catheterization), we performed a quality-control check by comparing our data with those of reports in acclimatized subjects at comparable altitude. The average resting alveolar  and
and  in our cohort corresponds well to measurements collected in acclimatized subjects at altitude (Table2, Rahn & Otis, 1949).
in our cohort corresponds well to measurements collected in acclimatized subjects at altitude (Table2, Rahn & Otis, 1949).
We utilized the non-invasive impedence cardiography (PhysioFlow Enduro) in order to make the key measurement of QT in each subject during rest, submaximal, and maximal exercise. While various reports have validated use of this equipment (Charloux et al. 2000; Richard et al. 2001; Tordi et al. 2004), this is not always the case (Siebenmann et al. 2014). In our study, the relationship between PhysioFlow QT and Carefusion-measured 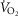 was linear, as expected, and was also in agreement with values predicted by the established relationship between QT and
was linear, as expected, and was also in agreement with values predicted by the established relationship between QT and  (QT ≈ 5 + 5
(QT ≈ 5 + 5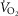 ; as illustrated in Barker et al. 1999) upon incremental increase during exercise at 4200 m. Therefore, the cardiac output data presented are supported not only by other reports regarding its accuracy but also by satisfying a known relationship using independent measures from the Carefusion
; as illustrated in Barker et al. 1999) upon incremental increase during exercise at 4200 m. Therefore, the cardiac output data presented are supported not only by other reports regarding its accuracy but also by satisfying a known relationship using independent measures from the Carefusion  system and PhysioFlow QT output. Again, supplemental material reporting these relationships is available.
system and PhysioFlow QT output. Again, supplemental material reporting these relationships is available.
A further limitation was to determine DMO2 (muscle O2 diffusion conductance) from values for venous  computed from the Fick principle, knowing
computed from the Fick principle, knowing 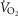 , cardiac output and arterial O2 concentration. This computation estimates mixed venous O2 levels, not those coming from the exercising muscle, which would be lower. This would then cause DMO2 to have been underestimated. However, leg blood flow must be lower than cardiac output. Since cardiac output was used in the calculation, this would per se have given an overestimate of DMO2. If we use reasonable estimates of non-exercising tissue metabolic rate and blood flow and subtract them from
, cardiac output and arterial O2 concentration. This computation estimates mixed venous O2 levels, not those coming from the exercising muscle, which would be lower. This would then cause DMO2 to have been underestimated. However, leg blood flow must be lower than cardiac output. Since cardiac output was used in the calculation, this would per se have given an overestimate of DMO2. If we use reasonable estimates of non-exercising tissue metabolic rate and blood flow and subtract them from 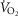 and QT, respectively, estimated muscle venous
and QT, respectively, estimated muscle venous 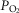 and O2 content would have been similar, but with leg blood flow less than cardiac output, DMO2 would have been proportionally lower than reported here. We felt that it was simpler and more transparent to use measured data and whole-body estimation of DMO2 than to guess at non-exercising
and O2 content would have been similar, but with leg blood flow less than cardiac output, DMO2 would have been proportionally lower than reported here. We felt that it was simpler and more transparent to use measured data and whole-body estimation of DMO2 than to guess at non-exercising  and blood flow and attempt correction of the data, but then to point out that absolute values reflect the whole-body transport process, not just the exercising muscles. However, the same computation was performed and simplifications used for all 30 subjects. Thus, the relative comparisons should be robust even if the absolute values of DMO2 are systematically in error.
and blood flow and attempt correction of the data, but then to point out that absolute values reflect the whole-body transport process, not just the exercising muscles. However, the same computation was performed and simplifications used for all 30 subjects. Thus, the relative comparisons should be robust even if the absolute values of DMO2 are systematically in error.
We knew, based on previous reports (Simonson et al. 2010), that [Hb] in this specific population spans a wide range, but no prior data regarding relationships with this trait and exercise capacity and/or O2 transport had been collected. Therefore, we did not know what if any relationships would be identified between [Hb], exercise capacity, and components of oxygen transport, and thus decided it would be premature to design studies to add on to basic exercise measurements – such as measures of blood volume, iron stores, cardiac function by ECHO, etc. Given the important insights presented here, next steps should include such assessments in an effort to understand the structure and functional basis of enhanced cardiac function and O2 extraction in addition to ventilatory control differences that were found.
Conclusion
At altitude >4000 m, Tibetan males with sea-level [Hb], compared to those with elevated [Hb], exhibit greater exercise capacity, higher cardiac output, and greater O2 diffusional conductance in muscle but not lung. These components as well as PaCO2 are further associated with exercise capacity. The physiological and genetic relationships among [Hb], cardiac function and muscle O2 diffusional conductance remain to be elucidated. Supplemental (100%) O2 given during exercise increases peak work rate in Tibetans by only a third of that reported in Caucasian lowlanders acclimatized to altitude (with no difference between high and low [Hb] groups). Acclimatized Han Chinese males performed similarly in terms of exercise capacity and components of O2 transport to the high [Hb] Tibetan group, suggesting a lack of adaptive changes in these groups. As indicated in many reports, the fact that healthy Andeans are polycythaemic at altitude, while many Amhara Ethiopians and Tibetans are not, suggests distinct evolutionary paths between these native highland groups.
Acknowledgments
We thank all high-altitude inhabitants who participated in this study and anonymous reviewers for helpful comments.
Glossary
- CMS
chronic mountain sickness
- DL
DM, diffusion capacity in lung, muscle
- Hb
haemoglobin
- HIF
hypoxia inducible factor
- O2
oxygen; peak
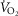 , volume of O2 utilized at peak exercise
, volume of O2 utilized at peak exercise- P50
partial pressure of O2 in the blood at which Hb is 50% saturated
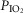
partial pressure of inspired O2
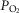
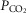 , partial pressure of O2, CO2
, partial pressure of O2, CO2- QT
cardiac output;
 , O2 saturation
, O2 saturation
Additional information
Competing interests
None declared.
Author contributions
Experiments were performed in the village of Maduo and The Center for High Altitude Research in Xining, Qinghai Province, China. P.D.W., T.S.S. and R.L.G. designed experiments. T.S.S., G.W., H.E.W., T.W., A.B., J.M.F., G.Q., F.G.B., M.Y., P.D.W. and R.L.G. performed data collection. T.S.S., G.W., and P.D.W. performed analysis and interpretation of data. All authors approved the final version of the manuscript.
Funding
National Institutes of Health (NIH) National Heart, Blood, and Lung Institute (NHBLI) P01 HL0981830-01A1; National Basic Research Program of China 2012CB518200; Program of International S&T Cooperation of China 0S2012GR0195; National Natural Science Foundation of China 30393133; NIH T32 HL098062-01A1; NIH K99 HL118215; Parker B. Francis Fellowship.
References
- Alkorta-Aranburu G, Beall CM, Witonsky DB, Gebremedhin A, Pritchard JK. Di Rienzo A. The genetic architecture of adaptations to high altitude in Ethiopia. PLoS Gen. 2012;8:e1003110. doi: 10.1371/journal.pgen.1003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker RC, Hopkins SR, Kellogg N, Olfert IM, Brutsaert TD, Gavin TP, Entin PL, Rice AJ. Wagner PD. Measurement of cardiac output during exercise by open-circuit acetylene uptake. J Appl Physiol. 1999;87:1506–1512. doi: 10.1152/jappl.1999.87.4.1506. [DOI] [PubMed] [Google Scholar]
- Beall CM. Tibetan and Andean patterns of adaptation to high-altitude hypoxia. Hum Biol. 2000;72:201–228. [PubMed] [Google Scholar]
- Beall CM. Andean, Tibetan, and Ethiopian patterns of adaptation to high-altitude hypoxia. Integr and Comp Bio. 2006;46:18–24. doi: 10.1093/icb/icj004. [DOI] [PubMed] [Google Scholar]
- Beall CM. Two routes to functional adaptation, Tibetan and Andean high-altitude natives. Proc Natl Acad Sci U S A. 2007;104(Suppl. 1)):8655–8660. doi: 10.1073/pnas.0701985104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM, Brittenham GM, Strohl KP, Blangero J, Williams-Blangero S, Goldstein MC, Decker MJ, Vargas E, Villena M, Soria R, et al. Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara. Am J Phys Anthropol. 1998;106:385–400. doi: 10.1002/(SICI)1096-8644(199807)106:3<385::AID-AJPA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M, et al. Natural selection on EPAS1 (HIF2α) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci U S A. 2010;107:11459–11464. doi: 10.1073/pnas.1002443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM, Decker MJ, Brittenham GM, Kushner I, Gebremedhin A. Strohl KP. An Ethiopian pattern of human adaptation to high-altitude hypoxia. Proc Natl Acad Sci U S A. 2002;99:17215–17218. doi: 10.1073/pnas.252649199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM, Song K, Elston RC. Goldstein MC. Higher offspring survival among Tibetan women with high oxygen saturation genotypes residing at 4000 m. Proc Natl Acad Sci U S A. 2004;101:14300–14304. doi: 10.1073/pnas.0405949101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard JL, Haas JD, Tufts D, Spielvogel H, Vargas E. Rodriguez C. Iron deficiency anemia and steady-state work performance at high altitude. J Appl Physiol. 1988;64:1878–1884. doi: 10.1152/jappl.1988.64.5.1878. [DOI] [PubMed] [Google Scholar]
-
Calbet JAL, Rådegran G, Boushel R, Søndergaard H, Saltin B. Wagner PD. Effect of blood haemoglobin concentration on
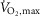 and cardiovascular function in lowlanders acclimatised to 5260 m. J Physiol. 2002;545:715–728. doi: 10.1113/jphysiol.2002.029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
and cardiovascular function in lowlanders acclimatised to 5260 m. J Physiol. 2002;545:715–728. doi: 10.1113/jphysiol.2002.029108. [DOI] [PMC free article] [PubMed] [Google Scholar] - Charloux A, Lonsdorfer-Wolf E, Richard R, Lampert E, Oswald-Mammosser M, Mettauer B, Geny B. Lonsdorfer J. A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise, comparison with the "direct" Fick method. Euro J Appl Physiol. 2000;82:313–320. doi: 10.1007/s004210000226. [DOI] [PubMed] [Google Scholar]
- Chen QH, Ge RL, Wang XZ, Chen HX, Wu TY, Kobayashi T. Yoshimura K. Exercise performance of Tibetan and Han adolescents at altitudes of 3417 and 4300 m. J Appl Physiol. 1997;83:661–667. doi: 10.1152/jappl.1997.83.2.661. [DOI] [PubMed] [Google Scholar]
- Curran LS, Zhuang J, Droma T, Moore LG. Superior exercise performance in lifelong Tibetan residents of 4400 m compared with Tibetan residents of 3658 m. Am J Phys Anthropol. 1998;105:21–31. doi: 10.1002/(SICI)1096-8644(199801)105:1<21::AID-AJPA3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Drummond MJ, Fujita S, Abe T, Dreyer HC, Volpi E. Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc. 2008;40:691–698. doi: 10.1249/MSS.0b013e318160ff84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL. Rose RJ. Cardiovascular and respiratory responses to submaximal exercise training in the thoroughbred horse. Pflugers Archiv. 1988;411:316–321. doi: 10.1007/BF00585121. [DOI] [PubMed] [Google Scholar]
- Ge RL, Chen QH, Wang LH, Gen D, Yang P, Kubo K, Fujimoto K, Matsuzawa Y, Yoshimura K, Takeoka M, et al. Higher exercise performance and lower VO2max in Tibetan than Han residents at 4700 m altitude. J Appl Physiol. 1994;77:684–691. doi: 10.1152/jappl.1994.77.2.684. [DOI] [PubMed] [Google Scholar]
- Ge RL, He Lun GW, Chen QH, Li HL, Gen D, Kubo K, Matsuzawa Y, Fujimoto K, Yoshimura K, Takeoka M, Kubo K. Kobayashi T. Comparisons of oxygen transport between Tibetan and Han residents at moderate altitude. Wild Environ Med. 1995;6:391–400. [Google Scholar]
- Hochachka PW. Mechanism and evolution of hypoxia-tolerance in humans. J Exp Biol. 1998;201:1243–1254. doi: 10.1242/jeb.201.8.1243. [DOI] [PubMed] [Google Scholar]
- Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S. Moore LG. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child Fetal Neonatal Ed. 2007;92:F372–377. doi: 10.1136/adc.2006.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser B, Marconi C, Amatya T, Basnyat B, Colombini A, Broers B. Cerretelli P. The metabolic and ventilatory response to exercise in Tibetans born at low altitude. Resp Physiol. 1994;98:15–26. doi: 10.1016/0034-5687(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Kayser B, Hoppeler H, Desplanches D, Marconi C, Broers B. Cerretelli P. Muscle ultrastructure and biochemistry of lowland Tibetans. J of Appl Physiol. 1996;81:419–425. doi: 10.1152/jappl.1996.81.1.419. (1), [DOI] [PubMed] [Google Scholar]
- Kelman GR. Digital computer subroutine for the conversion of oxygen tension into saturation. J Appl Physiol. 1966;21:1375–1376. doi: 10.1152/jappl.1966.21.4.1375. [DOI] [PubMed] [Google Scholar]
- Kelman GR. Digital computer procedure for the conversion of PCO2 into blood CO2 content. Resp Physiol. 1967;3:111–115. doi: 10.1016/0034-5687(67)90028-x. [DOI] [PubMed] [Google Scholar]
- Leon-Velarde F, Maggiorini M, Reeves JT, Aldashev A, Asmus I, Bernardi L, Ge RL, Hackett P, Kobayashi T, Moore LG, et al. Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol. 2005;6:147–157. doi: 10.1089/ham.2005.6.147. [DOI] [PubMed] [Google Scholar]
- Lorenzo FR, Huff C, Myllymäki M, Olenchock B, Swierczek S, Tashi T, Gordeuk V, Wuren T, Ri-Li G, McClain DA, Khan TM, Koul PA, Guchhait P, Salama ME, Xing J, Semenza GL, Liberzon E, Wilson A, Simonson TS, Jorde LB, Kaelin WGJr, Koivunen P. Prchal JT. A genetic mechanism for Tibetan high-altitude adaptation. Nature Genetics. 2014;46:951–956. doi: 10.1038/ng.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnis MJ, Koehle MS. Rupert JL. Evidence for a genetic basis for altitude illness: 2010 update. High Alt Med Biol. 2010;11:349–68. doi: 10.1089/ham.2010.1030. (4), [DOI] [PubMed] [Google Scholar]
- Monge CC, Arregui A. Leon-Velarde F. Pathophysiology and epidemiology of chronic mountain sickness. Intern J Sports Med. 1992;13(Suppl. 1)):S79–81. doi: 10.1055/s-2007-1024603. [DOI] [PubMed] [Google Scholar]
- Monge CC. Whittembury J. Chronic mountain sickness. Johns Hopkins Med J. 1976;139(Suppl.)):187–189. [PubMed] [Google Scholar]
- Moore LG. Human genetic adaptation to high altitude. High Alt Med Biol. 2001;2:257–279. doi: 10.1089/152702901750265341. [DOI] [PubMed] [Google Scholar]
- Moore LG, Curran-Everett L, Droma TS, Groves BM, McCullough RE, McCullough RG, Sun SF, Sutton JR, Zamudio S. Zhuang JG. Are Tibetans better adapted? Intern J of Sports Med. 1992;13(Suppl. 1)):S86–88. doi: 10.1055/s-2007-1024605. [DOI] [PubMed] [Google Scholar]
- Moore LG, Niermeyer S. Vargas E. Does chronic mountain sickness (CMS) have perinatal origins? Resp Physiol Neurobiol. 2007;158:180–189. doi: 10.1016/j.resp.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Niu W, Wu Y, Li B, Chen N. Song S. Effects of long-term acclimatization in lowlanders migrating to high altitude, comparison with high altitude residents. Euro J Appl Physiol Occ Physiol. 1995;71:543–548. doi: 10.1007/BF00238558. [DOI] [PubMed] [Google Scholar]
- Persson SG. Bergsten G. Circulatory effects of splenectomy in the horse. IV. Effect on blood flow and blood lactate at rest and during exercise. Zentralblatt fur Veterinarmedizin Reihe A. 1975;22:801–807. [PubMed] [Google Scholar]
- Petousi N. Robbins PA. Human adaptation to the hypoxia of high altitude, the Tibetan paradigm from the pregenomic to the postgenomic era. J Appl Physiol. 2014;116:875–884. doi: 10.1152/japplphysiol.00605.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk M, Tiemessen I, Hupperets MD, Kennedy BP, Powell FL, Hopkins SR. Wagner PD. Persistence of the lactate paradox over 8 weeks at 3800 m. High Alt Med Biol. 2003;4:431–443. doi: 10.1089/152702903322616182. [DOI] [PubMed] [Google Scholar]
- Reeves JT, Wolfel EE, Green HJ, Mazzeo RS, Young AJ, Sutton JR. Brooks GA. Oxygen transport during exercise at altitude and the lactate paradox, lessons from Operation Everest II and Pikes Peak. Exer Sport Sci Rev. 1992;20:275–296. [PubMed] [Google Scholar]
- Richard R, Lonsdorfer-Wolf E, Charloux A, Doutreleau S, Buchheit M, Oswald-Mammosser M, Lampert E, Mettauer B, Geny B. Lonsdorfer J. Non-invasive cardiac output evaluation during a maximal progressive exercise test, using a new impedance cardiograph device. Euro J Appl Physiol. 2001;85:202–207. doi: 10.1007/s004210100458. [DOI] [PubMed] [Google Scholar]
- Schuler B, Arras M, Keller S, Rettich A, Lundby C, Vogel J. Gassmann M. Optimal hematocrit for maximal exercise performance in acute and chronic erythropoietin-treated mice. Proc Natl Acad Sci U S A. 2010;107:419–423. doi: 10.1073/pnas.0912924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenmann C, Rasmussen P, Sorensen H, Zaar M, Hvidtfeldt M, Pichon A, Secher NH. Lundby C. Cardiac output during exercise: a comparison of four methods. Scand J Med Sci Sports. 2014;25:e20–27. doi: 10.1111/sms.12201. [DOI] [PubMed] [Google Scholar]
- Simonson TS, McClain DA, Jorde LB. Prchal JT. Genetic determinants of Tibetan high-altitude adaptation. Hum Gen. 2012;131:527–533. doi: 10.1007/s00439-011-1109-3. [DOI] [PubMed] [Google Scholar]
- Simonson TS, Wei G, Wagner HE, Wuren T, Bui A, Fine JM, Qin G, Beltrami FG, Yan M, Wagner PD. Ge RL. Increased blood-oxygen binding affinity in Tibetan and Han Chinese residents at 4200 m. Exper Physiol. 2014;99:1624–1635. doi: 10.1113/expphysiol.2014.080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, Bai Z, Lorenzo FR, Xing J, Jorde LB, Prchal JT. Ge R. Genetic evidence for high-altitude adaptation in Tibet. Science. 2010;329:72–75. doi: 10.1126/science.1189406. [DOI] [PubMed] [Google Scholar]
- Storz JF. Evolution. Genes for high altitudes. Science. 2010;329:40–41. doi: 10.1126/science.1192481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Scott GR. Cheviron ZA. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J Exp Biol. 2010;213:4125–4136. doi: 10.1242/jeb.048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SF, Droma TS, Zhang JG, Tao JX, Huang SY, McCullough RG, McCullough RE, Reeves CS, Reeves JT. Moore LG. Greater maximal O2 uptakes and vital capacities in Tibetan than Han residents of Lhasa. Resp Physiol. 1990;79:151–161. doi: 10.1016/0034-5687(90)90015-q. [DOI] [PubMed] [Google Scholar]
- Tordi N, Mourot L, Matusheski B. Hughson RL. Measurements of cardiac output during constant exercises, comparison of two non-invasive techniques. Intern J Sports Med. 2004;25:145–149. doi: 10.1055/s-2004-819949. [DOI] [PubMed] [Google Scholar]
- Tufts DA, Haas JD, Beard JL. Spielvogel H. Distribution of hemoglobin and functional consequences of anemia in adult males at high altitude. Am J Clin Nutr. 1985;42:1–11. doi: 10.1093/ajcn/42.1.1. [DOI] [PubMed] [Google Scholar]
- Vargas E. Spielvogel H. Chronic mountain sickness, optimal hemoglobin, and heart disease. High Alt Med Biol. 2006a;7:138–149. doi: 10.1089/ham.2006.7.138. [DOI] [PubMed] [Google Scholar]
- Vargas PE. Spielvogel H. Chronic mountain sickness, optimal hemoglobin, and heart disease. High Alt Med Biol. 2006b;7:138–149. doi: 10.1089/ham.2006.7.138. [DOI] [PubMed] [Google Scholar]
- Villafuerte FC, Cardenas R. Monge CC. Optimal hemoglobin concentration and high altitude, a theoretical approach for Andean men at rest. J Appl Physiol. 2004;96:1581–1588. doi: 10.1152/japplphysiol.00328.2003. [DOI] [PubMed] [Google Scholar]
-
Wagner PD. A theoretical analysis of factors determining
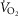 max at sea level and altitude. Respiration Physiology. 1996;106:329–343. doi: 10.1016/s0034-5687(96)00086-2. (3), [DOI] [PubMed] [Google Scholar]
max at sea level and altitude. Respiration Physiology. 1996;106:329–343. doi: 10.1016/s0034-5687(96)00086-2. (3), [DOI] [PubMed] [Google Scholar] - Wagner PD, Gale GE, Moon RE, Torrebueno JR, Stolp BW. Saltzman HA. Pulmonary gas-exchange in humans exercising at sea-level and simulated altitude. J Appl Physiol. 1986;61:260–270. doi: 10.1152/jappl.1986.61.1.260. [DOI] [PubMed] [Google Scholar]
- Wagner PD, Sutton JR, Reeves JT, Cymerman A, Groves BM. Malconian MK. Operation Everest II: pulmonary gas exchange during a simulated ascent of Mt. Everest. J Appl Physiol. 1987;63:2348–2359. doi: 10.1152/jappl.1987.63.6.2348. [DOI] [PubMed] [Google Scholar]
- Wagner PD. West JB. Effects of diffusion impairment on O2 and CO2 time courses in pulmonary capillaries. J Appl Physiol. 1972;33:62–71. doi: 10.1152/jappl.1972.33.1.62. [DOI] [PubMed] [Google Scholar]
- Winslow RM, Chapman KW, Gibson CC, Samaja M, Monge CC, Goldwasser E, Sherpa M, Blume FD. Santolaya R. Different hematologic responses to hypoxia in Sherpas and Quechua Indians. J Appl Physiol. 1989;66:1561–1569. doi: 10.1152/jappl.1989.66.4.1561. [DOI] [PubMed] [Google Scholar]
- Winslow RM, Monge CC, Brown EG, Klein HG, Sarnquist F, Winslow NJ. McKneally SS. Effects of hemodilution on O2 transport in high-altitude polycythemia. J Appl Physiol. 1985;59:1495–1502. doi: 10.1152/jappl.1985.59.5.1495. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang X, Wei C, Cheng H, Wang X, Li Y, Ge D, Zhao H, Young P, Li G. Wang Z. Hemoglobin levels in Qinghai-Tibet, different effects of gender for Tibetans vs. Han. J Appl Physiol. 2005;98:598–604. doi: 10.1152/japplphysiol.01034.2002. [DOI] [PubMed] [Google Scholar]
- Wu TY, Zhang Q, Jin B, Xu F, Cheng Q. Wan X. Chronic Mountain Sickness (Monge’s Disease), An Observation in Qinghai-Tibet plateau. Japan: Shinshu University Press; 1992. [Google Scholar]
- Wuren T, Simonson TS, Qin G, Xing J, Huff CD, Witherspoon DJ, Jorde LB. Ge RL. Shared and unique signals of high-altitude adaptation in geographically distinct Tibetan populations. PloS One. 2014;9:e88252. doi: 10.1371/journal.pone.0088252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZX, Pool JE, Xu X, Jiang H, Vinckenbosch N, Korneliussen TS, Zheng H, Liu T, He W, Li K, Luo R, Nie X, Wu H, Zhao M, Cao H, Zou J, Shan Y, Li S, Yang Q, Asan NiP, Tian G, Xu J, Liu X, Jiang T, Wu R, Zhou G, Tang M, Qin J, Wang T, Feng S, Li G, Huasang, Luosang J, Wang W, Chen F, Wang Y, Zheng X, Li Z, Bianba Z, Yang G, Wang X, Tang S, Gao G, Chen Y, Luo Z, Gusang L, Cao Z, Zhang Q, Ouyang W, Ren X, Liang H, Zheng H, Huang Y, Li J, Bolund L, Kristiansen K, Li Y, Zhang Y, Zhang X, Li R, Li S, Yang H, Nielsen R, Wang J. Wang J. Sequencing of 50 human exomes reveals adaptation to high altitude. Science. 2010;329:75–78. doi: 10.1126/science.1190371. [DOI] [PMC free article] [PubMed] [Google Scholar]