Abstract
Abuse of and addiction to psychostimulants remains a challenging clinical issue, yet no effective pharmacotherapy is available. Trace amine associated receptor 1 (TAAR 1) is increasingly recognized as a novel drug target that participates in the modulation of drug abuse. This review analyzed existing preclinical evidence from electrophysiological, biochemical to behavioral aspects regarding the functional interactions between TAAR 1 and dopaminergic system. TAAR 1 knockout mice demonstrate increased sensitivity to dopaminergic activation while TAAR 1 agonists reduce the neurochemical effects of cocaine and amphetamines, attenuate abuse- and addiction-related behavioral effects of cocaine and methamphetamine. It is concluded that TAAR 1 activation functionally modulate the dopaminergic activity and TAAR 1 agonists appear to be promising pharmacotherapies against psychostimulant addiction.
Keywords: TAAR 1, addiction, cocaine, methamphetamine, behavior
1. Introduction
Psychostimulant addiction remains among the most devastating disease in which compulsive drug-seeking and drug taking behaviors persist despite serious negative consequences. Although great progress has been made in understanding the neurobiology, biochemistry, and behavioral pharmacology of psychostimulant abuse, to date effective medicine is still lacking. One of the major problems with psychostimulant addiction is high rates of relapse even after prolonged drug abstinence (Ghitza, 2015; Marlatt, 1990). It is thought that dopaminergic, serotonergic and glutamatergic systems play important roles in reward-motivated behavior (Goldstein et al., 2009; Haleem, 2013; Quintero, 2013). A large body of preclinical and clinical studies have focused on these systems in the development of potential medications to treat psychostimulant addiction, but there is no treatment that directly modulates these systems and have substantially helped curb relapse. Studying alternative targets that indirectly modulate the dopaminergic, serotonergic or glutamatergic systems may provide a novel strategy.
Trace amine associated-receptors (TAARs) were discovered in 2001 with TAAR 1 as the first cloned and characterized member (Borowsky et al., 2001; Bunzow et al., 2001). Although all other TAARs serve predominantly or exclusively as chemosensory receptors in the main olfactory system (Liberles and Buck, 2006), TAAR 1 has been demonstrated to be a novel modulator of dopaminergic, serotonergic and glutamatergic activity and is the most studied member of TAARs (Bradaia et al., 2009; Leo et al., 2014; Lindemann et al., 2008; Revel et al., 2011a). TAAR 1 is largely located in the intracellular compartments both in neurons (Miller, 2011), in glial cells (Cisneros and Ghorpade, 2014) and in peripheral tissues (Grandy, 2007), and is a stimulatory G protein-coupled receptor that responds to so-called trace amines. Activation of TAAR 1 results in intracellular cAMP signaling that contributes to protein kinase A and protein kinase C phosphorylation of downstream targets (Berry, 2007; Grandy, 2007; Xie and Miller, 2007). Trace amines, including p-tyramine, β-phenylethylamine, tryptamine, and octopamine, represent a group of endogenous amines structurally closely related to the classical neurotransmitters such as dopamine, serotonin, and noradrenaline (Burchett and Hicks, 2006). Although trace amines exist in the vertebrate central nervous system at concentrations approximately 1000 times lower than those of catecholamines (Berry, 2004; Philips et al., 1978), previously being denoted as “false transmitters”, abnormal levels of trace amines have been implicated in schizophrenia, anxiety, depression, Parkinson’s disease, attention deficit hyperactivity disorder, drug abuse and dependence (Berry, 2007; Boulton, 1980; Greenshaw, 1984; Lapin, 1993; Narang et al., 2011; Shannon and Thompson, 1984). Since TAAR 1 was first discovered to be potently activated by trace amines, this receptor has been increasingly recognized as a potential therapeutic target of psychiatric disorders.
Accumulating evidence has suggested that TAAR 1 may play a key role in drug abuse and addiction. It has been hypothesized that TAAR 1 functions as a molecular “brake” to control the addiction-related effects of psychostimulants. However, many questions with regard to the underlying mechanisms remain unknown. In this review, we will primarily focus on the role of TAAR 1 in psychostimulant abuse and addiction. We will review results of studies using transgenic mice lacking Taar 1 (Taar 1−/− mice) or overexpressing Taar 1 (Taar 1 Tg mice), as well as TAAR 1 agonists and antagonists to elucidate the role of this target in psychostimulant abuse and addiction. We also briefly discussed future directions of this line of research.
2. TAAR 1 expression and distribution in the brain
In rodent and primate brain, there is an anatomical overlap between TAAR 1 expression and dopaminergic as well as serotonergic brain structures. Borowsky et al first detected a characteristic distribution of TAAR 1 mRNA in the mouse central nerves system using in situ hybridization histochemistry approach, with higher expression found in several monoaminergic cell groups such as the dorsal raphe, the locus coeruleus, and the ventral tegmental area (VTA) (Borowsky et al., 2001). Xie et al also reported a widespread distribution of TAAR 1 mRNA and protein in the rhesus monkey brain, including various regions throughout the monoaminergic systems (the dorsal and ventral caudate nucleus, putamen, substantia nigra, nucleus accumbens, VTA, locus coeruleus, amygdala, and raphe nucleus) (Xie et al., 2007). Similar observations were also made by Lindemann et al who analyzed the TAAR 1 expression in mice brain using LacZ signals. TAAR 1-positive cell bodies were detected in the VTA, the amygdala, the bed nucleus of the stria terminals, and the dorsal raphe nucleus (Lindemann et al., 2008). Because such brain regions as VTA, amygdala, nucleus accumbens and dorsal raphe nucleus are all critically involved in drug reinforcement and addiction, these anatomical observations are consistent with the notion that TAAR 1 might play a role in the control of addiction-related behavior.
3. TAAR 1 functionality: lessons learned from genetically-modified mice
The understanding of the physiological functions of TAAR 1 has been attempted through the generation of transgenic mice lacking TAAR 1. Taar 1−/− mice are generally healthy with no differences on the body weight, body temperature, locomotor activity, life span and other normal behaviors (e.g., nest-building behavior) as compared to wild type mice, although a deficit on prepulse inhibition was found in Taar 1−/− mice (Lindemann et al., 2008; Wolinsky et al., 2007). Moreover, Revel et al generated a transgenic mouse line overexpressing TAAR 1 in the brain (Taar 1 Tg mice) and they do not show overt behavioral abnormalities (Revel et al., 2012a). Thus, central TAAR 1 does not seem to be a vital system for the survival and general well-being of mice. However, more detailed studies revealed aberrant changes of the central nervous system, particular the mesolimbic system, as will be discussed below.
3.1 TAAR 1 and dopaminergic interaction: electrophysiological and neurochemical evidence
Electrophysiological recordings revealed burst firing frequency of dopaminergic neurons in the VTA (Lindemann et al., 2008; Revel et al., 2011a) and serotonergic neurons in the dorsal raphe nucleus (DRN) (Revel et al., 2011) and the firing frequency from Taar 1−/− mice was higher than wild type, suggesting that TAAR 1 tonically inhibits the firing frequency of dopaminergic or serotonergic neurons. Consistent with an increased firing rate of dopaminergic neuron observed in Taar 1−/− mice, recently developed selective TAAR 1 ligands (vide infra) also alter the firing rate of these neurons, with TAAR 1 agonists with higher efficacy reducing, while TAAR 1 agonists with lower efficacy and TAAR 1 antagonists enhancing the firing rate. For example, the selective TAAR 1 antagonist EPPTB increased the firing rate of dopaminergic neurons and prevented the p-tyramine-mediated inhibition of dopaminergic neuron firing (Bradaia et al., 2009). Besides, application of TAAR 1 partial agonist RO5203648 and RO5263397 also augmented the firing frequency of VTA dopaminergic and DRN serotonergic neurons in slices from wild type mice but not from Taar 1−/− mice (Bradaia et al., 2009; Revel et al., 2011; Revel et al., 2013). In contrast, both the endogenous agonist p-tyramine and the selective TAAR 1 agonist RO5166017 and RO5256390 inhibited the burst firing rate of VTA dopaminergic and DRN serotonergic neurons in wild-type but not in Taar 1−/− mice (Lindemann et al., 2008; Revel et al., 2011; Revel et al., 2013), which could be blocked by TAAR 1 antagonist EPPTB (Revel et al., 2011). Together, these findings mainly support the notion that TAAR 1 normally exerts an inhibitory tone on dopaminergic and serotonergic neurons. Unexpectedly, in mouse ectopically overexpressing TAAR 1 in the brain, elevated burst firing was observed in the VTA dopaminergic neurons and DRN serotonergic neurons (Revel et al., 2012a). It was found that ectopically expressing TAAR 1 in VTA GABA neurons functionally reduced the electrical activity of these neurons, which subsequently decreased the inhibitory inputs of GABA neurons to dopamine (DA) neurons (Revel et al., 2012a).
A wealth of evidence suggests that TAAR 1 is an important modulator of monoamine neurotransmission (Bradaia et al., 2009; Lindemann et al., 2005; Revel et al., 2012a; Revel et al., 2011). In both wild-type and Taar 1−/− mice, amphetamine produced a robust increase in striatal release of DA, norepinephrine (NE), and serotonin (5-HT), but this effect was significantly greater in Taar 1−/− mice than in wild type mice (Lindemann et al., 2008; Wolinsky et al., 2007). These findings support the notion that TAAR 1 functionally operates as a negative modulator of monoaminergic neurotransmission. The basal DA and NE extracellular levels in the nucleus accumbens (NAc) and 5-HT in the medial prefrontal cortex were significantly higher in the Taar 1 Tg mice than in the wild-type mice, which may be due to the elevated burst firing activity in the monoaminergic nuclei (Revel et al., 2012a). However, amphetamine had no effect on the release of DA and NA in the NAc of Taar 1 Tg mice, which partially explains the finding that amphetamine-induced a weaker locomotor hyperactivity in Taar 1 Tg mice as compared to their wild type counterparts.
Psychostimulants like cocaine, amphetamine and methamphetamine interact with dopamine transporter (DAT) to induce an enhancement of extracellular DA level, which is one of the major mechanisms that contribute to psychostimulant abuse and addiction (Desai et al., 2005; Salahpour et al., 2008; Xie and Miller, 2009). Therefore the DAT has been considered a potential drug target for the treatment of psychostimulant addiction. Ex vivo studies have shown that TAAR 1 is co-expressed with DAT in a subset of DA neurons in both rhesus monkey and mouse substantia nigra (Xie et al., 2007). There are evidence that TAAR 1 can modulate DAT function in in vitro cell culture experiments. Xie et al reported that β-phenethylamine (β-PEA) inhibited uptake and induced efflux of monoamines in thalamic synaptosomes of rhesus monkeys and wild-type mice, but not in synaptosomes of Taar 1−/− mice. Furthermore, the effect of β-PEA on efflux was blocked by transporter inhibitors in either the transfected cells or wild-type mouse synaptosomes (Xie and Miller, 2008). They also found that methamphetamine inhibited DA uptake, enhanced dopamine efflux, and induced DAT internalization by acting as a TAAR 1 agonist (Xie and Miller, 2009). However, more recent studies show inconsistent results. Leo et al. found that the DA clearance was not changed in Taar 1−/− mice as compared to their wild type counterparts (Leo et al., 2014), suggesting that the DAT function remained intact. In addition, no significant changes of dopamine uptake were observed in slices from Taar 1−/− mice, suggesting that the effect of TAAR 1 activation on DA-related function is independent of DAT (Leo et al., 2014). These apparent discrepancies may be attributable to the assays and tissues used in the studies. Xie et al. used mouse and monkey cellular synaptosome preparations with tissues from putamen and thalamus (Xie and Miller, 2008) while Leo et al. used fast scan cyclic voltammetry to measure DA uptake in mouse striatal slices (Leo et al., 2014).
Besides the inconsistent findings regarding TAAR 1-DAT interactions, recent reports suggest the existence of robust reciprocal interactions between TAAR 1 and D2R (Bradaia et al., 2009; Espinoza et al., 2011; Ledonne et al., 2010; Leo et al., 2014; Wolinsky et al., 2007). Firstly, TAAR 1 inactivation was found to enhance D2R activity. Using genetic approach, Wolinsky et al found that Taar 1−/− mice exhibited an increase in the high-affinity states of striatal D2R, which is indicative of DA supersensitivity and likely contributes to their enhanced response to amphetamine (Wolinsky et al., 2007). Similar results were observed using a pharmacological antagonist of TAAR 1, EPPTB (Bradaia et al., 2009). EPPTB increased the potency of the D2R agonist quinpirole and increased the affinity of DA at D2R (Bradaia et al., 2009). Secondly, D2R inactivation increased TAAR 1 activity. Espinoza et al found that D2R antagonists haloperidol, raclopride, and amisulpride all enhanced β-PEA/TAAR 1-mediated production of cAMP in a G protein-dependent and D2R-selective manner (Espinoza et al., 2011). These reciprocal interactions between TAAR 1 and D2R could be explained by the fact that both receptors can form functional heterodimers in human embryonic kidney 293 cells which can be disrupted by the D2R antagonist haloperidol. In support of this notion, haloperidol-induced catalepsy and striatal c-Fos expression are reduced in Taar 1−/− mice (Espinoza et al., 2011). Together, these data suggest a reciprocal inhibitory interaction between TAAR 1 and D2R which maintains the homeostasis of the dopaminergic system.
Interestingly, Revel et al observed an interaction between TAAR 1 and 5-HT1A autoreceptors (Leo et al., 2014). The 5-HT1A receptor is a subtype of 5-HT receptor that binds the endogenous neurotransmitter serotonin and mediates inhibitory neurotransmission. Activation of 5-HT1A could decrease impulsivity (Winstanley et al., 2005) and inhibit drug-seeking behavior (Carey et al., 2005; Muller et al., 2007). Activation of TAAR 1 with a TAAR 1 agonist, RO5166017, increased the potency of the 5-HT1A partial agonist ipsapirone, whereas blocking TAAR 1 by the TAAR 1 antagonist, EPPTB, resulted in a decrease in ipsapirone potency (Revel et al., 2011). These results suggest that TAAR 1 and 5-HT1A receptors are functionally coupled. Interestingly, this functional interaction is opposite to that with D2 receptors, and this differential modulation of TAAR 1 on D2 and 5-HT1A receptors could have important functional implications and offer new therapeutic possibilities.
Dopamine plays an important role in the initiation, maintenance and relapse/reinstatement of drug addiction (Shaham, Shalev et al. 2003). The evidence discussed above has shown that TAAR 1 plays a homoeostatic regulatory role in the dopaminergic system that prevents the excessive activity of DA neuron, presumably via the modulation of the burst firing of dopaminergic neurons and DAT or interaction with the D2 DA receptors. It is reasonable to hypothesize that TAAR 1 may be closely involved in the modulation of behavioral effects of drugs of abuse such as psychostimulants. Recently, Pei et al used fast cycling voltammetry to examine the effect of the TAAR 1 agonist, RO5203648, on cocaine-induced DA outflow from the rat nucleus accumbens slices (Pei et al., 2014). Cocaine elicited an enhancement in evoked DA outflow and RO5203648 significantly inhibited cocaine-induced DA efflux. This is consistent with the hypothesis and could explain the observations that TAAR 1 activation reduced various addiction-related behavioral effects of cocaine and methamphetamine (vide infra).
3.2 TAAR 1 and dopaminergic interaction: behavioral evidence
Taar 1−/− mice displayed a behavioral phenotype of supersensitivity to amphetamine and cocaine, as evidenced by drug-induced increases in both locomotor activity and rearing as compared with wild-type littermates (Lindemann et al., 2008; Wolinsky et al., 2007). In contrast, Taar Tg mice responded to amphetamine in an opposite manner and showed a reduced and delayed response to amphetamine as compared with wild-type mice, indicating that Taar Tg mice are hyposensitive to amphetamine (Revel et al., 2012a). Methamphetamine also produced similar behavioral outcome in Taar 1−/− mice (Achat-Mendes et al., 2012). In addition, behavioral sensitization induced by repeated amphetamine or methamphetamine exposure was more robust in Taar 1−/− mice than in wild-type mice (Achat-Mendes et al., 2012). In studies measuring the rewarding effects of drugs using a conditioned place preference (CPP) paradigm, Taar 1−/− mice acquired methamphetamine-induced CPP earlier than wild-type mice and exhibited higher CPP score during extinction training sessions, which suggests that the conditioned rewarding effect of methamphetamine is greater in Taar 1−/− mice (Achat-Mendes et al., 2012). In Taar 1−/− mice, animals drank more methamphetamine and exhibited insensitivity to the aversive property of methamphetamine as compared to the wild-type mice (Harkness et al., 2015), suggesting that TAAR 1 function modulates methamphetamine consumption. Interestingly, there was no difference between those two genotypes of mice in morphine CPP (Achat-Mendes et al., 2012), suggesting an interesting differentiation between morphine and psychostimulants. Opioid addiction and stimulant addiction are behaviorally and neurobiological distinct (Badiani et al., 2011), and future studies should disentangle this difference. For example, because pharmacological activation of TAAR 1 with agonists attenuates addiction-related behavioral effects of psychostimulants (vide infra), it would be important to see whether similar manipulations also attenuate addiction-related effects of morphine. All the data from Taar 1−/− mice and Taar Tg mice strongly suggest that TAAR 1 may play a critical role in the abuse-related behavioral effects of psychostimulants and that TAAR 1 may represent a novel target for the pharmacological intervention of psychostimulant addiction.
Recently, Sukhanov et al compared the behavioral effects of the nonselective dopamine receptor agonist apomorphine in wild-type and Taar 1−/− mice. Unlike cocaine or amphetamine, no behavioral supersensitivity to apomorphine was observed in Taar 1−/− mice. In contrast, it was found that apomorphine-induced stereotypies and climbing behaviors were lower in Taar 1−/− mice (Sukhanov et al., 2014). Furthermore, activation of TAAR 1 by RO5166017 increased climbing induced by co-activation of D1 and D2 dopamine receptor (Sukhanov et al., 2014), which lends further evidence to the functional interaction between TAAR 1 and dopamine receptors.
4 TAAR 1 ligands and the behavioral pharmacology of drugs of abuse
Recently, a number of pharmacologically highly selective TAAR 1 agonists were developed and their pharmacological effects on addiction-related behaviors have been reported, supporting the therapeutic potential of TAAR 1 (Table 1). All the TAAR 1 agonists are highly selective on TAAR 1 against a panel of more than 100 other receptors, enzymes, ion channels and binding sites. Readers interested in the detailed receptor binding and selectivity data of these ligands are referred to the cited references.
Table 1.
Modulation of TAAR 1 on behavioral effects of psychostimulants.
| Behavioral Model | Drug | Species | Treatment | Effect | Reference |
|---|---|---|---|---|---|
| Self-administration | Cocaine | Rats | RO5203648 | Attenuation of cocaine taking | (Revel et al., 2012i) |
| Rats | RO5203648 | Attenuation of context- and drug-induced relapse | (Pei et al., 2014) | ||
| Rats | RO5256390 | Attenuation of context- and drug-induced relapse | (Pei et al., 2014) | ||
| Rats | RO5263397 | Attenuation of cue- and drug-induced reinstatement | (Thorn et al., 2014a) | ||
| Methamphetamine | Rats | RO5263397 | Attenuation of methamphetamine taking | (Jing et al., 2015) | |
| Rats | RO5263397 | Attenuation of cue- and drug-induced reinstatement | (Jing et al., 2015) | ||
| Behavioral sensitization | Cocaine | Rats | RO5263397 | Attenuation of induction and expression of behavioral sensitization | (Thorn et al., 2014a; Thorn et al., 2014h) |
| Methamphetamine | Rats | RO5263397 | Attenuation of expression of behavioral sensitization | (Jing et al., 2015) | |
| CPP | Cocaine | Rats | RO5263397 | Attenuation of expression, but not development of cocaine CPP | (Thorn et al., 2014a) |
| Methamphetamine | Mice | Taar−/− | Magnitude of METH-induced CPP in Taar−/−>WT | (Achat-Mendes et al., 2012e) | |
| Acquisition time of METH-induced CPP: Taar−/−< WT | |||||
| Retention time of METH-induced CPP: Taar−/−> WT | |||||
| No difference in METH-induced reinstatement of CPP | |||||
| Morphine | Mice | Taar−/− | No difference in Magnitude of morphine-induced CPP | (Achat-Mendes et al., 2012e) | |
| Locomotion | d-amphetamine | Mice | Taar 1 Tg | Reduced response to AMPH | (Revel et al., 2012a) |
| d-amphetamine | Mice | RO5073012+ Taar 1 Tg | Enhancement of sensitivity to AMPH | (Revel et al., 2012a) | |
| Mice | RO5073012 | Attenuation of AMPH-induced hyperlocomotion | (Revel et al., 2012a) | ||
| Mice | Taar−/− | Enhancement of sensitivity to AMPH (lower dose) | (Wolinsky et al., 2007) | ||
| Mice | Taar−/− | Enhancement of sensitivity to AMPH | (Lindemann et al., 2008) | ||
| Mice | Taar−/− | AMPH-induced hyperactivity in Taar−/−>WT | (Achat-Mendes et al., 2012e) | ||
| Cocaine | Rats | RO5203648 | Attenuation of cocaine-induced hyperlocomotion | (Revel et al., 2012i) | |
| Mice | RO5203648 | Attenuation of cocaine-induced hyperlocomotion | (Revel et al., 2012i) | ||
| Mice | RO5203648+ DAT−/− | Attenuation of hyperlocomotion | (Revel et al., 2012i) | ||
| Mice | RO5203648+ DAT−/−/ Taar−/− | No effect on hyperlocomotion | (Revel et al., 2012i) | ||
| Mice | RO5166017 | Attenuation of cocaine-induced hyperlocomotion | (Revel et al., 2011l) | ||
| Mice | RO5166017+ DAT−/− | Attenuation of DAT−/−-induced hyperlocomotion | (Revel et al., 2011l) | ||
| Mice | RO5166017+ DAT−/−/ Taar−/− | No effect on hyperlocomotion | (Revel et al., 2011l) | ||
| Rats | RO5073012 | Attenuation of cocaine-induced hyperlocomotion | (Galley et al., 2012) | ||
| Mice | RO5166017 | Attenuation of cocaine-induced hyperlocomotion | (Revel et al., 2011l) | ||
| Mice | RO5166017+ Taar−/− | No effect on cocaine-induced hyperlocomotion | (Revel et al., 2011l) | ||
| Mice | RO5166017+ DAT−/− | Attenuation of DAT−/−-induced hyperlocomotion | (Revel et al., 2011a) | ||
| Mice | RO5166017 | Enhancement of climbing induced by co-activation of D1 and D2 dopamine receptor | (Sukhanov et al., 2014) | ||
| Mice | RO5256390 | Attenuation of cocaine, PCP, and L-687,414- induced hyperlocomotion | (Revel et al., 2013) | ||
| Mice | RO5263397 | Attenuation of cocaine, PCP, and L-687,414- induced hyperlocomotion | (Revel et al., 2013) | ||
| Mice | RO5203648 | Attenuation of cocaine-induced hyperlocomotion | (Revel et al., 2012i) | ||
| Rats | RO5203648 | Attenuation of cocaine-induced hyperlocomotion | (Revel et al., 2012i) |
4.1 TAAR 1 and behavioral sensitization to psychostimulants
In rodents, administration of psychostimulants such as cocaine and methamphetamine leads to hyperactivity (distance traveled and rearing) and stereotypic behavior as a result of enhanced dopaminergic activity. Treatment with various selective TAAR 1 agonists (RO5166017, RO5203648, RO5256390, RO5073012 and RO5263397) dose-dependently inhibited cocaine- or NMDA antagonists L-687,414- or phencyclidine-induced hyperlocomotion in wild-type mice but not in Taar 1−/− mice (Galley et al., 2012; Revel et al., 2011; Revel et al., 2012b; Revel et al., 2013). Besides, RO5166017 and RO5203648 also attenuated hyperlocomotion in DAT−/− mice. These data support the notion that activation of TAAR 1 inhibits psychostimulant-induced hyperlocomotion, and DAT is not involved in the effect of TAAR 1 on DA-related behavioral function (Revel et al., 2011; Revel et al., 2012b).
Repeated exposure to drugs of abuse enhances the behavioral response to these drugs, a phenomenon termed behavioral sensitization (Vezina, 2007). Behavioral sensitization is a long-lasting form of drug-associated neuroplasticity, which is implicated in certain aspects of drug addiction, including compulsive drug-seeking and -taking behaviors (De Vries et al., 1998; Robinson and Berridge, 2001). Thorn et al firstly reported the modulatory effect of a TAAR 1 agonist on repeated cocaine treatment-induced behavioral sensitization (Thorn et al., 2014b). Acute treatment with RO5263397 (3.2 and 10 mg/kg) had no effect on locomotor activity and did not significantly affect cocaine-induced hyperactivity. Daily co-administration of RO5263397 and cocaine for 7 days significantly blocked the induction of locomotor sensitization (Thorn et al., 2014b). Furthermore, RO5263397 attenuated the expression of locomotor sensitization elicited by a challenge dose of cocaine after 7 days of drug-free period. The attenuation was also demonstrated by the downward shift of the cocaine dose-effect curve by daily RO5263397 treatment (Thorn et al., 2014a). Recently, we also observed that RO5263397 dose-dependently attenuated the expression of behavioral sensitization to methamphetamine (Jing et al., 2014). These findings suggest that activation of TAAR 1 could modulate cocaine- and methamphetamine-induced behavioral plasticity, which may be indicative of the potential role of TAAR 1 agonists for altering psychostimulant-induced long-lasting behavioral maladaptation.
4.2 TAAR 1 and drug reward and reinforcement
CPP is a Pavlovian conditioning behavioral model that is widely used to study the rewarding effects of drugs (Tzschentke, 2007). The development and expression of CPP involve different neural mechanisms, and particularly the expression is thought to be related to context-induced relapse (Napier et al., 2013). We provided the first evidence for a critical role of TAAR 1 in mediating cocaine CPP (Thorn et al., 2014a). Administration of a TAAR 1 agonist RO5263397 significantly attenuated the expression, but not the development, of cocaine CPP, suggesting that TAAR 1 agonists can reduce cocaine reward and may be potentially useful against cocaine abuse (Thorn et al., 2014a).
Self-administration paradigm is considered the “gold standard” for the assessment of the reinforcing effects of a drug, in which an animal can obtain a drug injection by emitting an operant response (e.g., lever press or nose poke). This paradigm is widely used to evaluate potential medications for reducing drug intake. Revel et al was the first to report that RO5203648, a TAAR 1 partial agonist, dose-dependently reduced cocaine self-administration using a simple fixed ratio (FR) 1 schedule of reinforcement, but did not reduce sucrose intake, revealing a behaviorally specific suppression of cocaine intake (Revel et al., 2012b). In a more exhaustive study, Thorn et al applied behavioral economic analysis to evaluate the elasticity of cocaine demand curve. In contrast to FR 1 schedule, demand curve analysis involves systematically changing the FR up to a high FR that the subject fails to earn one reinforcer, thus it can measure how hard the subject is willing to work for one injection of cocaine (reinforcing effectiveness). In behavioral economic analysis, a more elastic demand curve indicates that the commodity (e.g., an injection of cocaine) is less essential such that the consumption decreases more quickly when the price (FR) increases (Hursh et al., 2005; Thorn et al., 2014a). We found that the TAAR 1 partial agonist, RO5263397, increased the elasticity of cocaine demand curve indicating that RO5263397 may be effective in attenuating the reinforcing effectiveness of cocaine. In a separate study, we also observed that RO5263397 significantly reduced methamphetamine self-administration (Jing et al., 2014). In a progressive ratio (PR) schedule of reinforcement, Pei et al reported that the TAAR 1 partial agonist, RO5203648, decreased cocaine-maintained responding in a dose- and time-dependent manner, and delayed the time to reach breakpoint for cocaine self-administration (Pei et al., 2014). In addition, RO5203648 was found to attenuate methamphetamine self-administration under a FR 1 schedule and did not maintain self-administration behavior in rats with a history of methamphetamine self-administration (Cotter et al., 2015). It is interesting to note that both TAAR 1 partial agonists studied thus far, RO5263397 and RO5203648, increased the dopamine firing rate in ex vivo brain slide recordings (Revel et al., 2012b; 2013), yet they consistently attenuated psychostimulant self-administration (Thorn et al., 2014a; Jing et al., 2014; Cotter et al., 2015). The causes of these discordant results are unclear, but it should not be a surprise to see this difference because the control of drug taking behavior requires the integration of the “reward circuitry” which includes many different mesolimbic brain regions. More work is needed to fully understand this discrepancy. Together, the above findings indicate that TAAR 1 plays an important role in mediating the rewarding and reinforcing properties of cocaine and methamphetamine and that pharmacological activation of TAAR 1 may reduce drug intake, an important therapeutic goal to control drug consumption.
4.3 TAAR 1 and drug relapse
A major obstacle in the treatment of drug addiction is the high relapse rate even after prolonged abstinence and recurrent cycles of abuse. This relapse to compulsive drug use can be triggered by various events, including re-exposure to drugs, or re-exposure to the stimuli previously associated with drug-taking or stress(Shaham et al., 2003). Pei et al applied a context-induced relapse model to examine the effect of TAAR 1 agonists on relapse to cocaine-seeking behavior (Pei et al., 2014). Two TAAR 1 agonists, RO5203648 and RO5256390, both dose-dependently suppressed cocaine-seeking after a 2-week period of forced abstinence from chronic cocaine self-administration. Similar results were also found in an extinction-reinstatement model with RO5203648. In our study, we found that the TAAR 1 agonist, RO5263397, attenuated both drug-associated cue- and cocaine prime-induced reinstatement of cocaine-seeking behavior (Thorn et al., 2014a) (Fig. 1). Importantly, a similar inhibitory effect of RO5263397 was found on the reinstatement of methamphetamine in which the administration of RO5263397 prevented both cue- and a priming injection of methamphetamine-induced reinstatement of methamphetamine-seeking behavior without altering cue-induced reinstatement of sucrose-seeking behavior (Jing et al., 2014) (Fig. 1). These data provide strong evidence that TAAR 1 agonists may be effective to prevent drug relapse, an exciting possibility in the clinical management of psychostimulant addiction.
Fig. 1.
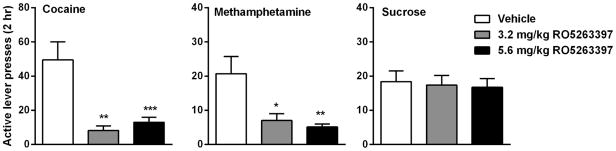
Effects of the TAAR 1 agonist RO5263397 on cue-induced reinstatement behavior in rats self-administering cocaine (left), methamphetamine (middle) or sucrose (right). * P < 0.05, ** P < 0.01, *** P < 0.001 as compared to vehicle-pretreated group. Data redrawn from Thorn et al. (2014) and Jing et al., (2015).
4.4 Other behavioral effects of TAAR 1 agonists
Although accumulating evidence suggests the promising effects of TAAR 1 agonists against various abuse- and addiction-related behavioral effects of cocaine and methamphetamine, more work is needed to rule out potentially important adverse effects of TAAR 1 activation. One important comparison is to examine whether TAAR 1 agonists also impact behavioral effects maintained by natural rewards (e.g., food and water) at doses that suppress drug-induced behaviors. This comparison can provide confidence that the effects on drug-induced behaviors are specific. Pei et al applied food self-administration procedure to investigate the effects of the TAAR 1 agonist, RO5203648, on responding for the natural reward (chocolate-flavored pellets) (Pei et al., 2014). Rats were re-trained to lever press for chocolate-flavored pellets after a cocaine self-administration history. RO5203648, at a dose that effectively inhibited relapse of cocaine-seeking behavior, did not affect food-maintained responding, suggesting the behavioral specificity under this condition (Pei et al., 2014). Revel et al reported that RO5203648 did not reduce sucrose intake but instead enhanced the reinforcing effects of sucrose (Revel et al., 2012i). Using a PR schedule of food reinforcement, it was found that RO5203648 significantly increased the total food earned, and dose-dependently enhanced the breakpoint (Pei et al., 2014), suggesting that although TAAR 1 agonists can reduce the reinforcing effects of drugs such as cocaine, they may enhance the reinforcing effects of natural rewards such as palatable food. Although the increase of the reinforcing value of palatable food (i.e., concern of overeating) might be considered undesirable in the general population, psychostimulants have well known appetite suppressant property and heavy psychostimulant users are often associated with malnutrition and weight loss (Glasner-Edwards et al., 2011; Hawks et al., 1969). Thus, this effect might be desirable in psychostimulant users. Importantly, RO5263397 has been shown to prevent olanzapine-induced body weight gain in rats (Revel, et al., 2013), suggesting that it does not seem to be a major concern. Recently, we employed extinction-reinstatement procedure to examine the effect of RO5263397 on cue-induced reinstatement of sucrose (chocolate-flavored sucrose pellets)-seeking behavior. At doses that effectively suppressed reinstatement of cocaine- and methamphetamine-seeking behavior, RO5263397 did not alter cue-induced reinstatement of sucrose seeking behavior (Jing et al., 2014). The differential modulation of the reinforcing effects of drugs is in agreement with the fact that both types of reinforcers have different neurobiological substrates and further suggests that TAAR 1 agonists do not generally suppress the motivational status but rather only specifically modulate drug-related motivational effects.
Learning and memory and drug addiction are modulated by the same neurotropic factors, share certain intracellular signaling cascades, and are associated with similar adaptations in neuronal morphology (Nestler, 2002). Revel et al assessed the effect of RO5256390 and RO5203648 on cognitive performance in monkeys using the object retrieval paradigm and in rats using an attentional set-shifting paradigm, two paradigms often used to assess the cognitive function. Both TAAR 1 agonists significantly enhanced the performance in these tasks (Revel et al., 2012b; Revel et al., 2013). Importantly, pharmacological MRI results in rats showed that TAAR 1 agonists enhanced brain activity in critical brain regions known to be involved in cognition such as prefrontal and temporal cortex, consistent with the behavioral data, and further suggest that TAAR 1 activation may be able to improve cognitive function. In light of the fact that drug addiction is closely related to aberrant learning and memory (Torregrossa et al., 2011), these findings suggest that activation of TAAR 1 can influence neuronal plasticity underlying drug-related aberrant learning and memory processes, thereby contributing to the attenuation of drug relapse.
5. Concluding statements
Taken together, the data reviewed here strongly support that TAAR 1 is implicated in the functional regulation of monoaminergic systems, especially dopaminergic system, and that TAAR 1 serves as a homeostatic “brake” system that is involved in the modulation of dopaminergic activity. Existing data provided robust preclinical evidence supporting the development of TAAR 1 agonists as potential treatment for psychostimulant abuse and addiction. In fact, selected TAAR 1 agonist is currently under phase II clinical trial for the treatment of schizophrenia (personal communications with Marius Hoener, Hoffmann-La Roche). If succeeded, the compound could potentially be quickly tested in patients suffering from psychostimulant addiction. TAAR 1 is discretely expressed in key brain regions involved in drug addiction, yet it is unknown of the neurocircuitry and the molecular mechanisms underlying the effects of TAAR 1 agonists against psychostimulant addiction. Future studies should begin to disentangle these critical neural mechanisms to provide further mechanistic support of developing TAAR 1 agonists as novel pharmacotherapies for drug addiction. Given that TAAR 1 is primarily located in the intracellular compartments and existing TAAR 1 agonists are proposed to get access to the receptors by translocation to the cell interior (Miller, 2011), future drug design and development efforts may need to take strategies of drug delivery into consideration (Rajendran et al., 2010).
Acknowledgments
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (Awards no. R01DA034806 and R21DA032837).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achat-Mendes C, Lynch LJ, Sullivan KA, Vallender EJ, Miller GM. Augmentation of methamphetamine-induced behaviors in transgenic mice lacking the trace amine-associated receptor 1. Pharmacol Biochem Behav. 2012;101:201–207. doi: 10.1016/j.pbb.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MD. Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J Neurochem. 2004;90:257–271. doi: 10.1111/j.1471-4159.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- Berry MD. The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev Recent Clin Trials. 2007;2:3–19. doi: 10.2174/157488707779318107. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton AA. Trace amines and mental disorders. The Canadian journal of neurological sciences. Can J Neurol Sci. 1980;7:261–263. doi: 10.1017/s0317167100023313. [DOI] [PubMed] [Google Scholar]
- Bradaia A, Trube G, Stalder H, Norcross RD, Ozmen L, Wettstein JG, Pinard A, Buchy D, Gassmann M, Hoener MC, Bettler B. The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc Natl Acad Sci USA. 2009;106:20081–20086. doi: 10.1073/pnas.0906522106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol. 2001;60:1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- Burchett SA, Hicks TP. The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Prog Neurobiol. 2006;79:223–246. doi: 10.1016/j.pneurobio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos E, Shanahan A, Muller CP, Huston JP. Evidence that the 5-HT1A autoreceptor is an important pharmacological target for the modulation of cocaine behavioral stimulant effects. Brain Res. 2005;1034:162–171. doi: 10.1016/j.brainres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Cisneros IE, Ghorpade A. Methamphetamine and HIV-1-induced neurotoxicity: role of trace amine associated receptor 1 cAMP signaling in astrocytes. Neuropharmacology. 2014;85:499–507. doi: 10.1016/j.neuropharm.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter R, Pei Y, Mus L, Harmeier A, Gainetdinov RR, Hoener MC, Canales JJ. The trace amine-associated receptor 1 modulates methamphetamine’s neurochemical and behavioral effects. Front Neurosci. 2015;9:39. doi: 10.3389/fnins.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci. 1998;10:3565–3571. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL. Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci. 2005;25:1889–1893. doi: 10.1523/JNEUROSCI.4778-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza S, Salahpour A, Masri B, Sotnikova TD, Messa M, Barak LS, Caron MG, Gainetdinov RR. Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor. Mol Pharmacol. 2011;80:416–425. doi: 10.1124/mol.111.073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley G, Stalder H, Goergler A, Hoener MC, Norcross RD. Optimisation of imidazole compounds as selective TAAR1 agonists: discovery of RO5073012. Bioorg Med Chem Lett. 2012;22:5244–5248. doi: 10.1016/j.bmcl.2012.06.060. [DOI] [PubMed] [Google Scholar]
- Ghitza UE. Needed Relapse-Prevention Research on Novel Framework (ASPIRE Model) for Substance Use Disorders Treatment. Front Psychiatry. 2015;6:37. doi: 10.3389/fpsyt.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson R. Bulimia nervosa among methamphetamine dependent adults: association with outcomes three years after treatment. Eat Disord. 2011;19:259–269. doi: 10.1080/10640266.2011.566149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Honorio Carrillo J, Maloney T, Woicik PA, Wang R, Telang F, Volkow ND. Dopaminergic response to drug words in cocaine addiction. J Neurosci. 2009;29:6001–6006. doi: 10.1523/JNEUROSCI.4247-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandy DK. Trace amine-associated receptor 1-Family archetype or iconoclast? Pharmacol Ther. 2007;116:355–390. doi: 10.1016/j.pharmthera.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenshaw AJ. beta-Phenylethylamine and reinforcement. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8:615–620. doi: 10.1016/0278-5846(84)90023-x. [DOI] [PubMed] [Google Scholar]
- Haleem DJ. Extending therapeutic use of psychostimulants: focus on serotonin-1A receptor. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:170–180. doi: 10.1016/j.pnpbp.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Harkness JH, Shi X, Janowsky A, Phillips TJ. Trace Amine-Associated Receptor 1 Regulation of Methamphetamine Intake and Related Traits. Neuropsychopharmacology. 2015:1–10. doi: 10.1038/npp.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawks D, Mitcheson M, Ogborne A, Edwards G. Abuse of methylamphetamine. Br Med J. 1969;2:715–721. doi: 10.1136/bmj.2.5659.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Galuska CM, Winger G, Woods JH. The economics of drug abuse: a quantitative assessment of drug demand. Mol Interv. 2005;5:20–28. doi: 10.1124/mi.5.1.6. [DOI] [PubMed] [Google Scholar]
- Jing L, Zhang Y, Li JX. Effects of the Trace Amine Associated Receptor 1 Agonist RO5263397 on Abuse-Related Behavioral Indices of Methamphetamine in Rats. Intl J Neuropsychopharmacol. 2014;18:1–7. doi: 10.1093/ijnp/pyu060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapin IP. Anxiogenic effect of phenylethylamine and amphetamine in the elevated plus-maze in mice and its attenuation by ethanol. Pharmacol Biochem Behav. 1993;44:241–243. doi: 10.1016/0091-3057(93)90305-d. [DOI] [PubMed] [Google Scholar]
- Ledonne A, Federici M, Giustizieri M, Pessia M, Imbrici P, Millan MJ, Bernardi G, Mercuri NB. Trace amines depress D(2)-autoreceptor-mediated responses on midbrain dopaminergic cells. Br J Pharmacol. 2010;160:1509–1520. doi: 10.1111/j.1476-5381.2010.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo D, Mus L, Espinoza S, Hoener MC, Sotnikova TD, Gainetdinov RR. Taar1-mediated modulation of presynaptic dopaminergic neurotransmission: role of D2 dopamine autoreceptors. Neuropharmacology. 2014;81:283–291. doi: 10.1016/j.neuropharm.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Ebeling M, Kratochwil NA, Bunzow JR, Grandy DK, Hoener MC. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics. 2005;85:372–385. doi: 10.1016/j.ygeno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H, Bettler B, Wettstein JG, Borroni E, Moreau JL, Hoener MC. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther. 2008;324:948–956. doi: 10.1124/jpet.107.132647. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Cue exposure and relapse prevention in the treatment of addictive behaviors. Addict Behav. 1990;15:395–399. doi: 10.1016/0306-4603(90)90048-3. [DOI] [PubMed] [Google Scholar]
- Miller GM. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J Neurochem. 2011;116:164–176. doi: 10.1111/j.1471-4159.2010.07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CP, Carey RJ, Huston JP, De Souza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog Neurobiol. 2007;81:133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Napier TC, Herrold AA, de Wit H. Using conditioned place preference to identify relapse prevention medications. Neurosci Biobehav Rev. 2013;37:2081–2086. doi: 10.1016/j.neubiorev.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narang D, Tomlinson S, Holt A, Mousseau DD, Baker GB. Trace Amines and Their Relevance to Psychiatry and Neurology: A Brief Overview. Klin Psikofarmakol B. 2011;21:73–79. [Google Scholar]
- Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;78:637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- Pei Y, Lee J, Leo D, Gainetdinov RR, Hoener MC, Canales JJ. Activation of the Trace Amine-Associated Receptor 1 Prevents Relapse to Cocaine Seeking. Neuropsychopharmacology. 2014;39:2299–2308. doi: 10.1038/npp.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips SR, Rozdilsky B, Boulton AA. Evidence for the presence of m-tyramine, p-tyramine, tryptamine, and phenylethylamine in the rat brain and several areas of the human brain. Biol Psychiatry. 1978;13:51–57. [PubMed] [Google Scholar]
- Quintero GC. Role of nucleus accumbens glutamatergic plasticity in drug addiction. Neuropsychiatr Dis Treat. 2013;9:1499–1512. doi: 10.2147/NDT.S45963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran L, Knolker HJ, Simons K. Subcellular targeting strategies for drug design and delivery. Nat Rev Drug Discov. 2010;9:29–42. doi: 10.1038/nrd2897. [DOI] [PubMed] [Google Scholar]
- Revel FG, Meyer CA, Bradaia A, Jeanneau K, Calcagno E, Andre CB, Haenggi M, Miss MT, Galley G, Norcross RD, Invernizzi RW, Wettstein JG, Moreau JL, Hoener MC. Brain-specific overexpression of trace amine-associated receptor 1 alters monoaminergic neurotransmission and decreases sensitivity to amphetamine. Neuropsychopharmacology. 2012a;37:2580–2592. doi: 10.1038/npp.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA, Metzler V, Chaboz S, Ozmen L, Trube G, Pouzet B, Bettler B, Caron MG, Wettstein JG, Hoener MC. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci USA. 2011;108:8485–8490. doi: 10.1073/pnas.1103029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Gainetdinov RR, Ferragud A, Velazquez-Sanchez C, Sotnikova TD, Morairty SR, Harmeier A, Groebke Zbinden K, Norcross RD, Bradaia A, Kilduff TS, Biemans B, Pouzet B, Caron MG, Canales JJ, Wallace TL, Wettstein JG, Hoener MC. Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics. Biol Psychiatry. 2012b;72:934–942. doi: 10.1016/j.biopsych.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Pouzet B, Mory R, Bradaia A, Buchy D, Metzler V, Chaboz S, Zbinden KG, Galley G, Norcross RD, Tuerck D, Bruns A, Morairty SR, Kilduff TS, Wallace TL, Risterucci C, Wettstein JG, Hoener MC. A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry. 2013;18:543–556. doi: 10.1038/mp.2012.57. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Salahpour A, Ramsey AJ, Medvedev IO, Kile B, Sotnikova TD, Holmstrand E, Ghisi V, Nicholls PJ, Wong L, Murphy K, Sesack SR, Wightman RM, Gainetdinov RR, Caron MG. Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc Natl Acad Sci USA. 2008;105:4405–4410. doi: 10.1073/pnas.0707646105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Thompson WA. Behavior Maintained under Fixed-Interval and 2nd-Order Schedules by Intravenous Injections of Endogenous Noncatecholic Phenylethylamines in Dogs. J Pharmacol Exp Ther. 1984;228:691–695. [PubMed] [Google Scholar]
- Sukhanov I, Espinoza S, Yakovlev DS, Hoener MC, Sotnikova TD, Gainetdinov RR. TAAR1-dependent effects of apomorphine in mice. Intl J Neuropsychopharmacol. 2014;17:1683–1693. doi: 10.1017/S1461145714000509. [DOI] [PubMed] [Google Scholar]
- Thorn DA, Jing L, Qiu Y, Gancarz-Kausch AM, Galuska CM, Dietz DM, Zhang Y, Li JX. Effects of the Trace Amine-Associated Receptor 1 Agonist RO5263397 on Abuse-Related Effects of Cocaine in Rats. Neuropsychopharmacology. 2014a;39:2309–2316. doi: 10.1038/npp.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Zhang C, Zhang Y, Li JX. The trace amine associated receptor 1 agonist RO5263397 attenuates the induction of cocaine behavioral sensitization in rats. Neurosci Lett. 2014b;566:67–71. doi: 10.1016/j.neulet.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol Learn Mem. 2011;96:609–623. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization, drug addiction and psychopathology in animals and humans. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1553–1555. doi: 10.1016/j.pnpbp.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky B, Seeman P, Branchek T, Gerald CP. The Trace Amine 1 receptor knockout mouse: an animal model with relevance to schizophrenia. Genes Brain Behav. 2007;6:628–639. doi: 10.1111/j.1601-183X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- Xie Z, Miller GM. Trace amine-associated receptor 1 is a modulator of the dopamine transporter. J Pharmacol Exp Ther. 2007a;321:128–136. doi: 10.1124/jpet.106.117382. [DOI] [PubMed] [Google Scholar]
- Xie Z, Westmoreland SV, Bahn ME, Chen GL, Yang H, Vallender EJ, Yao WD, Madras BK, Miller GM. Rhesus monkey trace amine-associated receptor 1 signaling: enhancement by monoamine transporters and attenuation by the D2 autoreceptor in vitro. J Pharmacol Exp Ther. 2007b;321:116–127. doi: 10.1124/jpet.106.116863. [DOI] [PubMed] [Google Scholar]
- Xie Z, Miller GM. Beta-phenylethylamine alters monoamine transporter function via trace amine-associated receptor 1: implication for modulatory roles of trace amines in brain. J Pharmacol Exp Ther. 2008;325:617–628. doi: 10.1124/jpet.107.134247. [DOI] [PubMed] [Google Scholar]
- Xie Z, Miller GM. A receptor mechanism for methamphetamine action in dopamine transporter regulation in brain. J Pharmacol Exp Ther. 2009;330:316–325. doi: 10.1124/jpet.109.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]


