Introduction
Stroke patients presenting within the 4.5-hour treatment window determined to have mild symptoms are excluded from thrombolysis in both clinical practice and randomized clinical trials.(1–4) Further, the variability in treatment with thrombolysis of mild stroke patients has been demonstrated in a large Specialized Program of Translational Research in Acute Stroke (SPOTRIAS) study.(5) This is in spite of growing evidence that a significant proportion of these patients go on to deteriorate neurologically and experience poor clinical outcomes.(2, 6–8) In the few studies that have reported magnetic resonance angiography (MRA) findings for this group, proximal vessel occlusion has been one of the strongest predictors of poor outcome,(7, 9, 10) with the largest and most recent reporting nearly 50–75% of patients with significant neurological deterioration consistent with vessel occlusion.(7)
In addition to occlusion site, diffusion-weighted (DWI) and perfusion-weighted (PWI) magnetic resonance imaging (MRI) can be invaluable in the work-up of stroke. Not only can lesion visualization be useful for excluding stroke mimics, (11) but the presence of significant ischemic penumbra, as estimated by a mismatch (MM) between hypoperfused regions on PWI and ischemic core on DWI has been shown to predict poor outcomes if early reperfusion is not achieved.(12–14) Recent evidence of a similar association specifically in patients with mild symptoms has also been described.(15) However, a complete understanding of how patients with mild symptoms differ from non-mild stroke patients is lacking. Standardization of thrombolysis decision making in mild stroke patients is also warranted.(5)
As such, using multimodal MRI, our purpose was to better characterize the findings of acute ischemic stroke patients with perfusion deficit presenting with mild symptoms in order to identify a clinically mild subpopulation that might benefit from IV thrombolysis.
Methods
Patients
This study is a retrospective analysis of data collected from patients who consented to an ongoing natural history study by the stroke section at the National Institute of Neurological Diseases and Stroke (NINDS). All eligible patients receive multimodal MRI. The appropriate ethics and institutional review boards approved the study. Consecutive patients presenting to Suburban Hospital in Bethesda, MD within 24 hours of time last seen normal (LSN) between September 2003 and April 2008 were considered for inclusion in the study. Patients were included if they had discharge diagnosis of ischemic stroke; screening MRI containing evaluable DWI, PWI, and time-of-flight (TOF) MRA performed prior to standard IV t-PA; perfusion deficit in MCA territory based on visual imaging confirmation; and admit NIHSS. Patients deemed to have stenosis without occlusion were excluded. Stroke with mild symptoms was defined as admit NIHSS score ≤ 4 and those with non-mild symptoms as admit NIHSS score > 4.
Imaging Acquisition
Imaging was performed using a 1.5-T (Twinspeed; General Electric) clinical MRI scanner. DWI and PWI series were acquired colocalized over the entire brain with a superior to inferior coverage of 14 cm. Typical imaging parameters for the spin-echo DWI echo-planar series included either 40–3.5-mm- or 20–7-mm-thick contiguous axial oblique sections with b = 0 and b = 1000 seconds/mm2, trace or isotropically weighted, TR/TE = 6000–7000/72–90 ms, acquisition matrix of 64 × 64–128 × 128, and FOV= 22 cm field of view. Typical imaging parameters for the gradient-recalled PWI echoplanar series included 20 contiguous axial oblique slices with single-dose gadolinium contrast injection of 0.1 mmol/kg through a power injector using 25 to 40 phase measurements TR/TE = 2000 to 2200/45 ms, acquisition matrix of 64x64 – 128x128, 20 7-mm slice thickness and FOV = 22-cm field of view. The intracranial 3D time-of-flight (TOF) MRA was acquired in the region of the Circle of Willis with parameters as follows: TR/TE = 39/6.9 ms, flip-angle = 25°; FOV = 24 x 18 cm; matrix of 224 x 160 for an in-plane resolution of approximately 1 mm, reconstructed to 92 axial images, 1.6 mm thick with a 0.8 mm overlap.
Imaging Analysis
DWI and PWI volumes were measured by two blinded, independent raters; one (M.L.) having years of experience and previously validated reliability,(16, 17) and the other (T.B.) trained over a month-long period reading multiple rounds of practice images and receiving detailed feedback. Readers were blinded to data from the clinical presentation including lateralization of the ischemic lesion and any other series from the screening MRI. Measurements were performed using Medical Imaging Processing and Visualization (MIPAV™), image analysis software with visualization, segmentation, and editing tools designed specifically for quantitative lesion measurement. Lesions were segmented on a slice-by-slice basis after optimally adjusting the window and level of the image without thresholding. DWI and PWI lesions were identified as hyperintense areas after excluding bilateral and susceptibility artifacts, as well as chronic lesions. The mean transit time (MTT) maps were calculated as the first moment of the time concentration curves divided by the zeroeth moment with no arterial input correction or deconvolution. The volumes were automatically calculated using the software by the planimetric method. The average volumes of the two readers were calculated and used in any subsequent analyses in the study. MM volume was calculated by subtracting the average DWI volume from the average PWI volume. If the quality of either the DWI or PWI or lateralization of the lesion was uncertain between the two readers, the case was sent to a third reader (L.L.L.) for the final measurement. Initial infarct growth velocity (IIGV) was calculated by dividing the final DWI measurement by the onset-to-image time as described by Olivot et al.(18).
Occlusion site was assigned independently by two vascular neurology fellows (J.S. and D.G.) mid-way through their fellowship which included daily interpretation of MRA images in a group setting and after demonstrating understanding of location definitions and proficiency with training images. Only TOF MRA images were used in an attempt to standardize occlusion classification. Fellows were blinded to data from the clinical presentation including lateralization of the ischemic lesion on the screening DWI and PWI, and read the MRA for occlusion location using Inteleviewer™, a PACS medical image viewing software. Images were read for most proximal occlusion which was classified as “proximal” if located in the intracranial carotid artery (ICA) or M1 segment, “distal” if located in the M2 segment or more distally or “MRA-negative” if no occlusion was detected. Occlusions located in the ICA were further categorized as carotid-T or saddle lesions depending on whether the occlusion appeared contiguous or not, respectively. Saddle lesions were excluded from analysis as the more proximal occlusion site may not be a true representation of these patients’ hemodynamic status as represented on PWI.(19) Images were read first independently by both readers and then by consensus. In cases where an agreement could not be reached, the images were read by a third reader (L.L.L.). If it was later determined that the MRA read did not agree with the correct index lesion side as determined by PWI, the readers were asked to re-evaluate the MRA images given the correct lateralization.
Statistical Analysis
Variable normality was assessed using the Kolmogorov-Smirnov test. Continuous variables were compared using the Mann Whitney U test, whereas the χ2 test was used for categorical variables. Comparison of variables among multiple groups was achieved using the Kruskal-Wallis test. Pairwise comparisons were analyzed using the Mann-Whitney U test with Bonferonni correction. Age and NIHSS were treated as continuous variables. Values were reported as n (%), median [interquartile range (IQR) 25–75], or mean (±standard deviation (SD)). Interrater agreement of the lesion volume measurements was assessed using Bland-Altman plots, in which the difference between two sets of measurements was plotted against the average.(20) The percent of measurements within two SDs of the mean difference was then determined. To determine the strength of correlation between the lesion volume measurements, Spearman’s coefficient was calculated. Statistical analysis was performed using the computing environment R.
Results
Patients
During the study period, 108 patients met initial inclusion criteria. From this group, patients were then excluded for the following reasons: presence of saddle lesion (n=9), no perfusion deficit in MCA territory determined after consensus of both independent readers or third reader (n=7) or presence of intracranial stent (n=1). Of the included patients, one was sent to the third reader for a tie-break read of the occlusion site. The occlusion site of two patients was re-evaluated after consensus read, due to incorrect lesion lateralization.
Ninety-one patients were included in the study (Table 1). The median age was 77 years [64–84.5], 55% were female and admit NIHSS score was 6 [3–13.5]. The time between LSN and baseline MRI was 165.4 [106.9–552.1] minutes with a majority of patients (n=56) imaged within 270 minutes (4.5 hours). No statistical difference was found between occlusion site and onset-to-baseline MRI. Twenty-three (25%) patients received IV t-PA. Fifty (55%) patients had a right-sided lesion. Categorized by occlusion status, 30 (33%) were “proximal”, 36 (39%) “distal” and 25 (27%) “MRA-negative” (Figure 1A). Perfusion volume differed significantly between all occlusion groups (p<0.001), with larger volumes associated with more proximal lesions (Figure 1B). Thirty-five (38%) patients had an admit NIHSS score ≤ 4, whereas 56 (62%) had an admit NIHSS score > 4. Of the 77 patients with documented discharge modified Rankin Score (mRS), thirty-six (47%) patients had a favorable clinical outcome defined by mRS ≤ 2, whereas 41 (53%) had a discharge mRS > 2. There was a trend for minor stroke patients treated with thrombolysis (88%, n=7/8) to more likely have a favorable clinical outcome versus untreated minor stroke patients (70%, n=16/23).
Table 1.
Baseline Clinical and Imaging Characteristics Associated with Mild and Non-Mild Symptoms in All Patients with Stroke*
| NIHSS <= 4 (n=35) | NIHSS > 4 (n=56) | P | |
|---|---|---|---|
| Age(y) | 76[59.5–83] | 77[66.75–85] | 0.3 |
| Sex (Female) | 14(40) | 36(64) | 0.04 |
| Admit NIHSS | 2[1–3] | 11[7–15.25)] | --- |
| Baseline PWI (mL) | 32.1[9–118.5] | 159.2[101.2–209.5] | <.001 |
| Baseline DWI (mL) | 2.56[.67–6.2] | 17.6[4.79–44.58] | <.001 |
| Baseline MM (mL) | 40.83[5.11–106.5] | 134[72.74–168.1] | <.001 |
| Onset-Baseline MRI Time (min) | 168[115.4–535] | 161.9[102.7–548.2] | 0.54 |
| Initial Growth Velocity (mL/hr) | 0.50[0.11–1.50] | 4.79[1.24–13.09] | <.001 |
| Hypertension | 22(63) | 41(73) | 0.42 |
| Diabetes Mellitus | 5(14) | 12(21) | 0.57 |
| Atrial Fibrillation | 9(26) | 28(50) | 0.04 |
| Hyperlipidemia | 17(49) | 21(38) | 0.88 |
| IV t-PA Treated | 6 (17) | 17(30) | 0.24 |
| Lesion Side (Right) | 22(63) | 28(50) | 0.33 |
| Occlusion Status | |||
| Proximal | 4(11) | 26(46) | .001 |
| Distal | 11(31) | 25(45) | 0.30 |
| MRA-Negative | 20(57) | 5(9) | <.001 |
Data are expressed as n (%) or median [IQR25–75].
Figure 1.
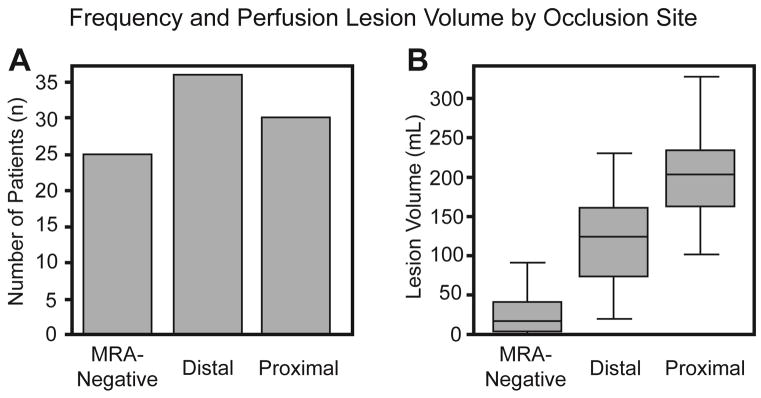
A: Baseline occlusion site distribution across all patients. B: Baseline PWI volume by occlusion site across all patients.
The median baseline DWI, PWI and MM volumes were 129.5 [42.29–185.1], 6.77 [1.62– 26.03], 98.11 [34.4–156.6] mL, respectively. The median IIGV was 2.36 mL/hr [0.375–6.98]. The lesion volume measurements of DWI and PWI between the two independent raters were strongly correlated (Spearman’s coefficient of 0.929 and 0.900, respectively). Bland-Altman plots were constructed for DWI and PWI (not shown). The mean difference between the DWI measurements was 7.3 (±10.4) with 92.31% of the measurements falling within two standard deviations of the mean difference. For the PWI measurements, the mean difference was 58.58 (±46.45) with 95.6% of measurements falling within the two standard deviations. Overall without stratification, mild patients had smaller DWI (2.56 mL [0.67–6.2] versus 17.6 mL [4.8–44.6], p<0.001), PWI (32.1 mL [9–118.5] versus 159.2 mL [101.2–209.5], p<0.001) and MM lesion volumes (40.8 mL [5.1–106.5] versus 134 mL [72.7–168.1], p<0.001) and were less likely to have MRA occlusion (42.9% versus 91.1%, p<0.001).
The following variables were associated with stroke with mild symptoms in univariate analysis: male sex (p=0.04), smaller PWI lesion volume (p<0.001), smaller DWI lesion volume (p<0.001), smaller MM volume (p<0.001) and absence of atrial fibrillation (p=0.04).
Comparison within Occlusion Site Groups
Table 2 shows data for patients further stratified by occlusion site. Among the 30 patients with proximal occlusion, 60% were women, with a median age of 78 years [66.5–86]. Median admit NIHSS score was 14 [6.25–15.75], DWI volume was 6.4 mL [2.35–41.19], PWI volume was 203.4 mL [162.9–232.6], MM volume was 162.8 mL [146.7–216.4], onset-to-MRI time was 128 minutes [83.52–483.1] and calculated IIGV was 3.91 mL/hr [0.75–7.54].
Table 2.
Baseline Clinical and Imaging Characteristics Associated with Mild and Non-Mild Symptoms in Patients with Proximal, Distal, and No Occlusion on MRA*
| Proximal
|
Distal
|
MRA-Negative
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| NIHSS <= 4 (n=4) | NIHSS > 4 (n=26) | p | NIHSS <= 4 (n=11) | NIHSS > 4 (n=25) | p | NIHSS <= 4 (n=20) | NIHSS > 4 (n=5) | p | |
| Age (y) | 82[71.5–83.75] | 76.5[66.5–86] | 1 | 80[56.5–82] | 78[68–83] | 0.68 | 70[60–85] | 74.5[60.5–81] | 0.89 |
| Sex (Female) | 3(75) | 10(38.5) | 1 | 6(54.5) | 17(68) | 0.69 | 5(25) | 3(60) | 0.33 |
| Admit NIHSS | 1.5[1–2.5] | 14.5[10–16.75] | --- | 3[2.5–3.5] | 10[7–14] | --- | 6[5–7] | 1[0.75–3] | --- |
| Baseline PWI (mL) | 207.3[171.5–237.3] | 203.4[162.9–224.6] | 0.98 | 107.8[55.42–150.7] | 129.5[97.64–160.2] | 0.34 | 18.98[17.37–30.67] | 12.57[3.23–42.14] | 0.87 |
| Baseline DWI (mL) | 4.11[1.56–10.43] | 7.29[2.94–44.64] | 0.36 | 3.58[2.79–8.15] | 32.13[17.48–46.38] | <.001 | 1.68[1.06–10.48] | 1.16[0.39–3.40] | 0.27 |
| Baseline MM (mL) | 192.7[153.8–232.5] | 162.8[146.7–214.3] | 0.54 | 101.1[51.77–142.9] | 85.88[65.63–121.9] | 0.95 | 17.3[16.31–17.57] | 8.66[1.16–41.1] | 1 |
| Onset-Baseline MRI Time (min) | 264.3[185–535.5] | 123.2[83.52–481.7] | 0.46 | 168[128.4–617.8] | 168.6[117.7–326.2] | 0.97 | 279.5[109.9–638.4] | 158.8[113.2–508.9] | 0.97 |
| Initial Growth Velocity (mL/hr) | 0.82[0.40–2.36] | 4.67[1.12–11.68] | 0.23 | 1.28[0.25–4.68] | 5.94[3.46–15.2] | 0.01 | 0.42[0.07–0.61] | 0.36[0.31–1.23] | .536 |
| Hypertension | 3(75) | 19(73.1) | 1 | 6(54.5) | 19(76) | 0.37 | 3(60) | 13(65) | 1 |
| Diabetes Mellitus | 0(0) | 7(26.9) | 0.58 | 2(18.2) | 4(16) | 1 | 1(20) | 3(15) | 1 |
| Atrial Fibrillation | 1(25) | 16(61.54) | 0.41 | 3(27.3) | 11(25) | 0.56 | 1(20) | 5(25) | 1 |
| Hyperlipidemia | 1(25) | 8(30.77) | 1 | 6(54.5) | 17(68) | 0.69 | 0(0) | 10(50) | 0.13 |
| IV t-PA Treated | 1(25) | 7(27) | 1 | 3(27) | 8(25) | 1 | 2(10) | 2(40) | 0.34 |
| Lesion Side (Right) | 2(50) | 13(50) | 1 | 7(63.6) | 13(52) | 0.78 | 13(65) | 2(40) | 0.61 |
Data are expressed as n (%) or median [IQR25–75].
Thirty-six patients, 64% female, had a distal occlusion site. Median age was 78.5 years [66.25–83]. Median admit NIHSS score for this group was 7 [4–11.25]. The DWI, PWI and MM volumes were 17.6 mL [6.37–41.04], 124.6 mL [73.78–160.9] and 91.99 mL [62.03–128.1], respectively. The onset-to-MRI time was 168.4 minutes [117.3–561.5] resulting in a calculated IIGV of 4.86 mL/hr [1.59–10.98].
There were 25 patients with no detectable occlusion site on MRA. Only 32% were female and the age was 74 years [60–84]. This group had an admit NIHSS score of 2 [1–4]. The DWI, PWI and MM volumes were 1.23 mL [0.43–3.42], 17.37 mL [3.72–42.1] and 16.31 mL [1.20–36.35], respectively. The onset-to-MRI time and IIGV was 165.4 minutes [109.9–509.2] and 0.39 mL/hr [0.09–0.70], respectively.
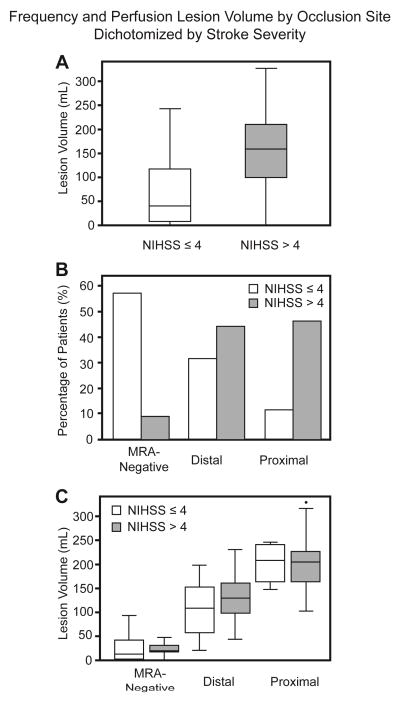
After grouping patients based on occlusion site, differences in PWI and MM volumes between mild stroke and non-mild stroke groups did not reach significance. Figure 2 (A–C) graphically depicts the occlusion site distributions and PWI volumes grouped by symptom severity and occlusion location. This relationship did not change when only the 56 patients imaged within 4.5 hours were considered (data not shown). No difference could be demonstrated for stroke risk factors including hypertension, diabetes mellitus, hyperlipidemia, and atrial fibrillation between the mild and non-mild groups when analyzed within the same occlusion site categories. DWI volumes and IIGV, however, were significantly larger in the non-mild group among distal occlusion site patients (p<0001 and p=.01, respectively).
Figure 2.
Baseline perfusion volumes and occlusion location distribution in stroke patients presenting with mild (white) and non-mild (gray) symptoms. A: Box-and-whisker plot showing perfusion volume by admit NIHSS cut-off. B: Histogram showing relative occlusion site frequency based on clinical severity. C: Box-and-whisker plot showing perfusion volume by NIHSS cut-off grouped by occlusion site.
Discussion
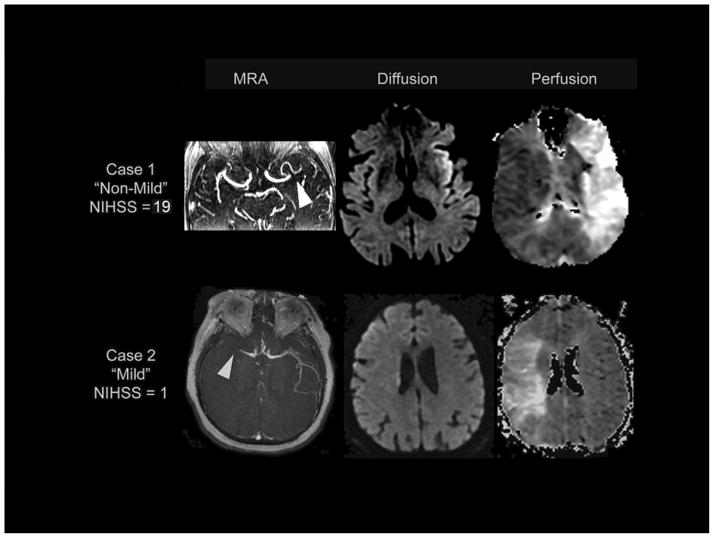
Our study revealed that, although stroke patients with mild symptoms had smaller DWI, PWI and MM volumes and were less likely to have conspicuous vessel occlusions on MRA, PWI and MM volumes were similar between the mild and non-mild groups when accounting for the presence and location of occlusion. Additionally, there was a trend for minor stroke patients treated with thrombolysis to more likely have a favorable clinical outcome versus untreated minor stroke patients. Figure 3 compares case examples of patients with the same occlusion site who presented with different admit NIHSS scores. Among patients with similar occlusion site, no other clinical or baseline imaging characteristic varied with symptom severity with the exception of DWI volume and IIGV in those patients with distal occlusion.
Figure 3.
Case 1: Patient presenting with non-mild symptoms, admit NIHSS=19; age=80 years; male; onset-to-image time=61 minutes; IIGV of 8.63 mL/hr with MRA showing evidence of left distal M1 occlusion (arrow) and large MM=210.27 mL. Case 2: Patient presenting with mild symptoms, admit NIHSS=1; age=81 years; female; onset-to-image time=62 minutes; IIGV of 6.09 mL/hr with MRA showing evidence of right distal M1 occlusion (arrow) and large MM=228.79 mL.
Stroke patients with mild symptoms represent a particularly vulnerable group. On one hand, the majority can expect a good outcome but are at risk of unnecessary treatment and its associated complications.(21) On the other hand, a significant proportion is at risk of clinical deterioration, but may not receive timely IV thrombolysis due to a progressing stroke which has yet to manifest clinically.(6–8, 15) Given that patients with mild symptoms tended to have smaller DWI volumes and slower IIGV in the distal occlusion group, one explanation is that the ischemic core spreads somewhat more slowly in these patients.(22) Recently, using the DEFUSE 2 cohort, Olivot et al showed that rapid IIGV was linked to imaging markers predictive of poor collateralization on cerebral angiogram and larger total infarct growth 5 days after onset.(18) This could also indicate that more tissue is salvageable in these patients when presenting within the treatment window. Although there was a trend toward smaller DWI and slower IIGV for mild patients in the proximal occlusion group as well, no significant difference could be demonstrated possibly due to small sample size. Thus, like previous reports, (9, 15, 23, 24) our study supports the utilization of multimodal including vascular imaging in this group. However, a particular strength of the current study is the direct comparison of the clinically mild stroke group with more severe patients, i.e. those traditionally considered for IV t-PA.
Interestingly, in our study, rates of IV t-PA thrombolysis did not differ significantly between severity groups. This is in contrast to other studies, (25, 26) and is likely due to the fact that patients routinely receive pre-treatment multimodal MRI at our institution. As such, patients with vascular occlusions and perfusion deficits are identified before being excluded from thrombolytic therapy. This may be a reflection of natural shifts in physician treatment practices when consistently given more complete imaging information. However, it could also indicate that the definition of stroke with mild symptoms was not consistent with that of the treating physicians and included more patients.
Although there is no consensus as to the most appropriate definition of stroke with mild symptoms, the NIHSS is a frequently implemented tool with a cut-off score of ≤ 3–5 most commonly used.(1, 3, 27) Our threshold choice of 4 to divide mild and non-mild patients aligned with previous literature while still allowing patients to populate each group for basic comparisons. While other studies have used more conservative definitions, (21, 28) we found patients with proximal occlusions and large PWI volumes even in patients presenting with an admit NIHSS as low as 1, and therefore, do not believe using another clinically-based definition would change the results of our study.
Our analysis is limited by several factors. First, our sample size is relatively small. Because patients in the mild group were less likely to have proximal occlusions and those in the non-mild group less likely to have no occlusion, we had only 4 and 5 patients in each of these groups, respectively. If a difference was present in these groups, we may not have achieved sufficient statistical power to detect it. However, given that no difference was found in the PWI and MM volumes of the distal occlusion group, which had more patients, we believe this is less likely. Second, the lack of follow-up data available for our patient population severely limited our analysis of clinical outcomes. Correlating clinical outcomes with imaging findings is important as other factors for which we did not assess such as collateralization may serve to protect mild patients from neurological deterioration and infarct progression despite large amounts of tissue at risk on imaging. Finally, this was a retrospective analysis that did not directly assess treatment success. The imaging protocol, in particular for the perfusion data, varied across the study period which contributed to the variability in the perfusion volume measurements. Further studies with a prospective design, including a randomized, controlled trial are needed to establish the efficacy of IV thrombolysis in stroke patients presenting with mild symptoms. To achieve adequate power, this study supports selection of patients based on vascular or perhaps perfusion imaging criteria. Of note, two new treatment trials studying the efficacy of thrombolysis in mild stroke patients are now underway.(29, 30)
In conclusion, the results of this study suggest that, while vascular occlusion is less common in patients with lower NIHSS, it is present in a significant proportion of this population and is associated with similar PWI and MM volumes in all patients, mild and non-mild. The literature suggests that these imaging parameters are powerful predictors of neurological deterioration and poor outcome for this population. By confirming the presence of a therapeutic target such as perfusion lesion or vessel occlusion before treatment decisions are made, it may be possible to better identify the population of patients presenting with mild symptoms that might benefit from t-PA. Our findings suggest that, regardless of symptom severity, patients with a clinically confirmed diagnosis of ischemic stroke meeting other criteria for t-PA should be considered for this therapy until advanced imaging is performed to better evaluate disease progression.
Acknowledgments
This research was made possible through the National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc, The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc. and Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit http://fnih.org/work/education-training-0/medical-research-scholars-program.
Footnotes
Author Contributions:
Mr. Brown contributed to manuscript drafting/revision, study concept/design and acquisition/statistical analysis/interpretation of data.
Dr. Luby contributed to manuscript drafting/revision, study concept/design and acquisition/statistical analysis/interpretation of data.
Dr. Shah contributed to manuscript revision and acquisition/interpretation of data.
Dr. Giannakidis contributed to manuscript revision and acquisition/interpretation of data.
Dr. Latour contributed to manuscript drafting/revision, study concept/design, statistical analysis/interpretation of data and study supervision.
Study Funding/Disclosures: This research was supported by the Division of Intramural Research of the National Institute of Neurological Disorders and Stroke, NIH.
Mr. Brown reports no disclosures.
Dr. Luby reports no disclosures.
Dr. Shah reports no disclosures.
Dr. Giannakidis reports no disclosures.
Dr. Latour reports no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albers GW, Clark WM, Madden KP, Hamilton SA. Atlantis trial: Results for patients treated within 3 hours of stroke onset. Alteplase thrombolysis for acute noninterventional therapy in ischemic stroke. Stroke; a journal of cerebral circulation. 2002;33:493–495. doi: 10.1161/hs0202.102599. [DOI] [PubMed] [Google Scholar]
- 2.Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from tpa therapy? An analysis of patient eligibility. Neurology. 2001;56:1015–1020. doi: 10.1212/wnl.56.8.1015. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The european cooperative acute stroke study (ecass) JAMA : the journal of the American Medical Association. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 4.Huisa BN, Raman R, Ernstrom K, Hemmen TM. Intravenous tissue plasminogen activator for patients with minor stroke. J Stroke Cerebrovasc Dis. 2012;21:732–6. doi: 10.1016/j.jstrokecerebrovasdis.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiley JZ, Khatri P, Khoury JC, Merino JG, Ford AL, Rost NS, et al. Variability in the Use of Intravenous Thrombolysis for Mild Stroke: Experience Across the SPOTRIAS Network. J Stroke Cerebrovasc Dis. 2013;22:318–22. doi: 10.1016/j.jstrokecerebrovasdis.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khatri P, Conaway MR, Johnston KC Acute Stroke Accurate Prediction Study I. Ninety-day outcome rates of a prospective cohort of consecutive patients with mild ischemic stroke. Stroke; a journal of cerebral circulation. 2012;43:560–562. doi: 10.1161/STROKEAHA.110.593897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nedeltchev K, Schwegler B, Haefeli T, Brekenfeld C, Gralla J, Fischer U, et al. Outcome of stroke with mild or rapidly improving symptoms. Stroke; a journal of cerebral circulation. 2007;38:2531–2535. doi: 10.1161/STROKEAHA.107.482554. [DOI] [PubMed] [Google Scholar]
- 8.Smith EE, Abdullah AR, Petkovska I, Rosenthal E, Koroshetz WJ, Schwamm LH. Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke; a journal of cerebral circulation. 2005;36:2497–2499. doi: 10.1161/01.STR.0000185798.78817.f3. [DOI] [PubMed] [Google Scholar]
- 9.Kim JT, Park MS, Chang J, Lee JS, Choi KH, Cho KH. Proximal arterial occlusion in acute ischemic stroke with low nihss scores should not be considered as mild stroke. PloS one. 2013;8:e70996. doi: 10.1371/journal.pone.0070996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajajee V, Kidwell C, Starkman S, Ovbiagele B, Alger JR, Villablanca P, et al. Early mri and outcomes of untreated patients with mild or improving ischemic stroke. Neurology. 2006;67:980–984. doi: 10.1212/01.wnl.0000237520.88777.71. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Almast J, Ekholm S. Lesions masquerading as acute stroke. Journal of Magnetic Resonance Imaging. 2013;37:15–34. doi: 10.1002/jmri.23647. [DOI] [PubMed] [Google Scholar]
- 12.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (defuse) study. Annals of neurology. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 13.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the echoplanar imaging thrombolytic evaluation trial (epithet): A placebo-controlled randomised trial. The Lancet. Neurology. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 14.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. Mri profile and response to endovascular reperfusion after stroke (defuse 2): A prospective cohort study. The Lancet. Neurology. 2012;11:860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asdaghi N, Hill MD, Coulter JI, Butcher KS, Modi J, Qazi A, et al. Perfusion mr predicts outcome in high-risk transient ischemic attack/minor stroke: A derivation-validation study. Stroke; a journal of cerebral circulation. 2013;44:2486–2492. doi: 10.1161/STROKEAHA.111.000208. [DOI] [PubMed] [Google Scholar]
- 16.Luby M, Bykowski JL, Schellinger PD, Merino JG, Warach S. Intra- and interrater reliability of ischemic lesion volume measurements on diffusion-weighted, mean transit time and fluid-attenuated inversion recovery mri. Stroke; a journal of cerebral circulation. 2006;37:2951–2956. doi: 10.1161/01.STR.0000249416.77132.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luby M, Warach S. Reliability of mr perfusion-weighted and diffusion-weighted imaging mismatch measurement methods. AJNR. American journal of neuroradiology. 2007;28:1674–1678. doi: 10.3174/ajnr.A0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivot JM, Mlynash M, Inoue M, Marks MP, Wheeler HM, Kemp S, et al. Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the defuse 2 cohort. Stroke; a journal of cerebral circulation. 2014;45:1018–1023. doi: 10.1161/STROKEAHA.113.003857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neumann-Haefelin T, Wittsack HJ, Fink GR, Wenserski F, Li TQ, Seitz RJ, et al. Diffusion- and perfusion-weighted mri: Influence of severe carotid artery stenosis on the dwi/pwi mismatch in acute stroke. Stroke; a journal of cerebral circulation. 2000;31:1311–1317. doi: 10.1161/01.str.31.6.1311. [DOI] [PubMed] [Google Scholar]
- 20.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 21.Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-pa stroke study group. The New England journal of medicine. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 22.Marks MP, Lansberg MG, Mlynash M, Olivot JM, Straka M, Kemp S, et al. Effect of collateral blood flow on patients undergoing endovascular therapy for acute ischemic stroke. Stroke; a journal of cerebral circulation. 2014;45:1035–1039. doi: 10.1161/STROKEAHA.113.004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexandrov AV, Felberg RA, Demchuk AM, Christou I, Burgin WS, Malkoff M, et al. Deterioration following spontaneous improvement : Sonographic findings in patients with acutely resolving symptoms of cerebral ischemia. Stroke; a journal of cerebral circulation. 2000;31:915–919. doi: 10.1161/01.str.31.4.915. [DOI] [PubMed] [Google Scholar]
- 24.Strbian D, Piironen K, Meretoja A, Sairanen T, Putaala J, Tiainen M, et al. Intravenous thrombolysis for acute ischemic stroke patients presenting with mild symptoms. International journal of stroke : official journal of the International Stroke Society. 2013;8:293–299. doi: 10.1111/j.1747-4949.2012.00808.x. [DOI] [PubMed] [Google Scholar]
- 25.Ingall TJ, O’Fallon WM, Asplund K, Goldfrank LR, Hertzberg VS, Louis TA, et al. Findings from the reanalysis of the ninds tissue plasminogen activator for acute ischemic stroke treatment trial. Stroke; a journal of cerebral circulation. 2004;35:2418–2424. doi: 10.1161/01.STR.0000140891.70547.56. [DOI] [PubMed] [Google Scholar]
- 26.Khatri P, Kleindorfer DO, Yeatts SD, Saver JL, Levine SR, Lyden PD, et al. Strokes with minor symptoms: An exploratory analysis of the national institute of neurological disorders and stroke recombinant tissue plasminogen activator trials. Stroke; a journal of cerebral circulation. 2010;41:2581–2586. doi: 10.1161/STROKEAHA.110.593632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith EE, Fonarow GC, Reeves MJ, Cox M, Olson DM, Hernandez AF, et al. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: Findings from get with the guidelines-stroke. Stroke; a journal of cerebral circulation. 2011;42:3110–3115. doi: 10.1161/STROKEAHA.111.613208. [DOI] [PubMed] [Google Scholar]
- 28.Park TH, Hong KS, Choi JC, Song P, Lee JS, Lee J, et al. Validation of minor stroke definitions for thrombolysis decision making. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2013;22:482–490. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Hill MD. Tnk-tpa evaluation for minor ischemic stroke with proven occlusion (tempo-1) 2013 http://clinicaltrials.gov/ct2/show/NCT01654445?term=TEMPO+coutts&rank=1.
- 30.A Study of the Efficacy and Safety of Activase (Alteplase) in Patients With Mild Stroke (PRISMS) 2014 http://clinicaltrials.gov/ct2/show/NCT02072226.