Introduction
Brain shifts due to dural opening, tumor resection evacuation of cystic components, osmotic shifts, drainage of cerebrospinal fluid (CSF), and other surgical effects lead to significant challenges to the use of conventional neuronavigational (nNav) systems1, which use preoperatively acquired images and frameless stereotaxy to depict the anatomical location and estimate the 3D extent of brain tumors1–3. Anatomical displacements of more than 10 mm distance can occur within an hour of dural opening especially when the tumor is large, cystic, deep, or in continuity with a CSF space, in which case the accuracy of conventional nNAv methods is severely affected [4, 10, 18, 19, 21, 23].
Therefore, in order to overcome the effect of intra-operative brain shifting, intra-operative MRI (IoMRI) was developed to compensate for this phenomenon [4, 8, 19, 22]. Intra-operative MRI appears to be an important tool that allows neurosurgeons to maximize the extent of surgical resection of gliomas, particularly for lower grade (non-enhancing) tumors [17]. Many studies have shown various advantages of IoMRI in surgical management of brain tumors, including its safety [11, 15, 24–28]. Yet the enthusiasm for applying IoMRI has been constrained by the lack of strong data to support clinically meaningful benefits to patients who have had surgery using IoMRI, and partly by the high cost of the OR suites that house IoMRI machines. Additional concerns include determining if IoMRI equally benefits all grades of gliomas.
In this study, we aim to objectively assess the impact of IoMRI on the outcomes of glioma resection in patients who were managed by the same surgeons and under the same setting, while adjusting for many prospective confounders.
Patients and Methods
Study design
This is a retrospective study examining 164 patients with glioma who underwent craniotomy for tumor resection by two primary neurosurgeons at the Brigham and Women’s Hospital between January 1, 2005 and December 31, 2009. Since the world’s first IoMRI machine at BWH had an unexpected irrecoverable failure in December 2006 and it was not replaced until installation of the current Advanced Multimodal Image Guided Operating (AMIGO) suite which was commissioned in 2011. This provides a unique opportunity for comparing outcomes in a group of patients who were managed with and without this technology in a single center by the same surgeons within a period of five to eight consecutive years. In order into evaluate the effect of ioMRI, patients were divided into two groups according to whether their surgery took place in the ioMRI or the conventional operating room, IoMRI and no-IoMRI groups.
Patient’s populations and data gathering
This study included only patients with newly diagnosed intracranial gliomas (age = 16 – 85 years). All 932 primary brain tumor surgical cases that were treated at BWH during the five year period were screened for this study. Since it can be difficult to differentiate recurrent tumor from radiation necrosis on imaging, patients with recurrent glioma were excluded from the study (431 cases). Similarly, all patients (27 cases) whose glioma had received prior treatment with radiotherapy were also excluded. Of the 474 surgery for primary glioma resection cases, 236 cases from 11 surgeons were further excluded for lack of case representation in the two surgical groups. These 11 surgeons only had eligible cases in one of the two surgical arms (IoMRI and non-IoMRI groups), but not in both arms. Since this is a potential source of bias into the analysis, as an individual surgeon decides when to stop glioma resection based on his or her perception in each case [43], we chose to limit this inter-operator variability by including only cases from surgeons who had cases in both IoMRI and non-IoMRI groups. Out of the remaining 238 cases we excluded 7 patients whose surgeries were planned using only CT images and 47 patients who had open or stereotatic biopsy. We included only those patients who had a pre-operative magnetic resonance imaging (MRI) within two weeks prior to surgery, and a post-operative MRI within 72 hours after surgery, excluding 7 patients who had post-operative MRI more than 4 week after glioma resection. Records of eight patients were not available and 5 other cases were excluded because their MRI images were not available for review. These exclusions resulted in 164 cases which were included in this study [Table 1].
Table 1.
Patients’ Baseline Parameters
| % of total | % of all | % in no-IoMRI | % in IoMRI | p-value | ||
|---|---|---|---|---|---|---|
| Age (years) | mean (±SD): 49.13(±17.08) | (n=164) | (n=89) | (n=75) | ||
| range 16 – 91 | ||||||
| Groups: (total =164) | 100 | 100 | - | - | 0.128 | |
| Young adults: 16 – 40 | 34 | 34 | 29 | 39 | ||
| Middle-aged: 40 – 65 | 48 | 48 | 47 | 49 | ||
| Elderly: >65 | 18 | 18 | 24 | 12 | ||
| Initial vol. (cm3) | Median: 25.91 | |||||
| range 0.48 – 286 | ||||||
| Groups: (total =164) | 100 | 100 | - | - | 0.179 | |
| Small lesions= 0.48 – 50 | 69 | 69 | 63 | 76 | ||
| Medium-sized lesions= 50.1 – 100 | 22 | 22 | 27 | 16 | ||
| Large lesions >100 | 9 | 9 | 10 | 8 | ||
| Adjuvant Treatment (total =134) | 100 | 82 | - | - | 0.547 | |
| Chemotherapy | 21 | 17 | 23 | 18 | ||
| Radiation | 10 | 8 | 13 | 7 | ||
| Chemo+Radiation | 41 | 33 | 40 | 42 | ||
| None | 28 | 23 | 24 | 33 | ||
| 1p19q codeletion (total =71) | 100 | 43 | - | - | 0.138 | |
| Codeleted | 45 | 19 | 34 | 50 | ||
| WHO Grade (total =164) | 100 | 100 | - | - | ||
| High grade | 65 | 65 | 73 | 56 | 0.022 | |
| Eloquent Brain area (total =164) | 100 | 100 | - | - | ||
| Involved | 94 | 57 | 49 | 67 | 0.026 | |
| Grade II | 35 | 35 | 27 | 44 | ||
| Grade III | 18 | 18 | 12 | 24 | ||
| Grade IV | 48 | 48 | 61 | 32 | ||
Based on the type of intra-operative image guidance employed for their glioma resection, 75 patients were grouped into the IoMRI group, (glioma resection with intra-operative MRI guidance in addition to standard neuronavigation), and 89 patients into non-IoMRI group(resection with standard intra-operative neuronavigation (nNav) guidance without IoMRI). The study was approved by the Partner’s Institutional Review Board.
Patients’ records, including five to eight years follow-up data were retrospectively reviewed to assess outpatient visits, discharge summaries, operative notes, histopathology reports, and imaging data. Parameters extracted from the patient records included the patients’ age, date of operation, tumor histology and W.H.O. grade, and eloquent area involvement. Death records were obtained from the social security death index (SSDI), a publicly accessible record of all registered deaths in the United States. General mortality was recorded as death from any cause that occurred between surgery and September 30, 2014.
Procedure
IoMRI Group
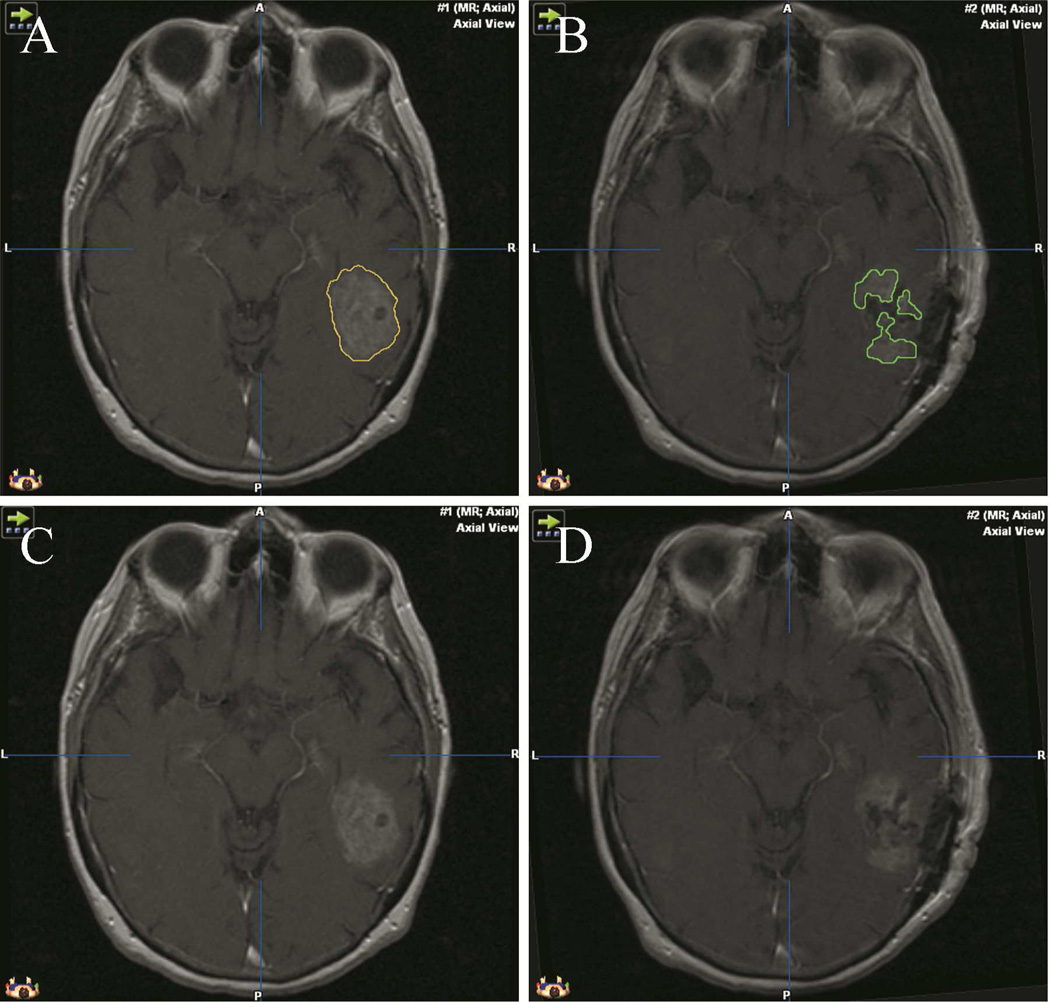
During surgery, patients’ heads were fixed using an MR-compatible carbon fiber Mayfield clamp (Ohio Medical Instruments, Cincinnati, Ohio) and imaged using the 0.5T MR imaging unit (Signa SP of GE Healthcare (then GE Medical Systems, Milwaukee, WI). Throughout the surgical procedure, images were taken at intervals determined by the surgeon. The initial and final image data sets were used to determine the initial (pre-op) and residual (post-op) tumor volumes, respectively [Fig.1]. Neuronavigation within the IoMRI was accomplished using an optical imaging system that was developed in-house and interfaced with 3D-slicer software (http://www.slicer.org/) [3, 20]. The software displayed updated images corresponding to position and angle of a pointing device.
Figure 1.
- pre-operative tumor segmentation
- post-operative residual tumor volume segmentation
- pre-operative tumor before segmentation
- post-operative residual tumor before segmentation
No-IoMRI Group
Patients in the non-IoMRI group underwent craniotomy without IoMRI guidance in the conventional neurosurgical operating room using either the VTI InstaTrak system (Visualization Technologies, Inc, Wilmington, MA) or the BrainLAB VectorVision neuronavigation system (Brainlab Feldkirchen, Germany). The pre-operative and post-operative MRI were used to assess the initial and the residual tumor volumes respectively (described below). All images were acquired using high field diagnostic MR scanners installed at BWH (various clinically approved scanners), and were transferred from the BWH imaging databank to a Windows console.
Tumor volume measurements
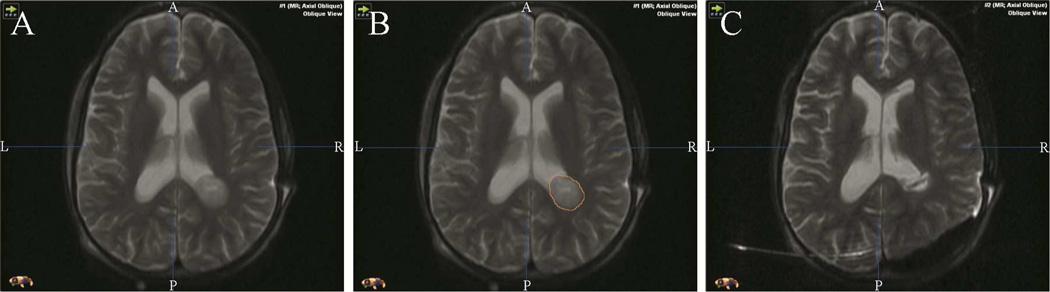
Tumor volumes were assessed from the patients’ pre-, intra-, and post- operative MRI images using the Brain Lab Iplannet surgical planning software (Brainlab Feldkirchen, Germany). All tumor volumes were measured in cubic centimeters. Depending on the characteristics of the tumor, T1 with and without contrast (gadolinium, several manufacturers), and/or T2 weighted images were reviewed for the assessment of tumor volumes. Pre-operative tumor volume was estimated by manual segmentation on axial, coronal and sagittal MRI slices [Figures 1 and 2] of the pre-operative MRI images. Similarly, residual tumor volume was measured on the corresponding post-operative MR images. Estimated extent of resection (EOR) was calculated by dividing the difference between the pre- and post- operative glioma volumes by the pre-operative glioma volume and multiplying by 100. Gross Total Resection (GTR) was defined as 100% EOR as seen on T2- weighted and FLAIR (fluid-attenuated inversion recovery) MRI sequences for non-enhancing tumors, or as seen on T1 weighted contrast-enhanced studies for contrast-enhancing tumors. The volumetric assessment was done by O.O and F.I, and reviewed by 2 experts; L.H (senior neuro-radiologist) and A.G (specialist/associate professor of neurosurgery and associate professor of radiology).
Figure 2.
- pre-operative tumor before segmentation pre-operative tumor segmentation
- pre-operative tumor segmentation
- post-operative residual tumor volume segmentation
All the gliomas were categorized as either involving eloquent brain area (E+) or sparing eloquent brain areas (E−). Eloquent brain areas were defined as the sensorimotor cortex, visual cortex, language areas, the insula, basal ganglia, corpus callosum, and the optic radiations.
Pathologic Review
All pathology was initially reviewed by the clinical neuropathology section at BWH, and were further reviewed for consistency by S.S who is an expert neuropathologist. Lesions were classified based on the W.H.O grading system, histopathologic type and cytogenetics including 1p19q gene codeletion.
Complications
Complications of interest were defined as surgery associated neurologic deficits (SAND) including new or worsening motor, language, sensory, memory, visual or cognitive deficits. These were assessed from pre-operative and post-operative outpatient clinic visit records as documented by the managing teams, including neurosurgery, oncology, and medical rehabilitation records. Permanent neurological deficit was defined as SAND that lasted beyond 3 months after surgery or till death (if less than 3 months after surgery), and peri-operative mortality was defined as death within 1 month of surgery.
Statistical Analysis
Standard summary statistics were used to summarize the demographic, baseline and observed outcome measures, with the Pearson chi-square p-values reported. The primary outcome variable was extent of tumor resection (EOR), and the secondary outcome variable was gross total resection (GTR). EOR was evaluated as a continuous variable of the pre-operative glioma volume, while GTR was evaluated as a binary outcome variable. Multivariate analysis by logistic regression was used to compare GTR and permanent SAND between the two surgical groups in order to control for differences in baseline parameters. Distribution of EOR was confirmed to follow a skewed pattern by the Shapiro–Wilk normality test (p<0.001); therefore the Wilcoxon Rank-Sum (Mann-Whitney) test was employed. Permanent SAND was evaluated as a binary outcome variable, using a multiple regression method. Overall survival was estimated by Kaplan-Meier based survival analysis including adjusted analysis in a multivariate cox-regression model. Significance level (α-value) was preset at 0.05 in all analyses, and two-sided p-values were reported. All statistical analysis was performed using STATA version 11.2 (StataCorp LP College Station, Texas USA).
Results
Patients and pathology
One-hundred and seven patients (65.24%) had high grade (WHO III and IV) lesions. Tumor grade and eloquent area involvement were unevenly distributed between IoMRI and no-IoMRI groups. 56% of loMRI group and 73% of no-loMRI group harbored WHO high grade lesions. Similarly, 67% of loMRI group and 49% of no-loMRI group involved eloquent brain area. Both comparisons were significantly different with p<0.05 [Table. 1]. Therefore these factors were included in our fitted models for adjusted analysis.
For 43% of the cases 1p19q chromosome codeletion status was available, and the distributions of this was not significantly different between the two surgical groups (p = 0.14). Use of non-surgical adjuvant therapies was confirmed in 59% of cases and non-use was confirmed in 23%.
Combined chemo/radiation therapy was the most commonly used adjuvant modality, employed in 33% of patients; while radiation only was the least used, found in 8% of patients; and chemotherapy only was included in management of 17% of the patients. Frequency of use of these adjuvant therapies was not different between the two surgical groups (p = 0.55) [Table 1].
Resection rate
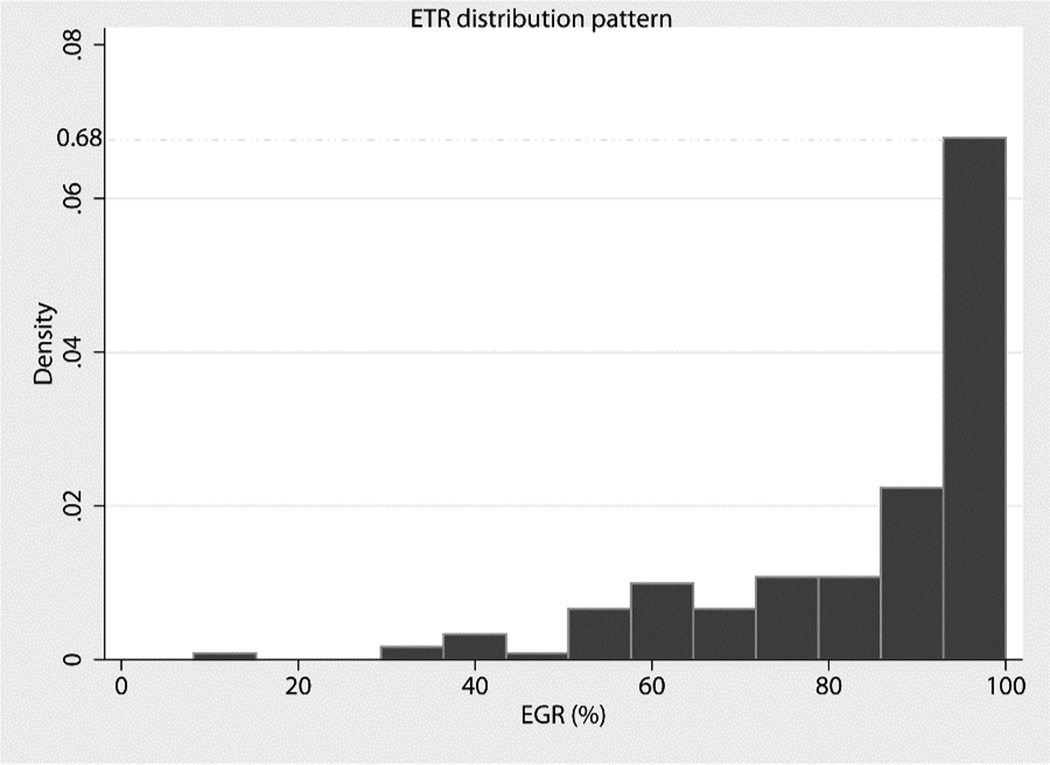
Median pre-operative tumor volume was 28.35cm3, range 0.48 – 286cm3. These were grouped into (i) small sized lesions with volume <50 cm3, (ii) medium-sized lesions, 50cm3<volume>100cm3, and (iii) large lesions with volume >100cm3. Lesion size distribution between IoMRI and no-IoMRI groups was not significantly different, (p = 0.179) [Table 1]. GTR was achieved in 56 cases, including 49% of patients in the IoMRI group and 21% of patients in the non-IoMRI group (p < 0.01). This remained significant after adjusting for the confounders of WHO grade and eloquent brain area involvement (p< 0.01) [Table.3]. Other significant predictors of GTR include WHO grade and involvement of eloquent brain area (p < 0.05) [Table 4]. Skewness of EOR distribution was confirmed by Shapiro-Wilk test for normality, (p < 0.01) [Figure 3]. Therefore EOR was compared between the two surgical groups by Wilcoxon rank-sum (Mann-Whiitney) test. Median EOR was 92% for IoMRI group and 89% for non-IoMRI group (p< 0.01). This remained significant within E− stratum (p = < 0.01), but was non-significant within E+ stratum (p = 0.29) [Table. 2]
Table 3.
GTR, permanent SAND and Censored data summary
| No-IoMRI (%) | IoMRI (%) | Total (%) | p-value | OR (95%CI) | ||
|---|---|---|---|---|---|---|
| crude | adjusted | |||||
| Permanent SAND | ||||||
| All | 12 (14) | 5 (7) | 17 (10) | 0.14 | 0.28 | 0.53 (0.17, 1.68) |
| E− | 6 (14) | 1 (4) | 7 (10) | 0.20 | 0.6 | 0.53 (0.05, 5.53) |
| E+ | 6 (14) | 4 (8) | 10 (11) | 0.36 | 0.35 | 0.51 (0.13, 2.05) |
| Gross Total Resection GTR | ||||||
| All | 19 (21) | 37(49) | 56 (34) | <0.01 | <0.01 | 5.21 (2.21, 12.27) |
| E− | 14 (31) | 21 (84) | 35 (50) | <0.01 | <0.01 | 7.42 (1.97, 27.9) |
| E+ | 5 (11) | 16(32) | 21 (22) | 0.02 | 0.03 | 3.36 (1.10, 10.29) |
| Censored | 3 (3) | 9 (12) | 12 (7) | 0.03 | - | - |
E+: eloquent brain area location of tumor
E− : tumor located outside eloquent brain area
IoMRI: used intra-operative magnetic resonance imaging
No-IoMRI: intra-operative magnetic resonance imaging was not used
Adjusted p-value: adjusted for eloquent brain area location of tumor, and WHO high grade tumor
Table 4.
Gross Total Resection (GTR) analysis
| Number of obs = 164 | LR chi2(4) = 47.61 | ||||
| Pseudo R2 = 0.23 | Prob > chi2 < 0.001 | ||||
| Gross total resection | Odds Ratio | z | P>|z| | [95% Conf. Interval] | |
| IoMRI_use | 5.20 | 3.77 | <0.01 | 2.21, 12.27 | |
| E+ | 0.20 | −3.62 | <0.01 | 0.08, 0.48 | |
| high grade | 0.34 | −2.74 | 0.06 | 0.15, 0.73 | |
| tumor size Group | 0.67 | −1.18 | 0.24 | 0.34, 1.30 | |
IoMRI: intra-operative MRI; E+: lesion location in eloquent brain area
IoMRI use and Tumor location in eloquent brain area were significantly associated with GTR
Figure 3.
Histogram of Extent of Tumor Resection (EOR) showing a skewed pattern of distribution in IoMRI and no-IoMRI groups, sktest p< 0.01
Table 2.
Extent of resection, EOR and Overall survival
| All | No-IoMRI | IoMRI | p-value | |
|---|---|---|---|---|
| EOR (%) | ||||
| Overall (Median) | 92 | 90 | 97 | <0.01 |
| Eloquent area | ||||
| Median | 89 | 89 | 92 | 0.29 |
| Non-eloquent area | ||||
| Median | 100 | 92 | 100 | <0.01 |
| Overall survival (months) Median | 60 | 39 | 90 | <0.01 |
| Eloquent area | ||||
| Median | 62 | 34 | 86 | <0.01 |
| Non-eloquent area | ||||
| Median | 60 | 46 | 92 | 0.02 |
EOR: extent of resection
IoMRI: used intra-operative magnetic resonance imaging
No-IoMRI: intra-operative magnetic resonance imaging was not used
Post-operative complications and survival
Incidence of post-operative complication in this study was generally low, with one mortality (0.6%) recorded within one month post-surgery and three (1.8%) within three months post-surgery. All cases with permanent SAND had pre-operative neurologic deficits, but these worsened and remained so beyond three months post-surgery. There were 7% and 14% cases with permanent SAND in the IoMRI and no-IoMRI groups, respectively. This was not statistically significant even after adjusting for the confounders (p = 0.3) [Table.2].
Median overall survival was 60 months, and was significantly higher in the IoMRI group than non-IoMRI group; Hazard Ratio = 0.50 (95% CI: 0.30 – 0.83, p < 0.01) [Table 5]. Other significant determinant of survival include WHO tumor grade (high vs. low WHO grade) (p < 0.01). A total of 12 international patients (7.02%) were lost to follow-up and thus were excluded from the survival estimates [Table 3].
Table 5.
Hazard ratio analysis for all-cause death
| Number of obs = 163 | LR chi2(3) = 69.67 | ||||
| No. of failures = 74 | Prob > chi2 < 0.01 | ||||
| Survival time | Haz Ratio | z | P>|z| | [95% Conf. Interval] | |
| IoMRI use | 0.43 | −3.20 | <0.01 | 0.26, 0.72 | |
| E+ | 1.02 | 0.07 | 0.95 | 0.62, 1.66 | |
| High grade | 13.62 | 5.03 | <0.01 | 4.92, 37.69 | |
IoMRI: intra-operative MRI; E+ : lesion location in eloquent brain area
IoMRI use and WHO high grade lesions were significantly associated with overall patient survival
Discussion
Our study showed an association of IoMRI with EOR in both the crude (unadjusted) analysis and among lesions located in non-eloquent brain areas. It also showed a significant difference in GTR between IoMRI and no-IoMRI groups in favor of IoMRI use for achieving GTR, even after adjusting for all identified confounders [Table 4]. This effect appeared to be higher among the E− gliomas (OR= 7), compared to the E+ gliomas (OR=3) [Table 3], although the difference was significant within both strata. Similarly, eloquent brain area involvement was also associated with lower GTR rate in the multivariate analysis, suggesting that this may be a negative predictor of GTR achievement [Table 4].. Similarly, this study demonstrated an association between IoMRI use and better overall survival, which was retained after adjusting for suspected confounders including the use of other nonsurgical adjuvant treatments. As expected, we found an association between overall death and WHO High Grade Gliomas, HGG (WHO grades III and IV) which implies an association with decreased survival [Table 5].
Relevance to previous studies
Our findings of association between EOR/GTR and overall patient survival support previous finding by Claus et al. [7], which suggests an association between extent of surgical resection and survival for neurosurgical patients who underwent surgery for low-grade glioma under intra-operative MRI guidance. GTR has also been shown to improve survival. In a randomized controlled study of 5-aminolevulinic acid in 243 patients with glioblastoma multiforme Stummer, Walter et al. [29], provided Level 2b evidence that survival depends on complete resection of enhancing tumor. Patients with complete and incomplete resections, as revealed by early MRI scans, were compared, and patients without residual tumor had increased survival (16.7 versus 11.8 mo, P < .001) [29].
Earlier studies supported resection as a major factor in survival after surgery for malignant gliomas [7, 14]. However some older studies presented conflicting data both for and against the use of aggressive surgical resection. Lacroix et al. [13] conducted a multivariate analysis of patients with GBM which demonstrated that >98% tumor resection was associated with 4.2 months extended survival (from 8.8 to 13 months) [1], suggesting that the presence of enhancing residual tumor was the single most important post-operative prognostic determinant of decreased survival in GBM. On the other hand, a study by Kowalczuk et al. examined 75 patients with high grade astrocytomas, and suggested that aggressive surgical management did not correlate with increased patient survival [12]. However, in a more recent study, Liang et al. demonstrated a positive correlation between extent of resection (EOR) and overall survival (OS), although the data was clearer in patients with low-grade gliomas (LGG) and still somewhat controversial in those with higher-grade tumors [5, 16].
Our findings also lend further support to existing level 1 and 2 data supporting the use of ioMRI improves surgical outcome. Wu et al. [31], in an interim analysis of a prospective, randomized, triple-blind, parallel-controlled trial with 142 patients, provided the first level 1 evidence for the application of 3.0T IoMRI in glioma surgery, both in low-grade and high-grade glioma. In this study, the median final tumor resection was 100% (range, 70.87%–100%; IQR, 100%-100%) in the IoMRI group, significantly different (p =0.001) from 100% resection (range, 51.81%– 100%; IQR, 87.77%–100%) in the control group. Median resection after the first intra-operative MRI scan did not differ between the IoMRI and the control groups (P = 0.93). In a similar study among eloquently located tumors, Senft et al. found that the combination of IoMRI guidance with multimodal neurophysiological monitoring allowed for extended resections in glioma surgery without inducing higher rates of neurological deficits, even in patients with tumors in eloquent areas [26]. Similarly, the post-operative complication rate in our study was low: 10.49% permanent SAND and one surgery-related mortality. Rate of permanent SAND was not significantly different between the two groups. Although we did not investigate for correlation between SAND and any structural feature such as post-operative diffusion weighted imaging (DWI) which may be an interesting area to investigate. This low SAND rate agrees with the established fact that most patients are neurologically stable or improved after either their first or second craniotomy [6]. It also means that the increased EOR achieved with IoMRI use was not at the cost of higher SAND. Although there was no comprehensive data on objective functional status of the patients (Karnofsky performance score) before and after the surgery in our study, overall, available data support the safe use of IoMRI and as an effective adjunct in glioma surgery [6, 8, 15, 16, 26, 31].
Challenges, Limitations and Strength
Although there were younger patients and lower grade tumors in the IoMRI group compared to the no iMRI group, we attempted to minimize the impact of these factors on our estimates via adjusted multivariate analysis. Also, our stringent inclusion criteria which aimed at minimizing possible selection bias resulted in a relatively small study sample size.
One major limitation of this study is the data quality. As a retrospective study, it was limited by the confines of available data that could be included for analysis, which reduced the sample size, limited available data and prevented some further analyses of interest (evaluation of the relationship between IoMRI use and GTR/EOR within the sub-strata of tumor class and age groups). Therefore, these same factors significantly challenge the internal and external validities of our estimates due to absence of data on many important aspects of the analysis including 1p19q codeletion (available only in 43% of cases), IDH mutation status and other oncopanel parameters [5], as well as pre- and post-operative Karnofsky performance score, which might provide better insight on the factors that inform the clinical outcome of patients with primary intracranial gliomas. 1p/19q tumor status is a known powerful predictor of patient survival[9] and a clinically useful marker of prognosis[2]. Similarly, IDH mutation, a known driver of oncogenes, is a promising subject of targeted therapies in these patients[30]. Another limitation is that tumor volumes of the patients of the IoMRI group were segmented on MR images obtained from a 0.5T midfield strength scanner, while the tumor volumes of the patients of the no-IoMRI group were segmented from MR images obtained from a high field 1.5T scanner. This could introduce some potential bias to the study. However Orrison et al., examined blindly interpreted normal and abnormal findings at 0.064T and 1.5T magnetic field strength MR scanners, and found that the sensitivity of brain tumor detection on the ultra-low field scanner was statistically indistinguishable from the sensitivity of tumor detection on the high field scanner [22].
This study has important strengths despite its limitations. One advantage is the spatial arrangement in time, which naturally separated the study population into two distinct cohorts while eliminating the surgeon bias to be more (or less) aggressive with either group. Unspecified, but confounding effects of improvements in clinical care over time would be expected to decrease the significance of our findings since the ioMRI was in use in the earlier time period of this study. Furthermore, this study was performed by various experts who handled the corresponding aspects of the study under a blinded set up (by blinding performers of tumor volumetry to both the patients’ records and the study design), thereby eliminating potential biases while maximizing expertise. Moreover, this study evaluated impact of IoMRI on overall survival of glioma patients over a relatively long follow up period of at least five years. This provided us the opportunity to assess survival over a relatively long time period, and assess its association with IoMRI use in glioma resection.
Conclusion
This study demonstrated a strong association between application of intra-operative MRI and increased rate of Gross Total Resection (GTR) of lesions located in both non-eloquent and eloquent brain areas. Using Intra-operative MRI is associated with improved extent of tumor resection and overall survival, without associated increased risk of permanent surgery-associated neurologic deficit over at least 5-year follow-up. Moreover, it also showed that glioma location within eloquent brain areas and WHO grade may be predictors of achieving GTR. However, more studies are required to further evaluate other factors that affect glioma’s resection rate and these patients’ survival.
Highlights.
-
-
This study has a long follow-up period of at least five years
-
-
Demonstrated strong association of intra-operative MRI (IoMRI) use with better optimization of gloima resection and better overall patient survival
-
-
Showed no association of IoMRI use with increased neurologic deficit
-
-
These associations appeared to be true after adjusting for both WHO-high grade and eloquent brain area located gliomas
Acknowledgements
The authors wish to acknowledge the following people for their valuable contributions to this work:
Kelly Doolin, M.S, Golby Lab, Department of Neurosurgery, Brigham and Women’s Hospital, Boston MA.
Edward Laws, MD. Director, Pituitary and Neuroendocrine Center; Professor, Harvard Medical School, Brigham and Women’s Hospital Boston.
Timothy Smith, MD. Pituitary Fellow Brigham and Women’s Hospital, Boston
Also, this project was supported by funding from:
The National Center for Research Resources and the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health through Grant Numbers P41EB015898, and R25 CA089017
Oversea Study Program of Guangzhou Elite Project
Abbreviations
- A.G
Alexandra Golby, MD
- A.O
Aysegul Ozdemir, MD
- E+
eloquent brain area involvement
- E−
eloquent brain area-sparing
- ETR
extent of tumor resection
- F.I
Fatih Incekara
- IoMRI
intra-operative magnetic resonance imaging
- IRB
institutional review board
- L.H
Liangge Hsu, MD
- nNav
neuronavigation
- O.O
Olutayo Olubiyi, MB.ChB, MPH
- P.B
Perter Black, MD
- S.S
Sandro Santagata, MD, Ph.D
- SAND
surgery associated neurologic deficit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Conflicts of interest:
The authors have no conflicts of interest to disclose.
References
- 1.Albert FK, et al. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery. 1994;34(1):45–60. doi: 10.1097/00006123-199401000-00008. discussion 60-10. [DOI] [PubMed] [Google Scholar]
- 2.Aldape K, Burger PC, Perry A. Clinicopathologic aspects of 1p/19q loss and the diagnosis of oligodendroglioma. Archives of pathology & laboratory medicine. 2007;131(2):242–251. doi: 10.5858/2007-131-242-CAOQLA. [DOI] [PubMed] [Google Scholar]
- 3.Archip N, et al. Non-rigid alignment of pre-operative MRI, fMRI, and DT-MRI with intra-operative MRI for enhanced visualization and navigation in image-guided neurosurgery. Neuroimage. 2007;35(2):609–624. doi: 10.1016/j.neuroimage.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett GH. The role of image-guided technology in the surgical planning and resection of gliomas. Journal of neuro-oncology. 1999;42(3):247–258. doi: 10.1023/a:1006138609201. [DOI] [PubMed] [Google Scholar]
- 5.Beiko J, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro-oncology. 2014;16(1):81–91. doi: 10.1093/neuonc/not159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang S, et al. Glioma Outcomes Investigators: Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg. 2003;98:1175–1181. doi: 10.3171/jns.2003.98.6.1175. [DOI] [PubMed] [Google Scholar]
- 7.Claus EB, et al. Survival rates in patients with low - grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103(6):1227–1233. doi: 10.1002/cncr.20867. [DOI] [PubMed] [Google Scholar]
- 8.DiMaio SP, et al. Image-guided neurosurgery at Brigham and Women's Hospital. Engineering in Medicine and Biology Magazine, IEEE. 2006;25(5):67–73. doi: 10.1109/memb.2006.1705749. [DOI] [PubMed] [Google Scholar]
- 9.Fallon KB, et al. Prognostic Value of 1p, 19q, 9p, 10q, and EGFR - FISH Analyses in Recurrent Oligodendrogliomas. Journal of Neuropathology & Experimental Neurology. 2004;63(4):314–322. doi: 10.1093/jnen/63.4.314. [DOI] [PubMed] [Google Scholar]
- 10.Gering DT, et al. An integrated visualization system for surgical planning and guidance using image fusion and an open MR. Journal of Magnetic Resonance Imaging. 2001;13(6):967–975. doi: 10.1002/jmri.1139. [DOI] [PubMed] [Google Scholar]
- 11.Hatiboglu MA, et al. Impact of Intraoperative High - Field Magnetic Resonance Imaging Guidance on Glioma Surgery: A Prospective Volumetric Analysis. Neurosurgery. 2009;64(6):1073–1081. doi: 10.1227/01.NEU.0000345647.58219.07. [DOI] [PubMed] [Google Scholar]
- 12.Kowalczuk A, et al. Quantitative imaging study of extent of surgical resection and prognosis of malignant astrocytomas. Neurosurgery. 1997;41(5):1028–1038. doi: 10.1097/00006123-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lacroix M, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. Journal of neurosurgery. 2001;95(2):190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 14.Laws E, et al. Springer; 2003. Surgical management of intracranial gliomas—does radical resection improve outcome? [DOI] [PubMed] [Google Scholar]
- 15.Leuthardt EC, et al. Use of movable high-field-strength intraoperative magnetic resonance imaging with awake craniotomies for resection of gliomas: preliminary experience. Neurosurgery. 2011;69(1):194–206. doi: 10.1227/NEU.0b013e31821d0e4c. [DOI] [PubMed] [Google Scholar]
- 16.Liang D, Schulder M. The role of intraoperative magnetic resonance imaging in glioma surgery. Surgical neurology international. 2012;3(Suppl 4):S320. doi: 10.4103/2152-7806.103029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammadi AM, et al. Use of High-Field Intraoperative Magnetic Resonance Imaging to Enhance the Extent of Resection of Enhancing and Nonenhancing Gliomas. Neurosurgery. 2014;74(4):339–350. doi: 10.1227/NEU.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 18.Mori S, et al. Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging. Annals of neurology. 2002;51(3):377–380. doi: 10.1002/ana.10137. [DOI] [PubMed] [Google Scholar]
- 19.Nabavi A, et al. Serial intraoperative magnetic resonance imaging of brain shift. Neurosurgery. 2001;48(4):787–798. doi: 10.1097/00006123-200104000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Nabavi A, et al. Image-guided therapy and intraoperative MRI in neurosurgery. Minimally Invasive Therapy & Allied Technologies. 2000;9(3–4):277–286. doi: 10.1080/13645700009169658. [DOI] [PubMed] [Google Scholar]
- 21.Nimsky C, et al. Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery. 2000;47(5):1070–1080. doi: 10.1097/00006123-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Orrison W, Jr, et al. Comparison of CT, low-field-strength MR imaging, and high-field-strength MR imaging. Work in progress. Radiology. 1991;181(1):121–127. doi: 10.1148/radiology.181.1.1887020. [DOI] [PubMed] [Google Scholar]
- 23.Roberts DW, et al. Intraoperative brain shift and deformation: a quantitative analysis of cortical displacement in 28 cases. Neurosurgery. 1998;43(4):749–758. doi: 10.1097/00006123-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Schneider JP, et al. Intraoperative MRI to guide the resection of primary supratentorial glioblastoma multiforme—a quantitative radiological analysis. Neuroradiology. 2005;47(7):489–500. doi: 10.1007/s00234-005-1397-1. [DOI] [PubMed] [Google Scholar]
- 25.Senft C, et al. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. The lancet oncology. 2011;12(11):997–1003. doi: 10.1016/S1470-2045(11)70196-6. [DOI] [PubMed] [Google Scholar]
- 26.Senft C, et al. Optimizing the extent of resection in eloquently located gliomas by combining intraoperative MRI guidance with intraoperative neurophysiological monitoring. Journal of neuro-oncology. 2012;109(1):81–90. doi: 10.1007/s11060-012-0864-x. [DOI] [PubMed] [Google Scholar]
- 27.Senft C, et al. Low field intraoperative MRI-guided surgery of gliomas: a single center experience. Clinical neurology and neurosurgery. 2010;112(3):237–243. doi: 10.1016/j.clineuro.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Senft C, et al. Usefulness of Intraoperative Ultra Low - Field Magnetic Resonance Imaging in Glioma Surgery. Neurosurgery. 2008;63(4):257–267. doi: 10.1227/01.NEU.0000313624.77452.3C. [DOI] [PubMed] [Google Scholar]
- 29.Stummer W. Extent of Resection and Survival in Glioblastoma Multiforme: Identification of and Adjustment for Bias. Neurosurgery. 2009;64(6):E1206. doi: 10.1227/01.NEU.0000346230.80425.3A. [DOI] [PubMed] [Google Scholar]
- 30.Wakimoto H, et al. Targetable signaling pathway mutations are associated with malignant phenotype in IDH-mutant gliomas. Clinical Cancer Research. 2014;20(11):2898–2909. doi: 10.1158/1078-0432.CCR-13-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J, et al. 142 3.0 T iMRI Guided Resection in Cerebral Glioma Surgery: Interim Analysis of a Prospective, Randomized, Triple-Blind, Parallel-Controlled Trial. Neurosurgery. 2013;60:167–167. doi: 10.1227/NEU.0000000000000372. [DOI] [PubMed] [Google Scholar]