Abstract
Interleukin-4 (IL-4), IL-5 and IL-13, the signature cytokines that are produced during type 2 immune responses, are critical for protective immunity against infections of extracellular parasites and are responsible for asthma and many other allergic inflammatory diseases. Although many immune cell types within the myeloid lineage compartment including basophils, eosinophils and mast cells are capable of producing at least one of these cytokines, the production of these “type 2 immune response-related” cytokines by lymphoid lineages, CD4 T helper 2 (Th2) cells and type 2 innate lymphoid cells (ILC2s) in particular, are the central events during type 2 immune responses. In this review, I will focus on the signaling pathways and key molecules that determine the differentiation of naïve CD4 T cells into Th2 cells, and how the expression of Th2 cytokines, especially IL-4 and IL-13, is regulated in Th2 cells. The similarities and differences in the differentiation of Th2 cells, IL-4-producing T follicular helper (Tfh) cells and ILC2s as well as their relationships will also be discussed.
Keywords: Type 2 T helper cells (Th2), Type 2 innate lymphoid cells (ILC2), Interleukin-4, Interleukin-13, Transcriptional regulation, GATA3
1. Introduction
CD4 T helper (Th) cells are the key players during adaptive immune responses1, 2. Almost thirty years ago, it was first recognized by Mossman and Coffman that there are different subsets of Th cells, namely type 1 T helper (Th1) and type 2 T helper (Th2) cells, which are critically involved in distinct types of immune responses3–5. With the discovery of a third major CD4 T effector cell lineage, Th17 cells that produce interleukin-17 (IL-17), and a regulatory T cell subset (induced regulatory T (iTreg) or peripherally induced regulatory T (pTreg)) that can be differentiated from naive CD4 T cells outside of the thymus, either in vitro or in vivo, the members of CD4 T cell family have expanded2, 6–9.
Th2 cells are the crucial lymphocytes during adaptive immune responses to the infections of extracellular parasites such as helminths; Th2 cells are also responsible for development of some asthmatic and allergic inflammatory diseases. By secreting IL-4, Th2 cells instruct B cells to produce IgG1 and IgE10; by producing IL-4 and IL-13, Th2 cells induce alternatively activated macrophage11. Th2 cells also recruit eosinophils via IL-5 production12 and directly act on epithelial cells and smooth muscle cells through IL-13 production13–15. Therefore, IL-4, IL-5 and IL-13 are the major effector cytokines produced by Th2 cells during type 2 immune responses. All three cytokine genes, Il4, Il5 and Il13, are located within a genomic segment, which is subjected to the regulation of locus control region (LCR) in the Rad50 gene16, 17.
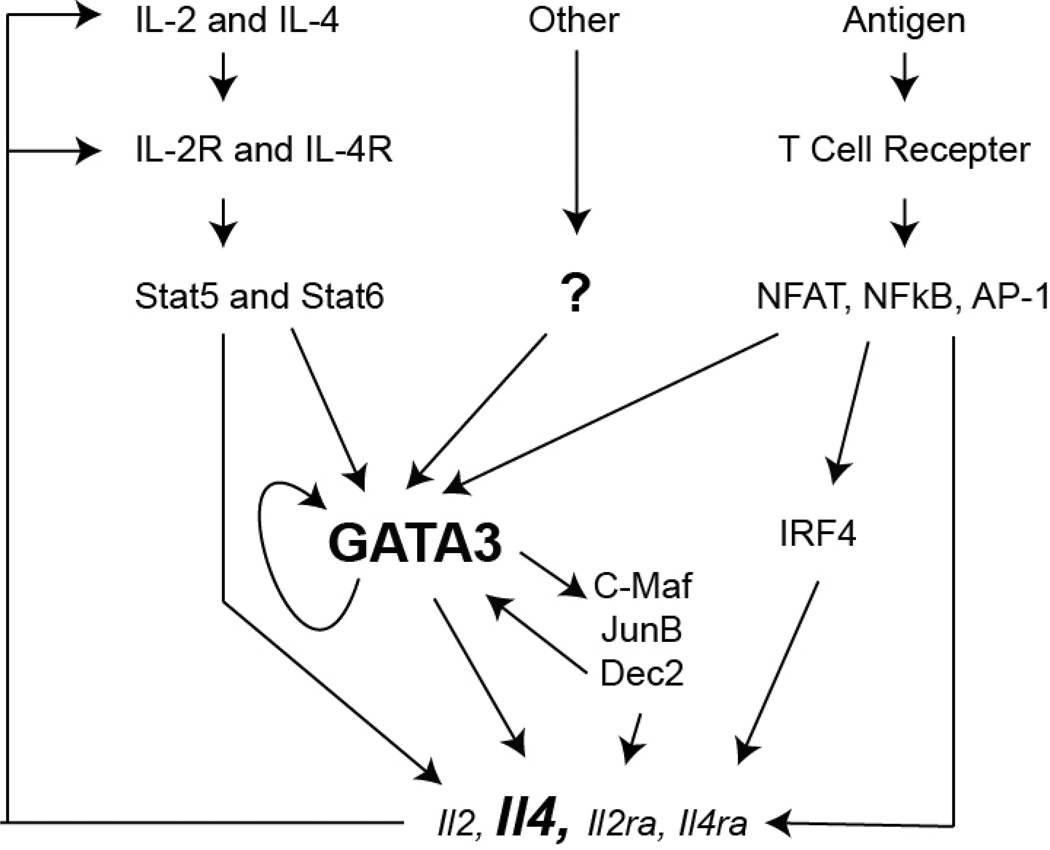
During CD4 T cell activation through T cell receptor (TCR)-mediated signaling and co-stimulation, cytokine signals received by the activated T cells are deterministic in T cell fate commitment. For example, together with TCR ligation, IL-4-mediated activation of the signal transducer and activator of transcription 6 (STAT6) plays an important role during Th2 cell differentiation 18–22, although IL-4-independent Th2 cell differentiation may occur in the absence of IL-4 signaling under certain conditions in vivo 23–27. Both IL-4-dependent and IL-4-independent Th2 differentiation requires the key transcription factor GATA-3 (Figure 1), which is responsible for epigenetic changes in many Th2-specific gene loci and for direct transcription activation28–31. In addition, IL-2-mediated activation of STAT5 is indispensable for the production of Th2 cytokines possibly through chromatin remodeling of the Th2 cytokine locus as well as maintaining GATA3 expression in already differentiated Th2 cells 18, 32–34. Therefore, GATA3 up-regulation and STAT5 activation are the two key events for Th2 cell differentiation. Th2 cell differentiation and the induction of Th2 cytokines are also regulated by many other transcription factors including NFAT, NFκb and AP-1 family members. Mechanisms for the reinforcement of Th2 cell differentiation include positive feedbacks, inhibition of other alternative lineage choices and selective growth of differentiated Th2 cells.
Figure 1. Transcriptional network and positive feedback regulation during Th2 cell differentiation.
TCR activation and cytokine-mediated signaling are critical during Th2 cell differentiation. TCR stimulation activates NFAT, NFκb and AP-1 family members, resulting in up-regulation of IRF4 expression, which has a general function in T cell activation. Low dose of antigen stimulation accompanied by the up-regulation of Th2 master regulator GATA3 favors Th2 cell differentiation. IL-4-mediated Stat6 activation and other signaling pathways such as Notch-mediated signaling are also capable of inducing GATA3 expression. GATA3 directly mediates epigenetic modifications at the Th2 cytokine locus and cytokine transcription. GATA3 also indirectly regulates Th2 cytokine expression by inducing other transcription factor some of which may further up-regulate GATA3 expression. GATA3 also regulates its own expression. IL-2-mediated Stat5 activation is another key event for Th2 cytokine production. Activated T cells are able to produce both IL-2 and IL-4, and to up-regulate IL-2 and IL-4 receptors, forming a powerful positive feedback loop.
Besides Th2 cells, other lymphoid cells including subsets of γδ T cells, NKT cells, T follicular helper (Tfh) cells and type 2 innate lymphoid cells (ILC2s) are also capable of producing IL-4 and/or IL-13. In fact, in the steady state, ILC2s are the major IL-5-producing cells 35,36. ILC2s exert similar functions as Th2 cells during type 2 immune responses 37,38. In fact, the production of IL-13 by T cells is dispensable for type 2 immunity suggesting that there is another importance source of IL-13, most likely from ILC2s 39. While this review will primarily focus on Th2 cell differentiation and the regulation of IL-4/IL-13 production in Th2 cells, the relationships among conventional Th2 cells, IL-4-producing Tfh cells and ILC2s, as well as the regulation of cytokine production in these cell subsets will be also discussed.
2. Signaling pathways involved in Th2 cell differentiation
2.1. IL-4-mediated signaling pathway
IL-4 promotes Th2 cell differentiation mainly by activating STAT6 through tyrosine phosphorylation20–22. Naïve STAT6-deficient CD4 T cells fail to up-regulate GATA3 expression and thus are not able to develop into Th2 cells in vitro even when IL-4 is exogenously provided. On the other hand, constitutively active STAT6 mutants are capable of replacing IL-4 in driving Th2 cell differentiation40, 41. In addition to up-regulating GATA3 expression, STAT6 is also responsible for chromatin remodeling at the Il4/Il13 LCR region16. The source of IL-4 during initial immune responses that lead to the differentiation of Th2 cells could be NKT cells42, basophils43, 44 and CD4 T cells themselves45, 46.
However, under certain circumstances, Th2 cell differentiation can occur in the absence of the IL-4/STAT6 pathway in vivo23–26, even though STAT6 is still important for Th2 cells migrating to the lung tissue26, for optimal expression of GATA347, and for the maintenance of Th2 memory cells24. IL-4-independent Th2 cell differentiation could be induced by low strength of TCR stimulation with co-stimulation, Notch signaling and/or IL-2-mediated STAT5 activation which will be discussed below.
2.2. TCR-mediated signaling pathway
Strength of TCR signaling is an important factor for determining the fate of T helper cell differentiation48. Although cytokine environment can often be the deterministic factor for T helper cell differentiation during infections, TCR signaling strength has a dominant effect over the Th1- or Th2-inducing adjuvants49–51. Naive CD4 T cells cultured in vitro with low dose peptide stimulation undergo IL-4/STAT6-independent induction of GATA3 within 24 hours, which is essential for the early IL-4 production by these cells45. GATA3 is not up-regulated when naïve CD4 T cells receive high dose peptide stimulation. TCR signaling also induces the activation of p38 kinase, which phosphorylates and regulates GATA3 function52. The inhibition of GATA3 up-regulation is due to a strong Erk activation by high levels of TCR activation. This is consistent with the finding that resting dendritic cells (DCs) preferentially induce differentiation of Th2 cells53. In fact, omega-1, an important glycoprotein in the Schistosoma mansoni egg, inhibits the activation of DCs thus limits the strength of T cell activation; this may be an important mechanism for how Schistosoma mansoni egg antigen induces Th2 responses in vivo54, 55. Similarly, some in vivo Th2 cell differentiation may depend on basophils, which serves as Th2-promoting antigen presenting cells possibly because of low expression levels of MHC class II on these cells44, 56, 57.
2.3. Co-stimulation of T cell activation
Although TCR co-stimulation mediated by CD28 is required for optimal T cell activation and Th2 cell differentiation, the CD28 effect is mainly through the induction of IL-2 production58. IL-2-STAT5 pathway, which will be further discussed below, is important for Th2 cell differentiation at least in vitro32, 33. Whether diminished Th2 responses in CD28-deficient mice are due to reduced IL-2 production is not clear59, 60. On the other hand, CTLA-4 is a negative regulator of Th2 cell differentiation61, 62. ICOS was originally thought to co-stimulate Th2 cell differentiation63, 64, however, ICOSL/ICOS interaction is also known to be important for other T helper cell differentiation, especially for Tfh cells9. In addition, OX40 co-stimulation promotes Th2 cell differentiation65, 66. Interestingly, TSLP induces the expression of OX40 ligand on DCs thus to promote Th2 response67.
T cell activation and co-stimulation also activate PI3K pathway, which leads to mTOR (mammalian target of rapamycin) activation; mTOR regulates Th cell fate determination68, 69. While mTORC1 signaling is important for Th1 and Th17 differentiation, mTORC2 signaling promotes Th2 cell differentiation partly through activating SGK1 to stabilize JunB protein (an important AP-1 transcription factor for Th2 cell differentiation)70–73. However, the dispensable role of mTORC1 during Th2 cell differentiation has been called into question. The conclusion that mTORC1 is dispensable for Th2 cell differentiation is based on the results obtained with Rheb-deficient cells (Rheb is considered as an important upstream activator of mTORC1)72, however, a study using Raptor-deficient cells (Raptor is the component of mTORC1) indicates that mTORC1 is also important for Th2 cell differentiation74. Rheb-deficient cells cultured under Th2 conditions divide slower than wild type cells and there is a Rheb-independent mTORC1 activation; these results are consistent with the notion that mTORC1 is required for metabolic reprogramming and cell proliferation during T cell activation. In addition, PI3K/mTOR pathway is involved in translational regulation of GATA3 protein75. Whether the dose of antigen affects preferential activation of either mTORC1 or mTORC2 is not known.
2.4. Notch-mediated signaling pathway
Notch signaling is involved in Th2 cell differentiation76–78. It seems that different notch ligand may have different function since Dll1/Notch interaction induces Th1 but Jag1/Notch interaction induces Th2 cell differentiation76. After activation, intracellular portion of Notch is translocated to the nucleus and forms a complex with CSL protein to induce gene expression. A Notch/CSL binding site is identified in the 3’ of the Il4 gene suggesting that Notch signaling may directly regulate IL-4 expression76. In addition, Notch/CSL complex binds to the distal promoter of Gata3 gene78. Therefore, Notch signaling may promote Th2 cell differentiation by inducing the expression of both GATA3 and IL-4. Notch signaling may also modulate co-stimulation by affecting PI3K pathway to allow naïve CD4 T cells to respond to low dose of antigen79. Furthermore, Notch-mediated signaling pathway regulates the expression of IL-2 and IL-2 receptor α chain79, 80; IL-2-mediated signaling pathway, discussed in detail below, is one of the key signaling pathways for Th2 cell differentiation.
2.5. STAT5-activating signaling pathways
STAT5 family includes STAT5A and STAT5B, which are encoded by two genetically linked genes81. While low amount of STAT5 activation is necessary and sufficient for the proliferation and survival of all the CD4 T cell subsets32, 82, high levels of STAT5 activation is essential for Th2 cell differentiation both in vitro and in vivo32, 33, 83, 84. Enforced expression of a constitutively active form of STAT5A in Th1 cells results in IL-4 production in these cells without up-regulating GATA3 expression33.
Several cytokines including IL-2, IL-7 and TSLP can activate STAT5 in T cells. TSLP, produced by epithelial cells, dendritic cells (DCs), and/or basophils, may mediate the initiation of Th2 responses in vivo43, 67, 85, 86. Although the major role of TSLP is to regulate the functions of dendritic cells67, 87, TSLP may also directly act on T cells to promote Th2 cell differentiation88, 89. Other potent STAT5 activators are IL-2 and IL-7. While IL-7 is capable of inducing and/or promoting Th2 differentiation in vitro, its function in vivo has not been carefully studied. IL-2 is mainly a product of activated T cells and thus may serve as an important autocrine cytokine during Th2 differentiation. However, due to a broader function of IL-2, including its effect on regulatory T cells, it is difficult to assess the precise role of IL-2 during Th2 cell differentiation in vivo.
It is known that TCR-mediated signaling transiently suppresses cytokine signaling during initial T cell activation90. Both IL-2/STAT5 and IL-4/STAT6 signaling pathways are inhibited 4–6 hours of T cell activation. Interestingly, IL-2-mediated STAT5 activation is detected at the early stages of T cell activation with low dose but not high dose of TCR stimulation45. As mentioned above, Notch signaling induces the expression of IL-2 and IL-2 receptor α chain during T cell activation. In fact, all five Th2-promoting signaling pathways triggered by antigen, co-stimulation, Notch, IL-4 and IL-2, respectively, may crosstalk with each other and collaborate to induce Th2 cell differentiation.
2.6. Th2 cell differentiation via a default program
IFNγ produced by Th1 cells is an important cytokine that promotes Th1 cell differentiation91–93. IFNγ is also produced by NK cells and/or ILC1s, which are the first responders at the innate phase of type 1 immune responses93–96. In addition, IL-12 produced by antigen presenting cells including DCs plays an important role in driving Th1 cell differentiation both in vitro and in vivo through activating STAT4 in CD4 T cells97–99. On the other hand, an equivalent DC-derived cytokine that induces Th2 cell differentiation has not been found. Instead, IL-2 and IL-4, two known cytokines driving Th2 cell differentiation, are mainly produced by T cells themselves. Thus, the determination of Th1 and Th2 cell fate appears to be asymmetrical. Even more interestingly, because GATA3 is critical for the development of CD4 T cells at multiple stages100, it is already expressed by naïve CD4 T cells28, 101. Furthermore, STAT5 is usually activated either by IL-7 at naïve stage or by IL-2 upon T cell activation.
Given that a Th2-biased differentiation program (basal GATA3 expression and STAT5 activation) has pre-existed in naïve CD4 T cells, IL-4/STAT6 signaling, which possibly serve as a reinforcement and/or amplification of Th2 responses, may not be necessary for Th2 cell differentiation, especially at early stages, in many situations in vivo. Indeed, Nippostrongylus brasiliensis and Schistosoma mansoni infection exclusively elicit IL-4-independent Th2 responses23–27. The requirement of IL-4/STAT6 during some in vivo Th2 responses such as in response to Trichuris muris infection102 may simply because such infection generates a mixture of Th1 and Th2 responses and IL-4 is required to suppress Th1 responses. Consistent with the notion that Th2 cell differentiation can occur through a default pathway when the major Th1-inducing signals are absent, mice deficient in either Th1 master regulator T-bet103 or STAT4/IFNγR doubly deficient mice developed Th2 responses in response to Toxoplasma gondii infection104, which usually induces robust Th1 responses. However, in the absence of IL-12 alone, T cells fail to default to a Th2 program105, suggesting that both IL-12 and IFNγ are involved in suppressing the endogenous Th2 program in T cells. Indeed, either IL-12 or IFNγ is able to induce T-bet expression104.
3. Key transcription factors required for Th2 cell differentiation
3.1. GATA3
GATA3 has been recognized as the master regulator of Th2 cells28–31. GATA3 is expressed by naïve CD4 T cells but its expression levels are induced during Th2 differentiation29, 106, 107. GATA3 is sufficient to induce the Th2 phenotype since retrovirus-mediated enforced expression of GATA3 in Th1 cultures induces IL-4 production and endogenous GATA3 expression106, 108. On the other hand, knocking down or deletion of GATA3 results in diminished Th2 cell differentiation, whether it is IL-4-dependent or IL-4-independent, both in vitro and in vivo28, 30, 31, 109.
GATA3 promotes Th2 cell differentiation through multiple mechanisms110. The key role of GATA3 is to directly act on the Il4/Il13 gene locus at various sites. Chromatin immune-precipitation followed by high throughput sequencing (ChIP-Seq) analysis with anti-GATA3 reveals genome wide pattern of GATA3 binding101. In Th2 cells, GATA3 binds to the promoters of the Il5 and the Il13 gene including the CGRE site in the Il13 distal promoter111, 112; GATA3 also binds to multiple sites at the LCR region in the Rad50113, 114 and several enhancer regulatory elements for the Il4 genes, such as DNase I hypersensitivity II (HSII)112, 115 and HSV sites116. GATA3 regulates epigenetic modifications in the Th2 cytokine locus during Th2 cell differentiation. However, in already differentiated Th2 cells, GATA3 is dispensable for IL-4 production although it is still essential for IL-5 and IL-13 production possibly because GATA3 is critical for activating the promoters of these two genes30. Besides its direct action on the Th2 cytokine locus, GATA3 also directly regulate many Th2 specific genes such as Il1rl1 encoding T1/ST2, the IL-33 receptor, and many chemokine receptor genes such as Ccr8101.
3.2. STAT5
As discussed above, a strong STAT5 activation is another key element for Th2 cell differentiation. Co-expression of GATA-3 and a constitutively active form of STAT5A in non-Th2 cells results in a larger proportion of IL-4-producing cells compared to that induced by either GATA3 or active STAT5A alone33. On the other hand, GATA3 has very limited IL-4-inducing capacity when STAT5 activation is inhibited by anti-IL-2 treatment32 and the active STAT5A is unable to induce IL-4 in Gata3-deficient CD4 T cells30. Therefore, both GATA3 induction and STAT5 activation are essential for Th2 cell differentiation; high STAT5 activation levels may reduce the requirement of GATA3 expression levels for inducing IL-4 expression117.
Mechanistically, STAT5 binds to the HSII and HSIII sites of the Il4 gene, through which it regulates the accessibility of these sites32, 33. ChIP-Seq results confirming STAT5 binding at the HSII site has also revealed another STAT5 binding at the LCR region, which may contribute to inducing IL-4118. Besides directly regulating the Il4/Il13 locus, IL-2/STAT5 induces the expression of IL-4Rα especially at the initial stage of Th2 cell differentiation so that activated T cells may respond to IL-4 to complete the differentiation process118.
3.3. Other transcription factors
In addition to GATA3 and STAT5, there are several other transcription factors that regulate IL-4 production and/or Th2 cell differentiation (Figure 1). IRF-4 is highly up-regulated during T cell activation and it is important for the differentiation and functions of virtually any CD4 T helper subsets including Th2 cells119–128. In Th2 cells, one important function of IRF4 is to bind to the Il4 promoter to regulate its expression121, 122.
It has been recently reported that c-Maf is highly expressed by Th17 cells126, 129, 130 and Tfh cells131; c-Maf regulates the expression of IL-10, IL-21 and IL-22. However, c-Maf was initially considered as a Th2 specific transcription factor by comparing its expression in Th1 and Th2 cells. Indeed, it controls the production of IL-4 but not other Th2 cytokines132. C-Maf directly binds to the promoter of the Il4 gene. C-Maf expression is negatively regulated by a LincRNA, linc-MAF-4, which is highly expressed in human Th1 cells133.
Klf13 collaborates with c-Maf in regulating IL-4 production in T cells134, 135. The action of c-Maf at the Il4 promoter also relies on JunB, which is critical for Th2 cell differentiation70. The induction of JunB expression partially depends on Dec2, another Th2-specific transcription factor136. Nfil3 (E4BP4), a transcription factor that is critical for the development of NK cells and ILCs137–142, is highly expressed by Th2 cells and responsible for regulating IL-10 production143. In addition, YY1 physically interacts with GATA3 and thus collaborates with GATA3 in chromatin remodeling and cytokine production144.
IL-4 production and Th2 cell differentiation can also be negatively regulated by many transcription factors. One typical example is Runx3, which binds to the HSIV site of the Il4 gene and suppresses IL-4 production145–147. Both Eomes, which is expressed by a subset of memory Th2 cells, and Sox4 that is induced by TGFβ suppress GATA3 activity and Th2 cytokine expression148, 149. PU.1, a critical transcription factor regulating myeloid and lymphoid cell development at early stages, is also expressed by subsets of T cells. In Th2 cells, PU.1 is preferentially expressed by IL-4 non-producers suggesting that PU.1 negatively regulates IL-4 production150.
GATA3 is expressed by some activated regulatory T cells (Tregs) at an intermediate level151–154, however, such cells do not produce IL-4 suggesting that Foxp3 may suppress the GATA3 function in inducing Th2 cytokines. Interestingly, reduced Foxp3 expression in Tregs results in Th2 cytokine production by these cells155. Such Foxp3 effect may be particularly important in humans since activated human effector T cells can transiently express Foxp3156, 157.
Therefore, Th2 cell differentiation and cytokine production depend on a network of many transcription factors, which consists of GATA3, STAT5, IRF4, c-Maf, JunB, Dec2, Klf13, E4BP4, YY1, Runx3 and PU.1 etc. Changes in the balance and/or the ratio of these transcription factors may lead to an alteration in Th2 cell fate determination.
4. Mechanisms of reinforcing Th2 cell differentiation
4.1. Positive feedback
A basic principle of T helper cell differentiation is positive feedback mechanism2. During Th2 cell differentiation, IL-4 produced by developing Th2 cells may instruct IL-4 non-producers to produce IL-4, and/or enhance the IL-4-producing capacity of the IL-4-producing cells in a paracrine and/or autocrine manner (Figure 1). Not only can IL-4 induce GATA3 expression and thus IL-4 production, it also enhances the sensitivity of developing Th2 cells to IL-4 stimulation by up-regulates IL-4Rα expression. Therefore, IL-4/GATA3/IL-4 and IL-4/IL-4R constitute two powerful positive feedback loops during Th2 differentiation.
It has been reported that GATA3 auto-regulates its expression108. Our ChIP-Seq data suggest that GATA3 binds to multiple regions at the Gata3 locus across 1 Mb length of DNA101. Therefore, GATA3 may regulate its own expression directly. However, in the absence of functional GATA3 protein, the truncated GATA3 mRNA expression is not reduced when T cells are activated in the presence of IL-4101. This result suggests that GATA3 auto-regulation is minimal when IL-4/STAT6 signaling is present. Nevertheless, GATA3 auto-regulation may be critical to sustain Th2 phenotype when IL-4/STAT6 signaling ceases. Among the GATA3 binding sites at the Gata3 gene, one co-localizes with a T cell specific enhancer for GATA3 expression158. Even more interesting, this enhancer region overlaps with the dual promoter that mediates the transcription of two long intergenic non-coding RNAs (LincRNAs) into opposite directions159. Whether the expression of these two LincRNAs is related to the “enhancer” activity needs to be further studied.
GATA3 may indirectly regulate itself by inducing other transcription factors such as Dec2 and IRF4; the expression of Dec2 and IRF4 is reduced when GATA3 is acutely deleted from Th2 cells101, 136. On the other hand, both Dec2 and IRF4 are able to enhance GATA3 expression levels in Th2 cells121, 136.
Positive feedback regulations are also found between the two critical pathways, IL-2/STAT5 and IL-4/STAT6/GATA3, during Th2 cell differentiation. Besides inducing CD25 (IL-2 receptor α chain) expression160, IL-2-mediated STAT5 activation up-regulates the expression of IL-4Rα during the initial T cell activation118. Furthermore, STAT5 activation is required to maintain GATA3 expression in differentiated Th2 cells34. On the other hand, GATA3 regulates optimal expression levels of CD25 by T effector cells as well as Tregs101, 151.
4.2. Suppression of other lineage fates
CD4 T cells can differentiate into different effector lineages including Th1, Th2 and Th17 cells. As mentioned above, IL-12/STAT4 and IFNγ/STAT1 pathways, both of which induce the master regulator T-bet, are important for Th1 cell differentiation92, 97, 98, 103, 161. Therefore, STAT1/STAT4 activation and T-bet induction are the key events during Th1 cell differentiation. Similarly, Th17 cell differentiation requires STAT3 activation and the induction of RORγt expression1, 8.
Th2 cell differentiation is accompanied by the suppression of other lineage fates. GATA3 deficiency leads to de-repression of STAT4 expression and enforced expression of GATA-3 down-regulates the expression of STAT4 in Th1 cells146, 162. Interestingly, Gata3 deletion during Th2 cell differentiation results in an IL-12/STAT4 and IFNγ/T-bet independent IFNγ production, which is mediated by Runx3/Eomesodermin (Eomes) pathway30, 145–147, 163. It has been shown that T-bet inhibits GATA-3 transcription107 and suppresses its function through protein-protein interaction164. T-bet and GATA3 co-expressing cells can be generated in response to parasite infection165. The physical interaction between GATA3 and T-bet may allow them to suppress each other’s function. Indeed, T-bet and GATA3 binding sites co-localize at many critical Th1- or Th2-specific genes166. Furthermore, GATA3 is able to silence Tbx21 (encoding T-bet) locus in Th2 cells by its direct binding to a site in the Tbx21 locus with suppressive epigenetic modifications; removing GATA3 results in a substantial reduction of such suppressive modifications at the binding site 101. Thus, GATA3 suppresses Th1 cell fate through multiple mechanisms110.
Strong STAT5 activation is necessary for Th2 differentiation as discussed above. Interestingly, constitutively active STAT5a suppresses T-bet expression when introduced into developing Th1 cells 33. STAT5 activation also suppresses Th17 differentiation and Tfh cell differentiation 167–171. Besides GATA3 and STAT5, another transcription factor, growth factor independent 1 (Gfi-1), which is preferentially expressed in Th2 cells, inhibits IFNγ and IL-17 production in Th1 and Th17 cells, respectively 172–174.
4.3. Selective growth of Th2 cells
IL-12 receptor β2 chain is selectively up-regulated during Th1 cell differentiation 175, thus, IL-12 plays an important role in selecting committed Th1 cells to grow 176. However, although IL-4 receptor α chain can be up-regulated by IL-4 177, naïve CD4 T cells as well as non-Th2 cells are also able to respond to IL-4 because of constitutive expression of IL-4 receptor on T cells 90. Therefore, IL-4 receptor up-regulation is not the major mechanism for selective outgrowth of Th2 cells in response to IL-4 stimulation. Nevertheless, IL-4 up-regulates Gfi-1 expression through STAT6 activation; Gfi-1 preferentially induces the expansion of GATA-3hi Th2 cells through its actions upstream and downstream of STAT5 activation172, 174.
In fully differentiated Th2 cells, IL-33 receptor α chain (T1/ST2 or IL-1R–like 1) is highly expressed178, 179. Since IL-33 is released during Th2 responses, IL-33 may play a role in promoting the survival and/or expansion of fully differentiated Th2 cells. Indeed, blocking IL-33 signaling results in attenuated eosinophilic airway inflammation and decreased responses to Schistosoma mansoni180, 181. Strikingly, continuous IL-33Rα expression in differentiated Th2 cells depends on both GATA3 expression and STAT5 activation34.
5. Chromatin remodeling and epigenetic modifications at the Il4/Il13 locus
The Il4 and Il13 genes, located at chromosome 5 in human and chromosome 11 in mouse, respectively, are flanked by the Rad50 and Kif3a genes. The locus control region (LCR) of the Il4-Il13 locus is within the 3’ of Rad50 gene113; the Il5 gene is on the other side of the Rad50. There are GATA3-mediated intrachromosomal interactions between the LCR and the Th2 cytokine gene promoters182. A potential regulatory element may reside within the Kif3a gene based on the specific-lineage epigenetic markers in Th2 cells in this region101. Transcriptional regulation of Il4/Il13 expression, like transcriptional regulation of any other lineage specific genes, highly depends on chromatin remodeling and epigenetic modifications17, 183, 184.
During Th2 differentiation, several Th2-specific DNase I hypersensitivity (HS) sites are induced at the Il4-Il13 locus185. These include the HSII site in the intron 2 of the Il4 gene112, 186, HSV and HSVa116 at 3’ of the Il4 coding region, conserved non-coding sequence 1 (CNS1)187, 188 which is located at the intergenic region of Il4 and Il13, and several HS sites within the LCR in the Rad50 gene113, 189. Interestingly, the HS IV site is accessible in both Th1 and Th2 cells185; Runx3 binds to this region in Th1 cells to suppress IL-4 production145, 190. Deletion of the RHS7 (HS7 site within the LCR in the Rad50) site results in reduced IL-4 and IL-13 production but not IL-5 in Th2 cells191. However, deletion of the RHS6 site or the whole LCR affects the expression of all three cytokines192, 193. The requirement of these elements to induce cytokine production seems to vary when different immunization protocols are followed192 suggesting that the LCR contains both IL-4-depedent and IL-4-independent regulatory elements, consist with the findings that STAT6 binds to both the RHS6 and RHS7 sites16, but GATA3 only binds to RHS5 and RHS6 not RHS7 of the LCR101. Activated STAT5 binds to the HSII site and induces the accessibility at this region33. ChIP-Seq analysis also indicates that GATA3 binds the HSII site101. Our unpublished data suggest GATA3 is responsible for the induction and maintenance of HSII accessibility.
Different histone modifications are associated with either gene activation or repression194. Tri-methylation and di-methylation at the lysine position 4 of histone 3 (H3K4me2 and 3) are usually indicative of active gene loci, whereas, H3K27me3 is a marker for repressed gene loci. The Il4/Il13 locus displays H3K4me3 modification in Th2 cells and H3K27me3 in Th1 cells195. MLL, a histone H3K4 methyltransferase, is important for maintaining H3K4 methylation at the Il4/Il13 locus in differentiated Th2 cells196. On the other hand, Ezh2, an H3K27 methyltransferase, is responsible for mediating H3K27me3 modification at the Il4/Il13 and Gata3 loci in Th1 cells197–199. Deletion of Ezh2 in T cells results in reduced H3K27me3 at the Tbx21 and Gata3 loci, and thus enhanced Th1 and Th2 differentiation. Ezh2 deletion also causes reduced stability of differentiated cells and enhances their plasticity. Besides histone methylation modifications, histone acetylation at the lineage-specific cytokine gene loci has also been described200.
Although GATA3 may regulate the expression of IL-5 and IL-13 through its direct binding to the promoters of the Il5 and Il13 genes30, 111, 201, 202, a main function of GATA3 in Th2 cell fate determination seems to mediate histone modifications at Th1 and Th2 specific gene loci including the Ifng and Il4/Il13 locus108, 203, 204. In the absence of GATA3, H3K27me3 marks around the GATA3 binding site at the Ifng locus is greatly reduced. On the other hand, H3K4me2 marks at the Il4/Il13 locus, especially at the LCR region where GATA3 binds, are diminished when GATA3 is absent. At a genome level, GATA3 binds to ∼2000 genes in Th2 cells101. When GATA3 is deleted, ∼100 genes among these 2000 genes have altered their expression level, however, alteration of histone modifications occurs at the loci of ∼900 genes101. This strongly indicates that GATA3-dependent alterations in epigenetic modifications is not a result of gene expression change.
DNA CpG methylation also affects gene expression205. Deletion of Dnmt-1, a DNA methyltrasferase, or of methyl CpG-binding domain protein-2 (MBD2), results in aberrant expression of IL-4 without affecting GATA3 expression206, 207. Interestingly, GATA3 inhibits the binding of MBD2 to methyl CpG. STAT5 activation is partially involved in de-methylation of the RHS7 site in the LCR during Th2 differentiation189.
Collectively, both GATA3 and STAT5 strongly bind to key regulatory elements of the Il4/Il13 locus including the HSII site112 and HS sites within the LCR191; both GATA-3 and STAT5 are involved in many aspects of chromatin remodeling at the Il4/Il13 locus including chromatin accessibility, histone modifications and DNA methylation.
6. Induction of IL-4 and IL-13 expression by Th2 cells
TCR-mediated signaling plays an important role in T helper cell differentiation as discussed above. Another critical function of TCR-mediated signaling is to induce cytokine production in already differentiated T helper cells. The most efficient way to stimulate Th2 cells to produce cytokines is through their TCRs or by chemicals such as PMA and ionomycin to mimic TCR-mediated signaling. TCR stimulation activates NFAT family members in a Ca2+ dependent manner208. Upon TCR re-stimulation of differentiated Th2 cells, NFAT translocates to the nucleus and binds to the Il4 promoter as well as HSVa region to regulate IL-4 expression122, 209. Besides inducing cytokine production, NFAT proteins are also involved in Th2 cell differentiation210.
T helper cells can also produce cytokines in response to cytokine stimulation. IL-18 together with IL-12 induces IFNγ production independent of TCR stimulation211, 212. Similarly, IL-33 in combination of STAT5 activators, such as IL-2, IL-7 and TSLP, induces IL-13 but not IL-4 expression in differentiated Th2 cells178, 179. In addition, Th2 cells express cysteinyl leukotriene receptor 1 and are able to respond to leukotrienes to produce IL-13 possibly through calcium-dependent pathway213, 214.
Whether Th2 cells are stimulated by TCR to produce IL-4 and IL-13, or by cytokines to produce IL-13, not all Th2 cells actually produce IL-4 and/or IL-13 upon stimulation. Although heterogeneity of Th2 cells can be a result of differential epigenetic status of each cytokine allele215, 216, the actual cytokine production in a given cell upon a single round of stimulation may be a stochastic event217. Heterogeneous activation of transcription factors such as NFAT or differential assembly of functional transcription factor complex needed for inducing cytokine production may be one of the main explanations217, 218. Thus, IL-4- and/or IL-13-non-producing cells identified after stimulation at one time may express these cytokines in response to another round of stimulation. Therefore, both cytokine-producing and non-producing cells may represent fully differentiated cells. Indeed, IL-4-producers and non-producers from a same Th2 culture express similar levels of GATA3 and have identical chromatin accessibility at the Il4/Il13 locus217. Interestingly, this may not be entirely true for IFNγ production in Th1 cells since low- and high-IFNγ-producing cells express different levels of T-bet and seem to have a quantitative memory to produce IFNγ219.
7. Relationship between Th2 cells and IL-4-producing Tfh cells
Tfh cells, many found in the germinal centers in the B cell follicle, are critical helper T cells in helping B cells produce antibodies and undergo immunoglobulin (Ig) class switching9. IL-4 is essential for the Ig class switching to IgE10, suggesting that some Tfh cells need to produce IL-4220–222. Indeed, IL-4-producing CD4 T cells are mainly Tfh cells222. Interestingly, Tfh cells do not produce IL-1347.
GATA3-deficient mice fail to generate type 2 response including IgE class switching30 suggesting that GATA3 is also required for Tfh cells to produce IL-4. However, Tfh cells containing IL-4-producers express low levels of GATA3 suggesting that small amount of GATA3 is sufficient for IL-4 production in Tfh cells47, 223. On the other hand, IL-13-producing Th2 cells are GATA3 high expressors47. GATA3 at a low level is sufficient to induce IL-4 but not IL-13 production when there is a strong STAT5 activation33. However, Tfh cells express low levels of IL-2Rα, and STAT5 activation is paradoxically suppressive for Tfh cell differentiation168, 224.
Since c-Maf is highly expressed by Tfh cells131 and it is important for IL-4 production in Th2 cells132, it is possible that high levels of c-Maf expression by Tfh cells may compensate low GATA3 expression and/or low STAT5 activity for inducing IL-4. Interestingly, deletion of a DNA segment containing both HSV and HSVA results in impaired IL-4 production in both Th2 cells and mast cells116, however, only deleting the CNS2 region at the HSV site dramatically affects IL-4 production in Tfh cells and in naïve CD4 T cells but has a small effect on IL-4 production in conventional effector Th2 cells in tissue225, 226. Consequently, IgE induction is completely abolished in CNS2 deficient mice. Furthermore, CNS2 region is much more active in Tfh cells225. Since Notch/CSL complex directly binds the CNS2 region76, Notch signaling may be constitutively active and critical for IL-4 production in Tfh cells.
The relationship between classical Th2 cells and IL-4-producing Tfh cells is still not certain. An in vitro study suggests that T cells can sequentially gain cytokine producing capacity and follicular helper phenotype227. That is, conventional Th2 cells can be converted to IL-4-producing Tfh cells. Similarly, Tfh cells can be further differentiated to produce IL-4. In a transfer model, it has been shown that activated T cells that have gained IL-4-producing capacity can later on display a Tfh cell phenotype in a B cell dependent manner221. However, this does not rule out the possibility that some Tfh cells gain IL-4-producing capacity after they have entered germinal center. Alternatively, activated T cells may gain IL-4-producing capacity and features of Tfh cells simultaneously, and their fate to become either classical effector Th2 cells or IL-4-producing Tfh cells may be determined during early T cell differentiation. Indeed, it has been shown that there is an early lineage commitment to either T effector cells or Tfh cells based on their Bcl6 and IL-2Rα expression two days after immunization168. Bcl6 is transiently induced during Th1 cell differentiation supporting the model of early fate determination228, however, whether Bcl6-expressed/expressing cells will become Tfh cells later is unknown.
IL-4-producing cells are mainly found in the B cell follicle whereas IL-13-producing cells are found in the tissue47; this suggests that Th2 effector cells and IL-4-producing Tfh cells may adopt distinct differentiation programs which involves regulation of GATA3 levels and other transcription factors collaborating with GATA3. Interestingly, it has also been shown that memory T helper cells may originate from Tfh cells229. A careful genome-wide assessment of the epigenetic status in conventional Th2 cells, IL-4-producing Tfh cells and Th2 memory cells may help understand the relationship between these cells.
8. Relationship between Th2 cells and ILC2
ILC2s are important players during type 2 immune responses particularly at early stages230–233 and their development requires transcription factors RORα234, 235 and GATA338, 236–240. Gfi-1, also highly expressed by ILC2s, is involved in the development and/or maintenance of ILC2s241; without Gfi-1, ILC2s aberrantly express IL-17 similar to the finding in Gfi-1-deficient T cells173. ILC2s not only contribute to the expulsion of helminths, they are also involved in allergic lung and skin inflammation242–246. ILC2s are also involved in adipocyte differentiation and energy metabolism35, 247, 248; whether Th2 cells have similar functions is an important question of interest.
When pharmacologically stimulated by cytokines such as IL-25 or IL-33, ILC2s are able to expel worms in the absence of adaptive immune system. During activation, ILC2s transiently produce IL-9, which in turn maintains ILC2 survival in an autocrine manner249, 250. IL-9 receptor is highly expressed on ILC2s and such expression depends on GATA338, 250. GATA3 is also critical for the expression of many ILC2-specific genes38, 47, including IL-5 and IL-13 that are also shared by Th2 cells, and GATA3 is indispensable for the maintenance of ILC2 cell numbers in vivo38, 237.
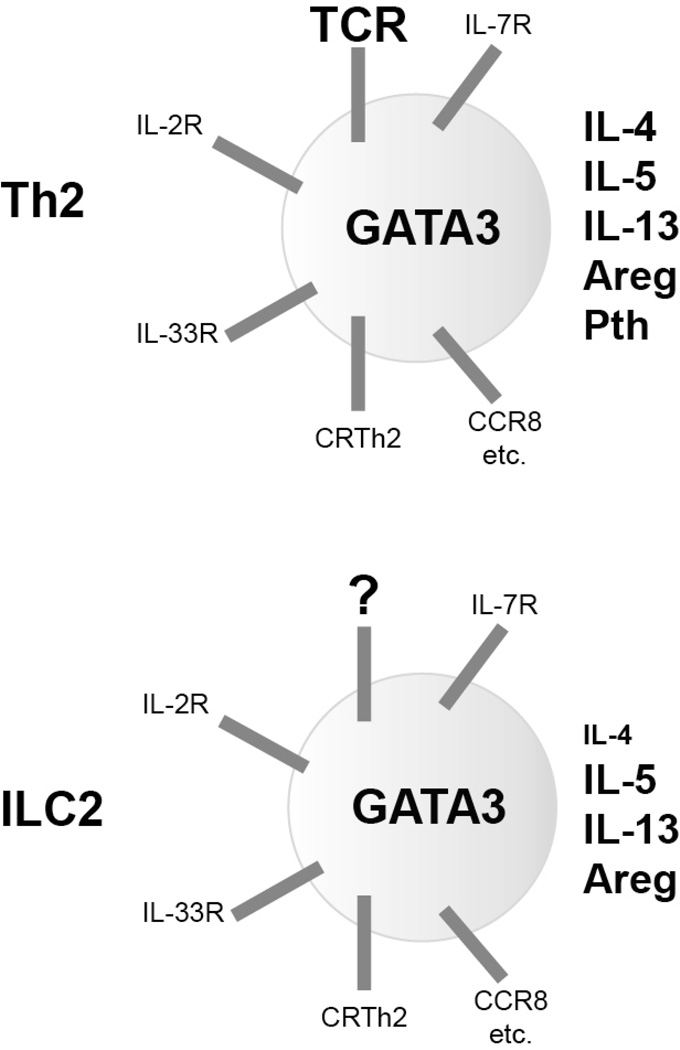
ILC2s and Th2 cells have identical functions and similar requirement of transcriptional machinery in cytokine production (Figure 2) 37, 251. However, due to the lack of an antigen receptor and toll-like receptor, ILC2s mainly respond to cytokines such as IL-33 to produce type 2 cytokines, IL-5 and IL-13, but not IL-4.
Figure 2. Similarity between Th2 cells and ILC2s.
Both Th2 cells and ILC2s are capable of producing a set of cytokines such as IL-5, IL-13 and Amphiregulin (Areg), although ILC2s produce less IL-4 and no parathyroid hormone (Pth). GATA3 is critical for the development, maintenance and functions of both Th2 cells and ILC2s. While ILC2s lack T cell receptor (TCR), they express cytokine receptors found on Th2 cells including IL-33 receptor (T1/ST2), IL-2 receptor and IL-7 receptor. Both Th2 cells and ILC2s produce IL-5 and IL-13 when stimulated through IL-33 and a Stat5 activator such as IL-2 and IL-7. Th2 cells and ILC2s also share expression of specific chemokine receptors including CRTh2, CCR8, CCR1 etc. Similar gene expression between Th2 cells and ILC2s and their dependence on GATA3 is consistent with their similar functions during type 2 immune responses.
ILC2s also respond to cysteinyl leukotrienes to produce cytokines252. Unlike IL-33, cysteinyl leukotriene D4 induces the expression of IL-4 in addition to IL-5 and IL-13. PGD2, a product of mast cells after IgE-mediated degranulation, can induce ILC2 migration and cytokine production through its interaction with CRTH2 expressed by ILC2s253. Interestingly, basophil-derived IL-4 is able to induce ILC2 proliferation245 and IL-13 production by ILC2s254; this direct effect of IL-4 on ILC2s to produce IL-13 requires further investigation and confirmation by other groups. On the other hand, lipoxin A4 and E-cadherin (a ligand for KLRG1) suppress cytokine production by ILC2s255, 256.
ILC2s and Th2 cells collaborate during type 2 immune responses257–260. In a lung inflammation model, papain-induced IL-33 activates ILC2s to produce IL-13, which induces the migration and activation of lung dendritic cells (DCs) to the draining lymph nodes260; these migratory DCs then induce Th2 cell differentiation in an IL-4-independent manner. Some ILC2s also express MHC class II through which activate T cells to produce IL-2, and IL-2 acts back on ILC2s to produce type 2 cytokines257, 258. IL-2 has been reported to induce ILC2 proliferation and cytokine release246, 249, 261. Co-culture ILC2s with naïve CD4 T cells results in ILC2 proliferation and cytokine production, which is mediated by IL-2 produced by T cells258. While it is clear that ILC2s are capable of inducing Th2 cell differentiation in vitro possibly through IL-4 production259, whether ILC2 and T cell direct interaction is critical for initiating and promoting Th2 cell differentiation in vivo, or this interaction mainly reflects collaborative relationship of ILC2s and Th2 cells at the effector stage requires further investigation.
9. Summary and conclusions
During Th2 cell differentiation, multiple signaling pathways result in two major critical events: up-regulation of GATA3 expression and activation of STAT5 proteins. Both GATA3 expression and STAT5 activation are essential for Th2 cell differentiation. GATA3 and STAT5 directly act on the Il4/Il13 locus through chromatin remodeling and epigenetic modifications. Several other transcription factors are also involved in Th2 cell differentiation. Th2 cell lineage commitment, determined by a network of transcription factors, is accompanied by blockage of alternative lineage fates and selective growth of already differentiated Th2 cells. Once Th2 cells are fully differentiated, the production of Th2 cytokines including IL-4, IL-5 and IL-13 requires re-stimulation by antigens through TCR, by cytokines such as IL-33, or possibly by other inflammatory molecules such as cysteinyl leukotrienes. Some transcription factors that are important for Th2 lineage commitment may also be involved in regulating Th2 cytokine production upon restimulation.
We have gained much knowledge of Th2 cell differentiation mainly from in vitro studies. However, our understanding of Th2 cell differentiation in vivo is far from complete. A particular example is that while IL-4/STAT6 pathway is absolutely necessary for in vitro Th2 cell differentiation, this pathway is only minimally involved in many in vivo Th2 responses. The complexity of Th2 responses in vivo is partially due to the involvement of other cytokines such as TSLP, IL-25 and IL-3385, 262, 263, and the participation of many immune cells including DCs, ILC2s, NKT cells, basophils, eosinophils and mast cells264. In the future, it is critical to further understand the relationship between conventional Th2 cells and IL-4-producing Tfh cells, and the functional similarity and difference between Th2 cells and ILC2s, particularly during type 2 immune responses in vivo. Two particular intriguing questions are: how GATA3 with various amounts differentially regulates IL-4 and IL-13 in conventional Th2 cells and Tfh cells, respectively; why Th2 cells express both IL-4 and IL-13 but ILC2s mainly produce IL-13 but not IL-4. By simultaneously studying Th2 cells and ILC2s, common and unique pathways/molecules that are involved in regulating type 2 immune responses will be identified; these pathways and molecules may be considered as drug targets for treating Th2- and/or ILC2-related diseases including allergy and asthma.
Acknowledgements
The work is supported by the Division of Intramural Research (DIR), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
The author declares no financial conflict of interest.
References
- 1.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annual review of immunology. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 4.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual review of immunology. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 5.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 6.Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annual review of immunology. 2012;30:733–758. doi: 10.1146/annurev-immunol-020711-075043. [DOI] [PubMed] [Google Scholar]
- 7.Abbas AK, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nature immunology. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 8.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 9.Crotty S. Follicular helper CD4 T cells (TFH) Annual review of immunology. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 10.Kopf M, et al. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 11.Gordon S. Alternative activation of macrophages. Nature reviews immunology. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 12.Coffman RL, Seymour BW, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science (New York, N.Y. 1989;245:308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 13.Kuperman DA, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nature medicine. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 14.Wynn TA. IL-13 effector functions. Annual review of immunology. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 15.Urban JF, Jr, et al. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 16.Lee DU, Rao A. Molecular analysis of a locus control region in the T helper 2 cytokine gene cluster: a target for STAT6 but not GATA3. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16010–16015. doi: 10.1073/pnas.0407031101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation of Th2 differentiation and Il4 locus accessibility. Annual review of immunology. 2006;24:607–656. doi: 10.1146/annurev.immunol.23.021704.115821. [DOI] [PubMed] [Google Scholar]
- 18.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. The Journal of experimental medicine. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 20.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 21.Shimoda K, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 22.Takeda K, et al. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 23.Jankovic D, et al. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol. 2000;164:3047–3055. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- 24.Finkelman FD, et al. Stat6 regulation of in vivo IL-4 responses. J Immunol. 2000;164:2303–2310. doi: 10.4049/jimmunol.164.5.2303. [DOI] [PubMed] [Google Scholar]
- 25.Min B, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. The Journal of experimental medicine. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 27.van Panhuys N, et al. In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12423–12428. doi: 10.1073/pnas.0806372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 29.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. The Journal of biological chemistry. 1997;272:21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 30.Zhu J, et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nature immunology. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 31.Pai SY, Truitt ML, Ho IC. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1993–1998. doi: 10.1073/pnas.0308697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cote-Sierra J, et al. Interleukin 2 plays a central role in Th2 differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- 34.Guo L, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proceedings of the National Academy of Sciences of the United States of America. 2009 doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nussbaum JC, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horiuchi S, et al. Genome-wide analysis reveals unique regulation of transcription of Th2-specific genes by GATA3. J Immunol. 2011;186:6378–6389. doi: 10.4049/jimmunol.1100179. [DOI] [PubMed] [Google Scholar]
- 37.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nature immunology. 2013;14:536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 38.Yagi R, et al. The transcription factor GATA3 is critical for the development of all IL-7Ralpha-expressing innate lymphoid cells. Immunity. 2014;40:378–388. doi: 10.1016/j.immuni.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. The Journal of experimental medicine. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurata H, Lee HJ, O'Garra A, Arai N. Ectopic expression of activated Stat6 induces the expression of Th2-specific cytokines and transcription factors in developing Th1 cells. Immunity. 1999;11:677–688. doi: 10.1016/s1074-7613(00)80142-9. [DOI] [PubMed] [Google Scholar]
- 41.Zhu J, Guo L, Watson CJ, Hu-Li J, Paul WE. Stat6 is necessary and sufficient for IL-4's role in Th2 differentiation and cell expansion. J Immunol. 2001;166:7276–7281. doi: 10.4049/jimmunol.166.12.7276. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. The Journal of experimental medicine. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nature immunology. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sokol CL, et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nature immunology. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamane H, Zhu J, Paul WE. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. The Journal of experimental medicine. 2005;202:793–804. doi: 10.1084/jem.20051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noben-Trauth N, Hu-Li J, Paul WE. IL-4 secreted from individual naive CD4+ T cells acts in an autocrine manner to induce Th2 differentiation. European journal of immunology. 2002;32:1428–1433. doi: 10.1002/1521-4141(200205)32:5<1428::AID-IMMU1428>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 47.Liang HE, et al. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nature immunology. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tao X, Constant S, Jorritsma P, Bottomly K. Strength of TCR signal determines the costimulatory requirements for Th1 and Th2 CD4+ T cell differentiation. J Immunol. 1997;159:5956–5963. [PubMed] [Google Scholar]
- 49.van Panhuys N, Klauschen F, Germain RN. T-cell-receptor-dependent signal intensity dominantly controls CD4(+) T cell polarization In Vivo. Immunity. 2014;41:63–74. doi: 10.1016/j.immuni.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nelson RW, et al. T Cell Receptor Cross-Reactivity between Similar Foreign and Self Peptides Influences Naive Cell Population Size and Autoimmunity. Immunity. 2014 doi: 10.1016/j.immuni.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tubo NJ, et al. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen CH, Zhang DH, LaPorte JM, Ray A. Cyclic AMP activates p38 mitogen-activated protein kinase in Th2 cells: phosphorylation of GATA-3 and stimulation of Th2 cytokine gene expression. J Immunol. 2000;165:5597–5605. doi: 10.4049/jimmunol.165.10.5597. [DOI] [PubMed] [Google Scholar]
- 53.Stumbles PA, et al. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. The Journal of experimental medicine. 1998;188:2019–2031. doi: 10.1084/jem.188.11.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinfelder S, et al. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) The Journal of experimental medicine. 2009;206:1681–1690. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Everts B, et al. Omega-1, a glycoprotein secreted by Schistosoma mansoni eggs, drives Th2 responses. The Journal of experimental medicine. 2009;206:1673–1680. doi: 10.1084/jem.20082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshimoto T, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nature immunology. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 57.Perrigoue JG, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nature immunology. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seder RA, Germain RN, Linsley PS, Paul WE. CD28-mediated costimulation of interleukin 2 (IL-2) production plays a critical role in T cell priming for IL-4 and interferon gamma production. The Journal of experimental medicine. 1994;179:299–304. doi: 10.1084/jem.179.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lenschow DJ, et al. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285–293. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- 60.Corry DB, Reiner SL, Linsley PS, Locksley RM. Differential effects of blockade of CD28-B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J Immunol. 1994;153:4142–4148. [PubMed] [Google Scholar]
- 61.Oosterwegel MA, et al. The role of CTLA-4 in regulating Th2 differentiation. J Immunol. 1999;163:2634–2639. [PubMed] [Google Scholar]
- 62.Bour-Jordan H, et al. CTLA-4 regulates the requirement for cytokine-induced signals in T(H)2 lineage commitment. Nature immunology. 2003;4:182–188. doi: 10.1038/ni884. [DOI] [PubMed] [Google Scholar]
- 63.Nurieva RI, et al. Transcriptional regulation of th2 differentiation by inducible costimulator. Immunity. 2003;18:801–811. doi: 10.1016/s1074-7613(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 64.Watanabe M, et al. ICOS-mediated costimulation on Th2 differentiation is achieved by the enhancement of IL-4 receptor-mediated signaling. J Immunol. 2005;174:1989–1996. doi: 10.4049/jimmunol.174.4.1989. [DOI] [PubMed] [Google Scholar]
- 65.Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. The Journal of experimental medicine. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohshima Y, et al. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–3345. [PubMed] [Google Scholar]
- 67.Ito T, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. The Journal of experimental medicine. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pollizzi KN, Powell JD. Regulation of T cells by mTOR: the known knowns and the known unknowns. Trends Immunol. 2015;36:13–20. doi: 10.1016/j.it.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nature reviews immunology. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li B, Tournier C, Davis RJ, Flavell RA. Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. The EMBO journal. 1999;18:420–432. doi: 10.1093/emboj/18.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee K, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nature immunology. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heikamp EB, et al. The AGC kinase SGK1 regulates TH1 and TH2 differentiation downstream of the mTORC2 complex. Nature immunology. 2014;15:457–464. doi: 10.1038/ni.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang K, et al. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity. 2013;39:1043–1056. doi: 10.1016/j.immuni.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cook KD, Miller J. TCR-dependent translational control of GATA-3 enhances Th2 differentiation. J Immunol. 2010;185:3209–3216. doi: 10.4049/jimmunol.0902544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amsen D, et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 77.Tanigaki K, et al. Regulation of alphabeta/gammadelta T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity. 2004;20:611–622. doi: 10.1016/s1074-7613(04)00109-8. [DOI] [PubMed] [Google Scholar]
- 78.Amsen D, et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27:89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laky K, Evans S, Perez-Diez A, Fowlkes BJ. Notch Signaling Regulates Antigen Sensitivity of Naive CD4(+) T Cells by Tuning Co-stimulation. Immunity. 2015;42:80–94. doi: 10.1016/j.immuni.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adler SH, et al. Notch signaling augments T cell responsiveness by enhancing CD25 expression. J Immunol. 2003;171:2896–2903. doi: 10.4049/jimmunol.171.6.2896. [DOI] [PubMed] [Google Scholar]
- 81.Lin JX, Leonard WJ. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene. 2000;19:2566–2576. doi: 10.1038/sj.onc.1203523. [DOI] [PubMed] [Google Scholar]
- 82.Moriggl R, et al. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999;10:249–259. doi: 10.1016/s1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- 83.Kagami S, et al. Stat5a regulates T helper cell differentiation by several distinct mechanisms. Blood. 2001;97:2358–2365. doi: 10.1182/blood.v97.8.2358. [DOI] [PubMed] [Google Scholar]
- 84.Kagami S, et al. Both stat5a and stat5b are required for antigen-induced eosinophil and T-cell recruitment into the tissue. Blood. 2000;95:1370–1377. [PubMed] [Google Scholar]
- 85.Zhou B, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nature immunology. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 86.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. The Journal of experimental medicine. 2006;203:269–273. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu YJ, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annual review of immunology. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 88.He R, et al. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 90.Zhu J, et al. Transient inhibition of interleukin 4 signaling by T cell receptor ligation. The Journal of experimental medicine. 2000;192:1125–1134. doi: 10.1084/jem.192.8.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Afkarian M, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nature immunology. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 92.Lighvani AA, et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martin-Fontecha A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nature immunology. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 94.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nature immunology. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 95.Goldszmid RS, et al. NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity. 2012;36:1047–1059. doi: 10.1016/j.immuni.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klose CS, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 97.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 98.Thierfelder WE, et al. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 99.Hsieh CS, et al. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science (New York, N.Y. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 100.Ho IC, Tai TS, Pai SY. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nature reviews immunology. 2009;9:125–135. doi: 10.1038/nri2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wei G, et al. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity. 2011;35:299–311. doi: 10.1016/j.immuni.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Else KJ, Finkelman FD, Maliszewski CR, Grencis RK. Cytokine-mediated regulation of chronic intestinal helminth infection. The Journal of experimental medicine. 1994;179:347–351. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 104.Zhu J, et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity. 2012;37:660–673. doi: 10.1016/j.immuni.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jankovic D, et al. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(−/−) setting. Immunity. 2002;16:429–439. doi: 10.1016/s1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 106.Ouyang W, et al. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 107.Usui T, et al. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. The Journal of experimental medicine. 2006;203:755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ouyang W, et al. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12:27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 109.Zhang DH, et al. Inhibition of allergic inflammation in a murine model of asthma by expression of a dominant-negative mutant of GATA-3. Immunity. 1999;11:473–482. doi: 10.1016/s1074-7613(00)80122-3. [DOI] [PubMed] [Google Scholar]
- 110.Yagi R, Zhu J, Paul WE. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int Immunol. 2011;23:415–420. doi: 10.1093/intimm/dxr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yamashita M, et al. Identification of a conserved GATA3 response element upstream proximal from the interleukin-13 gene locus. The Journal of biological chemistry. 2002;277:42399–42408. doi: 10.1074/jbc.M205876200. [DOI] [PubMed] [Google Scholar]
- 112.Tanaka S, et al. The enhancer HS2 critically regulates GATA-3-mediated Il4 transcription in T(H)2 cells. Nature immunology. 2011;12:77–85. doi: 10.1038/ni.1966. [DOI] [PubMed] [Google Scholar]
- 113.Fields PE, Lee GR, Kim ST, Bartsevich VV, Flavell RA. Th2-specific chromatin remodeling and enhancer activity in the Th2 cytokine locus control region. Immunity. 2004;21:865–876. doi: 10.1016/j.immuni.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 114.Lee GR, Fields PE, Griffin TJ, Flavell RA. Regulation of the Th2 cytokine locus by a locus control region. Immunity. 2003;19:145–153. doi: 10.1016/s1074-7613(03)00179-1. [DOI] [PubMed] [Google Scholar]
- 115.Hural JA, Kwan M, Henkel G, Hock MB, Brown MA. An intron transcriptional enhancer element regulates IL-4 gene locus accessibility in mast cells. J Immunol. 2000;165:3239–3249. doi: 10.4049/jimmunol.165.6.3239. [DOI] [PubMed] [Google Scholar]
- 116.Solymar DC, Agarwal S, Bassing CH, Alt FW, Rao A. A 3' enhancer in the IL-4 gene regulates cytokine production by Th2 cells and mast cells. Immunity. 2002;17:41–50. doi: 10.1016/s1074-7613(02)00334-5. [DOI] [PubMed] [Google Scholar]
- 117.Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell research. 2006;16:3–10. doi: 10.1038/sj.cr.7310002. [DOI] [PubMed] [Google Scholar]
- 118.Liao W, et al. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor alpha-chain expression. Nature immunology. 2008;9:1288–1296. doi: 10.1038/ni.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Murphy TL, Tussiwand R, Murphy KM. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nature reviews immunology. 2013;13:499–509. doi: 10.1038/nri3470. [DOI] [PubMed] [Google Scholar]
- 120.Huber M, Lohoff M. IRF4 at the crossroads of effector T-cell fate decision. European journal of immunology. 2014;44:1886–1895. doi: 10.1002/eji.201344279. [DOI] [PubMed] [Google Scholar]
- 121.Lohoff M, et al. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11808–11812. doi: 10.1073/pnas.182425099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rengarajan J, et al. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. The Journal of experimental medicine. 2002;195:1003–1012. doi: 10.1084/jem.20011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brustle A, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nature immunology. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 124.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Staudt V, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 126.Ciofani M, et al. A Validated Regulatory Network for Th17 Cell Specification. Cell. 2012 doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li P, et al. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature. 2012;490:543–546. doi: 10.1038/nature11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Glasmacher E, et al. A genomic regulatory element that directs assembly and function of immune-specific AP-1-IRF complexes. Science (New York, N.Y. 2012;338:975–980. doi: 10.1126/science.1228309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu J, et al. c-Maf regulates IL-10 expression during Th17 polarization. J Immunol. 2009;182:6226–6236. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rutz S, et al. Transcription factor c-Maf mediates the TGF-beta-dependent suppression of IL-22 production in T(H)17 cells. Nature immunology. 2011;12:1238–1245. doi: 10.1038/ni.2134. [DOI] [PubMed] [Google Scholar]
- 131.Bauquet AT, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature immunology. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim JI, Ho IC, Grusby MJ, Glimcher LH. The transcription factor c-Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity. 1999;10:745–751. doi: 10.1016/s1074-7613(00)80073-4. [DOI] [PubMed] [Google Scholar]
- 133.Ranzani V, et al. The long intergenic noncoding RNA landscape of human lymphocytes highlights the regulation of T cell differentiation by linc-MAF-4. Nature immunology. 2015 doi: 10.1038/ni.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kwon SJ, et al. KLF13 cooperates with c-Maf to regulate IL-4 expression in CD4+ T cells. J Immunol. 2014;192:5703–5709. doi: 10.4049/jimmunol.1302830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lai D, et al. KLF13 sustains thymic memory-like CD8(+) T cells in BALB/c mice by regulating IL-4-generating invariant natural killer T cells. The Journal of experimental medicine. 2011;208:1093–1103. doi: 10.1084/jem.20101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang XO, et al. Requirement for the basic helix-loop-helix transcription factor Dec2 in initial TH2 lineage commitment. Nature immunology. 2009;10:1260–1266. doi: 10.1038/ni.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kamizono S, et al. Nfil3/E4bp4 is required for the development and maturation of NK cells in vivo. The Journal of experimental medicine. 2009;206:2977–2986. doi: 10.1084/jem.20092176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gascoyne DM, et al. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nature immunology. 2009;10:1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]
- 139.Male V, et al. The transcription factor E4bp4/Nfil3 controls commitment to the NK lineage and directly regulates Eomes and Id2 expression. The Journal of experimental medicine. 2014;211:635–642. doi: 10.1084/jem.20132398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Geiger TL, et al. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. The Journal of experimental medicine. 2014;211:1723–1731. doi: 10.1084/jem.20140212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Seillet C, et al. Nfil3 is required for the development of all innate lymphoid cell subsets. The Journal of experimental medicine. 2014;211:1733–1740. doi: 10.1084/jem.20140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yu X, et al. The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. eLife. 2014;3 doi: 10.7554/eLife.04406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Motomura Y, et al. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nature immunology. 2011;12:450–459. doi: 10.1038/ni.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hwang SS, et al. Transcription factor YY1 is essential for regulation of the Th2 cytokine locus and for Th2 cell differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:276–281. doi: 10.1073/pnas.1214682110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Djuretic IM, et al. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nature immunology. 2007;8:145–153. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 146.Yagi R, et al. The transcription factor GATA3 actively represses RUNX3 protein-regulated production of interferon-gamma. Immunity. 2010;32:507–517. doi: 10.1016/j.immuni.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Naoe Y, et al. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the Il4 silencer. The Journal of experimental medicine. 2007;204:1749–1755. doi: 10.1084/jem.20062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Endo Y, et al. Eomesodermin controls interleukin-5 production in memory T helper 2 cells through inhibition of activity of the transcription factor GATA3. Immunity. 2011;35:733–745. doi: 10.1016/j.immuni.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 149.Kuwahara M, et al. The transcription factor Sox4 is a downstream target of signaling by the cytokine TGF-beta and suppresses T(H)2 differentiation. Nature immunology. 2012;13:778–786. doi: 10.1038/ni.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chang HC, et al. PU.1 expression delineates heterogeneity in primary Th2 cells. Immunity. 2005;22:693–703. doi: 10.1016/j.immuni.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 151.Wohlfert EA, et al. GATA3 controls Foxp3(+) regulatory T cell fate during inflammation in mice. The Journal of clinical investigation. 2011;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rudra D, et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nature immunology. 2012;13:1010–1019. doi: 10.1038/ni.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yu F, Sharma S, Edwards J, Feigenbaum L, Zhu J. Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nature immunology. 2015;16:197–206. doi: 10.1038/ni.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wan YSY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 156.Roncador G, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. European journal of immunology. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 157.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hosoya-Ohmura S, et al. An NK and T cell enhancer lies 280 kilobase pairs 3' to the gata3 structural gene. Mol Cell Biol. 2011;31:1894–1904. doi: 10.1128/MCB.05065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hu G, et al. Expression and regulation of intergenic long noncoding RNAs during T cell development and differentiation. Nature immunology. 2013;14:1190–1198. doi: 10.1038/ni.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim HP, Kelly J, Leonard WJ. The basis for IL-2-induced IL-2 receptor alpha chain gene regulation: importance of two widely separated IL-2 response elements. Immunity. 2001;15:159–172. doi: 10.1016/s1074-7613(01)00167-4. [DOI] [PubMed] [Google Scholar]
- 161.Vrisekoop N, Monteiro JP, Mandl JN, Germain RN. Revisiting thymic positive selection and the mature T cell repertoire for antigen. Immunity. 2014;41:181–190. doi: 10.1016/j.immuni.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Usui T, Nishikomori R, Kitani A, Strober W. GATA-3 suppresses Th1 development by downregulation of Stat4 and not through effects on IL-12Rbeta2 chain or T-bet. Immunity. 2003;18:415–428. doi: 10.1016/s1074-7613(03)00057-8. [DOI] [PubMed] [Google Scholar]
- 163.Pearce EL, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science (New York, N.Y. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 164.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science (New York, N.Y. 2005;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 165.Peine M, et al. Stable T-bet(+)GATA-3(+) Th1/Th2 hybrid cells arise in vivo, can develop directly from naive precursors, and limit immunopathologic inflammation. PLoS biology. 2013;11:e1001633. doi: 10.1371/journal.pbio.1001633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kanhere A, et al. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nature communications. 2012;3:1268. doi: 10.1038/ncomms2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 168.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. The Journal of experimental medicine. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ballesteros-Tato A, et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yamane H, Paul WE. Cytokines of the gamma(c) family control CD4+ T cell differentiation and function. Nature immunology. 2012;13:1037–1044. doi: 10.1038/ni.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhu J, Jankovic D, Grinberg A, Guo L, Paul WE. Gfi-1 plays an important role in IL-2-mediated Th2 cell expansion. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18214–18219. doi: 10.1073/pnas.0608981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhu J, et al. Down-regulation of Gfi-1 expression by TGF-beta is important for differentiation of Th17 and CD103+ inducible regulatory T cells. The Journal of experimental medicine. 2009;206:329–341. doi: 10.1084/jem.20081666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhu J, et al. Growth factor independent-1 induced by IL-4 regulates Th2 cell proliferation. Immunity. 2002;16:733–744. doi: 10.1016/s1074-7613(02)00317-5. [DOI] [PubMed] [Google Scholar]
- 175.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. The Journal of experimental medicine. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mullen AC, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science (New York, N.Y. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 177.Renz H, Domenico J, Gelfand EW. IL-4-dependent up-regulation of IL-4 receptor expression in murine T and B cells. J Immunol. 1991;146:3049–3055. [PubMed] [Google Scholar]
- 178.Hwang ES, White IA, Ho IC. An IL-4-independent and CD25-mediated function of c-maf in promoting the production of Th2 cytokines. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13026–13030. doi: 10.1073/pnas.202474499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xu D, et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. The Journal of experimental medicine. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lohning M, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. The Journal of experimental medicine. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Spilianakis CG, Flavell RA. Long-range intrachromosomal interactions in the T helper type 2 cytokine locus. Nature immunology. 2004;5:1017–1027. doi: 10.1038/ni1115. [DOI] [PubMed] [Google Scholar]
- 183.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nature reviews immunology. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 184.Kanno Y, Vahedi G, Hirahara K, Singleton K, O'Shea JJ. Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity. Annual review of immunology. 2012;30:707–731. doi: 10.1146/annurev-immunol-020711-075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Agarwal S, Rao A. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 186.Henkel G, et al. A DNase I-hypersensitive site in the second intron of the murine IL-4 gene defines a mast cell-specific enhancer. J Immunol. 1992;149:3239–3246. [PubMed] [Google Scholar]
- 187.Loots GG, et al. Identification of a coordinate regulator of interleukins 4, 13, and 5 by cross-species sequence comparisons. Science (New York, N.Y. 2000;288:136–140. doi: 10.1126/science.288.5463.136. [DOI] [PubMed] [Google Scholar]
- 188.Mohrs M, et al. Deletion of a coordinate regulator of type 2 cytokine expression in mice. Nature immunology. 2001;2:842–847. doi: 10.1038/ni0901-842. [DOI] [PubMed] [Google Scholar]
- 189.Kim ST, Fields PE, Flavell RA. Demethylation of a specific hypersensitive site in the Th2 locus control region. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17052–17057. doi: 10.1073/pnas.0708293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ansel KM, et al. Deletion of a conserved Il4 silencer impairs T helper type 1-mediated immunity. Nature immunology. 2004;5:1251–1259. doi: 10.1038/ni1135. [DOI] [PubMed] [Google Scholar]
- 191.Lee GR, Spilianakis CG, Flavell RA. Hypersensitive site 7 of the TH2 locus control region is essential for expressing TH2 cytokine genes and for long-range intrachromosomal interactions. Nature immunology. 2005;6:42–48. doi: 10.1038/ni1148. [DOI] [PubMed] [Google Scholar]
- 192.Williams A, et al. Hypersensitive site 6 of the Th2 locus control region is essential for Th2 cytokine expression. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6955–6960. doi: 10.1073/pnas.1304720110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Koh BH, et al. Th2 LCR is essential for regulation of Th2 cytokine genes and for pathogenesis of allergic asthma. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10614–10619. doi: 10.1073/pnas.1005383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Barski A, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 195.Wei G, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yamashita M, et al. Crucial role of MLL for the maintenance of memory T helper type 2 cell responses. Immunity. 2006;24:611–622. doi: 10.1016/j.immuni.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 197.Koyanagi M, et al. EZH2 and histone 3 trimethyl lysine 27 associated with Il4 and Il13 gene silencing in Th1 cells. The Journal of biological chemistry. 2005;280:31470–31477. doi: 10.1074/jbc.M504766200. [DOI] [PubMed] [Google Scholar]
- 198.Tumes DJ, et al. The polycomb protein Ezh2 regulates differentiation and plasticity of CD4(+) T helper type 1 and type 2 cells. Immunity. 2013;39:819–832. doi: 10.1016/j.immuni.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 199.Onodera A, et al. STAT6-mediated displacement of polycomb by trithorax complex establishes long-term maintenance of GATA3 expression in T helper type 2 cells. The Journal of experimental medicine. 2010;207:2493–2506. doi: 10.1084/jem.20100760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Avni O, et al. T-H cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nature immunology. 2002;3:643–651. doi: 10.1038/ni808. [DOI] [PubMed] [Google Scholar]
- 201.Siegel MD, Zhang DH, Ray P, Ray A. Activation of the interleukin-5 promoter by cAMP in murine EL-4 cells requires the GATA-3 and CLE0 elements. The Journal of biological chemistry. 1995;270:24548–24555. doi: 10.1074/jbc.270.41.24548. [DOI] [PubMed] [Google Scholar]
- 202.Kishikawa H, Sun J, Choi A, Miaw SC, Ho IC. The cell type-specific expression of the murine IL-13 gene is regulated by GATA-3. J Immunol. 2001;167:4414–4420. doi: 10.4049/jimmunol.167.8.4414. [DOI] [PubMed] [Google Scholar]
- 203.Seki N, et al. IL-4-induced GATA-3 expression is a time-restricted instruction switch for Th2 cell differentiation. J Immunol. 2004;172:6158–6166. doi: 10.4049/jimmunol.172.10.6158. [DOI] [PubMed] [Google Scholar]
- 204.Lee HJ, et al. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. The Journal of experimental medicine. 2000;192:105–115. doi: 10.1084/jem.192.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lee DU, Agarwal S, Rao A. Th2 lineage commitment and efficient IL-4 production involves extended demethylation of the IL-4 gene. Immunity. 2002;16:649–660. doi: 10.1016/s1074-7613(02)00314-x. [DOI] [PubMed] [Google Scholar]
- 206.Makar KW, et al. Active recruitment of DNA methyltransferases regulates interleukin 4 in thymocytes and T cells. Nature immunology. 2003;4:1183–1190. doi: 10.1038/ni1004. [DOI] [PubMed] [Google Scholar]
- 207.Hutchins AS, et al. Gene silencing quantitatively controls the function of a developmental trans-activator. Molecular cell. 2002;10:81–91. doi: 10.1016/s1097-2765(02)00564-6. [DOI] [PubMed] [Google Scholar]
- 208.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annual review of immunology. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 209.Agarwal S, Avni O, Rao A. Cell-type-restricted binding of the transcription factor NFAT to a distal IL-4 enhancer in vivo. Immunity. 2000;12:643–652. doi: 10.1016/s1074-7613(00)80215-0. [DOI] [PubMed] [Google Scholar]
- 210.Rengarajan J, Tang B, Glimcher LH. NFATc2 and NFATc3 regulate T(H)2 differentiation and modulate TCR-responsiveness of naive T(H)cells. Nature immunology. 2002;3:48–54. doi: 10.1038/ni744. [DOI] [PubMed] [Google Scholar]
- 211.Robinson D, et al. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 212.Yang J, Zhu H, Murphy TL, Ouyang W, Murphy KM. IL-18-stimulated GADD45 beta required in cytokine-induced, but not TCR-induced, IFN-gamma production. Nature immunology. 2001;2:157–164. doi: 10.1038/84264. [DOI] [PubMed] [Google Scholar]
- 213.Parmentier CN, et al. Human T(H)2 cells respond to cysteinyl leukotrienes through selective expression of cysteinyl leukotriene receptor 1. J Allergy Clin Immunol. 2012;129:1136–1142. doi: 10.1016/j.jaci.2012.01.057. [DOI] [PubMed] [Google Scholar]
- 214.Xue L, et al. Leukotriene E4 activates human Th2 cells for exaggerated proinflammatory cytokine production in response to prostaglandin D2. J Immunol. 2012;188:694–702. doi: 10.4049/jimmunol.1102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Guo L, Hu-Li J, Paul WE. Probabilistic regulation in TH2 cells accounts for monoallelic expression of IL-4 and IL-13. Immunity. 2005;23:89–99. doi: 10.1016/j.immuni.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 216.Guo L, et al. In TH2 cells the Il4 gene has a series of accessibility states associated with distinctive probabilities of IL-4 production. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10623–10628. doi: 10.1073/pnas.162360199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Guo L, Hu-Li J, Paul WE. Probabilistic regulation of IL-4 production in Th2 cells: accessibility at the Il4 locus. Immunity. 2004;20:193–203. doi: 10.1016/s1074-7613(04)00025-1. [DOI] [PubMed] [Google Scholar]
- 218.Kock J, et al. Nuclear factor of activated T cells regulates the expression of interleukin-4 in Th2 cells in an all-or-none fashion. The Journal of biological chemistry. 2014;289:26752–26761. doi: 10.1074/jbc.M114.587865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Helmstetter C, et al. Individual T helper cells have a quantitative cytokine memory. Immunity. 2015;42:108–122. doi: 10.1016/j.immuni.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature immunology. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zaretsky AG, et al. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. The Journal of experimental medicine. 2009;206:991–999. doi: 10.1084/jem.20090303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.King IL, Mohrs M. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. The Journal of experimental medicine. 2009;206:1001–1007. doi: 10.1084/jem.20090313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yusuf I, et al. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) J Immunol. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nurieva RI, et al. STAT5 protein negatively regulates T follicular helper (Tfh) cell generation and function. The Journal of biological chemistry. 2012;287:11234–11239. doi: 10.1074/jbc.M111.324046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Harada Y, et al. The 3' enhancer CNS2 is a critical regulator of interleukin-4-mediated humoral immunity in follicular helper T cells. Immunity. 2012;36:188–200. doi: 10.1016/j.immuni.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 226.Vijayanand P, et al. Interleukin-4 production by follicular helper T cells requires the conserved Il4 enhancer hypersensitivity site V. Immunity. 2012;36:175–187. doi: 10.1016/j.immuni.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lu KT, et al. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity. 2011;35:622–632. doi: 10.1016/j.immuni.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nakayamada S, et al. Early Th1 cell differentiation is marked by a Tfh celllike transition. Immunity. 2011;35:919–931. doi: 10.1016/j.immuni.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Luthje K, et al. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nature immunology. 2012;13:491–498. doi: 10.1038/ni.2261. [DOI] [PubMed] [Google Scholar]
- 230.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 231.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Price AE, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fallon PG, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. The Journal of experimental medicine. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Halim TY, et al. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity. 2012;37:463–474. doi: 10.1016/j.immuni.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 235.Wong SH, et al. Transcription factor RORalpha is critical for nuocyte development. Nature immunology. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hoyler T, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mjosberg J, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 238.Klein Wolterink RG, et al. Essential, dose-dependent role for the transcription factor Gata3 in the development of IL-5+ and IL-13+ type 2 innate lymphoid cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10240–10245. doi: 10.1073/pnas.1217158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yang Q, et al. T cell factor 1 is required for group 2 innate lymphoid cell generation. Immunity. 2013;38:694–704. doi: 10.1016/j.immuni.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Furusawa J, et al. Critical role of p38 and GATA3 in natural helper cell function. J Immunol. 2013;191:1818–1826. doi: 10.4049/jimmunol.1300379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Spooner CJ, et al. Specification of type 2 innate lymphocytes by the transcriptional determinant Gfi1. Nature immunology. 2013;14:1229–1236. doi: 10.1038/ni.2743. [DOI] [PubMed] [Google Scholar]
- 242.Chang YJ, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nature immunology. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 244.Monticelli LA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nature immunology. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kim BS, et al. Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol. 2014;193:3717–3725. doi: 10.4049/jimmunol.1401307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Roediger B, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nature immunology. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Brestoff JR, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2014 doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lee MW, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wilhelm C, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nature immunology. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Turner JE, et al. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. The Journal of experimental medicine. 2013;210:2951–2965. doi: 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lambrecht BN, Hammad H. The immunology of asthma. Nature immunology. 2015;16:45–56. doi: 10.1038/ni.3049. [DOI] [PubMed] [Google Scholar]
- 252.Doherty TA, et al. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Xue L, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133:1184–1194. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Motomura Y, et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40:758–771. doi: 10.1016/j.immuni.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 255.Barnig C, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Science translational medicine. 2013;5:174ra126. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Salimi M, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. The Journal of experimental medicine. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Oliphant CJ, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mirchandani AS, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J Immunol. 2014;192:2442–2448. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- 259.Drake LY, Iijima K, Kita H. Group 2 innate lymphoid cells and CD4+ T cells cooperate to mediate type 2 immune response in mice. Allergy. 2014;69:1300–1307. doi: 10.1111/all.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Halim TY, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Van Gool F, et al. Interleukin-5-producing group 2 innate lymphoid cells control eosinophilia induced by interleukin-2 therapy. Blood. 2014;124:3572–3576. doi: 10.1182/blood-2014-07-587493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Fort MM, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 263.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 264.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nature reviews immunology. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]