Abstract
The type 1 equilibrative nucleoside transporter (ENT1) is implicated in regulating the levels of extracellular adenosine ([AD]ex ). In the basal forebrain (BF) the levels of [AD]ex increase during wakefulness and closely correspond to the increases in the electroencephalogram (EEG) delta (0.75–4.5Hz) activity (NRδ) during subsequent non-rapid eye movement sleep (NREMS). Thus in the BF, [AD]ex serves as a biochemical marker of sleep homeostasis. Waking EEG activity in theta range (5–9Hz, Wθ) is also described as a marker of sleep homeostasis. An hour-by-hour temporal relationship between the Wθ and NRδ is unclear. In this study we examined the relationship between these EEG markers of sleep homeostasis during spontaneous sleep-wakefulness and during sleep deprivation (SD) and recovery sleep in the ENT1 gene knockout (KO) mouse. We observed that baseline NREMS amount was decreased during light period in ENT1 KO mice, accompanied by a weak correlation between Wθ of each hour and NRδ of its subsequent hour when compared to their wild-type (WT) littermates. Perfusion of low dose of adenosine into BF not only strengthened the Wθ –NRδ relationship, but also increased NREMS to match with the WT littermates suggesting decreased [AD]ex in ENT1 KO mice. However, the SD-induced [AD]ex increase in the BF and the linear correlation between the EEG markers of sleep homeostasis were unaffected in ENT1KO mice suggesting that during SD, sources other than ENT1 contribute to increase in [AD]ex. Our data provide evidence for a differential regulation of wakefulness-associated [AD]ex during spontaneous vs prolonged waking.
Graphical Abstract
Introduction
The levels of extracellular adenosine [AD]ex are partly regulated by nucleoside transporters, the bidirectional equilibrative nucleoside transporters (ENT1, 2, 3 and 4) driven by chemical gradient (Baldwin et al., 2004), and the concentrative nucleoside transporters (CNT1, 2, and 3) driven by sodium (and proton) electrochemical gradients (Gray et al., 2004). ENT1 is implicated in the regulation of [AD]ex in basal forebrain (BF) (Basheer et al., 2004; Brown et al., 2012). Blocking AD uptake within BF by ENT1 inhibitor, nitrobenzylthioinosine (NBMPR), increases [AD]ex and sleep (Porkka-Heiskanen et al., 1997). In rats, a decrease in NBMPR binding following acute sleep deprivation (SD) is attributed to the SD-induced increase in [AD]ex (Alanko et al., 2003). Besides the transport of [AD]ex via transporters, breakdown of extracellular adenosine triphosphate [ATP]ex released in response to increased neuronal activity as a cotransmitter from neurons or by gliotransmission, contribute to increased [AD]ex (Dunwiddie and Masino, 2001). Both, decreased re-uptake by transporters, and increased release/breakdown of gliotransmission-derived ATP are implicated in the regulation of [AD]ex in BF (Alanko et al., 2003, Halassa et al., 2009).
AD acts as a somnogenic factor in the BF consisting of cortically-projecting wake-active neurons (Basheer et al., 2004, Porkka-Heiskanen and Kalinchuk, 2011, Brown et al., 2012). The levels of [AD]ex are higher during wakefulness when compared to sleep (Porkka-Heiskanen et al., 1997, McKenna et al., 2003, Murillo-Rodriguez et al., 2004). SD further increase the [AD]ex within 3h in BF and by 5h in frontal cortex (Kalinchuk et al., 2011). In a recent study we reported that the SD-induced increase in BF [AD]ex depends on the neuronal induction of inducible nitric oxide synthase-dependent nitric oxide, not observed during spontaneous wakefulness, suggesting a potential difference in the mechanism of [AD]ex regulation during spontaneous wakefulness and SD (Kalinchuk et al., 2006b, Kalinchuk et al., 2010).
The increases in [AD]ex occurs during wakefulness. However, to date, its homeostatic effects are evaluated by the increases in the EEG delta activity (0.75–4.5Hz, NRδ) during subsequent non-rapid eye movement sleep (NREMS). In humans and rodents waking EEG activity in theta range (5–9Hz, Wθ) is also described as a marker of sleep homeostasis (Cajochen et al., 1995, Aeschbach et al., 1997, Finelli et al., 2000, Vyazovskiy and Tobler, 2005, Kalinchuk et al., 2015). A positive correlation exists between the rise rate of Wθ and the NRδ in NREMS (Finelli et al., 2000). In this study using an ENT1-gene knock-out mouse (ENT1KO) model (Choi et al., 2004), we sought to examine the role of ENT1 in spontaneous sleep-wake regulation. We observed that ENT1 is key in regulating a linear relationship between the hourly Wθ and the NRδ of the subsequent hour. We also examined the role of ENT1 on SD-induced increase in [AD]ex in the BF and homeostatic sleep response (HSR). We observed that SD-induced HSR was unaffected in ENT1KO mice with concurrent increase in BF [AD]ex. To our knowledge, this is the first study to measure [AD]ex in the BF of mice by microdialysis and to demonstrate that [AD]ex regulates the Wθ-NRδ relationship.
Materials and Methods
Animals
The ENT1KO mice were generated as described by Choi et al (2004). Briefly, the exons 2–4 of ENT1 gene were deleted in mice with C57BL/6J × 129X1/SvJ genetic background. The controls were the wild type (WT) littermates of the KO mice. Male adults (3–4 month old) mice were used in the study. Mice were housed in standard Plexiglas cages (room temperature 23.5–24.0 °C ; 12h light:12h dark cycle, lights on at 07:00A.M., ZT 0) with food and water provided ad libitum. Animal care and handling procedures were approved by the Association for Assessment and Accreditation of Laboratory Animal Care and Use Committee at Veterans Affairs Boston Healthcare System, Harvard University, and National Institutes of Health.
EEG Electrode and microdialysis cannula implantation
For electroencephalogram (EEG) recordings, mice were implanted with EEG and electromyogram (EMG) electrodes under general anesthesia (1–3% isoflurane inhalation). EEG electrodes (stainless-steel screws) were implanted epidurally over the frontal [anteroposterior (AP), +1.0 mm from Bregma; mediolateral (ML), 1.0 mm] and parietal (AP, +1.0 mm from lamda; ML, 1.0 mm) cortices. EMG recording electrodes (silver wires covered with Teflon) were implanted into neck muscles. A unilateral guide cannula for microdialysis probes (CMA 7 Guide Cannula, CMA Microdialysis, Solna, Sweden) was inserted targeting the dorsal margin of BF including substantia innominata, horizontal limb of diagonal band, and the magnocellular preoptic nucleus (AP, 0.0 mm; ML, 1.5 mm; dorsoventral, 5.0 mm) (Franklin and Paxinos, 2008). EEG, EMG electrodes and one guide cannula were attached to microconnector and fixed to the skull with dental cement. After surgery, analgesic (Ketofen, 5mg/kg) was given intramuscularly and each mouse was housed in individual cages and allowed to recover from surgery for one week.
EEG recording and analyses
One week after surgery, the mice were transferred to the recording cages in a sound-attenuated room for habituation with attached EEG recording cables for two days. Baseline EEG was monitored for a 24 h period starting at 7:00 A.M. The EEG/EMG signals were amplified and sampled at 100 Hz. EEG recordings (acquisition using Grass Gamma, version 4.3) were manually scored using the Rat Sleep Stager (version 3.2) in 10 s epochs for non-rapid eye movement (NREM) sleep, rapid eye movement (REM) sleep, and wakefulness. Time spent in each vigilance state was calculated and compared between WT littermates and ENT1 KO mice. The frequency and duration of NREMS and REM episodes were analyzed to determine the difference of sleep quality in ENT1 KO mice. Two established homeostatic sleep markers, EEG activity in NRδ (Borbely, 1982) and Wθ (Finelli et al., 2000, Vyazovskiy and Tobler, 2005, Kalinchuk et al., 2015), were determined using Fast Fourier Transform. Both NRδ and Wθ were normalized to its own baseline 24h mean values of NRδ and Wθ, respectively. To determine the relationship between the increases in Wθ with increasing duration of wakefulness, we performed correlation analysis between the time awake within each hour and the Wθ values for the same hour. Furthermore, to examine the relationship between the two homeostatic markers, we performed an hour-to hour analysis between previous hour Wθ and following hour NRδ.
Adenosine perfusion
Microdialysis probes (CMA 7, membrane length and outer diameter 1 mm and 0.24 mm, respectively, CMA Microdialysis, Solna, Sweden) were inserted into the guide cannula targeting BF and were connected to the programmable microinjection pump (CMA/100, CMA microdialysis), 12 h before the perfusion experiment. Decreased adenosine tone in ENT1 mice has been reported using an indrect measurement of a reduction in A1 receptor mediated inhibition of glutamate excitatory postsynaptic currents (EPSCs) (Choi et al., 2004; Nam et al., 2013). To determine if the decreased [AD]ex levels underlie the differences in Wθ-NRδ relationship during light period in ENT1KO mice, we perfused either artificial cerebrospinal fluid (aCSF, control) or 100μM AD into the BF. Previous studies showed that a 2–3-fold increase in [AD]ex during SD resulted in 50% increase in time spent in NREM sleep during the recovery period (Porkka-Heiskanen et al., 1997, Basheer et al., 1999, Kalinchuk et al., 2006a, Kalinchuk et al., 2006b). Similar (50%) increase in NREM sleep was also observed following reverse microdialysis of 300 μM AD into BF (Porkka-Heiskanen et al., 1997, Basheer et al., 1999, Kalinchuk et al., 2008). Our criteria was not to increase the sleep by 50% as seen after SD but to improve sleep quality to match that of the spontaneous sleep seen in WT mice. Consequently, we chose to perfuse AD that was 3-fold lower (100 μM) than the levels used to mimic SD. AD dissolved in aCSF (Harvard apparatus, Holliston, USA) was perfused for 3 h starting at 07:00 A.M. at 1 μL/min flow rate. For comparison, two kinds of baseline EEG were obtained. The EEG changes in response to AD perfusion during the 3h of perfusion and 3 h post perfusion were compared with the baseline time matched EEG recordings performed either with (1) no perfusion or with (2) aCSF perfusion.
Sleep deprivation
SD was done by gentle handling procedure (Franken et al., 1991), practiced routinely in our laboratory, and described previously (Basheer et al., 1999, Kalinchuk et al., 2008, Kalinchuk et al., 2010). The experimenter visually observes the animals and gently taps the cage or touches the animal with a soft brush at the EEG (appearance of low frequency high amplitude slow waves) and behavioral (inactive and curled posture with an attempt to close eyes) signs of sleep. In our laboratory we had tested the effect of our SD paradigm by examining the neuronal activation in stress related areas of amygdala and cingulate cortex using c-Fos immunohistochemistry. We had also measured cortisol in blood of sleep deprived rats. Both these markers of stress were not elevated in our animals (Kalinchuk et al., 2010). During SD, mice were allowed to consume food and water ad libitum. On the day of experiment, six-hour SD was performed from 10:00 A.M. to 04:00 P.M. Recovery sleep (RS) was allowed with EEG monitoring from 04:00 P.M. to 10:00 P.M.
In vivo adenosine measurement in BF
During SD, in vivo microdialysis was performed to collect samples every half hour to measure the changes in the [AD]ex level in BF. The samples were collected during pre-deprivation baseline (8:00 A.M – 10:00 A.M.), 6h SD (10:00 A.M. – 04:00 P.M.), and 2h recovery sleep (RS) (04:00 P.M. – 6:00 P.M.). The [AD]ex was measured by high performance liquid chromatography (HPLC) with UV detector (SPD-20A, Shimadzu, Columbia, MD, USA) as previously described (Porkka-Heiskanen et al., 1997). Values obtained from SD and RS were normalized to each animal’s own pre-deprivation baseline. The detection limit of the assays for adenosine was 0.8 nM (signal to noise ratio 2:1).
Real Time Polymerase Chain Reaction (RT-PCR)
Strategies based on the use of a specific gene KO mouse model are often questioned for the potential compensatory changes in the related gene expressions. Consequently it was important to examine the changes in the levels of gene expression at the mRNA level in brain. To evaluate any developmental compensatory changes in the expression of other nucleoside transporters including CNT2, CNT3, ENT2, ENT3, and ENT4, we examined the changes in their mRNA in the BF. The brains from the KO mice were collected at 10AM and RNA was extracted from dissected brain tissue samples of the BF from three mice by TRIzol® reagent method. Reverse transcription was done with Superscript II (Invitrogen) kit. RT-PCR of duplicated samples was performed using TaqMan Gene expression primer/probe (Applied Biosystems) for CNT2 (Mm04212034_m1), CNT3 (Mm00627874_m1), ENT2 (Mm00432817_m1), ENT3(Mm00469913_m1), ENT4 (Mm00525575_m1), and 18S ribosomal RNA (Cat. No.4333760F) as the endogenous control.
Statistics
To evaluate the statistical significance of the differences between the two groups (KO and WT), we used Student’s t-test or the nonparametric Mann–Whitney rank sum test. The correlation between the preceding hour Wθ activity and subsequent hour NRδ activity was analyzed using Pearson’s correlation coefficient. For RT-PCR data, relative quantification was done for each sample with comparative 2−ΔΔCT method (Livak and Schmittgen, 2001). To determine the statistical significance individual Ct values were converted to the linear for 2−Ct for comparison between WT and KO mice by the Student’s t-test. The data is presented as fold change with variability within each group. Statistical analysis was performed using SigmaPlot 11.0 Statistical software (Systat Software Inc.).
Results
Decreased sleep duration in ENT1 KO mice
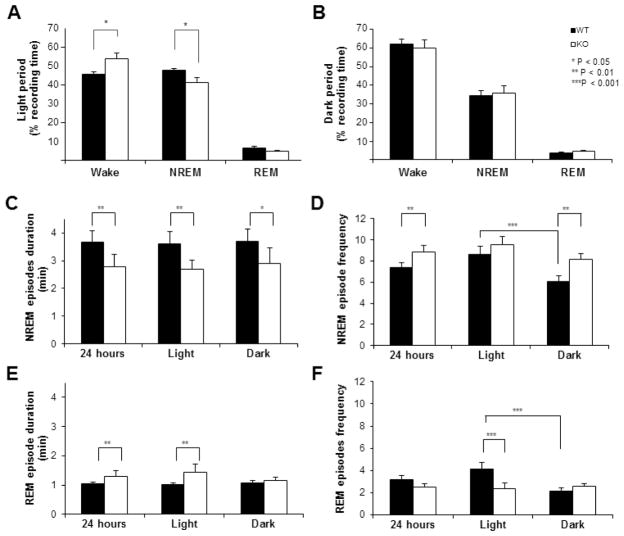
ENT1 KO mice showed decreased NREM sleep time (WT 48.0% vs KO 41.5%, t=2.3, df=10, P=0.03) and a concurrent increase in wakefulness (WT 45.6% vs KO 54.1%, t=2.9, df=10, P=0.01) during the light period with no change during the dark period (Figure 1A and B). Further analysis showed that while the mean duration of NREM sleep episodes was lower when compared to WT during both light (26.0% decrease, t=4.6, df=22, P<0.001) and dark periods (21.7% decrease, t=2.8, df=22, P=0.01), the frequency of NREM episodes was higher only during the dark period (34.4% increase, t=−3.03, df=22, P=0.006) with no change during the light period (Figure 1C and D). On the contrary, REM sleep episode duration was lower (40.2%, P<0.004) and frequency higher (42.7%, P<0.001) in KO mice (Figure 1E and F).
Figure 1.
Comparison of sleep profiles between ENT1 KO (KO) and wild type (WT) control mice. A and B, Time spent in wakefulness, NREM and REM sleep in KO and WT control mice. C and D, Frequency and duration of NREM sleep episodes. E and F, Frequency and duration of REM sleep episodes. Filled and open bars indicate WT and KO, respectively. Each bar represents mean value ± SEM (n=6). Statistical analyses were done by Student’s t-test (*P<0.05, **P<0.01, ***P<0.001).
Altered relationship between wake-theta (Wθ) and NREM delta (NRδ) in ENT1 KO
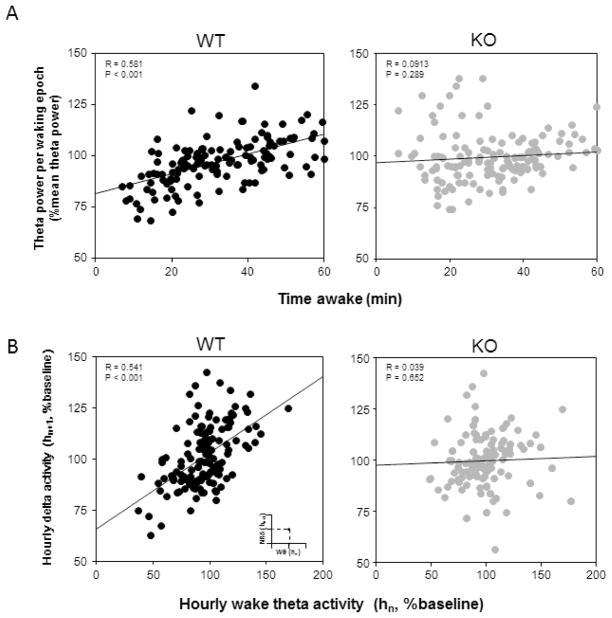
The increase in Wθ has been suggested to be a marker of sleep homeostasis. The higher the Wθ during wakefulness, more intense is the NRδ that follows wakefulness, thus suggesting a positive correlation between the two EEG parameters (Finelli et al., 2000, Vyazovskiy and Tobler, 2005, Kalinchuk et al., 2015). To test any changes in these homeostatic markers, first we examined the change in Wθ with increasing episode durations of spontaneous wakefulness (from 4min to 60 min durations). As shown in Figure 2A (N=6, each genotype, 24 data points for each mouse), there was a statistically significant positive correlation between the Wθ and durations of wakefulness in the WT mice (R=0.58, P<0.001). Such a positive correlation was absent in KO mice (R=0.09, P=0.28).
Figure 2.
Correlation of EEG parameters during spontaneous sleep-wake cycle in WT and ENT1 KO mice. A, Scatter plot of time awake in minute(s) and normalized theta power per waking epoch for the same time bin during a 24-hour sleep-wake cycle shows significant correlation in WT (black) but not in KO mice. B, Wake theta activity (Wθ) of each hour (hn, n=1 to 23) is plotted against NREM delta activity (NRδ) for the following hour (hn+1) (see inset). In WT mice (N=6, black dots) a significant linear correlation was observed but not in the KO (N=6, grey dots) mice. Theta and delta power are normalized to 24-hour mean theta and delta power, respectively. R, Pearson’s correlation coefficient; P, probability.
Next we examined the relationship between the two markers of sleep homeostasis, the Wθ during each (preceding) waking hour, and the NRδ in the following hour during 24 hours of spontaneous sleep-wakefulness. We observed that in an hour-by-hour EEG analysis, the relationship between the Wθ during the preceding hour of wakefulness and the NRδ during the following hour of NREM sleep showed a statistically significant positive correlation only in WT mice (R=0.54, P<0.001) but not in KO mice (R=0.03, P=0.65) (N=6, each genotype; 23 data points for each mouse, Figure 2B).
Increasing extracellular adenosine levels improves Wθ-NRδ relationship
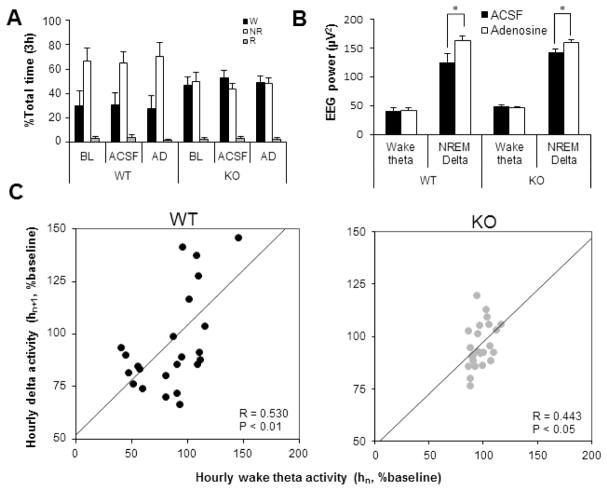
To determine the effect of enhancing the adenosine tone by increasing the [AD]ex on Wθ-NRδ relationship during light period, we perfused either aCSF (control) or 100μM adenosine, a concentration 3-fold lower than 300μM adenosine used to mimic the SD effects. The criteria for choosing this concentration was to correct any impairment in the adenosine mediated effects (Choi et al., 2004) while maintaining the time spent in sleep, wakefulness or REM sleep. Since a 3x higher concentration (300μm) of adenosine perfusion into BF increases sleep time by 50% and enhance NREM delta (Porkka-Heiskanen et al., 1997; Basheer et al., 1999), it was essential to use a concentration that did not result in similar increases in sleep quantity but increased it merely to match the levels observed in WT mice. The perfusion was carried out from 7AM to 10AM in undisturbed mice (N=4, each genotype). While no significant changes in the total sleep-wake time was observed in both WT and KO mice (Figure 3A), the NREM delta activity showed significant increases in both genotypes during the 3h period of adenosine infusion (Figure 3B). This change had a significant bearing on the Wθ-NRδ relationship during adenosine perfusion. Similar to WT mice, KO mice also showed a significant correlation between the Wθ in each hour and the NRδ in the following hour (R=0.53, P=0.008 for WT; R=0.44; P=0.03 for KO) (Figure 3C). However, the overall amount of REM sleep was unchanged.
Figure 3.
Sleep-wake profiles and EEG power changes during 100 μM adenosine perfusion for three hours during light period. A, Time spent in wake (black), NREM (white) and REM (grey) sleep was not significantly different during three conditions: baseline, ACSF and adenosine (AD) perfusion. B, Comparison of wake-theta (Wθ) and NREM delta activity between ASCF and AD perfusion for 3 hours from 7AM to 10AM. *P<0.05 by paired t-test. C, The scatter plot of normalized Wθ activity of each hour (hn, n=1 to 2) against normalized NRδ in the following hour (hn+1) during AD perfusion. A significant linear trend line is restored in KO mice (grey dots) by AD perfusion similar to that observed in WT (black dots) mice. R, Pearson’s correlation coefficient; P, probability.
Absence of ENT1 does not affect SD-induced [AD]ex and recovery sleep
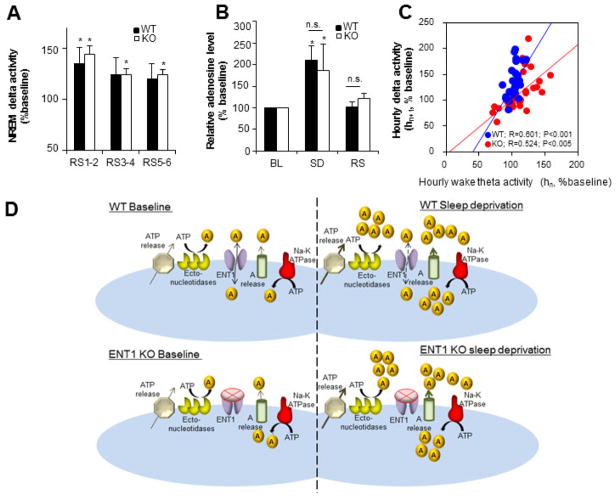
The NRδ during 6h of RS after 6h of SD showed significant increases in both the genotypes. The significant increase in NRδ was observed for entire 6h of RS in ENT1KO mice, whereas it was limited to first two hours of RS in WT mice (N=4, each genotype; Figure 4A). The [AD]ex levels in the BF showed significant and similar increases during SD in both genotypes when compared to within-subject baseline (Figure 4B). SD also restored the Wθ-NRδ relationship during 6h of recovery sleep in the KOs, similar to that of WT mice (N=5; R=0.60, P<0.001 for WT; R=0.52, P<0.005 for ENT1KO; Figure 4C).
Figure 4.
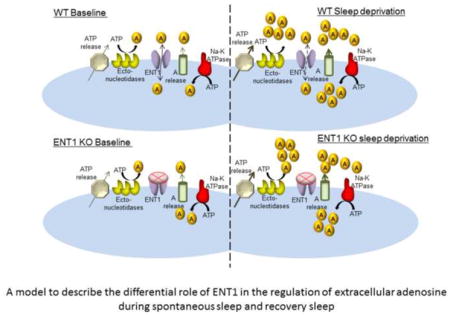
Effect of 6-hour Sleep deprivation (SD) on extracellular adenosine (AD) and recovery NREM delta activity. A, Delta power per NREM epoch during recovery sleep after 6-hour SD showed significant increase for the first two hours for WT (35.8% increase, black bar) and during entire 6 hours for ENT1 KO (44.8%, 24.3%, and 24.4%, open bars, respectively) when compared to the time-matched baseline day. Values are mean ± SEM normalized to baseline. *P < 0.05, Student’s t-test. B, Extracellular adenosine levels increased significantly in both WT (black bars) and ENT1 KO (open bar) mice (110% and 86%, respectively) during 6-hour SD. Values are mean ± SEM. *P <0.05, compared by repeated measure ANOVA. RS, recovery sleep; n.s., statistically non-significant. C, The scatter plot for Wθ of each hour (hn, n=1 to 5) against NRδ in the following hour (hn+1) during 6-hour RS showed statistically significant linear trends for both WT (blue dots) and KO (red dots) mice. Theta and delta power are normalized to 24-hour mean theta and delta power, respectively. R, Pearson’s correlation coefficient; P, probability. D. A model of the results show that in WT mice in basal conditions (spontaneous sleep-wakefulness) the small increases in the levels of extracellular AD are predominantly regulated by ENT1-dependent transport of intracellular AD whereas in the absence of ENT1 function in ENT1 KO mice the wake-associated increase in the levels are compromised (left top and bottom panels, respectively). During sleep deprivation, increased neuronal and glial release of ATP and increased vesicular release AD resulting from increased intracellular use of ATP by Na+K+ATPase contribute to significantly to the higher increase in [AD]ex overriding the need for ENT1-depenedent transport of AD (right top and bottom panels).
Absence of compensatory changes in other nucleoside transporters in ENT1KO mice
We measured the mRNA levels for the related nucleoside transporters CNT2, CNT3, ENT2, ENT3 and ENT4 in the BF of WT and ENT1 KO mice, using RT-PCR. As indicated in Table 1, the levels of mRNA of the transporters were not different between WT and ENT1 KO mice.
Table 1.
Relative Comparison of mRNA expression ENT1KO vs WT (ΔΔCt Method)
| Transporter | Fold Change | Significance | |
|---|---|---|---|
| WT | KO | ||
| CNT2 | 1.00±(0.5–2.2) | 2.7±(0.2–5.2) | NS |
| CNT3 | 1.00±(0.5–2.3) | 1.71±(0.5–2.0) | NS |
| ENT2 | 1.00±(0.5–2.0) | 1.6±(0.9–1.1) | NS |
| ENT3 | 1.00±(0.4–2.4) | 1.8±(1.0–1.8) | NS |
| ENT4 | 1.00±(0.5–2.5) | 1.5±(0.9–1.2) | NS |
Discussion
The results in this study demonstrate that: (i) The adenosine transport by equilibrative transporter ENT1 is important for spontaneous sleep-wake-associated changes in [AD]ex in the BF. (ii) The ENT1-dependent transport of [AD]ex during wakefulness is important for the regulation of NRδ and its linear relationship with the Wθ during light period. (iii) The presence of functional ENT1 is important in regulating [AD]ex during spontaneous sleep-wakefulness but not during SD. Together, our data shows that the mechanisms involved in the wakefulness-associated increase in [AD]ex in the BF during spontaneous wakefulness is different from that of during SD (see summary in model Figure 4D).
The decrease in the sleep time and shorter durations of sleep episodes in the ENT1 KO mice is attributable to an reduced levels of [AD]ex in basal conditions. These mice have increased glutamate neurotransmission attributed to decreased levels of [AD]ex and hence decreased inhibition of glutamate release via A1 adenosine receptor, a phenomenon reversed by the administration of A1 receptor agonists N6-CPA (Choi et al., 2004). The decrease in [AD]ex was also reported in decreased A2A receptor-mediated signaling in striatum (Choi et al., 2004, Chen et al., 2010, Nam et al., 2013). It is unlikely that decreased effect of adenosine is due to decreased A1 receptor expression since the A1 receptor density measured by receptor autoradiography in fact showed an increase in the ENT1KO mice (Choi et al., 2004).
The [AD]ex levels are critical in spontaneous sleep-wake regulation and show rapid state-dependent changes, being higher during wakefulness and lower during sleep, in feline BF (Porkka-Heiskanen et al., 1997). Similar measurements of spontaneous sleep-wake dependent changes in [AD]ex in mice could not be achieved due to short durations of sleep-wake episodes that yield insufficient volumes of microdialysates. [AD]ex, via its action on the presynaptic A1 adenosine receptors, inhibits glutamatergic input within BF area consisting of cortically projecting wake active neurons, resulting in sleep induction (Hawryluk et al., 2012, Yang et al., 2013). However, our observation that a low dose of AD (100μM) perfusion into BF resulted in increased NRδ KO mice to match the WT littermates suggests that an optimal level of [AD]ex is critical for increased quality of sleep.
We observed that the significant decrease in total NREM sleep in ENT1 KO mice was limited to the light period suggesting the functional significance of ENT1 to be greater during the light period. While the circadian regulation of ENT1 activity is not clear, its mRNA expression is higher within the first 3h of the light period (Alanko et al., 2003) when the NRδ intensity is also higher compared to 6h into the light period (Dash et al., 2009, Dworak et al., 2010), suggesting a preferential role for ENT1 in regulating [AD]ex during the earlier hours of the light period. Further, the absence of ENT1 function in KO mice decreases baseline NREM sleep time whereas the recovery sleep time following SD is not decreased. On the contrary, following SD, the recovery NRδ intensity in ENT1KO mice dissipates slower than that of WT littermates as shown in Figure 4A. The slow dissipation of NRδ intensity may be attributed to the absence of ENT1-dependent uptake of [AD]ex. We did not observe any change in other nucleoside transporter mRNA expression in the BF of ENT1KO, hence a compensatory increase in other transporters seems unlikely. Thus the observed effects on sleep-wake behavior and BF [AD]ex levels is consequential to the lack of ENT1 function.
Our data are also indicative of differential modes of regulation of [AD]ex during spontaneous sleep-wakefulness vs SD leading to enhanced homeostatic sleep pressure. The former is dependent on the presence of functional ENT1, but the latter is seemingly independent of ENT1. The wake-associated increase in [AD]ex differs significantly between spontaneous waking, (e.g., 30.6 ± 5.1 nM and 12.1 ± 2.3 nM, mean ± SEM, in cats and rats, respectively) and SD when the level are almost twice the basal levels (e.g., 58.9 ± 15.7 nM and 27.5 ± 8.5 nM in cats and rats, respectively) (Porkka-Heiskanen et al., 1997, Basheer et al., 1999). When compared to sleep, the increases in the BF [AD]ex during spontaneous wakefulness are of smaller magnitude. For example in cats the levels during waking is only ~20% higher (30.6 ± 5.1 nM) when compared to sleep (24.1 ± 4.4 nM) (Porkka-Heiskanen et al., 1997). In rats, the lowest level of [AD]ex in BF was reported to be 6.3±1.6 nM during the light period (between 10:00AM to 1:00PM) when the rats mostly sleep and the highest levels were 10.5 ± 1.9 nM during dark period when the rats are awake for ~65% of the time (McKenna et al., 2003). The magnitude of SD-induced increases in BF [AD]ex observed in this study (210 ± 60.1% in WT; 186 ± 33.2% in KO) is comparable to that previously reported in BF for cats (~200% and 140%) and rats (~225%) (Porkka-Heiskanen et al., 1997, Basheer et al., 1999, Porkka-Heiskanen et al., 2000) and thus is seemingly unaffected by ENT1 gene KO.
In summary, this study provides evidence in support of the role of ENT1 transporter in regulating the [AD]ex levels in BF during spontaneous sleep-wakefulness that is critical for the quality/quantity of NREM sleep and in maintaining a linear relationship between the Wθ and NRδ, the EEG markers of sleep homeostasis. During SD, reduced binding of AD to ENT1 and subsequent decrease in its uptake is suggested to contribute to increased [AD]ex in BF (Alanko et al., 2003). In accordance with the role of ENT1 in AD uptake, our data shows that the absence of ENT1-mediated uptake results in the presence of high levels of [AD]ex for longer period resulting in slow dissipation of NRδ (see the model Figure 4D). SD-induced increased neuronal activity affects the concentrations of cellular ATP leading to increased breakdown of intracellular ATP (Dworak et al., 2010) that would facilitate increased AD release. During increased neuronal activity, ATP is also released into extracellular space either as a co-transmitter from neurons or via gliotransmission, and its rapid breakdown, can contribute to AD increases (Halassa et al., 2009, Burnstock, 2013). Kalinchuk et al., (2006b) reported that an increase in nitric oxide (NO) via the induction of inducible NO synthase (iNOS) in BF neurons leads to [AD]ex increase. Neuronal induction of iNOS occurs only during SD and not during spontaneous wakefulness (Kalinchuk et al., 2010). Our data showing the SD-induced increase in [AD]ex and the homeostatic sleep response in ENT1KO mice suggest that the pathways such as ATP release and its breakdown to AD and/or NO-dependent increase in adenosine may be responsible for the increase in [AD]ex. Thus, the recovery sleep that follows SD is unaltered in ENT1 KO mice. In conclusion, the present study suggests that during spontaneous sleep-wakefulness, ENT1 is necessary for the linear relationship between EEG markers of waking and the following periods of NREMS, and the adenosine is a critical molecule for this regulation. The data also provides evidence for a differential regulation of [AD]ex during spontaneous vs prolonged wakefulness.
Highlights.
Equilibrative nucleoside transporter 1(ENT1) knockout mice have decreased non-rapid eye movement sleep (NREMS).
ENT1 mice display weak correlation between the hourly Wake EEG theta (Wθ) and NREMS delta (NRδ) of subsequent hour.
Adenosine infusion into basal forebrain restores the Wθ –NRδ correlation and NREMS.
ENT1 knockout mice show normal homeostatic sleep response following sleep deprivation.
The data suggest a differential regulation of adenosine during spontaneous and homeostatic sleep.
Acknowledgments
The work was supported by the Department of Veterans Affairs Medical Research Service Merit Award to RB and RWM, NINDS 079866 (RB), NIMH 39683(RWM), NIAAA 018779 (DSC). We thank Mrs. Farzana Pervin for excellent technical assistance and Diane Ghera and Dewayne Williams for help with animal care. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Dynamics of the human EEG during prolonged wakefulness: evidence for frequency-specific circadian and homeostatic influences. Neuroscience letters. 1997;239:121–124. doi: 10.1016/s0304-3940(97)00904-x. [DOI] [PubMed] [Google Scholar]
- Alanko L, Stenberg D, Porkka-Heiskanen T. Nitrobenzylthioinosine (NBMPR) binding and nucleoside transporter ENT1 mRNA expression after prolonged wakefulness and recovery sleep in the cortex and basal forebrain of rat. Journal of sleep research. 2003;12:299–304. doi: 10.1046/j.0962-1105.2003.00372.x. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Archiv : European journal of physiology. 2004;447:735–743. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- Basheer R, Porkka-Heiskanen T, Stenberg D, McCarley RW. Adenosine and behavioral state control: adenosine increases c-Fos protein and AP1 binding in basal forebrain of rats. Brain research Molecular brain research. 1999;73:1–10. doi: 10.1016/s0169-328x(99)00219-3. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Progress in neurobiology. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiological reviews. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Introduction to purinergic signalling in the brain. Advances in experimental medicine and biology. 2013;986:1–12. doi: 10.1007/978-94-007-4719-7_1. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Brunner DP, Krauchi K, Graw P, Wirz-Justice A. Power density in theta/alpha frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep. 1995;18:890–894. doi: 10.1093/sleep/18.10.890. [DOI] [PubMed] [Google Scholar]
- Chen J, Nam HW, Lee MR, Hinton DJ, Choi S, Kim T, Kawamura T, Janak PH, Choi DS. Altered glutamatergic neurotransmission in the striatum regulates ethanol sensitivity and intake in mice lacking ENT1. Behavioural brain research. 2010;208:636–642. doi: 10.1016/j.bbr.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, Diamond I, Bonci A, Messing RO. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci. 2004;7:855–861. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:620–629. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Sleep and brain energy levels: ATP changes during sleep. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:9007–9016. doi: 10.1523/JNEUROSCI.1423-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finelli LA, Baumann H, Borbely AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–529. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- Franken P, Dijk DJ, Tobler I, Borbely AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. New York: Elsevier Inc; 2008. [Google Scholar]
- Gray JH, Owen RP, Giacomini KM. The concentrative nucleoside transporter family, SLC28. Pflugers Archiv : European journal of physiology. 2004;447:728–734. doi: 10.1007/s00424-003-1107-y. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluk JM, Ferrari LL, Keating SA, Arrigoni E. Adenosine inhibits glutamatergic input to basal forebrain cholinergic neurons. Journal of neurophysiology. 2012;107:2769–2781. doi: 10.1152/jn.00528.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinchuk AV, Lu Y, Stenberg D, Rosenberg PA, Porkka-Heiskanen T. Nitric oxide production in the basal forebrain is required for recovery sleep. Journal of neurochemistry. 2006a;99:483–498. doi: 10.1111/j.1471-4159.2006.04077.x. [DOI] [PubMed] [Google Scholar]
- Kalinchuk AV, McCarley RW, Porkka-Heiskanen T, Basheer R. Sleep deprivation triggers inducible nitric oxide-dependent nitric oxide production in wake-active basal forebrain neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:13254–13264. doi: 10.1523/JNEUROSCI.0014-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinchuk AV, McCarley RW, Porkka-Heiskanen T, Basheer R. The time course of adenosine, nitric oxide (NO) and inducible NO synthase changes in the brain with sleep loss and their role in the non-rapid eye movement sleep homeostatic cascade. Journal of neurochemistry. 2011;116:260–272. doi: 10.1111/j.1471-4159.2010.07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinchuk AV, McCarley RW, Stenberg D, Porkka-Heiskanen T, Basheer R. The role of cholinergic basal forebrain neurons in adenosine-mediated homeostatic control of sleep: lessons from 192 IgG-saporin lesions. Neuroscience. 2008;157:238–253. doi: 10.1016/j.neuroscience.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinchuk AV, Porkka-Heiskanen T, McCarley RW, Basheer R. Cholinergic neurons of the basal forebrain mediate biochemical and electrophysiological mechanisms underlying sleep homeostasis. The European journal of neuroscience. 2015;41:182–195. doi: 10.1111/ejn.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinchuk AV, Stenberg D, Rosenberg PA, Porkka-Heiskanen T. Inducible and neuronal nitric oxide synthases (NOS) have complementary roles in recovery sleep induction. The European journal of neuroscience. 2006b;24:1443–1456. doi: 10.1111/j.1460-9568.2006.05019.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McKenna JT, Dauphin LJ, Mulkern KJ, Stronge AM, McCarley RW, Strecker RE. Nocturnal Elevation of Extracellular Adenosine in the Rat Basal Forebrain. Sleep Res Online. 2003;5:155–160. [Google Scholar]
- Murillo-Rodriguez E, Blanco-Centurion C, Gerashchenko D, Salin-Pascual RJ, Shiromani PJ. The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats. Neuroscience. 2004;123:361–370. doi: 10.1016/j.neuroscience.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Nam HW, Hinton DJ, Kang NY, Kim T, Lee MR, Oliveros A, Adams C, Ruby CL, Choi DS. Adenosine transporter ENT1 regulates the acquisition of goal-directed behavior and ethanol drinking through A2A receptor in the dorsomedial striatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:4329–4338. doi: 10.1523/JNEUROSCI.3094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Kalinchuk AV. Adenosine, energy metabolism and sleep homeostasis. Sleep medicine reviews. 2011;15:123–135. doi: 10.1016/j.smrv.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science (New York, NY) 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Tobler I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain research. 2005;1050:64–71. doi: 10.1016/j.brainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Yang C, Franciosi S, Brown RE. Adenosine inhibits the excitatory synaptic inputs to Basal forebrain cholinergic, GABAergic, and parvalbumin neurons in mice. Frontiers in neurology. 2013;4:77. doi: 10.3389/fneur.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]