Abstract
Flagellar calcium-binding protein (FCaBP) is a dually acylated Ca2+ sensor in the Trypanosoma cruzi flagellar membrane that undergoes a massive conformational change upon Ca2+ binding. It is similar to neuronal Ca2+ sensors, like recoverin, which regulate their binding partners through a calcium acyl switch mechanism. FCaBP is washed out of permeabilized cells with buffers containing EDTA, indicating Ca2+-dependent flagellar membrane association. We hypothesized that, like recoverin, FCaBP projects its acyl groups in the presence of Ca2+, permitting flagellar membrane and binding partner association and that it sequesters the acyl groups in low Ca2+, disassociating from the membrane and releasing its binding partner to perform a presumed enzymatic function. The x-ray crystal structure of FCaBP suggests that the acyl groups are always exposed, so we set out to test our hypothesis directly. We generated T. cruzi transfectants expressing FCaBP or Ca2+-binding mutant FCaBPE151Q/E188Q and recombinant wildtype and mutant proteins as well. Both FCaBP and FCaBPE151Q/E188Q were found to associate with lipid rafts, indicating the Ca2+-independence of this association. To our initial surprise, FCaBPE151Q/E188Q, like wildtype FCaBP, exhibited Ca2+-dependent flagellar membrane association, even though this protein does not bind Ca2+ itself [16]. One possible explanation for this is that FCaBPE151Q/E188Q, like some other Ca2+ sensors, may form dimers and that dimerization of FCaBPE151Q/E188Q with endogenous wildtype FCaBP might explain its Ca2+-dependent localization. Indeed both proteins are able to form dimers in the presence and absence of Ca2+. These results suggest that FCaBP possesses two distinct Ca2+-dependent interactions—one involving a Ca2+-induced change in conformation and another perhaps involving binding partner association.
Keywords: Calcium, flagellum, dimer, trypanosome
Graphical abstract
The central role of Ca2+ as a regulator of cellular activity is well established [1, 2]. Cell division, motility, gene expression and secretion are regulated by Ca2+ ions and the intracellular Ca2+ concentration must be precisely controlled during these processes. In T. cruzi, parasite proliferation and differentiation [3] and host cell invasion [4, 5] are regulated by Ca2+, and Ca2+-modulated cell signaling pathways similar to those in mammalian cells are present [6]. Moreover, T. cruzi possesses a highly specialized Ca2+-containing organelle, the acidocalcisome [7], which is believed to permit the amastigote stage of the parasite to survive in the low Ca2+ environment of the mammalian cell cytoplasm in which it replicates [8]. Intracellular Ca2+ signals are transduced into biological responses through their interaction with Ca2+-binding proteins (CaBPs) or Ca2+-binding domains in multidomain proteins. T. cruzi has a variety of proteins in this class of signal transducing molecules, including calmodulin [9], calmodulin-binding proteins [10] and calmodulin regulated enzymes [11, 12]. Despite the identification of several Ca2+-regulated processes in T. cruzi, the molecular mechanisms by which specific CaBPs transduce the Ca2+ signals remain to be elucidated.
Flagellar Ca2+ binding protein (FCaBP, TritrypID TcCLB.507491.151) is a 24 kDa highly immunogenic protein found in the flagellum of the protozoan parasite T. cruzi [13]. FCaBP specifically localizes to the flagellar plasma membrane via amino terminal myristoyl and palmitoyl modifications [14] and positively charged lysine residues [15]. FCaBP interacts with lipid raft microdomains [16], which are specifically enriched within the flagellar membrane of T. cruzi [17]. It has been hypothesized that interaction with lipid raft domains is responsible for either targeting or retention of some dually acylated proteins to the flagellar membrane. Additionally, FCaBP contains four EF hand Ca2+-binding motifs, the third and fourth of which bind Ca2+ [16, 18]. Based on its size, acylation, Ca2+ binding and membrane association, we hypothesized that FCaBP is a Ca2+ acyl switch protein. Such proteins undergo Ca2+-dependent membrane association by virtue of Ca2+-regulated extrusion or sequestration of a myristate moiety that mediates membrane binding [19].
The prototypical Ca2+-acyl switch protein is Recoverin (Rv), a myristoylated Ca2+ sensor in retinal rod cells that functions in cellular recovery from photoexcitation [20]. Rv has four EF hands but binds two Ca2+ ions [21] through the second and third EF hand domains [22]. Rv associates in a Ca2+ dependent manner via its amino terminal myristoyl group with the plasma membrane, where it binds to and inhibits the activity of rhodopsin kinase (RK) [23]. When the intracellular Ca2+ level drops upon retinal cell photoexcitation, Ca2+ dissociation from Rv leads to a conformational change and sequestration of the myristoyl group in a hydrophobic cleft [24]. Unable to associate with the membrane, Rv moves off the rod outer segment membrane, freeing RK to phosphorylate and inactivate rhodopsin, the first step in the cellular recovery phase. When the intracellular Ca2+ rises, Rv assumes its Ca2+-bound conformation and returns to the membrane, once again inactivating RK and completing the cycle.
The flagellar membrane localization of FCaBP requires several elements: dual N-terminal acylation with myristate and palmitate [14] and a cluster of nearby basic amino acids [15]. If FCaBP were in fact a protein like Rv that cycles on and off the flagellar membrane, we would expect its membrane association to be directly dependent on Ca2+ binding as well, as it is in Rv. To test this hypothesis, we employed T. cruzi transfectants expressing myc/his-tagged FCaBP or myc-tagged Ca2+ binding mutant FCaBPE151Q/E188Q, which is absolutely devoid of Ca2+ binding. FCaBP binds two molecules of Ca2+ and the E151Q and E188Q mutations disrupt binding at EF-hand domains 3 and 4, respectively [16]. The epitope tags are slightly different (myc and myc/his) because the transfectants were produced previously at different times for separate studies. Since protein localization is not affected by the tags, we did not generate a new transfectant so as to have matched tags. Immunofluorescence microscopy of these cell lines revealed that, like FCaBP-myc/his, the Ca2+ binding mutant FCaBPE151Q/E188Q-myc properly localizes to the flagellum (Fig. 1A), indicating that binding of Ca2+ via the high affinity sites is not required for localization.
Fig. 1.
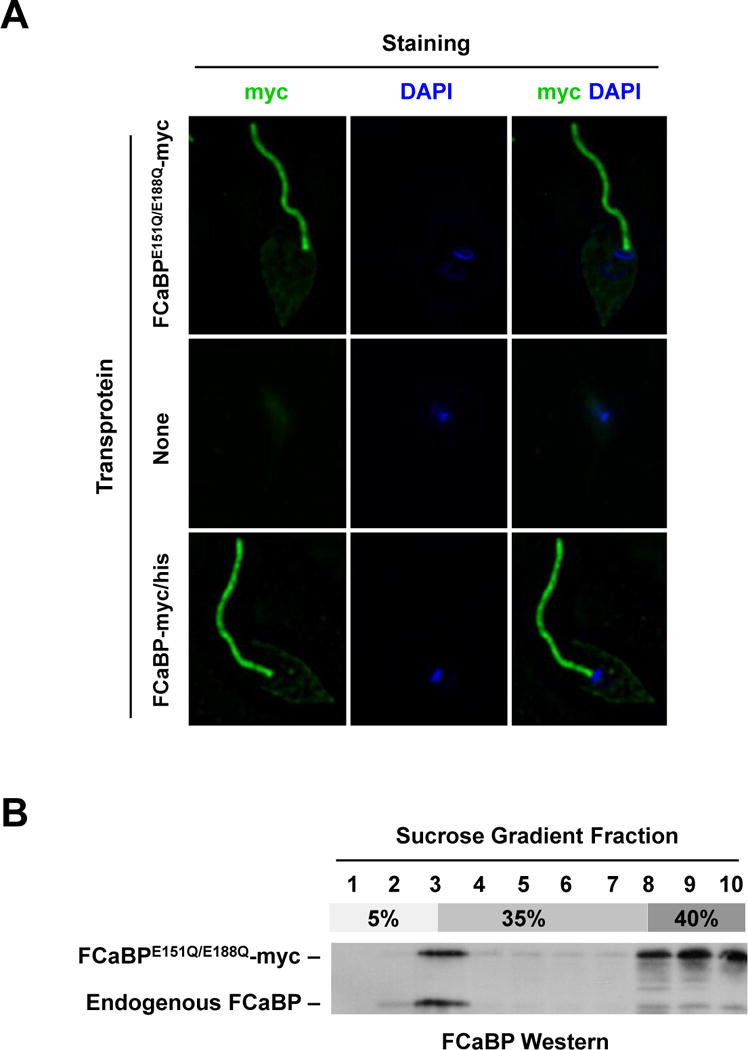
Mutation of the Ca2+ binding sites in FCaBP does not affect flagellar membrane localization or lipid raft association. (A) The flagellar localization of FCaBP is independent of Ca2+ binding. T. cruzi epimastigotes expressing a myc-tagged Ca2+-binding mutant of FCaBP (FCaBPE151Q/E188Q-myc), no transprotein (None) or wildtype myc/his-tagged FCaBP (FCaBP-myc/his) were analyzed by immunofluorescence microscopy using the myc-specific 9E10 monoclonal antibody. Parasite DNA was stained with DAPI. Both FCaBP-myc/his and FCaBPE151Q/E188Q-myc are flagellar. (B) FCaBP associates with lipid rafts independent of Ca2+ binding. T. cruzi epimastigotes expressing the FCaBP Ca2+-binding mutant (FCaBPE151Q/E188Q-myc) were lysed in ice-cold Triton X-100 and analyzed by discontinuous (5% – 35% – 40%) sucrose (Optiprep) gradient centrifugation and western blotting using FCaBP-specific antiserum. Both FCaBPE151Q/E188Q-myc and endogenous FCaBP float to fraction 3, which contains detergent-resistant membranes.
A major factor determining the localization of FCaBP to the flagellar membrane is its association with lipid rafts [15], which are highly enriched in the flagellar membrane [17]. Flagellar membrane association requires dual acylation by myristate and palmitate [14], and these two modifications are also required for lipid raft association [15]. To test whether Ca2+ binding is also required for lipid raft association, we tested whether FCaBPE151Q/E188Q-myc would float on a sucrose gradient. The FCaBPE151Q/E188Q-myc expressing cell line from Fig. 1A was lysed in ice-cold Triton X-100 and analyzed by a discontinuous sucrose density step gradient. Gradient fractions were collected, separated by SDS-PAGE and examined by western blotting using FCaBP-specific antiserum (Fig. 1B). Both the FCaBPE151Q/E188Q-myc transprotein and endogenous FCaBP floated to the 5%–35% interface, indicating that they associate with detergent-resistant membranes (lipid rafts).
One of the defining biophysical properties of FCaBP is that its flagellar membrane association is dependent on Ca2+. This can be assessed in vivo via a Ca2+ chelation slide assay, in which permeabilized trypanosomes are washed in buffers containing or lacking Ca2+ [14]. That FCaBPE151Q/E188Q localizes normally to the flagellar membrane and associates with lipid rafts does not mean that it would exhibit Ca2+-dependent membrane association. To test this, we analyzed FCaBPE151Q/E188Q-myc-expressing cells by the Ca2+ chelation slide assay (Fig. 2A). As was observed with endogenous FCaBP (left panels), FCaBPE151Q/E188Q-myc washed out of the cell upon Ca2+ chelation (right panels), indicating that direct Ca2+ binding by FCaBP is not necessary for the Ca2+-dependent membrane association. Paraflagellar rod (PFR) and FCaBP1–24-GFP (N-terminal acylated peptide fused to GFP) served as controls (middle panels). Despite its inability to bind Ca2+, FCaBP Ca2+-binding mutant surprisingly demonstrated Ca2+-dependent flagellar membrane localization, indicating a second level of Ca2+-modulation of protein localization and possible function. One possible explanation for the behavior of FCaBPE151Q/E188Q in the Ca2+ chelation slide assay is that this Ca2+ binding mutant might form dimers with endogenous wildtype FCaBP. A number of neuronal Ca2+ sensor proteins, (including Rv [25], GCAP2 [26], and DREAM [27, 28]) form dimers and even higher order oligomers. We analyzed both FCaBP and FCaBPE151Q/E188Q by size exclusion chromatography in the presence or absence of Ca2+ (Fig. 2B). Both proteins formed dimers independent of Ca2+.
Fig. 2.
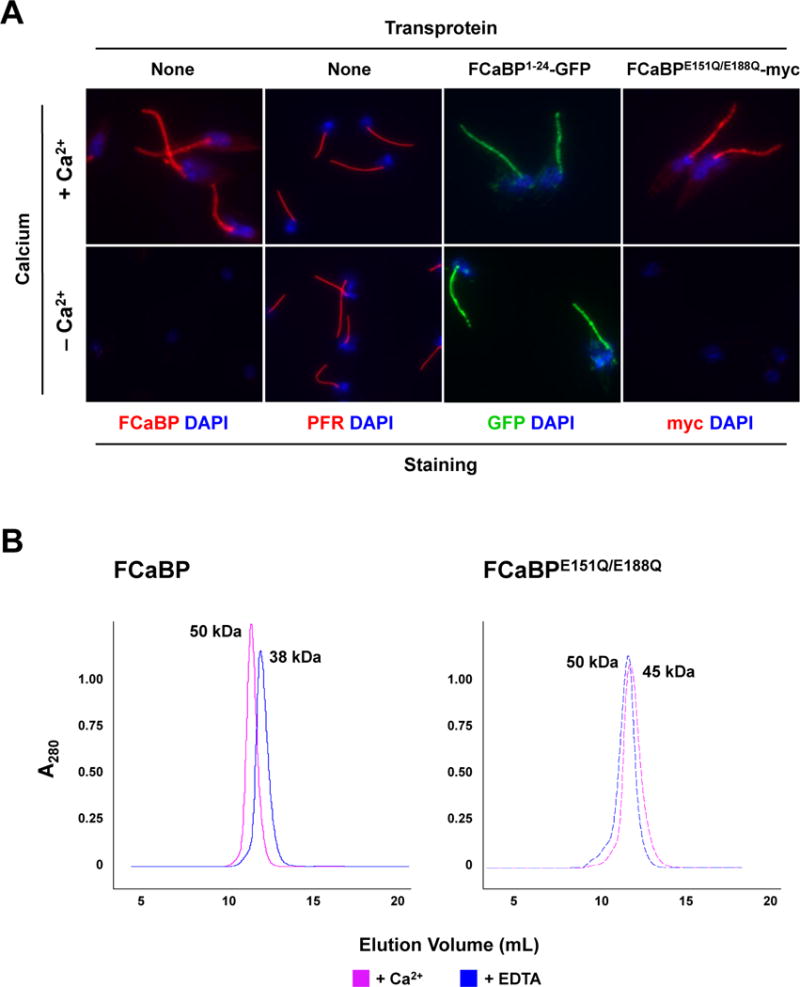
Mutation of the Ca2+ binding sites in FCaBP does not affect Ca2+-dependent flagellar membrane localization, possibly due to dimerization with endogenous wildtype FCaBP. (A) The flagellar retention of FCaBPE151Q/E188Q is Ca2+-dependent. Untransfected epimastigotes and transfectants expressing the N-terminal 24 amino acids of FCaBP fused to GFP (FCaBP1–24-GFP) or the FCaBP Ca2+-binding mutant FCaBPE151Q/E188Q-myc were permeabilized and washed with buffers containing or lacking (EDTA) Ca2+. Cells were then analyzed by direct fluorescence (GFP) or immunofluorescence microscopy with paraflagellar rod [PFR (T. cruzi PAR1)]- or FCaBP-specific antisera. DNA was stained with DAPI. Both endogenous FCaBP and FCaBPE151Q/E188Q-myc were washed out of the flagellum upon Ca2+ chelation. (B) Recombinant wildtype FCaBP and the Ca2+-binding mutant FCaBPE151Q/E188Q form dimers in a Ca2+-independent manner. Size exclusion chromatography of recombinant wildtype FCaBP and Ca2+ binding mutant FCaBPE151Q/E188Q were conducted in the presence of 2 mM CaCl2 (solid line) or 2 mM EDTA (dashed line). The absorbance at 280 nm is plotted vs elution volume. Both wildtype FCaBP and FCaBPE151Q/E188Q elute as dimers in both Ca2+-bound and Ca2+-free states. Multi-angle light scattering confirmed that FCaBP is a dimer in both Ca2+-free and Ca2+-bound states (not shown).
Our previous model of FCaBP regulation was largely based on Rv and other neuronal calcium sensors [16]. This model postulated that the flagellar membrane association and partner protein association of FCaBP are directly determined by Ca2+-binding by FCaBP. It further predicted that the FCaBP Ca2+-binding null mutant would not localize to the flagellum, since, like Rv, the protein might sequester the acyl groups and lose interactions with both the flagellar membrane and the binding partner. X-ray crystallography indicated that the acylated N-terminus of FCaBP is always exposed [29] and NMR studies of calflagin Tb24, a closely related flagellar Ca2+ sensor in the African trypanosome T. brucei, corroborated those findings [30, 31]. Dual acylation with myristate and palmitate [14], an N-terminal polybasic region [15], and lipid raft association [15, 17] are required for flagellar membrane association of FCaBP. Ca2+ is also required, but direct binding via the high affinity Ca2+ binding sites is not. This result may be explained by dimerization of FCaBPE151Q/E188Q to endogenous FCaBP, a possibility that can best be tested by analysis of FCaBPE151Q/E188Q behavior in a cell lacking endogenous FCaBP. This can now be explored experimentally with the advent of CRISPR-Cas9-mediated genome editing in T. cruzi [32]. Another intriguing possibility is that FCaBP participates in Ca2+-dependent partner protein association. While the association of Rv with RK is depending on Ca2+-binding by the high affinity sites in Rv, this may not be true of FCaBP. We found that both FCaBP and FCaBPE151Q/E188Q associate with proteins of 80-kDa and 30-kDa in a Ca2+-dependent manner [16]. Identification of these binding partners will permit this possibility to be tested directly.
Highlights.
-
►
FCaBP is a flagellar calcium sensor of Trypanosoma cruzi that displays calcium-dependent membrane association.
-
►
An FCaBP mutant that does not bind calcium still shows calcium-dependent membrane association.
-
►
FCaBP can form dimers, so the FCaBP calcium-binding mutant is still flagellar because it can dimerize with resident FCaBP.
Acknowledgments
This work was supported in part by research grants R01-GM93359 (DME) and R01-EY012347 (JBA) from the National Institutes of Health. DM was supported by predoctoral fellowships 0910101G and 11PRE5300002 from the American Heart Association.
References
- 1.Rasmussen H. The calcium messenger system (1) N Engl J Med. 1986;314:1094–101. doi: 10.1056/NEJM198604243141707. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen H. The calcium messenger system (2) N Engl J Med. 1986;314:1164–70. doi: 10.1056/NEJM198605013141807. [DOI] [PubMed] [Google Scholar]
- 3.Lammel EM, Barbieri MA, Wilkowsky SE, Bertini F, Isola EL. Trypanosoma cruzi: involvement of intracellular calcium in multiplication and differentiation. Exp Parasitol. 1996;83:240–9. doi: 10.1006/expr.1996.0070. [DOI] [PubMed] [Google Scholar]
- 4.Yakubu MA, Majumder S, Kierszenbaum F. Changes in Trypanosoma cruzi infectivity by treatments that affect calcium ion levels. Mol Biochem Parasitol. 1994;66:119–25. doi: 10.1016/0166-6851(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 5.Moreno SN, Silva J, Vercesi AE, Docampo R. Cytosolic-free calcium elevation in Trypanosoma cruzi is required for cell invasion. J Exp Med. 1994;180:1535–40. doi: 10.1084/jem.180.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Docampo R, Pignataro OP. The inositol phosphate/diacylglycerol signalling pathway in Trypanosoma cruzi. Biochem J. 1991;275:407–11. doi: 10.1042/bj2750407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Docampo R, de Souza W, Miranda K, Rohloff P, Moreno SN. Acidocalcisomes - conserved from bacteria to man. Nat Rev Microbiol. 2005;3:251–61. doi: 10.1038/nrmicro1097. [DOI] [PubMed] [Google Scholar]
- 8.Lu HG, Zhong L, de Souza W, Benchimol M, Moreno S, Docampo R. Ca2+ content and expression of an acidocalcisomal calcium pump are elevated in intracellular forms of Trypanosoma cruzi. Mol Cell Biol. 1998;18:2309–23. doi: 10.1128/mcb.18.4.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung SH, Swindle J. Linkage of the calmodulin and ubiquitin loci in Trypanosoma cruzi. Nucleic Acids Res. 1990;18:4561–9. doi: 10.1093/nar/18.15.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orr GA, Tanowitz HB, Wittner M. Trypanosoma cruzi: stage expression of calmodulin-binding proteins. Exp Parasitol. 1992;74:127–33. doi: 10.1016/0014-4894(92)90039-d. [DOI] [PubMed] [Google Scholar]
- 11.Ogueta SB, Solari A, Tellez-Inon MT. Trypanosoma cruzi epimastigote forms possess a Ca2+-calmodulin dependent protein kinase. FEBS Lett. 1994;337:293–7. doi: 10.1016/0014-5793(94)80212-2. [DOI] [PubMed] [Google Scholar]
- 12.Tellez-Inon MT, Ulloa RM, Torruella M, Torres HN. Calmodulin and Ca2+-dependent cyclic AMP phosphodiesterase activity in Trypanosoma cruzi. Mol Biochem Parasitol. 1985;17:143–53. doi: 10.1016/0166-6851(85)90013-1. [DOI] [PubMed] [Google Scholar]
- 13.Engman DM, Krause K-H, Blumin JH, Kim KS, Kirchhoff LV, Donelson JE. A novel flagellar Ca2+-binding protein in trypanosomes. J Biol Chem. 1989;264:18627–31. [PubMed] [Google Scholar]
- 14.Godsel LM, Engman DM. Flagellar protein localization mediated by a calcium-myristoyl/palmitoyl switch mechanism. EMBO J. 1999;18:2057–65. doi: 10.1093/emboj/18.8.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maric D, McGwire BS, Buchanan KT, Olson CL, Emmer BT, Epting CL, et al. Molecular determinants of ciliary membrane localization of Trypanosoma cruzi flagellar calcium-binding protein. J Biol Chem. 2011;286:33109–17. doi: 10.1074/jbc.M111.240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchanan KT, Ames JB, Asfaw SH, Wingard JN, Olson CL, Campana PT, et al. A flagellum-specific calcium sensor. J Biol Chem. 2005;280:40104–11. doi: 10.1074/jbc.M505777200. [DOI] [PubMed] [Google Scholar]
- 17.Tyler KM, Fridberg A, Toriello KM, Olson CL, Cieslak JA, Hazlett TL, et al. Flagellar membrane localization via association with lipid rafts. J Cell Sci. 2009;122:859–66. doi: 10.1242/jcs.037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maldonado RA, Mirzoeva S, Godsel LM, Lukas TJ, Goldenberg S, Watterson DM, et al. Identification of calcium binding sites in the trypanosome flagellar calcium acyl switch protein. Mol Biochem Parasitol. 1999;101:61–70. doi: 10.1016/s0166-6851(99)00055-9. [DOI] [PubMed] [Google Scholar]
- 19.Ames JB, Ishima R, Tanaka T, Gordon JI, Stryer L, Ikura M. Molecular mechanics of calcium-myristoyl switches. Nature. 1997;389:198–202. doi: 10.1038/38310. [DOI] [PubMed] [Google Scholar]
- 20.Kawamura S, Hisatomi O, Kayada S, Tokunaga F, Kuo CH. Recoverin has S-modulin activity in frog rods. J Biol Chem. 1993;268:14579–82. [PubMed] [Google Scholar]
- 21.Ames JB, Porumb T, Tanaka T, Ikura M, Stryer L. Amino-terminal myristoylation induces cooperative binding of calcium binding to recoverin. J Biol Chem. 1995;270:4526–33. doi: 10.1074/jbc.270.9.4526. [DOI] [PubMed] [Google Scholar]
- 22.Ames JB, Tanaka T, Ikura M, Stryer L. Nuclear magnetic resonance evidence for Ca2+ induced extrusion of the myristoyl group of recoverin. J Biol Chem. 1995;270:30909–13. doi: 10.1074/jbc.270.52.30909. [DOI] [PubMed] [Google Scholar]
- 23.Calvert PD, Klenchin VA, Bownds MD. Rhodopsin kinase inhibition by recoverin. Function of recoverin myristoylation. J Biol Chem. 1995;270:24127–9. doi: 10.1074/jbc.270.41.24127. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka T, Ames JB, Harvey TS, Stryer L, Ikura M. Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state. Nature. 1995;376:444–7. doi: 10.1038/376444a0. [DOI] [PubMed] [Google Scholar]
- 25.Myers WK, Xu X, Li C, Lagerstedt JO, Budamagunta MS, Voss JC, et al. Double electron-electron resonance probes Ca2+-induced conformational changes and dimerization of recoverin. Biochemistry. 2013;52:5800–8. doi: 10.1021/bi400538w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olshevskaya EV, Ermilov AN, Dizhoor AM. Dimerization of guanylyl cyclase-activating protein and a mechanism of photoreceptor guanylyl cyclase activation. J Biol Chem. 1999;274:25583–7. doi: 10.1074/jbc.274.36.25583. [DOI] [PubMed] [Google Scholar]
- 27.Osawa M, Dace A, Tong KI, Valiveti A, Ikura M, Ames JB. Mg2+ and Ca2+ differentially regulate DNA binding and dimerization of DREAM. J Biol Chem. 2005;280:18008–14. doi: 10.1074/jbc.M500338200. [DOI] [PubMed] [Google Scholar]
- 28.Lusin JD, Vanarotti M, Li C, Valiveti A, Ames JB. NMR structure of DREAM: Implications for Ca2+-dependent DNA binding and protein dimerization. Biochemistry. 2008;47:2252–64. doi: 10.1021/bi7017267. [DOI] [PubMed] [Google Scholar]
- 29.Wingard JN, Ladner J, Vanarotti M, Fisher AJ, Robinson H, Buchanan KT, et al. Structural insights into membrane targeting by the flagellar calcium-binding protein (FCaBP), a myristoylated and palmitoylated calcium sensor in Trypanosoma cruzi. J Biol Chem. 2008;283:23388–96. doi: 10.1074/jbc.M803178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Olson CL, Engman DM, Ames JB. NMR structure of the calflagin Tb24 flagellar calcium binding protein of Trypanosoma brucei. Protein Sci. 2012;21:1942–7. doi: 10.1002/pro.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Olson CL, Engman DM, Ames JB. 1H, 15N, and 13C chemical shift assignments of the calflagin Tb24 flagellar calcium binding protein of Trypanosoma brucei. Biomol NMR Assign. 2013;7:9–12. doi: 10.1007/s12104-012-9366-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng D, Kurup SP, Yao PY, Minning TA, Tarleton RL. CRISPR-Cas9-mediated single-gene and gene family disruption in Trypanosoma cruzi. MBio. 2014;6 doi: 10.1128/mBio.02097-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


