Abstract
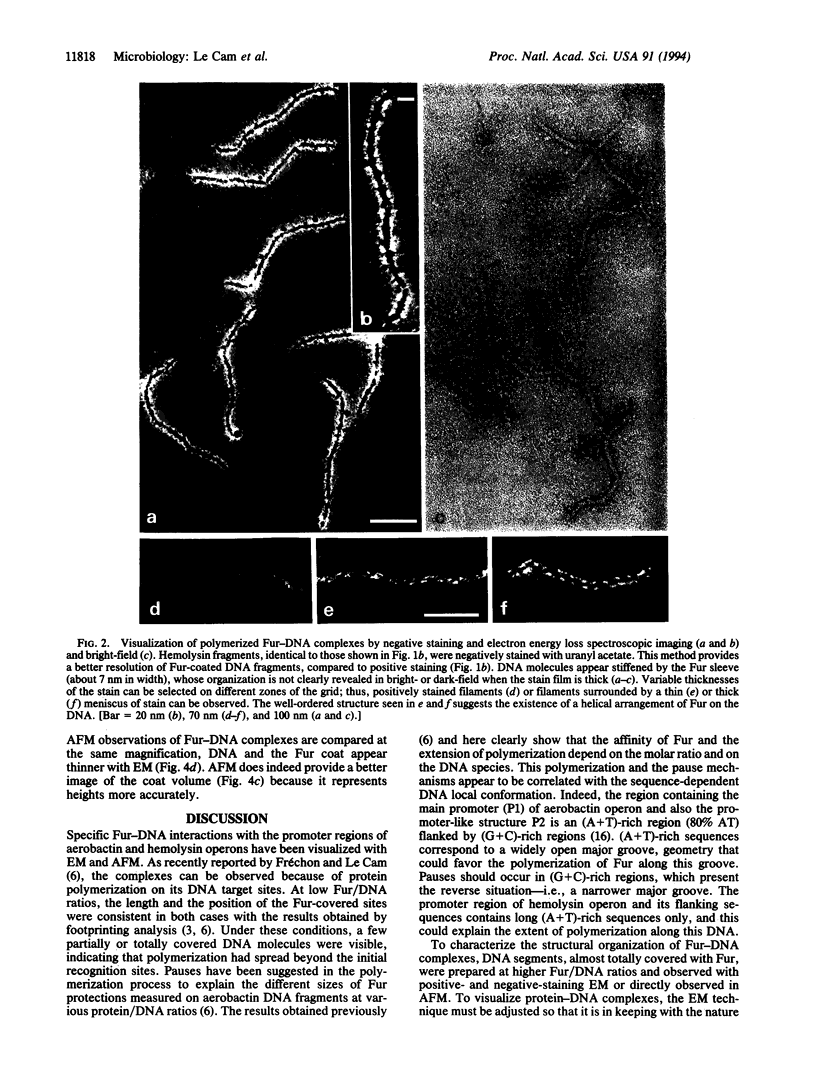
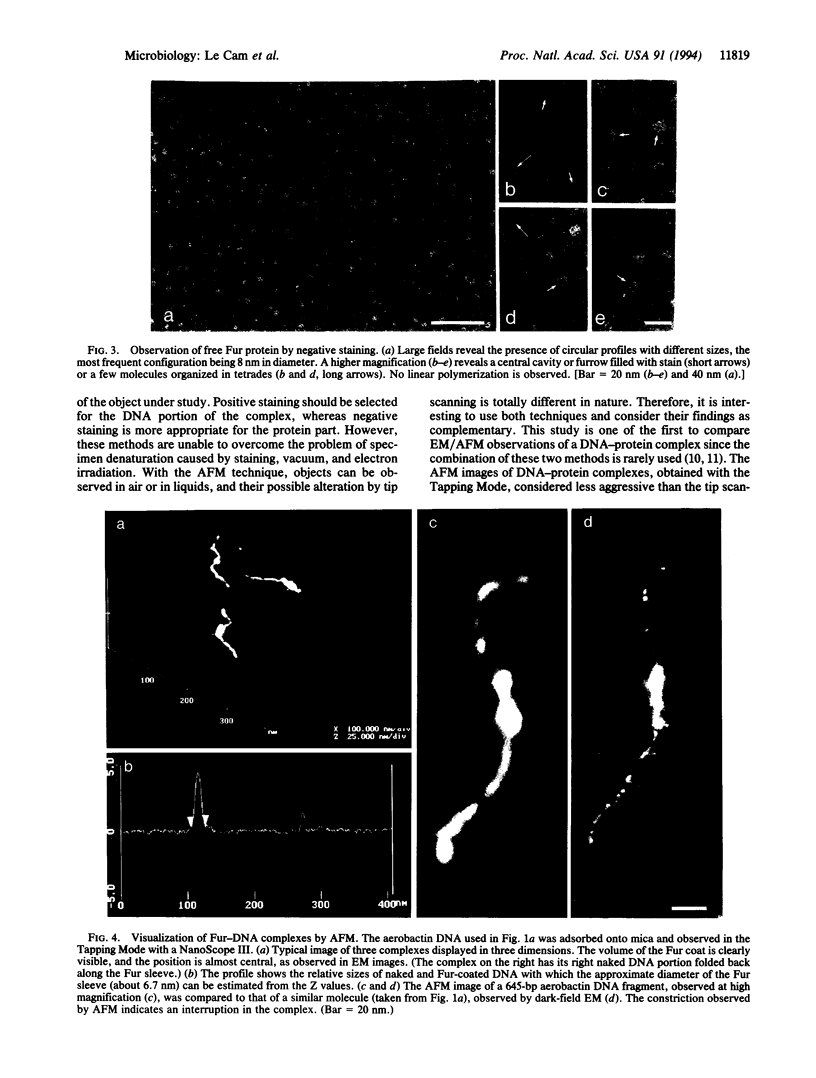
The Fur (ferric uptake regulation) protein is a global regulator that, in the presence of Fe2+, represses the expression of a number of iron-acquisition genes and virulence determinants such as toxins. Dark-field electron microscopy of positively stained Fur-DNA complexes in addition to atomic force microscopy allowed direct visualization of Fur interactions with the regulatory regions of aerobactin and hemolysin operons and provided complementary information about the structure of the complexes. According to the DNA used and the protein/DNA ratio, Fur binding to DNA results in partial or total covering of the fragments, indicating that the protein initiates polymerization along the DNA molecules at specific sites. Negative staining of Fur-DNA complexes revealed a well-ordered structure of the polymer suggesting a helical arrangement. Local rigidification of the DNA molecules resulting from Fur binding could be involved in the repression process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindereif A., Neilands J. B. Promoter mapping and transcriptional regulation of the iron assimilation system of plasmid ColV-K30 in Escherichia coli K-12. J Bacteriol. 1985 Jun;162(3):1039–1046. doi: 10.1128/jb.162.3.1039-1046.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. J., Dubochet J., Baudras A., Blazy B., Takahashi M. Electron microscope observation of a fibre structure formed by non-specific binding of cAMP receptor protein to DNA. J Mol Biol. 1981 Aug 15;150(3):435–439. doi: 10.1016/0022-2836(81)90558-1. [DOI] [PubMed] [Google Scholar]
- Delius H., Mantell N. J., Alberts B. Characterization by electron microscopy of the complex formed between T4 bacteriophage gene 32-protein and DNA. J Mol Biol. 1972 Jun 28;67(3):341–350. doi: 10.1016/0022-2836(72)90454-8. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- Fréchon D., Le Cam E. Fur (ferric uptake regulation) protein interaction with target DNA: comparison of gel retardation, footprinting and electron microscopy analyses. Biochem Biophys Res Commun. 1994 May 30;201(1):346–355. doi: 10.1006/bbrc.1994.1708. [DOI] [PubMed] [Google Scholar]
- Grünig H. M., Rutschi D., Schoch C., Lebek G. The chromosomal fur gene regulates the extracellular haemolytic activity encoded by certain hly plasmids. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Aug;266(1-2):231–238. doi: 10.1016/s0176-6724(87)80036-6. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. Fos-Jun heterodimers and Jun homodimers bend DNA in opposite orientations: implications for transcription factor cooperativity. Cell. 1991 Jul 26;66(2):317–326. doi: 10.1016/0092-8674(91)90621-5. [DOI] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. Selective DNA bending by a variety of bZIP proteins. Mol Cell Biol. 1993 Sep;13(9):5479–5489. doi: 10.1128/mcb.13.9.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cam E., Fack F., Ménissier-de Murcia J., Cognet J. A., Barbin A., Sarantoglou V., Révet B., Delain E., de Murcia G. Conformational analysis of a 139 base-pair DNA fragment containing a single-stranded break and its interaction with human poly(ADP-ribose) polymerase. J Mol Biol. 1994 Jan 21;235(3):1062–1071. doi: 10.1006/jmbi.1994.1057. [DOI] [PubMed] [Google Scholar]
- Le Cam E., Théveny B., Mignotte B., Révet B., Delain E. Quantitative electron microscopic analysis of DNA-protein interactions. J Electron Microsc Tech. 1991 Aug;18(4):375–386. doi: 10.1002/jemt.1060180406. [DOI] [PubMed] [Google Scholar]
- Murray M. N., Hansma H. G., Bezanilla M., Sano T., Ogletree D. F., Kolbe W., Smith C. L., Cantor C. R., Spengler S., Hansma P. K. Atomic force microscopy of biochemically tagged DNA. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3811–3814. doi: 10.1073/pnas.90.9.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzard G., Théveny B., Révet B. Electron microscopy mapping of pBR322 DNA curvature. Comparison with theoretical models. EMBO J. 1990 Apr;9(4):1289–1298. doi: 10.1002/j.1460-2075.1990.tb08238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhoffer E. C., Naranjo C. M., Bradley K. L., Fee J. A. Control of Escherichia coli superoxide dismutase (sodA and sodB) genes by the ferric uptake regulation (fur) locus. J Bacteriol. 1990 Apr;172(4):1930–1938. doi: 10.1128/jb.172.4.1930-1938.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Martín J., Espinosa M. Protein-induced bending as a transcriptional switch. Science. 1993 May 7;260(5109):805–807. doi: 10.1126/science.8387228. [DOI] [PubMed] [Google Scholar]
- Pérez-Martín J., Espinosa M. The RepA repressor can act as a transcriptional activator by inducing DNA bends. EMBO J. 1991 Jun;10(6):1375–1382. doi: 10.1002/j.1460-2075.1991.tb07657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding C. M. Homologous recombination: a universal recombination filament. Curr Biol. 1993 Jun 1;3(6):358–360. doi: 10.1016/0960-9822(93)90200-8. [DOI] [PubMed] [Google Scholar]
- Rees W. A., Keller R. W., Vesenka J. P., Yang G., Bustamante C. Evidence of DNA bending in transcription complexes imaged by scanning force microscopy. Science. 1993 Jun 11;260(5114):1646–1649. doi: 10.1126/science.8503010. [DOI] [PubMed] [Google Scholar]
- Roca A. I., Cox M. M. The RecA protein: structure and function. Crit Rev Biochem Mol Biol. 1990;25(6):415–456. doi: 10.3109/10409239009090617. [DOI] [PubMed] [Google Scholar]
- Stuiver M. H., Bergsma W. G., Arnberg A. C., van Amerongen H., van Grondelle R., van der Vliet P. C. Structural alterations of double-stranded DNA in complex with the adenovirus DNA-binding protein. Implications for its function in DNA replication. J Mol Biol. 1992 Jun 20;225(4):999–1011. doi: 10.1016/0022-2836(92)90100-x. [DOI] [PubMed] [Google Scholar]
- Wessel R., Ramsperger U., Stahl H., Knippers R. The interaction of SV40 large T antigen with unspecific double-stranded DNA: an electron microscopic study. Virology. 1992 Jul;189(1):293–303. doi: 10.1016/0042-6822(92)90705-t. [DOI] [PubMed] [Google Scholar]
- Yang J., Takeyasu K., Shao Z. Atomic force microscopy of DNA molecules. FEBS Lett. 1992 Apr 20;301(2):173–176. doi: 10.1016/0014-5793(92)81241-d. [DOI] [PubMed] [Google Scholar]
- Zingsheim H. P., Geisler N., Weber K., Mayer F. Complexes of Escherichia coli lac-repressor with non-operator DNA revealed by electron microscopy: two repressor molecules can share the same segment of DNA. J Mol Biol. 1977 Sep 25;115(3):565–570. doi: 10.1016/0022-2836(77)90171-1. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V., Giovannini F., Herrero M., Neilands J. B. Metal ion regulation of gene expression. Fur repressor-operator interaction at the promoter region of the aerobactin system of pColV-K30. J Mol Biol. 1988 Oct 20;203(4):875–884. doi: 10.1016/0022-2836(88)90113-1. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]