Abstract
Background
The success of an autologous fat graft depends in part on its total stromal vascular fraction (SVF) and adipose-derived stem cells (ASCs). However, variations in the yields of ASCs and SVF cells as a result of different harvesting techniques and donor sites are poorly understood.
Objective
To investigate the effects of adipose tissue harvesting technique and donor site on the yield of ASCs and SVF cells.
Methods
Subcutaneous fat tissues from the abdomen, flank, or axilla were harvested from patients of various ages by mechanical liposuction, direct surgical excision, or Coleman's technique with or without centrifugation. Cells were isolated and then analyzed with flow cytometry to determine the yields of total SVF cells and ASCs (CD11b−, CD45−, CD34+, CD90+, D7-FIB+). Differences in ASC and total SVF yields were assessed with one-way analysis of variance. Differentiation experiments were performed to confirm the multilineage potential of cultured SVF cells.
Results
Compared with Coleman's technique without centrifugation, direct excision yielded significantly more ASCs (P < .001) and total SVF cells (P = .007); liposuction yielded significantly fewer ASCs (P < .001) and total SVF cells (P < .05); and Coleman's technique with centrifugation yielded significantly more total SVF cells (P < .005), but not ASCs. The total number of SVF cells in fat harvested from the abdomen was significantly larger than the number in fat harvested from the flank or axilla (P < .05). Cultured SVF cells differentiated to adipocytes, osteocytes, and chondrocytes.
Conclusions
Adipose tissue harvested from the abdomen through direct excision or Coleman's technique with centrifugation was found to yield the most SVF cells and ASCs.
Autologous fat grafting is widely utilized for breast reconstruction and for repairing surface contour deformities.1-5 However, acceptance of the technique is limited by a wide range of issues related to the retention of the grafted fat at the operative site.2,5-9 Enriching fat grafts with adipose-derived stem cells (ASCs) before transplantation has been shown to improve the viability and outcome of the graft.10-13 In previous studies, authors have suggested that a number of factors, including fat-harvesting technique, donor site,14,15 patient age,15 and body mass index,16 influence the yield of stromal vascular fraction (SVF) cells, and thus ASCs, from adipose tissues. However, results from these studies are inconsistent and do not elucidate the relationship between harvesting procedure or other factors and the yield of ASCs from the SVF. A clearer understanding of whether different harvesting procedures or locations affect the yields of SVF cells and ASCs would improve the ways in which we select tissue sources for ASC- and SVF-rich fat grafts. Therefore, the purpose of the present study was to investigate the effects of harvesting technique and donor site on yields of ASCs and total SVF cells from adipose tissues harvested for fat grafting.
To isolate and quantify the yields of ASCs and SVF cells, we employed several current techniques for harvesting subcutaneous adipose tissue for clinical fat grafting.17,18 Coleman's technique, first proposed in 1994, is the most widely employed technique for harvesting subcutaneous adipose tissue for clinical fat grafting; liposuction, other syringe-based techniques, and excision are also utilized.18 Our findings have implications for the choice of harvesting technique and donor location for obtaining a high yield of ASCs for the purposes of clinical fat grafting and future ASC-based therapies.
MATERIALS AND METHODS
Fat Tissue Harvesting
All procedures were approved by MD Anderson's Institutional Review Board and performed in accordance with the institution's research guidelines by a single surgeon. Adipose tissue samples were harvested from 19 women undergoing reconstructive surgery after mastectomy at MD Anderson between October 2010 and May 2011. Patients provided their written informed consent to be included in the study. All patients seen at MD Anderson for reconstructive surgery were eligible for the study based on the following criteria. Inclusion criteria were (1) any sex, male or female; (2) age >21 years old, to be qualified as an adult per National Institutes of Health (NIH) guidelines; (3) any race and ethnic background; (4) patients presenting a complaint that required reconstructive surgery, but otherwise healthy; (5) patients would be eligible except under circumstances described in the exclusion criteria. Exclusion were (1) patients whose reconstructive surgery sequel did not result in incidental tissue; (2) patients who had received previous radiotherapy. Whenever possible, multiple harvest sites and harvest techniques were employed for each patient depending on the adipose tissue need and available fat for experiments. Fat tissue was harvested by Coleman's technique (manual harvest of fat aspirated with a 3-mm blunt cannula and a 10-mL syringe) with or without centrifugation of the harvested fat at 3200 rpm for 2-3 minutes; machine-assisted liposuction with a −750-mmHg vacuum at 100% negative pressure; or direct surgical excision. We also analyzed the blood–oil waste resulting from the centrifugation step after Coleman's procedure.
Fat Tissue Digestion and Cell Isolation
Direct surgical excision samples were weighed and minced, and phosphate-buffered saline (PBS) at a concentration of 1 g/mL was added to the samples. All harvested fat tissues were digested with 0.075% type IA collagenase (Sigma, St. Louis, MO) in sterile isotonic buffer at a ratio of 1 mL fat tissue to 2 mL collagenase for 2 hours. Blood–oil waste from fat centrifugation after performing Coleman's procedure was directly centrifuged at 200g for 5 minutes at 4°C without collagenase treatment. Cell pellets were obtained by filtering the digestion mixture through a 100-µm cell strainer (Millipore, Billerica, MA) and subjecting it to centrifugation at 200g for 5 minutes at 4°C. The cell pellets were reconstituted with 1 × red blood cell lysis buffer (Biolegend Inc., San Diego, CA) and incubated on ice for 5 minutes for purification. The purified pellets were reconstituted with 1% bovine serum albumin (Fisher Scientific, Pittsburgh, PA) in PBS or cultured in growth media.
Cell Characterization
Cells isolated from each tissue sample were counted with a Z1 Coulter Counter (Beckman Coulter, Brea, CA). For cell characterization, freshly isolated cells (1 × 105) in 100 µL of 1% bovine serum albumin were stained with the Aqua Dead Cell Stain Kit (Life Technologies, Grand Island, NY). The cells were then incubated with the primary antibodies for 30 minutes on ice, washed twice with PBS, and subjected to centrifugation at 200g for 5 minutes at 4°C. After performing centrifugation, the cells were suspended in 500 µL of 1% paraformaldehyde and subjected to flow cytometry analysis with an LSR II flow cytometer (BD, Franklin Lakes, NJ). Cell membrane marker expression levels were analyzed with the FlowJo software package version 7.6.5 (Tree Star, Inc., Ashland, OR).
Primary antibodies for cell membrane markers included mouse anti-human CD11b–phycoerythrin-cyanine 7, mouse anti-human CD34–phycoerythrin-cyanine 5, and mouse anti-human CD45–phycoerythrin–Texas red from Beckman Coulter and mouse anti-human CD90–Alexa Fluor 647 and mouse anti-human fibroblast/epithelial cell–phycoerythrin (D7-FIB) from ABD Serotec (Raleigh, NC). For control experiments, 1 × 105 cells from the same tissue samples were separately stained with the indicated isotype negative controls.
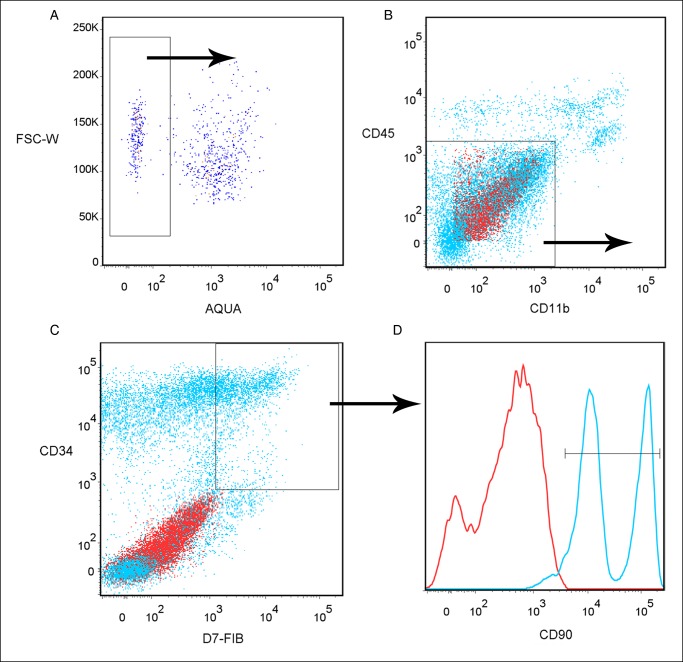
Aqua-negative total SVF cells that were CD11b-negative, CD45-negative, CD34-positive, D7-FIB–positive, and CD90-positive were considered to be ASCs (Table 1; Figure 1). Cells were counted by calculating the ratio of the frequency of positive staining to the initial number of total SVF cells. The numbers of SVF cells and ASCs were normalized to the original volume of fat tissue and reported as the number of cells per milliliter of fat tissue.
Table 1.
Selected Adipose Stem Cell Markers
| Antigen | Description | Stem Cell Status | Study |
|---|---|---|---|
| CD90 | Stromal cell marker | + | Gimble et al,36 Zuk et al,24 Yoshimura et al11 |
| CD34 | Bone marrow stem cell marker | +/− | Gimble et al,36 Zuk et al,24 Huss et al42 |
| D7-FIB | Fibroblast marker | + | Jones et al,20 English et al,22 Jones et al23 |
| CD11b | Macrophage-I cell marker | – | Gimble et al,36 Cao et al,28 Yoshimura et al43 |
| CD45 | Hematopoietic cell marker | − | Gimble et al,36 Cao et al,28 Yoshimura et al43 |
“+” and “−” refer to the known expression of the cell surface antigen in adipose stem cells.
Figure 1.
Representative analysis of stained cells isolated from fresh human adipose tissue. (A) Aqua Dead staining was absent in 31.5% of cells. (B) Most Aqua Live–stained cells (81.1%) did not express both CD11b and CD45. (C) Of the cells that did not express CD11b or CD45, 21.6% expressed both CD34 and D7-FIB. (D) Of the live CD11b−, CD45−, CD34+, D7-FIB+ cells, 95.4% also expressed CD90.
Primary Culture and Differentiation of ASCs
Freshly isolated cells were grown in α-minimum essential medium (Mediatech, Manassas, VA) containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 2 mM L-glutamine, 100 µg/mL penicillin, and 100 µg/mL streptomycin (Invitrogen); plated in T-25 culture flasks at a concentration of 1 × 105 cells/cm2; and incubated at 37°C in a 5% CO2 humidified incubator. ASC differentiation potential was evaluated with a GIBCO stem cell differentiation kit (Life Technologies). Briefly, ASCs of passage 3 were harvested and plated in three 12-well plates at 6000 cells/cm2 for 2 days. The cells were then transferred to adipogenic medium, osteogenic medium, or chondrogenic medium. Control cells were cultured in α-minimum essential medium supplemented with 10% fetal bovine serum. The medium for control and differentiation cells was changed every 3 days for 3 to 6 weeks. Adipogenesis of ASCs was assessed by Oil Red O staining. Osteogenesis was assessed by Alizarin Red S staining. Chondrogenesis was assessed by Safranin O staining.
Statistical Analysis
All data are presented as means ± standard deviations (SDs). Data were analyzed with Kruskal-Wallis one-way analysis of variance.19 To reduce the effect of different variables in the statistical analysis and to increase its power, we analyzed only the effect of harvesting technique on cell yields from samples obtained from the flanks of patients and the effect of anatomical location on cell yields from samples prepared with the Coleman technique. The Bonferroni approach was utilized to adjust multiple comparisons and set the overall false discovery rate at 0.05. All statistical analyses were performed with SAS software program version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Effect of Harvesting Technique on the Yield of SVF Cells and ASCs
Nineteen women undergoing breast reconstruction following mastectomy were included in the study. The patients' mean body mass index and mean age were 27 kg/m2 (range, 23-31 kg/m2; SD, ±4) and 51.2 years (range, 41.7-61.7 years; SD, ±9.5), respectively. Thirty-four flank, 7 axilla, and 8 abdomen adipose tissue samples were harvested by means of multiple techniques, including 19 instances of Coleman's technique with centrifugation, 18 instances of Coleman's technique without centrifugation, 9 instances of direct excision, and 7 instances of liposuction; 5 blood–oil discards from fat centrifugation following Coleman's technique were also collected.
A comparison of the yields of SVF cells and ASCs by direct excision, liposuction, Coleman's technique without centrifugation, or Coleman's technique with centrifugation, and from the blood and oil waste of adipose tissues harvested from patients' flanks is illustrated in Figure 2. One-way analysis of variance revealed that the type of harvesting technique was significantly associated with the numbers of total SVF cells and ASCs yielded (P < .001).
Figure 2.
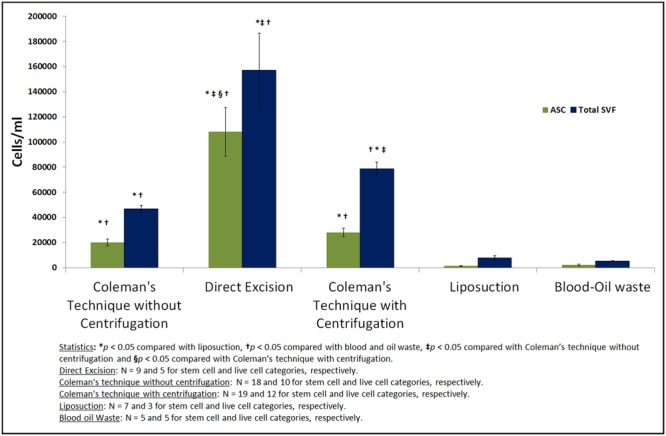
Quantitative analysis of total stromal vascular fraction (SVF) cell and adipose-derived stem cell (ASC) yields of adipose tissues harvested from the flank by means of different harvesting techniques. Error bars show the standard error of the mean (SEM) and Ns indicate the numbers of samples. Compared with Coleman's technique without centrifugation, direct excision and Coleman's technique with centrifugation both yielded significantly more total SVF cells. Compared with liposuction, Coleman's technique without centrifugation yielded significantly more total SVF cells. The number of ASCs obtained utilizing direct excision was significantly higher than the numbers of ASCs obtained from blood–oil waste or utilizing liposuction or Coleman's technique with or without centrifugation. The numbers of total SVF cells and ASCs obtained from blood–oil waste were similar to those obtained utilizing liposuction.
Pair-wise comparisons revealed significant differences between the numbers of total SVF cells and ASCs obtained with different approaches. The numbers of ASCs and total SVF cells obtained with direct excision were significantly higher than those obtained with Coleman's technique without centrifugation (P < .001 and P < .007, respectively). The number of total SVF cells obtained with Coleman's technique with centrifugation was significantly higher than that obtained with Coleman's technique without centrifugation (P = .003); however, the difference between the numbers of ASCs obtained utilizing Coleman's technique with or without centrifugation did not differ significantly. Compared with Coleman's technique without centrifugation, liposuction and blood–oil waste yielded significantly fewer ASCs (P < .001 and P < .035, respectively) and total SVF cells (P = .024 and P = .008, respectively). The numbers of SVF cells and ASCs obtained utilizing liposuction and the numbers of those obtained from blood–oil waste did not differ significantly.
Effect of Donor Site on the Yield of Total SVF Cells and ASCs
The yields of ASCs and total SVF cells from the axilla, abdomen, and flank utilizing Coleman's technique are shown in Figure 3. Analysis of variance revealed that harvest location was significantly associated with the number of SVF cells (P < .001), but not ASCs yielded. Compared with fat harvested from the axilla or flank, fat harvested from the abdomen yielded significantly more SVF cells (P < .05).
Figure 3.
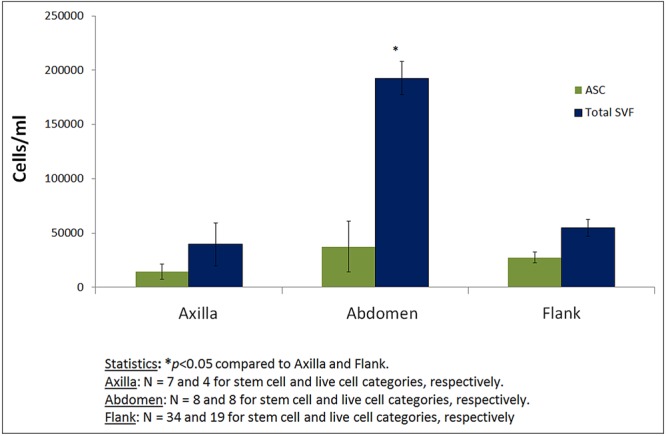
Quantitative comparison of total stromal vascular fraction (SVF) cell and adipose-derived stem cell (ASC) yields of fat tissue harvested from different locations. Error bars show the standard error of the mean (SEM), and Ns indicate the numbers of samples. Coleman's technique was utilized to isolate the cells. The number of total SVF cells obtained from adipose tissue harvested from the abdomen was significantly higher than the numbers of total SVF cells obtained from adipose tissue harvested from the flank or axilla.
Multilineage Differentiation Potential of Cultured ASCs
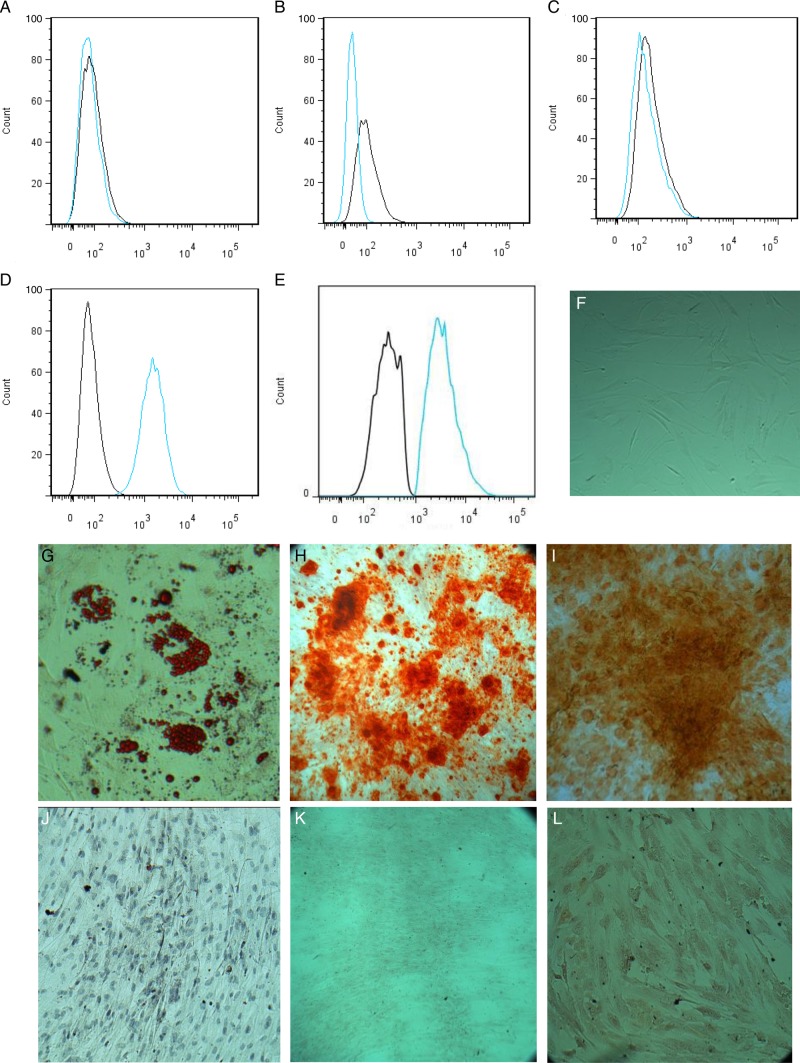
Flow cytometry analysis findings are illustrated in Figure 4. ASCs at passage 3 had fibroblast or spindle-like morphologies (Figure 4F). The vast majority of cells expressed CD90 (99.8%) and D7-FIB (96.7%), but not CD45 (0.03%), CD11b (0.73%), or CD34 (0.79%) These results were consistent with the findings of other studies.3,4
Figure 4.
Characteristics of cultured adipose-derived stem cells (ASCs). Flow cytometry analyses revealed that the cultured cells were (A) CD11b− (0.73%), (B) CD45− (0.03%), (C) CD34− (0.79%), (D) CD90+ (99.82%), and (E) D7FIB+ (96.7%). (F) At third passage, the cells had a spindle-like morphology. (G-I) Differentiation of ASCs to adipocytes (G and J, Oil Red O staining), osteocytes (H and K, Alizarin red S staining), and chondrocytes (I and L, Safranin O staining) in vitro. (J-L) ASCs cultured in basic medium served as controls.
Cells cultured in adipogenic medium differentiated to adipocytes at 3 weeks and displayed characteristic multiple intracellular bright white oil droplets. Oil Red O staining revealed red vesicles in the cells cultured with adipogenic medium (Figure 4G), but not cells cultured with basic medium (Figure 4J). ASCs cultured in osteogenic medium differentiated to osteoblasts at 3 weeks. Alizarin Red S staining revealed calcification deposits on ASCs cultured in osteogenic medium (Figure 4H), but not cells cultured in control medium (Figure 4K). ASCs cultured in chondrogenic medium differentiated to chondrocytes at 6 weeks. Safranin O staining revealed glycosaminoglycan in the differentiated chondrocytes (Figure 4I), but did not reveal cartilaginous extracellular matrix in cells cultured in basic control medium (Figure 4L). Together, these results indicate that ASCs have a mesenchymal stem cell phenotype and the potential for multilineage differentiation.
DISCUSSION
In the present study, we found that harvesting technique and donor site affect the initial numbers of total SVFs and ASCs obtained from adipose tissue. Among the harvesting techniques evaluated, direct excision yielded the most ASCs and total SVF cells, whereas liposuction yielded the fewest number of cells. Coleman's technique with or without centrifugation yielded more ASCs than liposuction did but fewer ASCs than direct excision did. Coleman's technique with centrifugation yielded more total SVF cells, but not ASCs, than did Coleman's technique without centrifugation. The total SVF yield from donor sites in the abdomen was greater than that from donor sites in the flank or axilla. ASC yield did not differ significantly among donor sites.
In addition to traditional markers such as CD90, CD34, and CD29, D7FIB was also utilized to identify a pure stem cell population (Table 1). Results from several studies have shown that the D7FIB+, CD45– marker is more effective than traditional markers, such as the CD90+, CD105+ marker, for the identification of distinct stem cell populations in bone marrow,20-22 adipose tissue, and degenerative joint tissues.23 As expected, compared with fresh isolated cells, cultured cells had higher percentages of CD11b–, CD45–, CD90+, D7FIB+ ASCs that differentiated into mesenchymal lineages. Some researchers have suggested that CD34+ ASCs are capable of differentiating into all mesenchymal stem cell lineages24-26 and endothelial cells,27,28 and others have maintained that CD34– ASCs lack the potential to differentiate into endothelial cells and vascularize in vivo.29,30 Other authors have classified SVF cells as pericytes and endothelial progenitor cells or as ASCs to investigate specific precursor cells in the SVF, but these authors also acknowledged that the chosen isolation procedures and the specific differentiation capacity of the cells varied substantially.30-34 In the present study, fresh isolated total SVF cells included a significant number of CD34+, D7FIB+ ASCs, whereas cultured and passaged total SVF cells were almost all D7FIB+ but CD34–, which suggests that the culture process caused the loss of CD34 expression. These findings are consistent with those of other studies, in which culture conditions caused the loss of CD34 expression in ASCs and CD34– ASCs retained their multilineage differentiation potential.24-26,35,36
Factors such as an excision-based fat-harvesting technique and high-density adipose tissue are expected to contribute to higher yields of total SVF cells and ASCs. This observation is reflected in our study, in which surgical excision yielded more ASCs than Coleman's technique (with or without centrifugation) or liposuction and more total SVF cells than Coleman's technique without centrifugation. Table 2 summarizes our results and compares them with those of some previous studies. The trends for total SVF cells and ASCs in our study were similar to those in some previous studies, but also different from trends in others. For instance, the trends for total SVF cells in our study were similar to those in studies by Schreml et al37 and Pu et al,6 but not to those in the study by Faustini et al.38 However, unlike these previous studies, our study showed that ASC yield varied with harvesting technique. We speculate that the density of fat tissue may play a role in the yield of total SVF cells and ASCs. Fat tissues obtained by different harvesting techniques have different densities: Liposuction samples are less viscous than those harvested utilizing Coleman's technique without centrifugation, which in turn are less viscous than samples obtained utilizing Coleman's technique with centrifugation, and surgically excised fat is solid tissue. Thus, surgical excision, which provides tissue with a high fat density, is expected to yield more SVF cells and ASCs than Coleman's technique or liposuction would, and this was the case in the present study. Similarly, excision and Coleman's technique (with or without centrifugation) yielded more cells than liposuction did.
Table 2.
Effect of Harvesting Technique and Postharvest Processing on Yields of Different Cells from Adipose Tissues in the Present and Previous Studies
| Study | Effect of Harvesting Technique and Postharvest Processing |
|---|---|
| Present study | ASCs: excision > Coleman (with or without centrifugation) > liposuction Total SVFs: excision > Coleman with centrifugation > Coleman without centrifugation > liposuction |
| Faustini et al38 | ASCs: excision = liposuction Total SVFs: excision = liposuction |
| Schreml et al37 | ASCs: excision = liposuction Total SVFs: excision > liposuction |
| Pu et al6 | Adipocytes: Coleman > liposuction |
| Kurita et al44 | Adipocytes: liposuction plus centrifugation > liposuction ASCs: liposuction plus centrifugation > liposuction |
ASC, adipose-derived stem cell; SVF, stromal vascular fraction.
However, Coleman's technique with centrifugation unexpectedly yielded more total SVF cells, but not ASCs, than Coleman's technique without centrifugation. These findings are in agreement with those of previous studies15,39 showing that the yield of ASCs per milliliter of fat tissue utilizing Coleman's technique with centrifugation is not significantly different from that utilizing Coleman's technique without centrifugation. Because we employed the same protocols for both harvesting techniques, the differences we observed are likely not due to the in vitro processing of the cells. Therefore, factors in addition to collagenase treatment, aqueous fraction of the adipose tissue volume, and centrifugation may affect the ASC yield. A centrifugation step, as expected, would increase the cell density and thus increase cell yields. However, the increase in total SVF cells but not ASCs seems to indicate an inherent difference in the ways in which SVF cells and ASCs are harvested. Coleman's technique with centrifugation and Coleman's technique without centrifugation may differ only in terms of the centrifugation step. Based on our experience with centrifugation in routine cell cultures, it is highly unlikely that a centrifugation step would damage cell integrity. Thus, we believe that the denser fat grafts obtained with Coleman's technique may undergo higher shear stresses when they are extruded from the syringe and that these stresses affect ASC yields. Factors such as the diameter of syringe and force applied by the surgeon may affect the shear stresses. The higher total SVF yield utilizing Coleman's technique with centrifugation might be explained by the presence of nontissue cells in the total SVF, such as those in the blood–oil milieu, which may not be subject to such high shear stresses during centrifugation and extrusion. The finding is important because the non-ASC fraction of the SVF may play an important role in promoting regeneration by improving the microenvironment. In addition to this non-ASC fraction of the SVF, other important microenvironment participants such as growth factors may play a role in mediating effective wound healing response. The microenvironment factors present in adipose tissue and the decanted blood–oil milieu are poorly understood and warrant future investigation.
In this study, we mainly assessed cells residing in the stromal tissue and not the mature adipocytes found in the oil that was discarded during SVF isolation following collagenase treatment and centrifugation. However, we also assessed the cell yields of the blood–oil waste discarded following Coleman's technique with centrifugation and found that subjecting this waste to a second centrifugation yielded a significant number of ASCs and total SVF cells. This approach may provide an additional source of cells for enriching fat grafts.
We also compared the cell yields of tissue obtained from different donor sites and found that fat tissues from the abdomen yielded more SVF cells, but not ASCs, than fat tissues from the axilla or flank did. Our results and those of previous studies are summarized in Table 3. We found that fat tissues harvested from the abdomen yielded more total SVF cells than fat tissues harvested from the flank or axilla did, as Faustini et al reported38; however, Fraser et al40 reported the opposite finding. Similar to Oedayrajsingh-Varma et al,39 we found that ASC yield did not vary with harvest site, whereas Jurgens et al15 and Padoin et al41 noted higher yields of ASCs from the abdomen. Adipose tissues harvested from different locations yielded different amounts of cells, but the reason for this remains unknown.
Table 3.
Effect of Donor Site Location on the Yields of Different Cells from Adipose Tissues in the Present and Previous Studies
| Study | Effect of Donor Site Location |
|---|---|
| Present study | ASCs: no difference Total SVFs: abdomen > axilla/flank |
| Jurgens et al15 | ASCs: abdomen > flank |
| Fraser et al40 | Total SVFs: flank > abdomen |
| Oedayrajsingh-varma et al39 | ASCs: abdomen = hip = mamma |
| Faustini et al38 | Total SVFs: abdomen > back > kneea |
| Padoin et al41 | ASCs: lower abdomen > inner thigh/knee |
ASC, adipose-derived stem cell; SVF, stromal vascular fraction. aFor male but not for female.
The present study is the first, to our knowledge, to identify an association between ASCs and total SVF yields based on adipose tissue density. This new understanding will further help surgeons select and identify the ideal source for harvesting cells from adipose tissues and optimize harvesting techniques for autologous fat grafting. However, our study was limited by patient demographics such as sex, age, body mass index, race, and other confounding factors that may influence cell yield. Additional considerations such as the availability of fat tissue in patients with low body mass indexes and the feasibility of performing surgical procedures based on reconstructive need limited the range of fat harvesting techniques that could be performed for the same patient. Another potential limitation of the study was the narrow scope of the statistical analysis, which considered the effect of harvesting technique on cell yields from samples obtained from the flanks of patients and the effect of anatomical location on cell yields from samples prepared with the Coleman technique, but did not consider relationships between other harvest sites and techniques. More patient samples and thus a larger study are needed to sufficiently power a statistical analysis of all variables, including harvesting technique and location, and we are currently in the process of conducting such a study. Despite the limitations of analyzing a focused set of populations, we found that total SVF and ASC yields were significantly associated with harvesting technique and donor site. Finally, our study did not include patients who had received previous radiotherapy, which limits the applicability of our findings given the large number of patients who receive radiotherapy before undergoing reconstructive surgery. Our future studies will include irradiated patients to better understand the effect of radiation on aesthetic outcomes and stem cell numbers.
CONCLUSIONS
Our findings suggest that donor site and harvesting technique affect the yields of total SVF cells and ASCs. Based on our findings, the ideal method to maximize the yields of total SVF cells and ASCs is to harvest adipose tissue from the abdomen by means of a direct excision technique. Although it yielded fewer SVF cells and ASCs than direct excision did, Coleman's technique with centrifugation—which offers the advantage of being an easier technique for fat grafting—is also a favorable method for performing aesthetic procedures and obtaining SVF cells and ASCs from adipose tissue. A comprehensive follow-up study would ensure better control over the aforementioned factors as well as better correlation between reconstructive graft outcomes and ASC and total SVF cell yields in individual patients. Our findings also confirm that CD34–, D7FIB+ ASCs indeed have multilineage differentiation potential despite the loss of CD34 expression owing to further expansion in culture.
Disclosures
The authors have no financial interests to declare in relation to the content of this article. The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
This work was funded in part by the National Institutes of Health (NIH) grant R21EB007587-02. This work was also supported by the NIH through MD Anderson′s Cancer Center Support grant CA016672. The awarded funding was only applied to direct or indirect costs, including research supplies and personnel based on NIH guidelines.
Acknowledgments
We dedicate this study to the memory of Dr. Elisabeth K. Beahm. We thank the personnel of MD Anderson's Flow Cytometry Core Facility for assisting in flow cytometry and Joseph Munch in MD Anderson's Department of Scientific Publications for editing the manuscript.
REFERENCES
- 1.Meier JD, Glasgold RA, Glasgold MJ. Autologous fat grafting: long-term evidence of its efficacy in midfacial rejuvenation. Arch Facial Plast Surg. 2009;11:24-28. [DOI] [PubMed] [Google Scholar]
- 2.Eremia S, Newman N. Long-term follow-up after autologous fat grafting: analysis of results from 116 patients followed at least 12 months after receiving the last of a minimum of two treatments. Dermatol Surg. 2000;26:1150-1158. [PubMed] [Google Scholar]
- 3.Locke MB, de Chalain TM. Current practice in autologous fat transplantation: suggested clinical guidelines based on a review of recent literature. Ann Plast Surg. 2008;60:98-102. [DOI] [PubMed] [Google Scholar]
- 4.Rosing JH, Wong G, Wong MS, et al. Autologous fat grafting for primary breast augmentation: a systematic review. Aesthetic Plast Surg. 2011;35:882-890. [DOI] [PubMed] [Google Scholar]
- 5.Spear SL, Wilson HB, Lockwood MD. Fat injection to correct contour deformities in the reconstructed breast. Plast Reconstr Surg. 2005;116:1300-1305. [DOI] [PubMed] [Google Scholar]
- 6.Pu LL, Coleman SR, Cui X, et al. Autologous fat grafts harvested and refined by the Coleman technique: a comparative study. Plast Reconstr Surg. 2008;122:932. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen A, Pasyk KA, Bouvier TN, et al. Comparative study of survival of autologous adipose tissue taken and transplanted by different techniques. Plast Reconstr Surg. 1990;85:378-386; discussion 87-89. [PubMed] [Google Scholar]
- 8.Niechajev I, Sevcuk O. Long-term results of fat transplantation: clinical and histologic studies. Plast Reconstr Surg. 1994;94:496-506. [DOI] [PubMed] [Google Scholar]
- 9.Ersek RA. Transplantation of purified autologous fat: a 3-year follow-up is disappointing. Plast Reconstr Surg. 1991;87:219-227; discussion 28. [PubMed] [Google Scholar]
- 10.Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375-3382. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32:48-55; discussion 56-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimura K, Sato K, Aoi N, et al. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34:1178-1185. [DOI] [PubMed] [Google Scholar]
- 13.Castro-Govea Y, De La Garza-Pineda O, Lara-Arias J, et al. Cell-assisted lipotransfer for the treatment of parry-romberg syndrome. Arch Plast Surg. 2012;39:659-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schipper BM, Marra KG, Zhang W, et al. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg. 2008;60:538-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jurgens WJ, Oedayrajsingh-Varma MJ, Helder MN, et al. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res. 2008;332:415-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Harmelen V, Skurk T, Rohrig K, et al. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int J Obes Relat Metab Disord. 2003;27:889-895. [DOI] [PubMed] [Google Scholar]
- 17.Shiffman MA, Mirrafati S. Fat transfer techniques: the effect of harvest and transfer methods on adipocyte viability and review of the literature. Dermatol Surg. 2001;27:819-826. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman MR, Bradley JP, Dickinson B, et al. Autologous fat transfer national consensus survey: trends in techniques for harvest, preparation, and application, and perception of short- and long-term results. Plast Reconstr Surg. 2007;119:323-331. [DOI] [PubMed] [Google Scholar]
- 19.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:583-621. [Google Scholar]
- 20.Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford). 2008;47:126-131. [DOI] [PubMed] [Google Scholar]
- 21.Jones EA, Kinsey SE, English A, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349-3360. [DOI] [PubMed] [Google Scholar]
- 22.English A, Jones EA, Corscadden D, et al. A comparative assessment of cartilage and joint fat pad as a potential source of cells for autologous therapy development in knee osteoarthritis. Rheumatology (Oxford). 2007;46:1676-1683. [DOI] [PubMed] [Google Scholar]
- 23.Jones EA, English A, Henshaw K, et al. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004;50:817-827. [DOI] [PubMed] [Google Scholar]
- 24.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y, Liu T, Song K, et al. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26:664-675. [DOI] [PubMed] [Google Scholar]
- 27.Nakagami H, Maeda K, Morishita R, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542-2547. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y, Sun Z, Liao L, et al. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370-379. [DOI] [PubMed] [Google Scholar]
- 29.Miranville A, Heeschen C, Sengenes C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. [DOI] [PubMed] [Google Scholar]
- 30.Sengenes C, Lolmede K, Zakaroff-Girard A, et al. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J Cell Physiol. 2005;205:114-122. [DOI] [PubMed] [Google Scholar]
- 31.Khan WS, Tew SR, Adesida AB, et al. Human infrapatellar fat pad-derived stem cells express the pericyte marker 3G5 and show enhanced chondrogenesis after expansion in fibroblast growth factor-2. Arthritis Res Ther. 2008;10:R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin G, Garcia M, Ning H, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suga H, Matsumoto D, Eto H, et al. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009;18:1201-1210. [DOI] [PubMed] [Google Scholar]
- 34.Zannettino AC, Paton S, Arthur A, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413-421. [DOI] [PubMed] [Google Scholar]
- 35.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362-369. [DOI] [PubMed] [Google Scholar]
- 37.Schreml S, Babilas P, Fruth S, et al. Harvesting human adipose tissue-derived adult stem cells: resection versus liposuction. Cytotherapy. 2009;11:947-957. [DOI] [PubMed] [Google Scholar]
- 38.Faustini M, Bucco M, Chlapanidas T, et al. Nonexpanded mesenchymal stem cells for regenerative medicine: yield in stromal vascular fraction from adipose tissues. Tissue Eng Part C Methods. 2010;16:1515-1521. [DOI] [PubMed] [Google Scholar]
- 39.Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, et al. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8:166-177. [DOI] [PubMed] [Google Scholar]
- 40.Fraser JK, Wulur I, Alfonso Z, et al. Differences in stem and progenitor cell yield in different subcutaneous adipose tissue depots. Cytotherapy. 2007;9:459-467. [DOI] [PubMed] [Google Scholar]
- 41.Padoin AV, Braga-Silva J, Martins P, et al. Sources of processed lipoaspirate cells: influence of donor site on cell concentration. Plast Reconstr Surg. 2008;122:614-618. [DOI] [PubMed] [Google Scholar]
- 42.Huss R. Isolation of primary and immortalized CD34-hematopoietic and mesenchymal stem cells from various sources. Stem Cells. 2000;18:1-9. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64-76. [DOI] [PubMed] [Google Scholar]
- 44.Kurita M, Matsumoto D, Shigeura T, et al. Influences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr Surg. 2008;121:1033-1041; discussion 42-43. [DOI] [PubMed] [Google Scholar]