Abstract
Peripheral neuropathy is a major complication associated with diabetes and central neuropathy characterized by Alzheimer’s disease-like features in the brain is associated with increased dementia risk for patients with diabetes. Although glucose uptake into the cells of the nervous system is insulin-independent, contribution of impaired insulin support is clearly recognized to play a role, however not yet fully understood, in the development of neuropathy. In this study, we assessed the direct role of insulin on the PNS and CNS of insulin-dependent type 1 diabetic rats. Fresh sciatic nerve and hippocampus from control and diabetic rats were incubated with varied ex vivo concentrations of insulin and phosphorylation levels of insulin receptor and GSK3β were assessed by Western blot analysis. Both sciatic nerve and hippocampus from type 1 diabetic rats were highly responsive to exogenous insulin with a significantly increased phosphorylation of insulin receptor and GSK3 compared to tissues from control rats. Further, sustained in vivo insulin delivery, not sufficient to restore normal blood glucose, normalized the activation of both insulin receptor and GSK3 in both PNS and CNS tissues. These results suggest that the insulin-signaling pathway is responsive to exogenous insulin in the nervous system of insulin-deficient type 1 diabetic rats and that constant insulin delivery restore normal nerve function and may protect peripheral and central nervous system from damage.
Keywords: Diabetes, Hippocampus, Insulin, Insulin receptor, GSK3, Sciatic nerve
Peripheral neuropathy is the most common complications associated with long-term diabetes mellitus and develops in more than half of all diabetic patients. In addition, several studies have demonstrated a co-incidence between diabetic complications and impaired function of the central nervous system (CNS) (Ryan et al., 1993, Ferguson et al., 2003), suggesting that the brain is susceptible to the same processes that underlie other complications of diabetes. However, the pathologic mechanisms leading to damage of the peripheral and central nervous systems (PNS and CNS) are not yet understood. One of the proposed mechanisms is the contribution of impaired insulin support.
Insulin exhibits a number of properties independent of regulation of glucose uptake in both the PNS and CNS. It has neurotrophic effects on peripheral nerves to control survival, maintenance, repair and regeneration (Apfel, 1999) and plays a role in plasticity, neuroprotection and cognition in the brain (reviewed in (Gasparini et al., 2002). In type 1 diabetes, insulin replacement therapy by repeated injections, or subcutaneous implanted insulin pumps is titrated against maintenance of glycemic control, not the neurotrophic properties of insulin. The lack of insulin and/or fluctuations of exogenous insulin levels in type 1 diabetes results in aberrant insulin signaling pathway activity in neurons and/or associated glial cells that can impair function and structure of both the PNS and CNS.
In both the PNS and CNS, binding of insulin to its receptor at the plasma membrane triggers several signaling pathways including those mediated by phospholipase Cγ, mitogen-activated protein kinase and phosphatidylinositol 3-kinase (PI3K) (Taniguchi et al., 2006). The PI3K-AKT (also known as protein kinase B) pathway involves the downstream glycogen synthase kinase-3 (GSK3), an enzyme whose activity is down-regulated by phosphorylation (Sutherland et al., 1993, Cross et al., 1995). GSK3 regulates many aspects of cellular structure, function and survival while dysregulation of GSK3 activity has been linked to a number of pathologic conditions (Jope and Johnson, 2004). The substrates of GSK3 include a variety of metabolic, signaling and structural proteins and transcription factors, a number of which are highly pertinent to the nervous system.
In this study, we investigated how insulin impacts the insulin-signaling cascade in both the PNS and CNS during type 1 diabetes. We have found that insulin triggers an enhanced activation of the insulin receptor in both the sciatic nerve and the hippocampus from diabetic rats, resulting in increased phosphorylation of GSK3. Further, we have shown that this involves the PI3K/AKT pathway and that the hyperactivation of the insulin-signaling pathway was prevented by sustained insulin administration to normalize blood glucose from onset of diabetes. Even when insulin levels were not sufficient to normalize glycemia, there was normalization of activation patterns in tissue from both the PNS and CNS. This suggests not only a similarity between the two nervous systems but also that constant low insulin administration, via subcutaneous pump, can normalize responses separately from the effects on glucose levels during type 1 diabetes.
Experimental Procedures
Animals
Adult female Sprague-Dawley rats were purchased from Harlan Industries (Placentia, CA, USA). Animals were housed 2–3 per cage with free access to food and water and maintained in a vivarium approved by the American Association for the Accreditation of Laboratory Animal Care. All animal studies were carried out according to protocols approved by the Institutional Animal Care and Use Committee of the University of California San Diego.
Induction of type 1 diabetes
Insulin-deficient diabetes was induced in 2-month old Sprague-Dawley rats following an overnight fast by intraperitoneal (i.p.) injection of streptozotocin (STZ, Sigma. St, Louis, MO, USA) at 50 mg/kg dissolved in 0.9% sterile saline. Hyperglycemia was confirmed using a strip-operated reflectance meter in a blood sample obtained by tail prick 4 days and again 4 weeks after STZ injection and in another sample collected at the conclusion of the study. Only rats with blood glucose level > 270 mg/dl were considered diabetic.
Experimental groups
Several rat cohorts have been carried out to provide sufficient material for the individual experiments that consist of time courses, age-matched comparison of control versus diabetic rats and an insulin in vivo experiment that included diabetic rats implanted with subcutaneous insulin pellet. For each experiment, rats were maintained diabetic for 8 weeks before sacrifice. For the insulin in vivo experiment, 3 groups of diabetic rats were maintained: one group received no treatment while 1 group of diabetic rats was implanted subcutaneously with 1/3 of an insulin pellet (Linplant, Linshin Canada Inc, Toronto, Canada), corresponding to 0.3U insulin/24hr, to partially correct blood glucose levels (diabetic partial insulin group) and the third group was implanted subcutaneously with 1 full pellet, corresponding to 2U insulin/24hr, to completely restore normoglycemia (diabetic full insulin group). Insulin pellets were implanted at the onset of diabetes and replaced after 40 days or earlier if blood glucose levels were increasing. Each day of dissection, only 2 rats were sacrificed in order to rapidly dissect hippocampus and sciatic nerve and ensure prompt processing and viability of tissues.
Ex vivo insulin incubation
Rats were killed by isoflurane and decapitation. Brains were dissected within 1 minute and hippocampi were placed in ice-cold oxygenated modified Krebs buffer (125 mM NaCl pH7.35, 3 mM KCL, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.2mM NaH2PO4, 25 mM NaHCO3, 5 or 25 mM glucose), before being sliced into 450 μm thick sections with a McIlwain Tissue Chopper (Ted Pella Inc., Redding, CA, USA). Sciatic nerves were dissected, had their epineurium removed and were cut in 1 cm long pieces placed in ice-cold oxygenated modified Krebs buffer. Slices of hippocampus and pieces of sciatic nerve were then placed in a 12-well plate containing 2 ml of oxygenated Krebs buffer at 37°C. After 10 min equilibration, insulin (0, 0.1, 1 or 10 nM) was added to the wells for varied time (1, 15, 30 min). For AKT inhibitor experiments, 10 μM AKT inhibitor IV (EMD, Temecula, CA, USA) was added to the well 1 min prior to the addition of insulin.
Tissue preparation for Western blot analysis
At the end of the incubation, sciatic nerve and slices of hippocampus were homogenized in buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.5% Triton X, 1 mM EDTA, protease inhibitor cocktail). Homogenates were centrifuged at 13,000 g for 30 min and supernatants were stored in aliquots at −80°C. Protein concentration was assessed using the bicinchoninic acid method (BCA protein assay kit, Pierce, Rockford, IL, USA).
Western blotting
Aliquots of sciatic nerve or hippocampus homogenates were boiled in Laemmli LDS sample buffer (Invitrogen, Carlsbad, CA, USA). Up to 20 μg of total extract protein were separated on 4–12% SDS-PAGE Bis-Tris gels (Novex, Invitrogen, Carlsbad, CA, USA) and immunoblotted onto nitrocellulose. To maximize the number of proteins analyzed by Western blot, membranes were cut along the molecular weight markers into strips containing the proteins of interest. Blot strips were incubated with antibodies against phosphorylated-insulin receptor (phosphorylated Ser 972, 1/700, Upstate, Temecula, CA, USA), insulin receptor (1/200, Chemicon International, Temecula, CA, USA), phosphorylated-GSK3β (phosphorylated-Ser9; 1/1000, Cell Signaling technology, USA) or actin (1/5000, Sigma) followed by the corresponding secondary antibodies tagged with infrared dyes (IRDye, 1/15000, LI-COR Biosciences, Lincoln, NE, USA). Blots were developed using the Odyssey Fc infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA). For each animal, band intensities were normalized by calculating the ratio of the intensity of the band corresponding to primary antigen of interest to the intensity of the band corresponding to actin. The average of the intensities ratio for the control group (tissue without insulin) is then used to calculate the percent of phosphorylation, as indicated on graphs, for all the groups.
Statistical analysis
Data are represented as mean ± SEM. Comparison between groups were performed using two-way ANOVA followed by Dunnett’s post hoc test, or by one-way ANOVA followed by Tukey’s post hoc test.
Results
Time course of activation of insulin signaling in tissues from control rats
Sciatic nerve
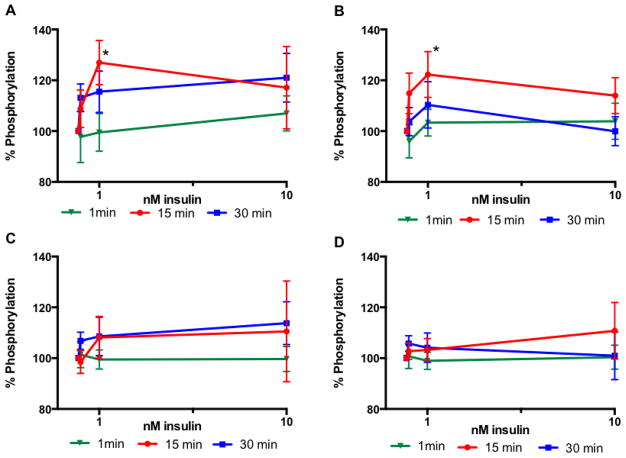
To determine insulin concentrations and incubation times, desheathed sciatic nerves from control rats were incubated with insulin at 0, 0.1, 1 or 10 nM for 1, 15 or 30 min. Phosphorylation of insulin receptor and GSK3β followed a similar pattern with an increase at 0.1 and 1 nM insulin that plateaued or decreased at 10 nM insulin at both 15 and 30 min time point (Fig 1A, B). One-minute incubation did not lead to significant phosphorylation of insulin receptor or GSK3β (Fig 1A, B). Fifteen minute incubation lead to a significant (p<0.05) increase in phosphorylation of both proteins at 1nM insulin. Since 10nM insulin did not activate the insulin receptor further than the activation induced by 1nM and because 1 nM insulin corresponds to the half-maximally effective concentration (ED50) for neurite outgrowth in neuronal cell culture (Fernyhough et al., 1989), 1 nM as well as 0.1 nM insulin, which correspond to physiological level, were selected with a 15 min incubation for all subsequent experiments.
Figure 1.
Time course of activation of the insulin-signaling pathway in sciatic nerve (A, B) and hippocampus (C, D) from control rats. Levels of phosphorylation of insulin receptor (A, C) and GSK3β (B, D) after 1 min (closed triangle), 15 min (closed circle) or 30 min (closed square). Data are mean ± sem, n=6–9. *p<0.05 vs 0 nM insulin by two-way ANOVA followed by Dunnett’s post hoc test.
Hippocampus
Similar experiments to those conducted with sciatic nerves were performed with hippocampal slices from control rats. Similar patterns were observed, however without reaching significance (Fig 1C, D). Given an estimated physiological insulin levels of 0.03 to 1.3 nM in the brain (Talbot et al., 2012), the same conditions (0.1 and 1 nM insulin and 15 min) as those determined for the sciatic nerve were used for all subsequent experiments.
Insulin signaling mechanisms are intact in diabetic tissues
Sciatic nerve
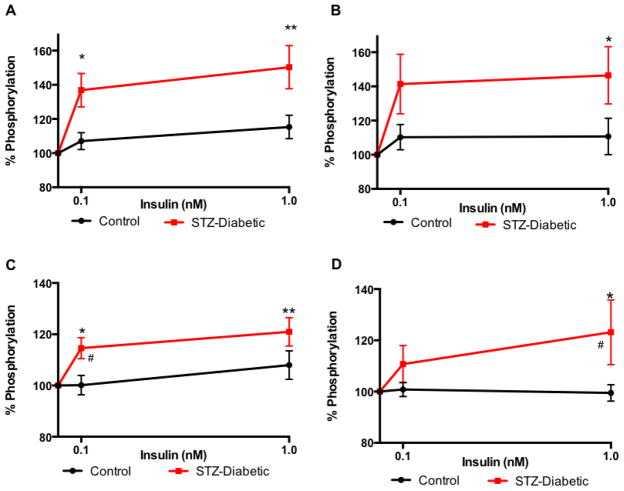
Activation of insulin receptor was significantly increased in the sciatic nerve of diabetic rats after incubation with 0.1 or 1 nM insulin (p<0.05 and p<0.01, respectively) compared to sciatic nerve from control rats carried out in parallel (Fig 2A). GSK3β was phosphorylated to a significantly elevated level (*p<0.05) in the sciatic nerve of diabetic rats when incubated with 1 nM insulin and compared to that of control rats (Fig 2B).
Figure 2.
Increased sensitivity of insulin receptor and GSK3β to ex vivo insulin in sciatic nerve (A, B) and hippocampus (C, D) from diabetic rats (closed square). Levels of phosphorylation of insulin receptor (A, C) and GSK3β (B, D) after 15 min incubation with varied concentration of insulin (0, 0.1, 1 nM). Data are mean ± sem, n=5–10. *p<0.05, **p<0.01, vs 0 nM insulin by two-way ANOVA followed by Dunnett’s post hoc test, # p<0.05, vs control (closed circle) at the same time point by two-way ANOVA followed by Tukey’s post hoc test.
Hippocampus
Similarly, activation of insulin receptor was significantly increased in the hippocampus of diabetic rats after incubation with 0.1 or 1 nM insulin (p<0.05 and p<0.01, respectively) compared to hippocampus from control rats in the same conditions (Fig 2C). When incubated with 1 nM insulin, GSK3β was phosphorylated to a significantly higher level (*p<0.05) in the hippocampus of diabetic rats compared to that of control rats (Fig 2D).
Signaling via the PI3K/AKT pathway
Sciatic nerve
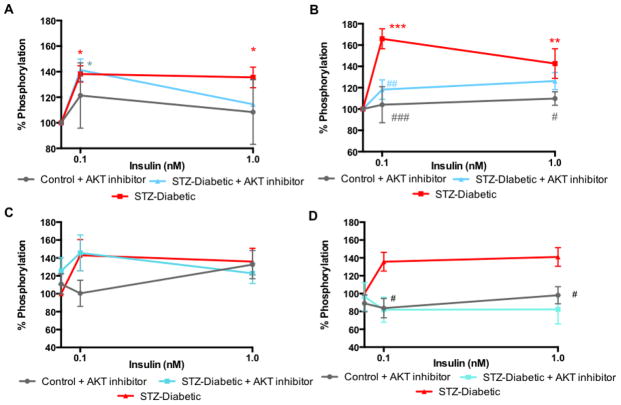
To confirm which insulin-signaling pathway was stimulated in the PNS, sciatic nerves were incubated with AKT inhibitor IV prior to addition of insulin. Inhibition of AKT had no effect on the activation of insulin receptor by insulin (Fig 4A) but significantly (p<0.01) prevented the phosphorylation of GSK3β at 0.1 nM insulin (Fig 4B) in the sciatic nerve of diabetic rats. AKT inhibition had no effect on insulin receptor or GSK3β phosphorylation in sciatic nerve from control rats (Fig 4A, B).
Figure 4.
Effect of AKT inhibitor on insulin receptor (A, C) and GSK3β (B, D) phosphorylation in sciatic nerve (A. B) and hippocampus (C, D) from diabetic rats (close square). Levels of phosphorylation of insulin receptor (A, C) and GSK3β (B, D) after 15 min incubation with varied concentration of insulin (0, 0.1, 1 nM) ± 10 μM AKT inhibitor (close triangle). Data are mean ± sem, n=3–7. *p<0.05, **p<0.01, ***p<0.001 vs 0 nM insulin by two-way ANOVA followed by Dunnett’s post hoc test, # p<0.05, ##p<0.01, ###p<0.001 vs diabetic (closed square) at the same time point by two-way ANOVA followed by Tukey’s post hoc test.
Hippocampus
To confirm which insulin-signaling pathway was stimulated in the CNS, hippocampus slices were incubated with AKT inhibitor IV prior to addition of insulin. Inhibition of AKT had no effect on the activation of insulin receptor by insulin (Fig 4C) but significantly (p<0.05) prevented the phosphorylation of GSK3β (Fig 4D) in the hippocampus of diabetic rats at both 0.1 and 1 nM insulin. Similar to the results seen in the sciatic nerve, AKT inhibition did no affect insulin receptor or GSK3β phosphorylation in hippocampus from control rats (Fig 4C, D).
Effect of in vivo insulin on insulin receptor activation
In the in vivo insulin experiment, diabetic rats implanted with a full pellet had normal blood glucose (not significantly different from levels in control rats) while animals implanted with a 1/3 of a pellet still displayed hyperglycemia, however it was significantly lower than diabetic rats without an insulin implant (blood glucose of control: 96±5###, diabetic: 560±14***, diabetic partial insulin: 430±43**,##, diabetic full insulin: 53±16 mg/dl###, **p<0.01, ***p<0.001, one-way ANOVA followed by Tukey’s post hoc test compared to control group and ## p<0.01, ### p<0.01 one-way ANOVA followed by Tukey’s post hoc test compared to diabetic group). Only untreated diabetic rats had a significantly lower weight at the end of the study (body weight of control: 264±5###, diabetic: 217±7***, diabetic partial insulin: 239±5#, diabetic full insulin: 251±5 mg/dl###, ***p<0.001, one-way ANOVA followed by Tukey’s post hoc test compared to control group and # p<0.05, ### p<0.01 one-way ANOVA followed by Tukey’s post hoc test compared to diabetic group).
Sciatic nerve
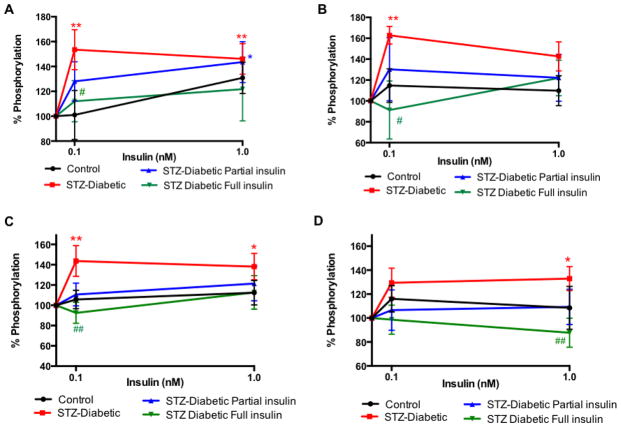
Constant insulin delivery that did not normalize glycemia but significantly reduced blood glucose from onset of diabetes did partially prevent the insulin receptor activation by ex vivo insulin (0.1 nM) stimulation (Fig 5A) in sciatic nerve from diabetic rats. Sustained insulin delivery that normalized blood glucose for 8 weeks of diabetes significantly prevented activation of insulin receptor by ex vivo insulin at 0.1 nM (#p<0.05) (Fig 4A). Similar patterns were observed for GSK3β phosphorylation (Fig 5B).
Figure 5.
Effect of sustained insulin delivery in vivo on insulin receptor (A, C) and GSK3β (B, D) phosphorylation in sciatic nerve (A, B) and hippocampus (C, D) from diabetic rats (close square). Levels of phosphorylation of insulin receptor (A, C) and GSK3β (B, D) after 15 min incubation with varied concentration of insulin (0, 0.1, 1 n M) for tissues from control (close circle), diabetic (close square), diabetic implanted with partial insulin pellet (upright triangle) or full insulin pellet (downward triangle) rats. Data are mean ±sem, n=4–7. *p<0.05, **p<0.01 vs 0 nM insulin by two-way ANOVA followed by Dunnett’s post hoc test, # p<0.05, ##p<0.01 vs diabetic (closed square) at the same time point by two-way ANOVA followed by Tukey’s post hoc test.
Hippocampus
Sustained insulin delivery that did not normalize glycemia but significantly reduced blood glucose from onset of diabetes did prevent the insulin receptor activation by ex vivo insulin stimulation (Fig 5C) in hippocampus from diabetic rats. Constant insulin delivery that normalized blood glucose for 8 weeks of diabetes significantly prevented activation of insulin receptor by ex vivo insulin at 0.1 nM (##p<0.01) (Fig 5C). Similar results were observed for GSK3β phosphorylation (Fig 5D).
Changes in phosphorylation levels observed were not due to changes in total insulin receptor levels as insulin receptor protein levels normalized to actin protein levels were not significantly different in all the insulin incubation conditions within the time frame studied in either the sciatic nerve or the hippocampus from control and diabetic rats (Table 1).
Table 1.
Insulin receptor protein levels in hippocampus and sciatic nerve from control and diabetic rats after 15 min incubation. Data are expressed as percent of the intensity of the band corresponding to insulin receptor over the intensity of the band corresponding to actin and referenced against tissue incubated without insulin (0 nM). Data are mean ± SEM, n=4–7.
| Sciatic nerve | Hippocampus | |||
|---|---|---|---|---|
| Insulin | Control | Diabetic | Control | Diabetic |
| 0 nM | 100 | 100 | 100 | 100 |
| 0.1 nM | 98.2 ± 9.6 | 99.7 ± 10.5 | 90.8 ± 4.5 | 92.6 ± 3.5 |
| 1 nM | 94.1 ± 3.1 | 103.3 ± 2.1 | 102.5 ± 6.8 | 97.4 ± 2.3 |
Discussion
Using ex vivo stimulation by insulin, we have shown that both the sciatic nerve and hippocampal neurons, and/or their associated glia, of type 1 diabetic rats are not resistant, but rather highly responsive to exogenous insulin. We have also shown that phosphorylation of GSK3 was prevented by sustained insulin delivery that normalize blood glucose but also to some extent by constant insulin delivery which was not sufficient to restore normal blood glucose. This suggests that treatment with constant insulin may prevent both peripheral and central diabetic neuropathy through the normalization of GSK3 activity, thus attenuating the detrimental effects of activated GSK3 on synaptic plasticity and proteins such as the microtubule associated protein tau (Jolivalt et al., 2008, King et al., 2013).
In the PNS, insulin receptors are present in Schwann cells, endothelial cells, pericytes and neurons (Sugimoto et al., 2000, Sugimoto et al., 2002, Brussee et al., 2004). In the CNS, insulin receptors are present in all regions but are especially prevalent in the olfactory bulb, hypothalamus, cerebral cortex and hippocampus (Havrankova et al., 1978a, Havrankova et al., 1978b, Havrankova et al., 1981, Havrankova et al., 1983, Le Roith et al., 1983, Plum et al., 2005). These receptors are localized to astrocytes (Rajasekar et al., 2014) and at the synapse where they regulate neurotransmitter release and receptor recruitment, playing a role in synaptic plasticity (Jonas et al., 1997, Wan et al., 1997). In adult primary sensory neurons in culture, insulin promotes neurite outgrowth (Fernyhough et al., 1993) and, via local cutaneous delivery, promotes regrowth of epidermal axons (Guo et al., 2011). We have shown that in both the CNS and the PNS of insulin-deficient diabetic mice, disruption of the insulin-signaling pathway was associated with increased GSK3 activity, tau phosphorylation and Aβ levels (Jolivalt et al., 2008, Jolivalt et al., 2012). In this ex vivo study, we have shown that in both the PNS and CNS, the insulin-signaling pathway, despite being disrupted in insulin-deficient diabetes, can be activated by exogenous insulin. Firstly, physiological doses of insulin, 0.1 and 1 nM, were able to activate the insulin receptor in sciatic nerve and hippocampus from control rats, leading to activation of AKT and phosphorylation (deactivation) of GSK3 within 15 min as it was shown for activation of hypothalamic PI3K (Niswender et al., 2003). Activation of the insulin-signaling pathway, including AKT participation, has been recently demonstrated in non-diabetic mice receiving systemic injection of insulin (Grote et al., 2013b). In conditions similar to the ones outlined in this study, insulin evoked a dose-dependent activation of the insulin-signaling pathway in control human and rat brains exposed to ex vivo insulin (1 and 10 nM) (Talbot et al., 2012). Because of the similarity of pathways, including AKT and GSK3, and cross-reaction of insulin on (insulin-like growth factor-1 (IGF-1) receptors, we cannot exclude a possible role of IGF-1 receptors with exogenous insulin. However, here we showed activation of insulin receptors in both sciatic nerve and hippocampus, in contrast to IGF-1’s lack of effect in the CNS of diabetic rats (Piriz et al., 2009), suggesting that the effects observed in this study are due to insulin activating the insulin receptor pathway but this need to be confirmed in further studies. In the sciatic nerve and hippocampus of type 1 diabetic rats, we have shown that not only is insulin signaling preserved when physiological levels of insulin activate the insulin-signaling pathway via AKT/GSK3, but also that the insulin-evoked activation of the pathway is enhanced when compared to tissues from control rats. These results are in contrast with a recent study showing that the sciatic nerve from type 2 diabetic mice develops insulin resistance after intrathecal administration of insulin (Grote et al., 2013a), suggesting that systemic insulin resistance is paralleled in the PNS and may contribute to the pathogenic mechanisms of diabetic neuropathy in type 2 diabetes. Similarly, insulin resistance was described in the hippocampus of high fructose diet fed rats that developed systemic peripheral insulin resistance (Wu et al., 2014). Recently, it was also shown that human Alzheimer’s disease (AD) brains subjected to ex vivo stimulation by insulin at doses similar to those used in our study displayed insulin resistance (Talbot et al., 2012), supporting the term “brain insulin resistant state” to describe AD brains (de la Monte and Tong, 2014). In type 1 diabetic conditions of insulin deficiency, the basal activation of the insulin receptor/AKT/GSK3 pathway is reduced in the sciatic nerve and hippocampus of type 1 diabetic STZ mice (Jolivalt et al., 2008, Jolivalt et al., 2012). However, when exposed to exogenous insulin, both PNS and CNS responses to insulin were enhanced compared to control rats. Supporting our data, several studies showed that trace amounts of insulin can exert ameliorating effects on nerve dysfunction independent of glycemic level (Singhal et al., 1997, Huang et al., 2003, Brussee et al., 2004, Toth et al., 2006). How this enhanced response to insulin affects neuronal functions is still to be determined and further studies are ongoing.
In order to further study the effect of insulin on the sciatic nerve and hippocampus, rats were implanted with subcutaneous insulin pellets that normalized or partially reduced blood glucose. Interestingly, when the sciatic nerve and hippocampus of these rats were subjected to ex vivo stimulation by insulin, a blunted effect was observed compared to diabetic rats naïve to insulin. The activating effect of insulin observed on untreated diabetic PNS and CNS was significantly reduced with sustained insulin delivery not only at a level normalizing glycemia but also when minimally decreasing blood glucose levels. This suggests that the diabetic nerve develops either a form of insulin resistance in long-term insulin therapy or that there is a normalization of function to control levels. In our study, after 8 weeks of a constant normalizing dose of insulin, phosphorylation levels of insulin receptor and GSK3 are significantly decreased compared to diabetic animals but not to control groups, suggesting that a longer insulin therapy might induce nerve insulin resistance as shown on type 2 diabetic mice (Grote et al., 2013a) or high fructose fed rats (Wu et al., 2014). Longer insulin therapy will be necessary to assess if the PNS and CNS of insulin-deficient rodents will develop insulin resistance. The later view of normalization of function suggests that constant insulin delivery, even at a level not sufficient to fully correct hyperglycemia, may be beneficial to maintain normal functions of the nervous system, both peripherally and centrally. Sustained low dose insulin delivered via subcutaneous implants alleviated painful diabetic neuropathy in type 1 diabetic rats despite persistent hyperglycemia (Hoybergs and Meert, 2007), in contrast to intermittent insulin injection that did not normalize tactile allodynia nor blood glucose (Calcutt et al., 1996). Normalization of insulin’s effect at the PNS or CNS level suggests that insulin contributes to the normalization of functions such as maintenance of neuronal mitochondrial functions (Huang et al., 2003, Chowdhury et al., 2010), support of memory functions (Jolivalt et al., 2008, McNay et al., 2010) and nerve regeneration (Guo et al., 2011).
Conclusion
While complete restoration of regulated circulating insulin levels would be the ideal therapy, this goal is not currently attained in most diabetic patients as insulin delivery is intermittent and largely titrated to the needs of glycemic control rather than neuronal support and protection. The Diabetes Control and Complications Trial (DCCT) studies showed that intensive glycemic control with an external insulin pump compared to 1–2 daily insulin injections can delay development of neuropathy (DCCT, 1993, Martin et al., 2014). Our data supports the approach that constant insulin delivery, even at low doses that do not restore normal blood glucose, may protect peripheral and central nerves from neuropathy.
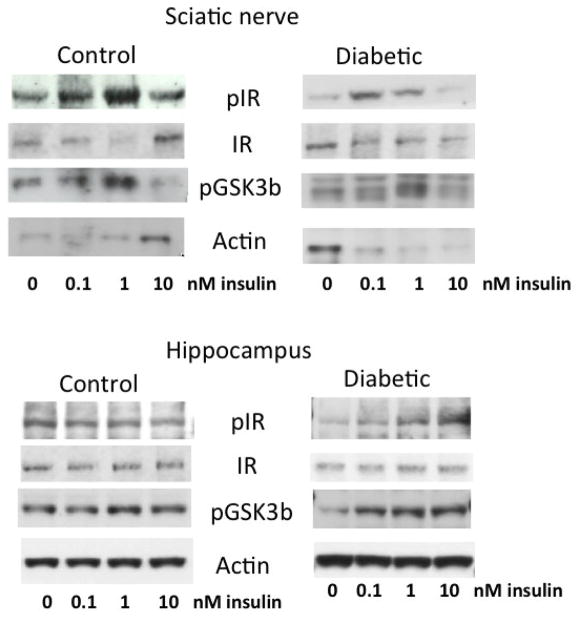
Figure 3.
Representative Western blot images for phosphorylated insulin receptors (pIR), insulin receptor (IR), phosphorylated GSK3β (pGSK3β) and actin in the sciatic nerve (upper panels) and hippocampus (lower panels) from control and diabetic rats incubated with 0 to 1nM insulin for 15 min.
Highlights.
In insulin-deficient diabetes, the CNS is susceptible to the same changes as the PNS.
Ex vivo insulin triggers an enhanced activation of the insulin receptor in both the PNS and CNS from diabetic rats.
Low dose insulin therapy in diabetic rats is sufficient to restore impaired insulin signaling to normal level and function.
Acknowledgments
This work was funded by a Juvenile Diabetes Research Foundation Career Development Award and National Institutes of Health grant AG039736 to CGJ.
Glossary
- AD
Alzheimer’s disease
- CNS
Central nervous system
- IGF-1
Insulin-like growth factor-1
- GSK3
Glycogen synthase kinase 3
- PI3K
phosphatidylinositol 3-kinase
- PNS
peripheral nervous system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apfel SC. Neurotrophic factors and diabetic peripheral neuropathy. Eur Neurol. 1999;41(Suppl 1):27–34. doi: 10.1159/000052077. [DOI] [PubMed] [Google Scholar]
- Brussee V, Cunningham FA, Zochodne DW. Direct insulin signaling of neurons reverses diabetic neuropathy. Diabetes. 2004;53:1824–1830. doi: 10.2337/diabetes.53.7.1824. [DOI] [PubMed] [Google Scholar]
- Calcutt NA, Jorge MC, Yaksh TL, Chaplan SR. Tactile allodynia and formalin hyperalgesia in streptozotocin-diabetic rats: effects of insulin, aldose reductase inhibition and lidocaine. Pain. 1996;68:293–299. doi: 10.1016/s0304-3959(96)03201-0. [DOI] [PubMed] [Google Scholar]
- Chowdhury SK, Zherebitskaya E, Smith DR, Akude E, Chattopadhyay S, Jolivalt CG, Calcutt NA, Fernyhough P. Mitochondrial respiratory chain dysfunction in dorsal root ganglia of streptozotocin-induced diabetic rats and its correction by insulin treatment. Diabetes. 2010;59:1082–1091. doi: 10.2337/db09-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- DCCT . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Tong M. Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem Pharmacol. 2014;88:548–559. doi: 10.1016/j.bcp.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJ, Wardlaw J, Deary IJ, Frier BM. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes. 2003;52:149–156. doi: 10.2337/diabetes.52.1.149. [DOI] [PubMed] [Google Scholar]
- Fernyhough P, Mill JF, Roberts JL, Ishii DN. Stabilization of tubulin mRNAs by insulin and insulin-like growth factor I during neurite formation. Brain Res Mol Brain Res. 1989;6:109–120. doi: 10.1016/0169-328x(89)90044-2. [DOI] [PubMed] [Google Scholar]
- Fernyhough P, Willars GB, Lindsay RM, Tomlinson DR. Insulin and insulin-like growth factor I enhance regeneration in cultured adult rat sensory neurones. Brain Res. 1993;607:117–124. doi: 10.1016/0006-8993(93)91496-f. [DOI] [PubMed] [Google Scholar]
- Gasparini L, Netzer WJ, Greengard P, Xu H. Does insulin dysfunction play a role in Alzheimer’s disease? Trends Pharmacol Sci. 2002;23:288–293. doi: 10.1016/s0165-6147(02)02037-0. [DOI] [PubMed] [Google Scholar]
- Grote CW, Groover AL, Ryals JM, Geiger PC, Feldman EL, Wright DE. Peripheral nervous system insulin resistance in ob/ob mice. Acta Neuropathol Commun. 2013a;1:15. doi: 10.1186/2051-5960-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote CW, Ryals JM, Wright DE. In vivo peripheral nervous system insulin signaling. J Peripher Nerv Syst. 2013b;18:209–219. doi: 10.1111/jns5.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Kan M, Martinez JA, Zochodne DW. Local insulin and the rapid regrowth of diabetic epidermal axons. Neurobiol Dis. 2011;43:414–421. doi: 10.1016/j.nbd.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Havrankova J, Brownstein M, Roth J. Insulin and insulin receptors in rodent brain. Diabetologia. 1981;20(Suppl):268–273. [PubMed] [Google Scholar]
- Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978a;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- Havrankova J, Roth J, Brownstein MJ. Insulin receptors in brain. Adv Metab Disord. 1983;10:259–268. doi: 10.1016/b978-0-12-027310-2.50014-1. [DOI] [PubMed] [Google Scholar]
- Havrankova J, Schmechel D, Roth J, Brownstein M. Identification of insulin in rat brain. Proc Natl Acad Sci U S A. 1978b;75:5737–5741. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoybergs YM, Meert TF. The effect of low-dose insulin on mechanical sensitivity and allodynia in type I diabetes neuropathy. Neurosci Lett. 2007;417:149–154. doi: 10.1016/j.neulet.2007.02.087. [DOI] [PubMed] [Google Scholar]
- Huang TJ, Price SA, Chilton L, Calcutt NA, Tomlinson DR, Verkhratsky A, Fernyhough P. Insulin prevents depolarization of the mitochondrial inner membrane in sensory neurons of type 1 diabetic rats in the presence of sustained hyperglycemia. Diabetes. 2003;52:2129–2136. doi: 10.2337/diabetes.52.8.2129. [DOI] [PubMed] [Google Scholar]
- Jolivalt CG, Calcutt NA, Masliah E. Similar pattern of peripheral neuropathy in mouse models of type 1 diabetes and Alzheimer’s disease. Neuroscience. 2012;202:405–412. doi: 10.1016/j.neuroscience.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivalt CG, Lee CA, Beiswenger KK, Smith JL, Orlov M, Torrance MA, Masliah E. Defective insulin signaling pathway and increased glycogen synthase kinase-3 activity in the brain of diabetic mice: parallels with Alzheimer’s disease and correction by insulin. J Neurosci Res. 2008;86:3265–3274. doi: 10.1002/jnr.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas EA, Knox RJ, Smith TC, Wayne NL, Connor JA, Kaczmarek LK. Regulation by insulin of a unique neuronal Ca2+ pool and of neuropeptide secretion. Nature. 1997;385:343–346. doi: 10.1038/385343a0. [DOI] [PubMed] [Google Scholar]
- Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- King MR, Anderson NJ, Guernsey LS, Jolivalt CG. Glycogen synthase kinase-3 inhibition prevents learning deficits in diabetic mice. J Neurosci Res. 2013;91:506–514. doi: 10.1002/jnr.23192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roith D, Hendricks SA, Lesniak MA, Rishi S, Becker KL, Havrankova J, Rosenzweig JL, Brownstein MJ, Roth J. Insulin in brain and other extrapancreatic tissues of vertebrates and nonvertebrates. Adv Metab Disord. 1983;10:303–340. doi: 10.1016/b978-0-12-027310-2.50017-7. [DOI] [PubMed] [Google Scholar]
- Martin CL, Albers JW, Pop-Busui R, Group DER. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:31–38. doi: 10.2337/dc13-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010;93:546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–231. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- Piriz J, Torres-Aleman I, Nunez A. Independent alterations in the central and peripheral somatosensory pathways in rat diabetic neuropathy. Neuroscience. 2009;160:402–411. doi: 10.1016/j.neuroscience.2009.02.047. [DOI] [PubMed] [Google Scholar]
- Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005;16:59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Rajasekar N, Dwivedi S, Nath C, Hanif K, Shukla R. Protection of streptozotocin induced insulin receptor dysfunction, neuroinflammation and amyloidogenesis in astrocytes by insulin. Neuropharmacology. 2014;86:337–352. doi: 10.1016/j.neuropharm.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Ryan CM, Williams TM, Finegold DN, Orchard TJ. Cognitive dysfunction in adults with type 1 (insulin-dependent) diabetes mellitus of long duration: effects of recurrent hypoglycaemia and other chronic complications. Diabetologia. 1993;36:329334. doi: 10.1007/BF00400236. [DOI] [PubMed] [Google Scholar]
- Singhal A, Cheng C, Sun H, Zochodne DW. Near nerve local insulin prevents conduction slowing in experimental diabetes. Brain Res. 1997;763:209–214. doi: 10.1016/s0006-8993(97)00412-5. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Murakawa Y, Sima AA. Expression and localization of insulin receptor in rat dorsal root ganglion and spinal cord. J Peripher Nerv Syst. 2002;7:44–53. doi: 10.1046/j.1529-8027.2002.02005.x. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Murakawa Y, Zhang W, Xu G, Sima AA. Insulin receptor in rat peripheral nerve: its localization and alternatively spliced isoforms. Diabetes Metab Res Rev. 2000;16:354–363. doi: 10.1002/1520-7560(200009/10)16:5<354::aid-dmrr149>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Sutherland C, Leighton IA, Cohen P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296 (Pt 1):15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Toth C, Brussee V, Martinez JA, McDonald D, Cunningham FA, Zochodne DW. Rescue and regeneration of injured peripheral nerve axons by intrathecal insulin. Neuroscience. 2006;139:429–449. doi: 10.1016/j.neuroscience.2005.11.065. [DOI] [PubMed] [Google Scholar]
- Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- Wu HW, Ren LF, Zhou X, Han DW. A high-fructose diet induces hippocampal insulin resistance and exacerbates memory deficits in male Sprague-Dawley rats. Nutr Neurosci. 2014 doi: 10.1179/1476830514Y.0000000133. [DOI] [PubMed] [Google Scholar]