Abstract
A prior study (Bruder et al., 1997) found left hemisphere advantage for verbal dichotic listening was predictive of clinical response to cognitive behavioral therapy (CBT) for depression. This study aimed to confirm this finding and to examine the value of neuropsychological tests, which have shown promise for predicting antidepressant response. Twenty depressed patients who subsequently completed 14 weeks of CBT and 74 healthy adults were tested on a Dichotic Fused Words Test (DFWT). Patients were also tested on the National Adult Reading Test to estimate IQ, and word fluency, choice RT, and Stroop neuropsychological tests. Left hemisphere advantage on the DFWT was more than twice as large in CBT responders than non-responders, and was associated with improvement in depression following treatment. There was no difference between responders and non-responders on neuropsychological tests. The results support the hypothesis that the ability of individuals with strong left hemisphere dominance to recruit frontal and temporal cortical regions involved in verbal dichotic listening predicts CBT response. The large effect size, sensitivity and specificity of DFWT predictions suggests the potential value of this brief and inexpensive test as an indicator of whether a patient will benefit from CBT for depression.
Keywords: Depression, CBT, lateralization, dichotic listening, neuropsychological tests
1. Introduction
Cognitive behavioral therapy (CBT) is an evidence-based treatment for depression but, like medication, it is effective in only 40% to 60% of patients having a major depressive disorder (MDD) (DeRubeis et al., 2005; Siegle et al., 2011). This has led to research to identify predictors to aid in selecting a treatment that will benefit a patient. Although there are encouraging findings for potential neuroimaging biomarkers (Siegle et al., 2012; McGrath et al., 2013), these tests are expensive and may be difficult to implement in clinical settings. Although studies have raised the possibility of developing inexpensive behavioral tests for predicting response to CBT, the findings of early studies using self-report measures of beliefs or attitudes were conflicting (Rude & Reham, 1991; Sotsky et al., 1991). There has, however, been less research on whether performance on neuropsychological tests of cognitive abilities might predict CBT response. Studies using self-administered tests as estimates of IQ have reported conflicting findings. Hagga et al. (1991) measured vocabulary and abstraction scales in depressed patients, but found no relationship between IQ estimates and CBT outcome. More recently, Fournier et al. (2009) derived IQ scores from the Shipley-Hartford Living Scale and found higher estimates of intelligence before CBT or antidepressant medications were associated with lower depression ratings at end of treatment.
Cognitive therapies are highly verbal treatments that involve self-monitoring and reevaluation of negative thoughts, emotions and cognitive distortions, which may be mediated by cognitive skills in which verbal abilities play an important role. Treatment success may therefore depend on verbal skills and activation of the left hemisphere, which is dominant for language processing in most right-handed individuals (Otto, Yeo and Dougher, 1987). In our initial study (Bruder et al., 1997), we found that clinical response to CBT for depression was related to left-hemisphere advantage for verbal dichotic listening. Different consonant-vowel syllables were simultaneously presented to the left and right ears, and the difference in accuracy of performance between ears provided an index of perceptual asymmetry (PA). Depressed patients who responded to 16 weekly CBT sessions (n=15) had more than twice the right ear advantage than non-responders (n=12). Patients with right ear accuracy greater than healthy controls had a 75% response rate, whereas those with less than normal right ear accuracy had only a 20% response rate. Given the largely contralateral projections between each ear and hemisphere, this is consistent with the hypothesis that patients with greater left-hemisphere advantage for verbal processing benefit more from CBT than other depressed patients. In contrast, there was no difference between CBT responders and non-responders in PA for a non-verbal dichotic listening test, which further supports left hemisphere dominance for verbal processing as a key predictor of response to CBT for depression. It is, however, important to demonstrate that this predictor is reliable and the findings replicable.
The main purpose of the present study was to attempt to replicate our findings in a new sample of depressed patients using a Dichotic Fused Words Test (DFWT), which yields a robust left hemisphere advantage in healthy adults (Wexler and Halwes, 1983). In this test words that rhyme (e.g., coat, goat) are simultaneously presented to right and left ear. These words fuse into a single percept and accuracy for reporting the word in the right or left ear provides a measure of PA. This test has been shown to yield valid estimates of hemispheric lateralization for speech as determined by intracarotid amobarbital injections (Zatorre, 1989; Fernandes and Smith, 2000), and has high test-retest reliability (r=.85; Wexler and Halwes, 1983). Patients having a depressive disorder were tested on the DFWT prior to receiving CBT, and healthy adults were tested to provide normative data. We previously found that depressed patients who respond to an antidepressant had a larger left hemisphere advantage than non-responders on the DFWT, and the mean PA for healthy adults can serve as a meaningful criterion for predicting treatment response (Bruder et al., 1996; 2004; 2007). Based on our prior findings, we hypothesized that CBT responders would have stronger left hemisphere advantage than non-responders, and patients with nominally above average left hemisphere advantage would be predicted to response to CBT.
A secondary purpose was to examine performance on selected neuropsychological tests that have shown promise for predicting therapeutic response to antidepressants. Specifically, we used word fluency, psychomotor speed, and Stroop tests, which have been associated with response to antidepressants (Dunkin et al., 2000; Taylor et al., 2006; Gorlyn et al., 2008; Bruder et al., 2014). The National Adult Reading Test (NART) was also administered to provide estimates of premorbid intelligence (Bright et al., 2002). We examined whether or not these tests would also predict response to CBT, and in particular, evaluated the hypothesis that response to CBT depends on general verbal skills or cognitive abilities assessed by these tests.
2. Methods
2.1 Participants
Outpatients at the Depression Evaluation Service of the New York State Psychiatric Institute were recruited for this study before beginning a clinical trial of CBT and healthy controls were recruited from the New York metropolitan area. The patients met the DSM-IV-TR criteria for MDD (n=16) or dysthymia (n=4), and one of the dysthymic patients had comorbid anxiety disorders (panic disorder and social phobia),1 as determined by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First et al.,1995). Additionally, patients scored at least a 13 or higher at baseline on the Beck Depression Inventory (BDI-II; A.T. Beck et al., 1996). Exclusion criteria for patients were lifetime or current diagnoses of schizophrenia or any other psychotic disorder, major depressive disorder with psychotic or catatonic features, bipolar disorder, organic mental disease, or current substance abuse or dependence in the last 6 months (except nicotine or caffeine dependence). Patients with a prior history of treatment failure with CBT or who were currently receiving any form of psychotherapy or counseling were also excluded. Three patients (two who subsequently responded to CBT and one non-responder) were permitted to participate in the study while on concurrent antidepressant medication, but their doses were stabilized for a minimum of three months prior to study entry. Patients who clinically worsened during treatment could also be considered for medication. One patient (a non-responder) began an antidepressant after the 8th week of CBT.2 All participants were required to be in good general medical and neurological health. This was established in patients via physical examination as part of the initial screening. Participants were excluded if they had an unstable medical or neurological condition or a hearing loss greater than 30 dB at 500, 1000 or 2000 Hz. The healthy controls were tested on the DFWT as part of a separate study in our laboratory over the same time frame as the patients and were screened using the Structured Clinical Interview for DSM-IV Axis I Disorders, Nonpatient Edition (First et al., 1996) to exclude those with Axis I disorders, except for nicotine dependence. All participants were right-handed and fluent in English. The New York State Psychiatric Institute Institutional Review Board (IRB) approved the protocol. All patients provided informed consent prior to enrolling in the study.
2.2 Treatment Protocol
Twenty patients completed 14 weekly 50 minute sessions of CBT free of charge. Therapy sessions used a CBT manual protocol for depression (Emery, 2000). The study author (RK) is a CBT therapist who has been in clinical practice for 25 years, and has been providing CBT sessions in different studies at New York State Psychiatric Institute (NYSPI) for the past 8 years. Her sessions are rated by the Beck Institute in Philadelphia on a regular basis and have reached adherence. CBT was provided by 3 graduate students of Clinical Psychology who were at the last phase of their PhD program and had extensive clinical experience in externships before joining the study. They were selected and screened by the study author (RK) and the head psychologist of NYSPI (L. Mufson). Before seeing patients in the study, all therapists received a treatment manual and training consisting of a short theoretical course and role playing in a clinical setting. Under the supervision of the study author (RK), they were assigned at least 3 training cases to ensure their clinical skills before working with study participants. During the study, the therapists received weekly individual supervision from the study author who evaluated their sessions based on their reports and audiotape of their sessions to ensure adherence to the treatment manual.
2.3 Assessment of Treatment Response
Clinical response was based on ratings from the 17-item Hamilton Depression Rating Scale (HAM-D17; Hamilton, 1960) by an independent evaluator who was blind to the dichotic listening and neurocognitive test data, but who knew the treatment being given. The evaluator has a master degree in clinical and counseling psychology and was specifically trained for this role. Patients showing a reduction in HAM-D17 ≥ 50% from baseline to completion of CBT were considered to be treatment responders. Of 20 patients who completed the 14 weeks of CBT, 11 (55%) were responders and 9 were non-responders. As shown in Table 1, the responders and non-responder groups did not significantly differ in gender, age, or education, and all were right-handed as indicated by laterality quotient (LQ) scores on the Edinburgh Inventory (Oldfield, 1971). There was no difference between responders and non-responders in pre-treatment ratings on the HAM-D17 or BDI-II, and responders showed significantly lower post-treatment depression ratings than non-responders.
Table 1.
Characteristics of Treatment Responders and Non-Responders
| Variable | Responders | Non-Responders | Statistics |
|---|---|---|---|
| Gender (men/women) | 5/6 | 6/3 | χ2(1) = 0.90 |
| Age (in years) | |||
| M | 39.9 | 38.4 | t(18) = 0.22 |
| SD | 15.2 | 13.8 | |
| Education (in years) | |||
| M | 15.6 | 16.2 | t(18) = 0.52 |
| SD | 2.1 | 3.6 | |
| Handedness LQ | |||
| M | 84.4 | 78.3 | t(18) = 0.68 |
| SD | 15.8 | 24.2 | |
| Pre-Treatment HAM-D17 | |||
| M | 16.3 | 14.0 | t(18) = 0.94 |
| SD | 5.0 | 5.9 | |
| Post-Treatment HAM-D17 | |||
| M | 4.0 | 12.9 | t(18) = 4.17** |
| SD | 2.9 | 6.3 | |
| Pre-Treatment BDI | |||
| M | 31.1 | 33.8 | t(18) = 0.61 |
| SD | 8.6 | 11.2 | |
| Post-Treatment BDIa | |||
| M | 7.3 | 20.8 | t(17) = 3.09** |
| SD | 5.8 | 12.4 | |
Note. LQ = Laterality Quotient on Edinburgh Inventory; HAM-D17 = Hamilton Depression Scale; BDI= Beck Depression Inventory-II.
Responders: n=10; Non-Responders: n=9.
p<.01
2.4 Procedures
Before beginning CBT, all 20 patients received the DFWT, 18 received the NART and 17 received the other neuropsychological tests.
The DFWT consists of 15 different single-syllable word pairs in which each member of every pair differs from the other only in the initial consonant (e.g., coat, goat). All words begin with one of six stop consonants (b, d, p, t, g, k) and are natural speech spoken by a male voice. When word pairs are presented dichotically, the members fuse into a single percept. Participants indicate the word they heard by marking a line through it on an answer sheet that has four possible responses, both members of the dichotic pair and two other words differing from the dichotic stimuli only in the initial consonant. Following practice trials, each participant received four 30-item blocks for a total of 120 trials. Orientation of headphones was reversed after the first and third quarters to control for channel differences and ear of presentation. The test was presented via a matched pair of TDH-49 headphones at a comfortable level of 75 dB sound pressure level (SPL) to a participant seated in a sound-attenuated booth and total test time was about 20 minutes.
The neuropsychological tests selected were previously used in our studies in depressed patients (Taylor et al., 2006; Gorlyn et al., 2008; Keilp et al., 2008; Bruder et al., 2014). Training and supervision of the research assistants who administered the tests was done by one of the authors (JGK), a Clinical Psychologist and neuropsychologist by training. The non-computerized tests included: (1) word fluency test using a written version of the Controlled Oral Word Association Test (Benton et al., 1983), in which participants had one minute to write down as many words as possible that began with each of three letters (FAS). It was used instead of the usual oral version so as to match the test used in our study of predictors of response to antidepressants (Bruder et al., 2014), and is likely to reflect writing speed as well as word fluency. (2) NART, which provided a premorbid IQ estimate (Bright et al., 2002). Two computerized tests were presented on a Macintosh laptop with PsyScope programming language (Keilp et al., 2005). A 4-choice reaction time task was adapted from Thorne et al. (1985). The participant sees a black screen with 4 white squares arranged in a windowpane pattern. A red “X” appears in one of the squares, and the subject responds by pressing one of four buttons to indicate the position of the X. Following the response, the X disappears and then reappears in the same or different square. The participant is instructed to “catch the X” by pressing the correct buttons as the task progresses. The dependent measure is median reaction time on correct trials. A computerized Stroop test used single item presentation and a button press response (Keilp et al., 2008). Three conditions were given in a blocked fashion in a fixed order: (1) Word Condition—identify the color names in black letters; (2) Color Condition—identify color of a string of four X’s displayed in one of three colors; and (3) Color/Word Condition—indentify display color of the stimulus containing an incongruous color name, ignoring the text. Auditory feedback was provided for all responses: correct (beep) and incorrect (Buzz). Word and Color blocks included 45 stimulus trials and Word/Color blocks included 90 trials. Median reaction time on correct trials is the dependent measure.
2.5 Statistical Analyses
The number of correct responses in the DFWT was computed for the right and left ear presentations. These scores were used to compute a standard measure of perceptual asymmetry (PA), where PA= 100(Right Correct-Left Correct)/(Right Correct+Left Correct). Differences between CBT responders and non-responders in PA on DFWT and in accuracy or RT scores on the neuropsychological tests were examined with independent t-tests. No analyses of accuracy scores on the DFWT were performed because the members of dichotic pairs fuse into a single percept and subjects are essentially 100% correct for reporting the word heard in either ear.
The potential value of PA scores on the DFWT for predicting treatment response to CBT was examined using a χ2 test to compare the response rate of patients with PA scores above versus below the mean score for healthy controls, and sensitivity, specificity, positive predictive value, and negative predictive value were also computed. Pearson’s correlation and Spearman’s rho examined the association between pre-treatment PA scores for patients on DFWT and percent change in their HAM-D17 ratings after CBT. A logistic regression analysis was also performed to examine the significance of continuous PA scores for predicting response to CBT (responder/non-responder) when controlling for patient age, education, and handedness LQ.3
3. Results
3.1 DFWT
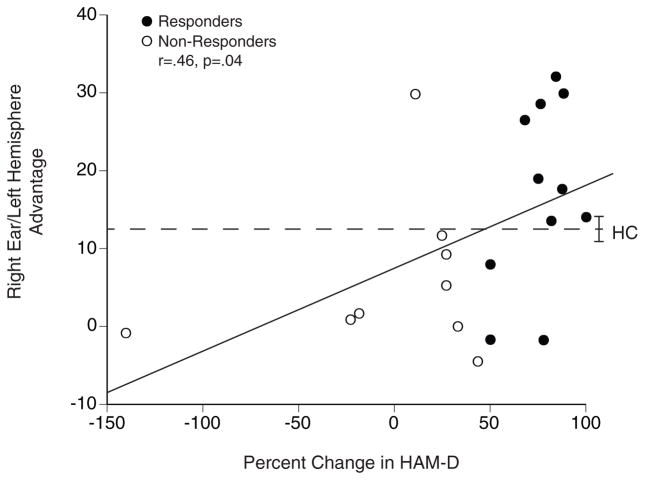
The left hemisphere advantage for CBT responders (Mean= 16.9, SD= 11.9) was significantly greater than for non-responders (Mean= 5.9, SD= 10.3; t [18] = 2.17, p<.05), which corresponds to a large effect size (Cohen’s d= .99). This is also evident in Figure 1, which shows the left hemisphere advantage for individual responders and non-responders plotted as a function of the percent change in HAM-D17 ratings from before to after CBT. Larger left hemisphere advantage before treatment was associated with greater improvement in HAM-D17 depression ratings following CBT (Pearson’s r= .46, Spearman’s rho= .47, p<.05). This correlation was maintained after excluding the patient in Figure 1 who did extremely poor following treatment (r= .45, p=.05).
Figure 1.
Relationship between pretreatment right ear (left hemisphere) advantage and percent change in HAM-D17 in CBT responders and non-responders; dashed line is mean for 74 healthy controls (HC) with brackets indicating the standard error of mean.
The dashed line labeled HC in Figure 1 gives the mean left hemisphere advantage for 74 right-handed healthy controls (32 men and 42 women, with mean age= 32.0, SD= 10.3 and education= 15.6, SD= 2.1).4 As in our prior studies, the normative PA value for controls was used as a cutoff for predicting response to CBT, with those having PA > HC predicted to be responders and those having PA < HC predicted to be non-responders. Patients with left hemisphere advantage > HC had 89% (8 out of 9) response rate to CBT, whereas those < HC had only a 27% (3 out of 11) response rate, χ2 (1, n= 20) = 7.59, p<.01. This corresponds to a positive predictive value of 89% and negative predictive value of 73%. Also, the percentage of responders with scores > HC was 73% (sensitivity) and the percentage of non-responders with scores < HC was 89% (specificity).
A logistic regression analysis was also used to examine the value of continuous PA scores on the DFWT for predicting treatment response, when controlling for patient age, education, and handedness LQ. A model including PA and handedness LQ scores significantly improved prediction of treatment response over a constant, χ2 (1, n= 20) = 7.26, p<.01, while age and education did not enter into the equation. PA was a significant predictor of treatment response, Wald test (1, n=20)= 4.43, p<.05, and handedness LQ approached a conventional level of significance, Wald test (1, n=20)= 3.53, p= .06. Using this logistic model, 9 of 11 CBT responders were correctly predicted to be responders (sensitivity of 82%) and 8 of 9 non-responders were predicted to be non-responders (specificity of 89%). Overall, 85% of patients were correctly classified by the logistic regression equation.
3.2 Neuropsychological Tests
Table 2 gives the pretreatment performance of patients who received neuropsychological tests. There was no difference between responders and non-responders on the NART estimate of premorbid IQ or on the other verbal tests, i.e., word fluency or Stroop word reading RT. Nor was there a difference in choice RT or Stroop interference effect between responders and non-responders.
Table 2.
Performance in Neurocognitive Tests
| Test | Responders | Non-Responders | Statistics |
|---|---|---|---|
| Word Fluency (Total Correct) | |||
| n | 8 | 9 | t (15) = 0.12 |
| M | 36.2 | 35.7 | |
| SD | 8.5 | 11.9 | |
| NART (IQ Estimate) | |||
| n | 9 | 9 | t (16) = 1.02 |
| M | 107.7 | 112.8 | |
| SD | 12.0 | 9.0 | |
| Choice RT (ms) | |||
| n | 9 | 8 | t (15) = 0.85 |
| M | 553 | 504 | |
| SD | 147 | 89 | |
| Stroop Word RT (ms) | |||
| n | 9 | 8 | t (15) = 0.83 |
| M | 624 | 585 | |
| SD | 113 | 69 | |
| Stroop Color RT (ms) | |||
| n | 9 | 8 | t (15) = 0.86 |
| M | 633 | 598 | |
| SD | 79 | 90 | |
| Stroop Interference (ms) | |||
| n | 9 | 8 | t (15) = 0.68 |
| M | 194 | 157 | |
| SD | 124 | 97 | |
Note: NART = National Adult Reading Test; RT = Reaction Time.
4. Discussion
This study confirmed our finding of greater left hemisphere advantage for verbal dichotic listening in depressed patients who respond to CBT when compared to non-responders (Bruder et al., 1997). In both this and our prior study, CBT responders had more than twice the left hemisphere advantage than non-responders. Pretreatment PA scores of individuals on the DFWT were predictive of improvement in HAM-D17 depression ratings following CBT. Patients with nominally above average left hemisphere advantage had 89% response rate to CBT, whereas those with nominally below average left hemisphere advantage had only 27% response rate. This is comparable to our prior findings for a dichotic syllable test. The DFWT has the added advantage of yielding valid estimates of hemispheric lateralization for speech with high test-retest reliability (Wexler and Halwes, 1983; Zatorre, 1989; Fernandes and Smith, 2000).
Reappraisal of negative thoughts is a central component of CBT, which involves verbal cognitive processes for which the left hemisphere is dominant in most right-handed individuals. A study measuring fMRI in healthy adults during different emotional regulation tasks found that reappraisal activated left ventrolateral prefrontal cortex (Price et al., 2013), which is consistent with evidence of left prefrontal and temporal activation during reappraisal of negative emotions (Ochsner et al., 2004; Silvers et al., 2014). The extent to which individual differences in cortical activation during reappraisal are related to clinical response to CBT for depression has, however, received little attention. One study (Deldin and Chiu, 2005) measured resting EEG in depressed patients and healthy controls who were administered a brief, one-time introduction to cognitive restructuring. Individuals who reported an increase in happiness scores following this introduction (so called “responders”) showed greater overall cortical activity (less alpha) than non-responders, with depressed “responders” having relatively greater right than left frontal activity. Their findings do not necessarily conflict with our findings. Although their cognitive restructuring task was based on the techniques of CBT, it is not clear that findings for this brief intervention generalize to results for standard CBT, and treatment response was based on improvement in happiness scores and not depressive symptoms. Also, our finding of greater left hemisphere advantage for dichotic words in responders to CBT is likely related to processes in more posterior temporoparietal regions, where right ear advantage for dichotic words or syllables has been linked to relatively greater activity (less resting EEG alpha) over the left hemisphere (Davidson and Hugdahl, 1996; Bruder et al., 2001), and responders to a SSRI showed an alpha asymmetry indicative of relatively greater left hemisphere activity (Bruder et al., 2008). Note that Davidson and Hugdahl (1996) found that larger right ear advantage for dichotic syllables was also associated with less activity (greater alpha) over left than right prefrontal region. Thus, the greater right ear advantage in CBT responders could reflect both greater left temporoparietal and right prefrontal activation.
Individuals with greater left hemisphere language lateralization may be better able to recruit cortical regions critical for success in CBT. Although sample size was small and neuropsychological tests were limited, we found no difference in performance of CBT responders and non-responders on tests assessing verbal skills, i.e., word fluency and word reading RT, or on the NART estimate of premorbid IQ. This provides no support for the hypothesis that general verbal skill or cognitive ability predicts therapeutic response to CBT. Rather, our findings support the hypothesis that individuals with left hemisphere language dominance have greater ability to access frontal and temporal regions involved in cognitive reappraisal. The lack of a difference between CBT responders and non-responders on measures of psychomotor speed (e.g., word fluency and choice RT), which have been reported to differentiate responders and non-responders to antidepressants (Taylor et al., 2006; Gorlyn et al., 2008; Bruder et al., 2014), suggests some degree of specificity of these tests for predicting response to antidepressants.
We have also found enhanced left hemisphere advantage on the DFWT in responders to the antidepressants fluoxetine (Bruder et al., 1996) or bupropion (Bruder et al., 2007), which suggests that it would not provide differential prediction of response to CBT and antidepressants. The dichotic syllable test used in our prior CBT study (Bruder et al., 1997) differs from the DFWT, in that, the subject reports the syllable heard in each ear, which provides a separate measure of accuracy for each side. The larger left hemisphere advantage for dichotic syllables in CBT responders compared to non-responders was due to better right ear accuracy in responders. Given the contralateral advantage of projections from ear to auditory cortex, this is indicative of greater left hemisphere dominance for language in responders. In a study using the same dichotic listening tasks (Bruder et al., 1990), patients who responded to a tricyclic antidepressant also tended to have larger left hemisphere advantage for syllables, but this was due to their poorer left ear accuracy when compared to non-responders. Moreover, tricyclic responders differed from non-responders in failing to show a left ear advantage for dichotic complex tones, which is consistent with right hemisphere dysfunction. In contrast, there was no difference between CBT responders and non-responders for dichotic complex tones (Bruder et al., 1996). This argues that the basis for the dichotic listening differences between responders and non-responders may be different for CBT and antidepressants.
The DFWT predicted response to CBT with a large effect size and with good sensitivity and specificity. The potential value of this test, as compared to neuroimaging, is that it can be administered by an assistant or technician with minimal training and equipment in an office or clinical setting at little expense. It would not, however, by itself be of value for differentiating between patients who are likely to respond to CBT as opposed to an antidepressant. It may be necessary to use a dichotic syllable test, which yields separate measures of right and left ear accuracy, to indicate whether the patient shows heightened right ear accuracy reflecting increased left hemisphere dominance or poorer left ear accuracy reflecting right hemisphere deficit.
One could ask whether the weak or absent left hemisphere advantage in CBT non-responders is irrevocably a bad prognostic sign or whether the underlying mechanism might be overcome by an intervention that enhances engagement of the left hemisphere. For instance, Triggs et al. (2010), in a randomized control trial of rTMS coupled with a social intervention for refractory depression, found that right cranial stimulation was the most effective treatment. Also, could a behavioral therapy that engages the right hemisphere be more effective in depressed patients without a left hemisphere advantage for dichotic words or syllables?
This study has several limitations. First, sample size is small and future studies would benefit by using a larger number of depressed patients. The findings do, however, replicate those of our prior study (Bruder et al., 1997), which had larger numbers of CBT responders and non-responders, and the relatively high sensitivity and specificity in both studies indicate good prediction of response for individual patients. Second, although a standard CBT manual was used (Emery, 2000), it was administered by graduate student therapists. They were, however, well trained and closely supervised by an experienced CBT therapist. Moreover, results are mixed in regard to therapist’s experience as a predictor of psychotherapy outcome (Christensen and Jacobson, 1994; Blatt et al., 1996; Tallman and Bohart, 1999), and the obtained 55% CBT response rate is typical of that seen in the field (DeRubeis et al., 2005; Siegle et al., 2011). This may also indicate that our findings are not limited to highly trained clinicians in research settings and may generalize to clinical settings. Third, another possible limitation is our use of the PA norm for healthy controls as a cutoff for predicting CBT response. About half of patients would be expected to fall above or below the mean PA for controls. This cutoff value was not, however, meant for differentiating depressed patients versus controls. It was chosen a priori based on its success in predicting treatment response in our prior research and the norm is not an arbitrary cutoff but rather characterizes patients as to whether their language lateralization is above or below normal. Also, a logistic regression analysis including continuous PA and handedness LQ scores yielded predictions of CBT response comparable to those seen using the PA norm as a cutoff. Lastly, neuroimaging or electrophysiological methods might provide more direct measures of language lateralization of the brain. The DFWT has, however, been found to provide a valid measure of language lateralization, and could be administered in clinical settings to provide a brief and inexpensive predictor of CBT response.
Highlights.
Replicated larger left hemisphere dominance for speech in CBT responders than non-responders
Larger left hemisphere advantage was associated with greater improvement following CBT
Neuropsychological tests of verbal ability or premorbid IQ did not predict response to CBT
Dichotic fused words test predictions of CBT response had high sensitivity and specificity
Acknowledgments
This research was supported in part by a grant from the National Institute of Mental Health (MH36295).
Footnotes
We have not found a difference in DFWT performance between patients having a MDD and those having “pure dysthymia” (Bruder et al., 2012). In agreement with the overall results, the 2 dysthymic patients who were responders had a larger left hemisphere advantage (PA= 8.0 and 28.6) than the 2 dysthymic patients who were non-responders (PA= 1.7 and −0.8), with correct prediction of CBT response in 3 of these 4 cases. Also, predictions of CBT response remained essentially the same when only the 16 patients having a MDD were included. Of the patients having a MDD, 7 of 8 with PA scores above the mean for HC responded to CBT, whereas only 2 of 8 with PA scores below the mean for HC responded to treatment. This is a positive predictive value of 88% and a negative predictive value of 75%, which agrees with the results for the total sample, including patients having dysthymia.
The PA scores for the 4 patients who received medication during the course of CBT were well within the range of scores for the other patients and correctly predicted their response to CBT. The 2 medicated patients who responded had PA scores above the mean for HC (14.0 and 29.9), while the 2 medicated non-responders were below the mean for HC (0.87 and 9.2). Inclusion of these medicated patients did not adversely affect the findings of the study.
Although some reduction of predictive accuracy may occur when using logistic regression with small samples (Cohen et al., 2003), the results of this analysis are in accord with those seen for the χ2 and correlation analyses.
The controls are comparable in gender, age and education to the patients. There was no difference in left hemisphere advantage on the DFWT between male (M=10.7, SD=13.6) and female (M=13.5, SD=15.9) controls, t (72) = 0.79, ns. Also, their PA scores on DFWT were not significantly correlated with age (r= −.01, ns) or education (r= .08, ns).
Conflict of Interest
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory— II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. 3. AJA Associates; Iowa City: 1983. [Google Scholar]
- Blatt SJ, Sanislow CA, Zuroff DC, Pilkonis PA. Characteristics of effective therapists: Further analyses of data from the National Institute of Mental Health treatment of depression collaborative research program. Journal of Consulting and Clinical Psychology. 1996;64:1276–1284. doi: 10.1037//0022-006x.64.6.1276. [DOI] [PubMed] [Google Scholar]
- Bright P, Jaldow E, Kopelman MD. The National Adult Reading Test as a measure of premorbid intelligence: a comparison with estimates derived from demographic variables. Journal of the International Neuropsychological Society. 2002;8:847–854. doi: 10.1017/s1355617702860131. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Alvarenga JE, Alschuler D, Abraham K, Keilp JG, Hellerstein DJ, Stewart JW, McGrath PJ. Neurocogntive predictors of antidepressant clinical response. Journal of Affective Disorders. 2014;166:108–114. doi: 10.1016/j.jad.2014.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Otto MW, McGrath PJ, Stewart JW, Fava M, Rosenbaum JF, Quitkin FM. Dichotic listening before and after fluoxetine treatment for major depression: relations of laterality to therapeutic response. Neuropsychopharmacology. 1996;15:171–179. doi: 10.1016/0893-133X(95)00180-L. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, Hellerstein D, Alvarenga JE, Alschuler D, McGrath PJ. Abnormal functional brain asymmetry in depression: Evidence of biologic commonality between major depression and dysthymia. Psychiatry Research. 2012;196:250–254. doi: 10.1016/j.psychres.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Sedoruk JP, Stewart JW, McGrath PJ, Quitkin FM, Tenke CE. Electroencephalographic alpha measures predict therapeutic response to a selective serotonin reuptake inhibitor antidepressant: Pre- and post-treatment findings. Biological Psychiatry. 2008;63:1171–1177. doi: 10.1016/j.biopsych.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, McGrath PJ, Deliyannides D, Quitkin FM. Dichotic listening tests of functional brain asymmetry predict response to fluoxetine in depressed women and men. Neuropsychopharmacology. 2004;29:1752–1761. doi: 10.1038/sj.npp.1300519. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, Mercier MA, Agosti V, Leite P, Donovan S, Quitkin FM. Outcome of cognitive-behavioral therapy for depression: relation of hemispheric dominance for verbal processing. Journal of Abnormal Psychology. 1997;106:138–144. doi: 10.1037//0021-843x.106.1.138. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, Schaller JD, McGrath PJ. Predicting therapeutic response to secondary treatment with bupropion: dichotic listening tests of functional brain asymmetry. Psychiatry Research. 2007;15:137–143. doi: 10.1016/j.psychres.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, Tenke CE, McGrath PJ, Leite P, Bhattacharya N, Quitkin FM. Electroencephalographic and perceptual asymmetry differences between responders and nonresponders to an SSRI antidepressant. Biological Psychiatry. 2001;49:416–425. doi: 10.1016/s0006-3223(00)01016-7. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, Voglmaier MA, Harrison WM, McGrath P, Tricamo MA, Quitkin FM. Cerebral laterality and depression: relations of perceptual asymmetry to outcome of treatment with tricyclic antidepressants. Neuropsychopharmacology. 1990;3:1–10. [PubMed] [Google Scholar]
- Christensen A, Jacobson NS. Who (or what) can do psychotherapy? The status and challenge of nonprofessional therapies. Psychological Science. 1994;5:8–14. [Google Scholar]
- Cohen J, Cohen P, West S, Aiken L. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Erlbaum Associates; Mahwah, New Jersey: 2003. [Google Scholar]
- Deldin PJ, Chiu P. Cognitive restructuring and EEG in major depression. Biological Psychology. 2005;70:141–151. doi: 10.1016/j.biopsycho.2005.01.003. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, O’Reardon JP, Lovett ML, Gladis MM, Brown LL, Gallop R. Cognitive therapy vs medications in the treatment of moderate to severe depression. Archives of General Psychiatry. 2005;62:409–416. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- Dunkin JJ, Leuchter AF, Cook IA, Kasl Godley JE, Abrams M, Rosenberg-Thompson S. Executive dysfunction predicts nonresponse to fluoxetine in major depression. Journal of Affective Disorders. 2000;60:13–23. doi: 10.1016/s0165-0327(99)00157-3. [DOI] [PubMed] [Google Scholar]
- Emery G. Overcoming depression: A cognitive behavior protocol for the treatment of depression. New Harbinger Publications; Oakland, CA: 2000. [Google Scholar]
- Fernandes MA, Smith ML. Comparing the fused dichotic words test and the intracarotid amobarbital procedure in children with epilepsy. Neuropsychologia. 2000;38:1216–1228. doi: 10.1016/s0028-3932(00)00035-x. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. The structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P, Verson 2) American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Nonpatient Edition (SCID-NP) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1996. [Google Scholar]
- Fournier JC, DeRubeis RJ, Shelton RC, Hollon SD, Amsterdam JD, Gallop R. Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. Journal of Consulting and Clinical Psychology. 2009;77:775–787. doi: 10.1037/a0015401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlyn M, Keilp JG, Grunebaum MF, Taylor BP, Oquendo MA, Bruder GB, Stewart JW, Mann JJ. Neuropsychological characteristics as predictors of SSRI treatment response in depressed subjects. Journal of Neural Transmission. 2008;115:1213–1219. doi: 10.1007/s00702-008-0084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurological and Neurosurgical Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaga DAF, DeRubeis RJ, Stewart BL, Beck AT. Relationship of intelligence with cognitive therapy outcome. Behavioral Research Therapy. 1991;29:277–281. doi: 10.1016/0005-7967(91)90118-m. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Gorlyn M, Oquendo MA, Burke AK, Mann JJ. Attention deficit in depressed suicide attempters. Psychiatry Research. 2008;159:7–17. doi: 10.1016/j.psychres.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilp JG, Sackeim HA, Mann JJ. Correlates of trait impulsiveness in performance measures and neuropsychological tests. Psychiatry Research. 2005;135:191–201. doi: 10.1016/j.psychres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, Craddock RC, Mayberg HS. Toward a neuroimaging treatment selection biomarker for major depressive disorder. Journal of the American Medical Association Psychiatry. 2013;70:821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: Neural systems supporting the cognitive down- and up- regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Otto MW, Yeo RA, Dougher MJ. Right hemisphere involvement in depression: Toward a neuropsychological theory of negative affective experiences. Biological Psychiatry. 1987;22:1201–1215. doi: 10.1016/0006-3223(87)90028-x. [DOI] [PubMed] [Google Scholar]
- Price RB, Paul B, Schneider W, Siegle GJ. Neural correlates of three neurocognitive intervention strategies: A preliminary step towards personalized treatment for psychological disorders. Cognitive Therapy Research. 2013;37:657–672. doi: 10.1007/s10608-012-9508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rude SS, Rehm LP. Response to treatments for depression: The role of initial status on targeted cognitive and behavioral skills. Clinical Psychology Review. 1991;11:493–514. [Google Scholar]
- Siegle GJ, Steinhauer SR, Friedman ES, Thompson WS, Thase ME. Remission prognosis for cognitive therapy for recurrent depression using the pupil: Utility and neural correlates. Biological Psychiatry. 2011;69:726–733. doi: 10.1016/j.biopsych.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson WK, Collier A, Berman SR, Feldmiller J, Thase ME, Friedman ES. Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenul cingulate activity for determining depression outcome in cognitive therapy across studies, scanners and patient characteristics. Archives of General Psychiatry. 2012;69:913–924. doi: 10.1001/archgenpsychiatry.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Weber J, Wager TD, Ochsner KN. Bad and worse: neural systems underlying reappraisal of high- and low-intensity negative emotions. Social Cognitive and Affective Neuroscience, Advance Access. 2014 doi: 10.1093/scan/nsu043. Published Online April 9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotsky SM, Glass DR, Shea MT, Pilkonis PA, Collins JF, Elkin I, Watkins JT, Imber SD, Leber WR, Moyer J, Oliveri ME. Patient predictors of response to psychotherapy and pharmacotherapy: Findings in the NIMH Treatment for Depression Collaborative Research Program. American Journal of Psychiatry. 1991;148:997–1008. doi: 10.1176/ajp.148.8.997. [DOI] [PubMed] [Google Scholar]
- Tallman K, Bohart AC. The client as a common factor: Clients as self-healers. In: Hubble MA, Duncan BL, Miller SD, editors. The heart and soul of change: What works in therapy. American Psychological Association; Washington, DC: 1999. pp. 91–131. [Google Scholar]
- Taylor BP, Bruder GE, Stewart JW, McGrath PJ, Halperin J, Ehrlichman H, Quitkin FM. Psychomotor slowing as a predictor of fluoxetine nonresponse in depressed outpatients. American Journal of Psychiatry. 2006;163:73–78. doi: 10.1176/appi.ajp.163.1.73. [DOI] [PubMed] [Google Scholar]
- Thone DR, Genser SG, Sing HC, Hegge FW. The Walter Reed performance assessment battery. Neurobehavioral Toxicology. 1985;7:415–418. [PubMed] [Google Scholar]
- Triggs WJ, Ricciuti N, Ward HE, Cheng J, Bowers D, Goodman WK, Kluger BM, Nadeau SE. Right and left dorsolateral pre-frontal rTMS treatment of refractory depression: a randomized, sham-controlled trial. Psychiatry Research. 2010;178:467–474. doi: 10.1016/j.psychres.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Halwes T. Increasing the power of dichotic methods. The fused rhymed words test. Neuropsychologia. 1983;21:59–66. doi: 10.1016/0028-3932(83)90100-8. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ. Perceptual asymmetry on the dichotic fused words test and cerebral speech lateralization determined by the carotid sodium amytal test. Neuropsychologia. 1989;27:1207–1219. doi: 10.1016/0028-3932(89)90033-x. [DOI] [PubMed] [Google Scholar]