Abstract
Clinical trials of intranasal administration of oxytocin for treating psychiatric problems have yielded mixed results. To conduct a quantitative review of placebo-controlled clinical trials of intranasally-administered oxytocin (OT) for psychiatric symptoms, manual and electronic searches using PubMed and PsycINFO were conducted. Of 1,828 entries, 16 placebo-controlled studies totaling 330 participants were included in the analysis. The overall placebo-controlled effect size was moderately strong (Hedges’ g = 0.67) and robust as suggested by the fail-safe N and funnel plot analysis. OT reduced symptoms of depression, anxiety, autism/repetitive behaviors, psychotic symptoms, and general psychopathology. In the combined sample, symptom reduction was moderated by frequency of administration. Publication year and diagnostic category did not moderate the effect of OT on the clinical outcome measures. We conclude that intranasal administration of OT is a potentially useful intervention for reducing psychiatric symptoms. However, more studies are needed to determine the best treatment target and to identify the mechanism of treatment change.
Keywords: oxytocin, therapy, depression, anxiety, autism, psychosis
1. Introduction
Oxytocin (OT) is a neuropeptide that is synthesized in the paraventricular and supraoptic nuclei of the hypothalamus. It acts as a central neurotransmitter, as well as a peripheral hormone. Some of the peripheral effects of OT release include uterine contraction during childbirth and lactation. In addition, OT has been shown to be involved in social attachment (Carter, 1998; Insel, 2000), trust (Kosfeld et al., 2005), and the processing of social information (Kirsch et al., 2005; Guastella et al., 2008), among other aspects of social behaviors (MacDonald and MacDonald, 2010). Other studies suggest that OT may have general anxiolytic effects (Heinrichs et al., 2009).
A number of factors appear to moderate the effects of intranasal OT on subjective experiences and behaviors. These include the person’s sex, genotype for the OT receptor gene, attachment style and early childhood experiences, and the perception of whether a social partner is perceived as being a member of the in-group or out-group (for review, see Bakermans-Kranenburg and van Ijzendoorn, 2013). A recent meta-analysis examined the effects of intranasal administration of OT on face recognition and trust (van Ijzendoorn and Bakermans-Kranenburg, 2012). This study included 13 studies that evaluated face recognition, 8 studies that examined in-group trust and 10 studies investigating out-group trust. The results suggested that intranasal OT administration might enhance the recognition of facial expressions of emotions and might elevate the level of in-group trust. No effect was found for out-group trust. However, the effect size estimates were not robust, because the Trim-and-Fill analysis indicated the presence of a publication bias and the fail-safe N was below the Rosenthal criterion.
Given the effect of intranasal administration of OT on social behaviors and emotions, it has been suggested that OT might be beneficial for treating various psychiatric disorders, including social anxiety disorder, autism, and schizophrenia (Insel, 2010; Meyer-Lindenberg et al., 2011). However, the literature on the potential therapeutic value of intranasal OT for psychiatric symptoms remains inconclusive. The goal of the present study was to conduct a quantitative review of the effect of intranasally-administered OT on psychiatric symptoms. In contrast to previous meta-analyses that examined intranasal OT’s effects on any outcome in psychiatric populations (Bakermans-Kranenburg and van Ijzendoorn, 2013), we included only placebo-controlled studies that had outcome measures for psychiatric symptoms. We tested the hypothesis whether intranasal administration of OT leads to a reduction of psychiatric symptoms in psychiatric clinical populations. It should be noted that we use the term psychiatric symptoms in a general way, recognizing that this term reflects different problems in different populations.
2. Methods
2.1. Search
A search was conducted in PubMed and PsycInfo for original, peer-reviewed studies published between the first available year and April 25, 2014. The following search terms were used: oxytocin AND diagnos* OR disorder* OR psychiat* OR psychopath*. In addition, manual searches were conducted for relevant studies using reference lists of published papers.
2.2. Selection
Studies were selected by two of the authors (A.F. and D.B.) and were included in the present study if they met the following inclusion criteria: 1) involved the administration of intranasal OT; 2) included a psychiatric clinical sample; 3) included at least one clinical measure of psychiatric symptoms, behavior, or subjective mood or anxiety; 4) provided sufficient data for performing a meta-analysis of effect sizes.
Studies were excluded if they met the following exclusion criteria: 1) the study was a review, meta-analysis, qualitative study, animal study, or case study; 2) the paper was a correction to a published article or a comment or letter to the journal editor; 3) the study did not involve administration of OT; 4) the study did not measure clinical symptoms; 5) the study did not include clinical samples or involved clinical but non-psychiatric samples (such as individuals diagnosed with diabetes, fibromyalgia, obesity, or genetic disorders); and 6) the study involved a route of administration other than intranasal administration.
2.3. Validity Assessment
To evaluate the quality of included studies, we used the Effective Public Health Practice Project criteria (Thomas et al., 2004). Following the EPHPP Quality Assessment Tool for Quantitative Studies, we evaluated the quality of included studies on each of the following criteria: (a) selection bias, (b) study design, (c) confounders, (d) blinding, (e) data collection methods, (f) withdrawals and drop-outs, (g) intervention integrity, and (h) analyses. A score of 1 (strong), 2 (moderate), or 3 (weak) was assigned for each component criterion, and a global rating was assigned for each study depending on how many “weak” ratings were given in total (where “1” reflected a strong study with no weak ratings, “2” reflected a moderate study with only one weak rating, and “3” reflected a weak study with two or more weak ratings). Two independent raters evaluated these criteria and any disagreements were resolved through discussion.
Rosenthal’s fail-safe N was calculated in order to address potential publication bias (Rosenthal and Rubin, 1988; Rosenthal, 1991). The fail-safe N reflects the number of unpublished studies that would be needed to make the effect size estimate non-significant, and must be greater than 5K + 10, where K represents the number of studies in the pooled analysis. To further assess potential publication bias, we generated a funnel plot, and used the Trim-and-Fill procedure (Duval and Tweedie, 2000), which addresses outliers in the data as well as the sample size of studies to examine whether negative or positive trials are under- or over-represented.
2.4. Data Extraction
Numerical data were extracted from selected studies in order to compute effect sizes using a standardized metric. Clinical outcomes relevant to psychiatric behaviors and symptomatology (pre- and post- means and standard deviations) for patient groups were extracted where available. In cases where relevant data were not reported in published studies, the corresponding authors were contacted to supply the required data. Population characteristics (participant psychopathology, treated sample size), drug dosage and frequency of administration, and study variables (study design, clinical outcome measures) were extracted.
2.5. Study Characteristics
The characteristics of included studies are shown in Table 1. These studies included data for 330 (27.3% female) patients who were administered intranasal OT. The most common disorder studied was schizophrenia and schizoaffective disorder (n = 7), followed by social anxiety disorder (n = 2), obsessive-compulsive disorder (n = 2), autism spectrum disorders (n = 2), substance dependence (n = 2), and depression (n = 1). All included studies provided data for continuous clinical outcome measures before and after OT administration, and all included studies were placebo-controlled.
Table 1.
Study Characteristics
| Study | EPHPP Score |
Total Sample Size |
Study Design |
Diagnosis | % Females |
OT Dosage |
Frequency of OT administration |
Clinical Measures |
Effect Sizes (Hedges’ g)a |
p- value |
|---|---|---|---|---|---|---|---|---|---|---|
| Depression: Hedges’ g = 0.39, p = 0.04 | ||||||||||
| Averbeck et al. (2012) | 3 | 21 | Within-subject | Schizophrenia | 0/21 (0%) | 24 IU | Once | BMISb | 0.48 | 0.12 |
| den Boer and Westenberg (1992) | 1 | 12 (6 OT) | Between-subject | OCD | 9/12 (75.0%) | 18 IU- 54 IU | Four times per day for six weeks | HDS | 0.28 | 0.60 |
| Epperson et al. (1996) | 3 | 7 | Within-subject | OCD | 3/7 (42.9%) | 160 IU- 320 IU | Four times per day for one week | BDI | 0.82 | 0.12 |
| Labuschagne et al. (2012) | 3 | 18 | Within-subject | SAD | 0/18 (0%) | 24 IU | Once | POMS depression subscale | 0.15 | 0.64 |
| Anxiety: Hedges’ g = 0.42, p < .001 | ||||||||||
| den Boer and Westenberg (1992) | 1 | 12 (6 OT) | Between-subject | OCD | 9/12 (75.0%) | 18 IU- 54 IU | Four times per day for six weeks | STAIb | 0.58 | 0.29 |
| Epperson et al. (1996) | 3 | 7 | Within-subject | OCD | 3/7 (42.9%) | 160 IU- 320 IU | Four times per day for one week | YBOCSb | 0.57 | 0.27 |
| Gibson et al. (2012) | 1 | 19 (10 OT) | Between-subject | Schizophrenia | 3/19 (15.8%) | 24 IU | Twice daily for six weeks | PANSS anxiety subscale | 0.11 | 0.80 |
| Guastella et al. (2009) | 3 | 25 (12 OT) | Between-subject | SAD | 0/25 (0%) | 24 IU | Once a week for four weeks | LSASb | 0.23 | 0.55 |
| Labuschagne et al. (2012) | 3 | 18 | Within-subject | SAD | 0/18 (0%) | 24 IU | Once | STAIb | 0.33 | 0.31 |
| MacDonald et al. (2013) | 2 | 17 | Within-subject | Depression | 0/17 (0%) | 40 IU | Once | STAIb | 0.57 | 0.09 |
| McRae-Clark et al. (2013) | 3 | 16 (8 OT) | Between-subject | Substance dependence | 4/16 (25.0%) | 40 IU | Once | Subjective anxiety Likert scaleb | 0.36 | 0.45 |
| Pedersen et al. (2011) | 1 | 20 (11 OT) | Between-subject | Schizophrenia | 3/20 (15.0%) | 24 IU | Twice daily for two weeks | PANSS anxiety subscale | 0.17 | 0.70 |
| Pedersen et al. (2013) | 2 | 11 (7 OT) | Between-subject | Substance dependence | 2/11 (18.2%) | 24 IU | Twice daily for three days | POMS tension/anxiety subscaleb | 1.61 | 0.02 |
| Autism/Repetitive Behaviors: Hedges’ g = 0.37, p = 0.11 | ||||||||||
| Anagnostou et al. (2012) | 2 | 19 (10 OT) | Between-subject | Autism | 3/19 (15.9%) | 24 IU | Twice daily for six weeks | RBS-Rb | 0.52 | 0.25 |
| Dadds et al. (2014) | 1 | 38 (19 OT) | Between-subject | Autism | 0/38 (0%) | 12 IU- 24 IU dependent on weight | Once daily for four consecutive days | CARSb | 0.22 | 0.49 |
| Epperson et al. (1996) | 3 | 7 | Within-subject | OCD | 3/7 (42.9%) | 160 IU- 320 IU | Four times per day for one week | YBOCS | 0.57 | 0.27 |
| Psychotic Symptoms: Hedges’ g = 0.75, p = 0.02 | ||||||||||
| Davis et al. (2014) | 1 | 24 (13 OT) | Between-subject | Schizophrenia | 0/24 (0%) | 40 IU | Twice per week for six weeks | CAINS | 0.04 | 0.92 |
| Feifel et al. (2010) | 3 | 15 | Within-subject | Schizophrenia | 3/15 (20.0%) | 40 IU | Twice daily for three weeks | PANSSb | 0.36 | 0.32 |
| Gibson et al. (2012) | 1 | 19 (10 OT) | Between-subject | Schizophrenia | 3/19 (15.8%) | 24 IU | Twice daily for six weeks | PANSSb | 0.94 | 0.04 |
| Lee et al. (2013) | 1 | 28 (13 OT) | Between-subject | Schizophrenia | 8/28 (28.6%) | 20 IU | Twice daily for three weeks | SANS | 0.40 | 0.28 |
| Modabbernia et al. (2013) | 1 | 40 (20 OT) | Between-subject | Schizophrenia | 7/40 (17.5%) | 20 IU- 40 IU | 20 IU twice daily for first week, then 40 IU twice daily for 7 weeks | PANSSb | 2.19 | 0.00 |
| Pedersen et al. (2011) | 1 | 20 (11 OT) | Between-subject | Schizophrenia | 3/20 (15.0%) | 24 IU | Twice daily for two weeks | PANSSb | 0.61 | 0.17 |
| General Psychopathology: Hedges’ g = 0.79, p < .001 | ||||||||||
| Davis et al. (2014) | 1 | 24 (13 OT) | Between-subject | Schizophrenia | 0/24 (0%) | 40 IU | Twice per week for six weeks | BPRSb | 0.79 | 0.07 |
| Feifel et al. (2010) | 3 | 15 | Within-subject | Schizophrenia | 3/15 (20.0%) | 40 IU | Twice daily for three weeks | PANSS general psychopathology subscale | 0.40 | 0.27 |
| Gibson et al. (2012) | 1 | 19 (10 OT) | Between-subject | Schizophrenia | 3/19 (15.8%) | 24 IU | Twice daily for six weeks | PANSS general psychopathology subscale | 0.76 | 0.10 |
| Lee et al. (2013) | 1 | 28 (13 OT) | Between-subject | Schizophrenia | 8/28 (28.6%) | 20 IU | Twice daily for three weeks | BPRSb | 1.07 | 0.01 |
| Modabbernia et al. (2013) | 1 | 40 (20 OT) | Between-subject | Schizophrenia | 7/40 (17.5%) | 20 IU- 40 IU | 20 IU twice daily for first week, then 40 IU twice daily for 7 weeks | PANSS general psychopathology subscale | 1.08 | 0.00 |
| Pedersen et al. (2011) | 1 | 20 (11 OT) | Between-subject | Schizophrenia | 3/20 (15.0%) | 24 IU | Twice daily for two weeks | PANSS general psychopathology subscale | 0.52 | 0.24 |
All effect-sizes are placebo-controlled.
Denotes the primary outcome measure from the study that was used toward the calculation of overall effect size.
Note. OCD = Obsessive-Compulsive Disorder; SAD = Social Anxiety Disorder Clinical Measures: BDI = Beck Depression Inventory; BMIS = Brief Mood Inventory Scale; BPRS = Brief Psychiatric Rating Scale; CARS = Childhood Autism Rating Scale; CAINS = Clinical Assessment Interview for Negative Symptoms; HDS = Hamilton Depression Scale; LSAS = Liebowitz Social Anxiety Scale; PANSS = Positive and Negative Syndrome Scale; POMS = Profile of Mood States; RBSR = Repetitive Behavior Scale- Revised; SANS = Scale for the Assessment of Negative Symptoms; STAI = State Trait Anxiety Inventory; YBOCS = Yale Brown Obsessive Compulsive Scale.
2.6. Quantitative Data Synthesis
Hedges’ g and its 95% confidence interval were used to estimate effect sizes for clinical outcome measures (Hedges and Olkin, 1985). Hedges’ g is a variation of Cohen’s d that accounts for sample size bias, and may be interpreted using Cohen’s (1998) convention as small (0.2), medium (0.5), and large (0.8). We calculated placebo-controlled effect sizes using the following formula:
where Δ̅ is the mean pre- to post-treatment change, SD is the standard deviation of post-treatment scores, n is the sample size, and CONT refers to the control condition. In addition, pre- and post-treatment measure correlations were needed to calculate the pre-post effect sizes. Given that this correlation was not available from study reports, we followed recommendations by Rosenthal (1993) to assume a conservative estimation of r = 0.7.
Effect size estimates were pooled across studies to obtain an overall effect size. If a study contributed more than one outcome measure, the primary outcome measure for that study was selected to contribute toward the calculation of the overall effect size (see Table 1, superscript b). Thus, participants were not counted twice in the overall effect size estimate. Data were also examined for statistical outliers. All effect sizes were within 2 standard deviations of the mean effect size. Finally, the random-effects model, rather than the fixed-effects model, was chosen to calculate effect size estimates given the heterogeneity of the studies (Hedges and Vevea, 1998; Moses et al., 2002).
2.7. Effect on Psychiatric Symptoms
To examine the effect of OT on psychiatric symptoms, including depression, anxiety, autism/repetitive behaviors, psychotic symptoms, and other symptoms, we computed separate effect sizes for each symptom type. The clinical measures used in the studies included the following: for depression-specific outcomes—Beck Depression Inventory (Beck et al., 1961), Hamilton Depression Scale (Hamilton, 1967), Brief Mood Inventory Scale (Mayer and Gaschke, 1988), Profile of Mood States depression subscale (McNair et al., 1971); for anxiety-specific outcomes—Liebowitz Social Anxiety Scale (Liebowitz, 1987), Yale-Brown Obsessive Compulsive Scale (Goodman et al., 1989), State Trait Anxiety Inventory (Spielberger et al., 1983), Positive and Negative Syndrome Scale anxiety subscale (Kay et al., 1987), Profile of Mood States tension/anxiety subscale (McNair et al., 1971); for autism/repetitive behaviors outcomes—Repetitive Behavior Scale – Revised (Bodfish et al., 2000), Childhood Autism Rating Scale (Schopler et al., 1980), Yale-Brown Obsessive Compulsive Scale (Goodman et al., 1989); for psychotic symptom outcomes—Clinical Assessment Interview for Negative Symptoms (Kring et al., 2013), Positive and Negative Syndrome Scale (Kay et al., 1987), Scale for the Assessment of Negative Symptoms (Buchanan et al., 2007); and, for general psychopathology measures—Brief Psychiatric Rating Scale (Overall and Gorham, 1962), Positive and Negative Syndrome Scale general psychopathology subscale (Kay et al., 1987). When multiple outcome measure for specific psychiatric symptoms were available, the most valid measure was selected.
2.8. Moderator Analyses
We examined whether the clinical outcome effect sizes varied as a function of study characteristics (study year, study design, study quality, dose frequency) or clinical characteristics (diagnostic category). For categorical moderators, we computed separate effect sizes for each group. For continuous moderators, we used meta-regression analyses to compute unstandardized regression coefficients. To evaluate the statistical significance of moderator effects, we used Cochran’s Q test of heterogeneity. We conducted all analyses using the software program Comprehensive Meta-Analysis, Version 2 (Borenstein et al., 2005).
3. Results
3.1. Trial Flow
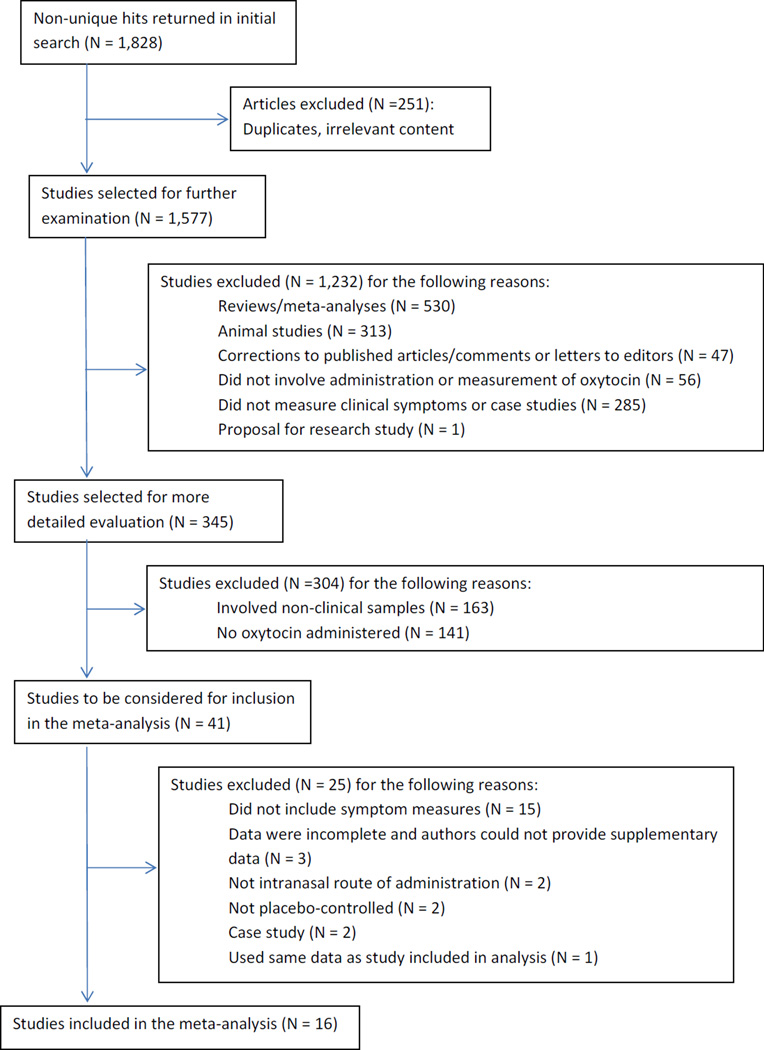
The study selection process is shown in Figure 1. Of 1,828 entries identified for potential inclusion, 16 published articles met all inclusion criteria.
Figure 1.
Flow diagram of study selection process.
3.2. Study Characteristics
As shown in Table 1, the global ratings of study quality ranged from 1 to 3 with a median of 2 (M = 1.93, SD = 0.93). The inter-rater reliability was 89%. Disagreements between raters were resolved after discussion.
3.3. Quantitative Data Analysis
3.3.1. Placebo-controlled effect size
Based on the 16 studies included in the current meta-analysis, the placebo-controlled Hedges’ g was 0.67 (95% CI: 0.42–0.93, z = 5.15, p < .001) for improving psychiatric symptoms. The results are depicted in Table 1.
3.3.2. Publication bias
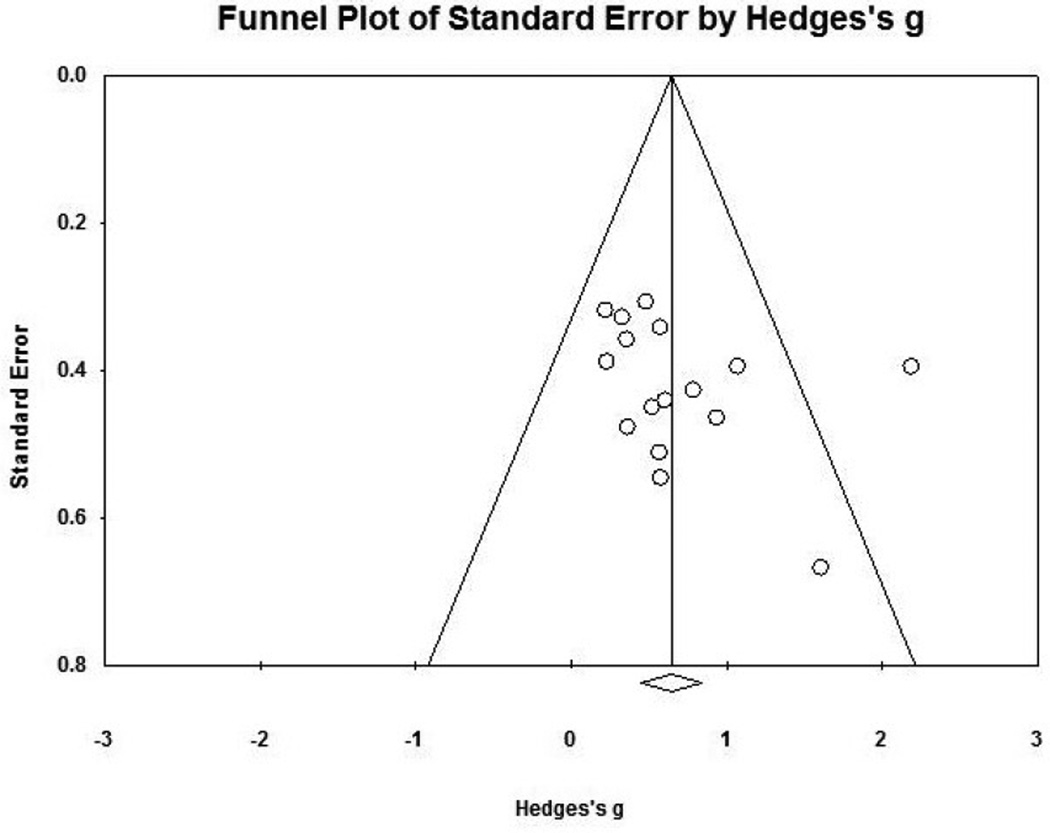
The observed effect size for clinical measures corresponded to a z value of 6.64. Using an alpha level of .05, the fail-safe N for clinical measures was 168, which indicated that 168 studies with non-significant findings would be required to nullify the effect. Because this number is greater than 5K + 10, where K is the number of studies included in the meta-analysis, the effect size can be interpreted as statistically robust. Moreover, using the Trim-and-Fill procedure (Figure 2), 0 studies would need to fall to the left of the mean (i.e., have an effect size smaller than the mean) and 3 studies would need to fall to the right of the mean (i.e., have an effect size larger than the mean) in order to make the plot symmetrical. This suggests that the computed effect size is a conservative estimate. Assuming a random-effects model, the imputed mean effect size would be Hedges’ g = 0.79 (95% CI: 0.55–1.04), which is within the range of the observed effect size. Based on these tests (i.e., the Trim-and-Fill Procedure and the fail-safe N), it is unlikely that the observed effect size was primarily a result of publication bias.
Figure 2.
Funnel plot.
3.3.3. Effect on psychiatric symptoms
Effect sizes reported in studies that examined the effect on depression, anxiety, autism/repetitive behaviors, psychotic symptoms, and other symptoms were not robust, as suggested by fail-safe N analyses ranging from 0 to 27.
3.3.4. Moderator analyses
The moderator analyses revealed that publication year (B = 0.01, SE = 0.02, p = 0.58) and study quality did not moderate clinical outcome effect sizes (B = −0.23, SE = 0.23, p = 0.07). We further compared effect sizes for clinical outcomes across study design. The Cochran’s Q test revealed no significant difference between effect sizes (χ2Interaction= 0.88, p = 0.35). Regardless of whether studies used between-subject or within-subject crossover designs, effect sizes were moderate in magnitude (between-subject Hedges’ g = 0.76, 95% CI: 0.52–1.01, z = 6.07, p < .001; within-subject Hedges’ g = 0.44, 95% CI: 0.11–0.77, z = 2.61, p = 0.008). Furthermore, clinical outcome effect sizes were not moderated by diagnostic category, as the Cochran’s Q test showed no significant difference between effect sizes (χ2Interaction= 24.33, p = 0.06). For the combined sample, frequency of OT administration (B = 0.006, SE = 0.003, p = 0.01) moderated the effect. However, when examining the dose-effect relationship for specific psychiatric symptoms, we found no effect for mood, anxiety, autism or general psychopathology.
4. Discussion
A number of recent experimental studies have reported short-term and beneficial effects of intranasal OT on social behaviors and emotions. Less is known about the potential therapeutic value of OT. We identified 16 placebo-controlled studies examining the effect of intranasal administration of OT on psychiatric symptoms in 330 clinical patients. The results showed that OT had a moderately strong effect (Hedges’ g = 0.67) across different psychiatric symptoms. This effect was robust as suggested by examining the funnel plot and a fail-safe N of 168, suggesting that 168 studies with non-significant findings would be required to nullify the effect. The effect cannot be easily explained by the placebo effect, because the effect size was placebo-controlled and it was indistinguishable from the uncontrolled pre-post effect sizes. Moreover, publication year, study design, and diagnostic category did not moderate the effect of OT on the clinical outcome measures.
This study warrants further investigation of OT as a potential therapeutic compound for improving certain psychiatric symptoms. However, many more adequately powered and well-controlled studies are needed before any drawing conclusions about the clinical utility and efficacy of OT. Moreover, the mechanism of OT needs to be further examined. Interestingly, OT was not more effective for anxiety symptoms than other psychiatric symptoms, including depression, autism, and psychotic symptoms. In fact, OT showed relatively strong and dose-dependent effects for improving psychotic symptoms (Hedges’ g = 0.75). This is consistent with a recent meta-analysis showing a modest effect of intranasal OT on psychotic symptoms in patients diagnosed with psychosis or schizophrenia (Gumley, Braehler, and Macbeth, 2014). We found no significant dose dependency for mood, anxiety, autism or general psychopathology. Because the majority of the studies reported here were conducted in patients with schizophrenia, it is possible that these effects might have skewed the results (e.g., Gumley et al., 2014).
On a related note, the frequency of administration moderated the effect of OT in the combined sample. Therefore, it is possible that the therapeutic effect can only be achieved through frequent OT administration. This might initiate a change in the body’s regulatory system of OT, OT receptors, and/or OT production, which in turn might affect certain psychiatric symptoms. This is consistent with the notion that long-term treatment with OT can lead to different results compared to short-term administration (Bales et al., 2013; Huang et al., 2014). In case OT shows therapeutic potential, future studies will then need to examine its optimal dosing.
Although the results suggest that OT may be beneficial for reducing psychiatric symptoms in clinical populations, it remains unclear which psychiatric disorders or symptoms are most responsive. The studies that we identified targeted a range of psychiatric symptoms, including depression, anxiety, autism/repetitive behaviors, psychotic symptoms, and general psychopathology. Therefore, the analyses were based on a relatively heterogeneous group of disorders and relatively small-sized samples. This is an important limitation. The results suggest that the beneficial effects of OT are not limited to enhancing the processing of social information (Kirsch et al., 2005; Guastella et al., 2008) and social behaviors (MacDonald and MacDonald, 2010). Furthermore, the efficacy of OT cannot simply be reduced to a general anxiolytic effect (Heinrichs et al., 2009), because OT also improved other psychiatric symptoms. Nevertheless, our results indicate that intranasal OT had an anxiolytic effect across a range of psychiatric populations, especially for patients with obsessive-compulsive disorder, depression, and social anxiety disorder. This raises the question if and how the pro-social effects of intranasal OT are associated with any anxiolytic properties in psychiatric patients (Churchland and Winkielman, 2012). Future studies should control for anxiety to clarify its role in OT’s effects. Moreover, the interpretation of these results are complicated by the fact that there are a number of factors moderating the effect of intranasal OT, including the person’s sex, genotype for the OT receptor gene, attachment style and early childhood experiences, etc. (Bakermans-Kranenburg and van Ijzendoorn, 2013).
In sum, the results of this meta-analysis suggest that intranasal OT administration has clinical potential, but more studies are needed to determine the treatment indication and symptom targets. The primary limitation of this study includes the relatively small number of trials and subjects per trial, and the under-representation of women in these trials. It is also worth noting that most studies included in this study were conducted on patients diagnosed with schizophrenia, which may have skewed results. Finally, the measures that were used for the calculation of an effect size were obviously limited by the measures reported in a particular study.
A critical area for future research is to examine the mechanism through which OT acts and to examine whether these effects are maintained at long-term follow-up. Another important question for future research is to examine the interaction with other pharmacological interventions. Finally, a meta-analysis cannot replace a large-scale, high-quality randomized controlled trial on the effect of OT on specific psychiatric symptoms. Thus, our recommendation is to conduct a large-scale placebo-controlled trial to examine intranasal OT on specific psychiatric symptoms. Together with studies examining the mechanism of change, these results would be of great clinical significance, especially if the effects of OT are long-lasting and associated with an acceptable side-effect profile.
Highlights.
To examine the effects of oxytocin in clinical populations, we conducted a meta-analysis.
Of 1,828 entries, 16 placebo-controlled studies were included in the analysis.
The effect size was moderately strong, but should be judged as preliminary.
Further studies of oxytocin as a clinical intervention are warranted.
Acknowledgments
Dr. Hofmann receives support from NIH/NCCIH (R01AT007257), NIH/NIMH (R01MH099021, R34MH099311, R34MH086668, R21MH102646, R21MH101567, K23MH100259), and the Department of the Army for work unrelated to the studies reported in this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
S. G. H. developed the study concept and study design and wrote the manuscript. A. F. and D. N. B conducted the article search, data extraction, and study quality ratings. A. F. conducted the data analysis. All authors contributed to the writing of the manuscript and approved of the final version of the manuscript for submission.
Conflict of Interest
All authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
References
Note. References listed with and asterisk (*) refer to studies included in the meta-analysis.
- *.Anagnostou E, Soorya L, Chaplin W, Bartz J, Halpern D, Wasserman S, Wang AT, Pepa L, Tanel N, Kushki A, Hollander E. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Molecular Autism. 2012;3:16–25. doi: 10.1186/2040-2392-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Averbeck BB, Bobin T, Evans S, Shergill SS. Emotion recognition and oxytocin in patients with schizophrenia. Psychological Medicine. 2012;42:259–266. doi: 10.1017/S0033291711001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van I Jzendoorn MH. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Translational Psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, Yun CR, Solomon M, Jacob S, Mendoza SP. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biological Psychiatry. 2013;74:180–188. doi: 10.1016/j.biopsych.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. Journal of Autism and Developmental Disorders. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive meta-analysis, version 2. Englewood, NJ: Biostat, Inc.; 2005. [Google Scholar]
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. American Journal of Psychiatry. 2007;164:1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Churchland PS, Winkielman P. Modulating social behavior with oxytocin: how does it work? What does it mean? Hormones and Behavior. 2012;61:392–399. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1998. [Google Scholar]
- Cyranowski JM, Hofkens TL, Frank E, Seltman H, Cai HM, Amico JA. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosomatic Medicine. 2008;70:967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Dadds MR, MacDonald E, Cauchi A, Williams K, Levy F, Brennan J. Nasal oxytocin for social deficits in childhood autism: a randomized controlled trial. Journal of Autism and Developmental Disorders. 2014;44:521–531. doi: 10.1007/s10803-013-1899-3. [DOI] [PubMed] [Google Scholar]
- *.Davis MC, Green MF, Lee J, Horan WP, Senturk D, Clarke AD, Marder SR. Oxytocin-augmented social cognitive skills training in schizophrenia. Neuropsychopharmacology. 2014;39:2070–2077. doi: 10.1038/npp.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.den Boer JA, Westenberg HGM. Oxytocin in obsessive compulsive disorder. Peptides. 1992;13:1083–1085. doi: 10.1016/0196-9781(92)90010-z. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- *.Epperson CN, McDougle CJ, Price LH. Intranasal oxytocin in obsessive-compulsive disorder. Biological Psychiatry. 1996;40:547–549. doi: 10.1016/0006-3223(96)00120-5. [DOI] [PubMed] [Google Scholar]
- *.Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W, Lefebvre M, Gonzales J, Hadley A. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biological Psychiatry. 2010;68:678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- *.Gibson CM. An investigation of the effects of oxytocin on social cognition and social functioning in schizophrenia [dissertation]*** Chapel Hill (NC): University of North Carolina at Chapel Hill; 2012. [Google Scholar]
- Goldman M, Marlow-O’Connor M, Torres I, Carter CS. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophrenia Research. 2008;98:247–255. doi: 10.1016/j.schres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Archives of General Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Green L, Fein D, Modahl C, Feinstein C, Waterhouse L, Morris M. Oxytocin and autistic disorder: alterations in peptide forms. Biological Psychiatry. 2001;50:609–613. doi: 10.1016/s0006-3223(01)01139-8. [DOI] [PubMed] [Google Scholar]
- *.Guastella AJ, Howard AL, Dadds MR, Mitchell P, Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34:917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Gumley A, Braehler C, Macbeth A. A meta-analysis and theoretical critique of oxytocin and psychosis: Prospects for attachment and compassion in promoting recovery. British Journal of Clinical Psychology. 2014;53:42–61. doi: 10.1111/bjc.12041. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. New York: Academic Press; 1985. [Google Scholar]
- Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychological Methods. 1998;3:486–504. [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Frontiers in Neuroendocrinology. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Huang H, Michetti C, Busnelli M, Managò F, Sannino S, Scheggia D, Giancardo L, Sona D, Murino V, Chini B, Scattoni ML, Papaleo F. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology. 2014;39:1102–1114. doi: 10.1038/npp.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Toward a neurobiology of attachment. Review of General Psychology. 2000;4:176–185. [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keri S, Kiss I, Kelemen O. Sharing secrets: oxytocin and trust in schizophrenia. Social Neuroscience. 2009;4:287–293. doi: 10.1080/17470910802319710. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. American Journal of Psychiatry. 2013;170:165–172. doi: 10.1176/appi.ajp.2012.12010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ. Medial frontal hyperactivity to sad faces in generalized social anxiety disorder and modulation by oxytocin. International Journal of Neuropsychopharmacology. 2012;15:883–896. doi: 10.1017/S1461145711001489. [DOI] [PubMed] [Google Scholar]
- *.Lee MR, Wehring HJ, McMahon RP, Linthicum J, Cascella N, Liu F, Bellack A, Buchanan RW, Strauss GP, Contoreggi C, Kelly DL. Effects of adjunctive intranasal oxytocin on olfactory identification and clinical symptoms in schizophrenia: results from a randomized double blind placebo controlled pilot study. Schizophrenia Research. 2013;145:110–115. doi: 10.1016/j.schres.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz MR. Social phobia. Modern Problems in Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- MacDonald K, MacDonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harvard Review of Psychiatry. 2010;18:1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- *.MacDonald K, MacDonald TM, Brüne M, Lamb K, Wilson MP, Golshan S, Feifel D. Oxytocin and psychotherapy: a pilot study of its physiological, behavioral and subjective effects in males with depression. Psychoneuroendocrinology. 2013;38:2831–2843. doi: 10.1016/j.psyneuen.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Gaschke YN. The experience and meta-experience of mood. Journal of Personality and Social Psychology. 1988;55:102–111. doi: 10.1037//0022-3514.55.1.102. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- *.McRae-Clark AL, Baker NL, Maria MM, Brady KT. Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology. 2013;2228:623–631. doi: 10.1007/s00213-013-3062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- *.Modabbernia A, Rezaei F, Salehi B, Jafarinia M, Ashrafi M, Tabrizi M, Hosseini SM, Tajdini M, Ghaleiha A, Akhondzadeh S. Intranasal oxytocin as an adjunct to risperidone in patients with schizophrenia. CNS Drugs. 2013;27:57–65. doi: 10.1007/s40263-012-0022-1. [DOI] [PubMed] [Google Scholar]
- Moses LE, Mosteller F, Beuhler JH. Comparing results of large clinical trials to those of meta-analyses. Statistics in Medicine. 2002;21:793–800. doi: 10.1002/sim.1098. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- *.Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Leserman J, Jarskog LF, Penn DL. Intranasal oxytocin reduces psychotic symptoms and improves theory of mind and social perception in schizophrenia. Schizophrenia Research. 2011;13:50–53. doi: 10.1016/j.schres.2011.07.027. [DOI] [PubMed] [Google Scholar]
- *.Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampoy-Polevoi A, Casey RL, Fender T, Garbutt JC. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcoholism, Clinical, and Experimental Research. 2013;37:484–489. doi: 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. Meta-analytic procedures for social research, revised edition. Thousand Oaks, CA: Sage Publications; 1991. [Google Scholar]
- Rosenthal R. Meta-analytic procedures for social research. Newbury Park, CA: Sage Publications; 1993. [Google Scholar]
- Rosenthal R, Rubin DB. Comment: assumptions and procedures in the file drawer problem. Statistical Science. 1988;3:120–125. [Google Scholar]
- Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Marechal P, Pequeux C, Ansseau M, Legros JJ. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32:407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS) Journal of Autism and Developmental Disorders. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologist Press; 1983. [Google Scholar]
- Thomas BH, Ciliska RN, Dobbins RN, Micucci BA. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews on Evidence Based Nursing. 2004;1:176–184. doi: 10.1111/j.1524-475X.2004.04006.x. [DOI] [PubMed] [Google Scholar]
- Tops M, van Peer JM, Korf J, Wijers AA, Tucker DM. Anxiety, cortisol, and attachment predict plasma oxytocin. Psychophysiology. 2007;44:444–449. doi: 10.1111/j.1469-8986.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Van Ijzendoorn MH, Bakermans-Kranenburg MJ. A sniff of trust: meta-analysis of the effects of intranasal oxytocin administration on face recognitions, trust to in-group, and trust to out-group. Psychoneuroendocrinology. 2012;37:438–443. doi: 10.1016/j.psyneuen.2011.07.008. [DOI] [PubMed] [Google Scholar]