Abstract
Background
In a previous study, higher concordance to the MIND diet, a hybrid Mediterranean-DASH diet, was associated with slower cognitive decline. In this study we related these three dietary patterns to incident Alzheimer’s disease.
Methods
We investigated the diet-AD relations in a prospective study of 923 participants, ages 58 to 98 years, followed on average 4.5 years. Diet was assessed by a semi-quantitative food frequency questionnaire.
Results
In adjusted proportional hazards models, the second (HR=0.65, 95% CI 0.44, 0.98) and highest tertiles (HR=0.47, 95% CI 0.26, 0.76) of MIND diet scores had lower rates of AD versus tertile 1 whereas only the third tertiles of the DASH (HR=0.61, 95% CI 0.38, 0.97) and Mediterranean (HR=0.46, 95% CI 0.26, 0.79) diets were associated with lower AD rates.
Conclusion
High adherence to all three diets may reduce AD risk. Moderate adherence to the MIND diet may also decrease AD risk.
Keywords: cognition, Alzheimer disease, nutrition, diet, epidemiological study, aging
Introduction
Dietary patterns have been associated with protective relations to cognitive decline and incident dementia in epidemiological studies.1;2 Encouraging support for these findings was recently provided by reports of secondary analyses of two dietary intervention trials. In the PREDIMED trial,3 participants at high vascular risk were randomized to dietary counseling of either the Mediterranean diet (supplemented with either extra-virgin olive or mixed nuts) or a low-fat control diet. After 6.5 years of nutritional intervention, those randomized to the Mediterranean diet had significantly higher scores on the Mini-Mental State Examination (MMSE) and Clock Drawing Test (CDT) compared to the control diet participants. In the second trial,4 124 overweight participants with elevated blood pressure were randomized to the DASH diet (Dietary Approaches to Stop Hypertension) alone or in combination with exercise and caloric restriction, or to a usual diet control group. After 4 months of the intervention, the participants on the DASH diet exhibited greater improvements in psychomotor speed compared with the usual diet control.
The results of these dietary intervention trials provide evidence that dietary patterns may reduce the risk of dementia. However, whereas both the cultural-based Mediterranean diet and the blood pressure-lowering DASH diet have demonstrated protective effects on cardiovascular conditions that can adversely affect brain health, their dietary components may not specifically capture the levels and types of foods shown to optimize brain health. In a previous study, we described a hybrid of the Mediterranean-DASH diets, called MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) that emphasizes the dietary components and servings linked to neuroprotection and dementia prevention. Similar to the Mediterranean and DASH diets, the MIND diet score emphasizes natural plant-based foods and limited intakes of animal and high saturated fat foods but uniquely specifies consumption of berries and green leafy vegetables, and does not specify high fruit consumption (3-4 servings/d in the DASH and Mediterranean diets), high dairy (2+ servings/d in DASH), high potato consumption (2 servings/d in the Mediterranean) or greater than 1 fish meal per week (>6 meals/week in the Mediterranean). The MIND diet score was associated with a slower rate of cognitive decline equivalent to 7.5 years of younger age among the participants in the top third of MIND diet scores compared with the lowest third.5 In this study, we examined the relative associations of the MIND, DASH, and Mediterranean diets to the risk of developing incident Alzheimer’s disease (AD).
Methods
Study Population
The study was conducted among participants of the Rush Memory and Aging Project (MAP), a study of volunteers living in retirement communities and senior public housing units in the Chicago area. The ongoing open cohort study began in 1997 and includes annual clinical neurological examinations as previously described.6 From 2004-February 2013, the MAP study participants were invited to complete food frequency questionnaires. Over the course of the diet study, 1,545 older persons enrolled in the MAP study, 80 died and 159 withdrew before the diet study began, leaving 1306 participants eligible for the analyses of diet and incident AD. Of these, 1068 completed the dietary questionnaires of which 923 had at least two neuropsychological assessments and were clinically determined not to have AD at the baseline. The Institutional Review Board of Rush University Medical Center approved the study, and all participants gave written informed consent.
Alzheimer Disease
Clinical diagnosis of probable AD was determined at each annual evaluation as previously described.7 Briefly, the AD diagnosis was made by an experienced clinician using data from a structured neurological examination and medical history, cognitive performance testing, and with the assistance of an algorithmically-based rating of cognitive impairment. The AD diagnosis was based on criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association8 which require a history of cognitive decline with impairment in memory and at least one other cognitive domain. According to these diagnostic criteria, there were 144 incident cases of AD and 14 incident cases of non-Alzheimer’s type dementia which were analyzed as non-cases.
Diet Scores
The diet scores were computed from responses to a semi-quantitative food frequency questionnaire (FFQ), a modified version of the Harvard FFQ that was validated for use in older Chicago community residents.9 Participants were asked to report usual frequency of intake over the previous 12 months of 144 food items. Nutrient levels and total energy for each food item were based either on natural portion sizes (e.g. slice of bread) or according to age- and sex-specific portion sizes from national dietary surveys. Table 1 lists the dietary components and maximum scores for each diet.
Table 1.
Dietary component servings and maximum scores for the DASH, Mediterranean and MIND diet scores
| DASHa | MedDietb | MIND | |||
|---|---|---|---|---|---|
| DASH components | Max Score |
Mediterranean Diet components |
Max Score |
MIND components |
Max Score |
| Total Grains ≥7/d | 1 | Nonrefined Grains >4/d | 5 | Whole Grains ≥3/d | 1 |
| Vegetables ≥4/d | 1 | Vegetables >4/d | 5 | Green Leafy ≥6/wk | 1 |
| Potatoes >2/d | 5 | Other Vegetables ≥1/d | 1 | ||
| Fruits ≥4/d | 1 | Fruits >3/d | 5 | Berries ≥2/wk | 1 |
| Dairy ≥2/d | 1 | Full-fat Dairy ≤10/wk | 5 | ||
| Meat, poultry & fish ≤ 2/d |
1 | Red meat ≤ 1/wk | 5 | Red Meats and products <4/wk |
1 |
| Fish >6/wk | 5 | Fish ≥1/wk | 1 | ||
| Poultry ≤3/wk | 5 | Poultry ≥2/wk | 1 | ||
| Nuts, seeds & legumes ≥4/wk |
1 | Legumes, nuts & beans >6/wk |
5 | Beans >3/wk | 1 |
| Nuts ≥5 /wk | 1 | ||||
| Fast/fried food <1/wk | 1 | ||||
| Total Fat ≤ 27% of kcal | 1 | ||||
| Saturated Fat ≤ 6% of kcal |
1 | ||||
| Olive oil ≥1/d | 5 | Olive Oil primary oil | 1 | ||
| Butter, margarine <1T/d | 1 | ||||
| Cheese<1/wk | 1 | ||||
| Sweets ≤ 5/wk | 1 | Pastries, sweets <5/wk | 1 | ||
| Sodium ≤ 2400mg/d | 1 | ||||
| Alcohol < 300mL/d but >0 | 5 | Alcohol/wine 1/d |
1 | ||
| TOTAL DASH Score | 10 | TOTAL MedDiet Score | 55 | Total MIND Score | 15 |
Epstein DE, Sherwood A, Smith PJ, Craighead L, Caccia C, Lin P, Babyak MA, Johnson JJ, Hinderliter A, Blumenthal JA. Determinants and consequences of adherence to the Dietary Approaches to Stop Hypertension Diet in African-American and White adults with high blood pressure: Results from the ENCORE Trial. J Acad Nutr Diet 2012;112:1763-73.
Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity among healthy adults; the accuracy of the MedDietScore. Prev.Med 2007 Apr;44(4):335-40
The MIND diet score has 15 dietary components including 10 brain healthy food groups (green leafy vegetables, other vegetables, nuts, berries, beans, whole grains, fish, poultry, olive oil and wine) and 5 unhealthy food groups (red meats, butter and stick margarine, cheese, pastries and sweets, and fried/fast food). Olive oil consumption was scored 1 if identified by the participant as the primary oil usually used at home and 0 otherwise. For all other diet score components we summed the frequency of consumption of each food item portion associated with that component and then assigned a concordance score of 0, 0.5, or 1. (Table 1) The total MIND diet score was computed by summing over all 15 of the component scores.
The DASH diet scoring,10 was based on 7 food groups and 3 dietary components (total fat, saturated fat and sodium), each scored 0, 0.5, or 1, and summed for a total score ranging from 0 (lowest) to 10 (highest) diet concordance. The MedDiet Score was computed based on scoring described by Panagiotakos and colleagues.11 The scoring uses serving quantities of the traditional Greek Mediterranean diet as the comparison metric. It includes 11 dietary components each scored 0 to 5 that are summed for a total score ranging from 0 to 55 (highest dietary concordance). We have found protective relations of this MedDiet Score and cognitive decline in both the MAP study2 and the Chicago Health and Aging Project.12
Covariates
Non-dietary variables in the analyses were obtained from structured interview questions and measurements at the participants’ baseline clinical evaluations. Age (in years) was computed from self-reported birth date and date of the baseline cognitive assessment. Education (years) is self-reported years of regular schooling. APOE- genotyping was performed using high throughput sequencing as previously described. Participation in cognitively stimulating activities was computed as the average frequency rating, based on a 5-point scale, of different activities (e.g. reading, playing games, writing letters, visiting the library).13 Physical activity (hours per week) was computed from self-reported minutes spent within the previous two weeks on five activities: walking for exercise, yard work, calisthenics, biking, and water exercise.14 Depressive symptoms (number) was assessed by a modified 10-item version of the Center for Epidemiological Studies-Depression scale.15 Body Mass Index (weight in kg/height in m2) was computed from measured weight and height and modeled as two indicator variables, BMI≤20 and BMI ≥30. Hypertension history was determined by self-reported medical diagnosis, measured blood pressure (average of 2 measurements ≥160 mmHg systolic or ≥90 mmHg diastolic) or current use of hypertensive medications. Myocardial infarction history was based on self-reported medical diagnosis or use of cardiac glycosides (e.g. lanoxin, digitoxin). Diabetes history was determined by self-reported medical diagnosis or current use of diabetic medications. Medication use was based on interviewer inspection. Clinical diagnosis of stroke was based on clinician review of self-reported history, neurological examination and cognitive testing history.16
Statistical Analyses
We used proportional hazards models in SAS© to investigate the relationship between diet scores and time in years to diagnosis of Alzheimer’s disease. We first examined the relations of the three dietary pattern scores in separate age-adjusted and basic-adjusted models. The basic model included potential confounders with the most established evidence for association with Alzheimer disease: age, sex, education, participation in cognitively stimulating activities, physical activity, and APOE-ε4. Total energy intake was also included as a potential confounder in the basic model because of its relevance to diet. Further analyses added covariates to the basic-adjusted models: 1) cardiovascular conditions, which have high likelihood of mediating the diet effects on dementia, and 2) depression and weight measures which may act as effect mediators but in addition have complex cause and effect relations with dementia. The dietary scores were modeled both as continuous variables and in tertiles in each of these models with similar results. We present the results of the tertile analyses to enable comparison of the dietary score associations with AD given the different dietary score ranges. We also report the p-value for linear trend based on a categorical variable of the tertiles with records in each tertile scored at the tertile median. Effect modification was investigated for the MIND diet score and each covariate by including a multiplicative term between the diet score and the potential effect modifier in the basic-adjusted model and testing at p<0.05.
RESULTS
A total of 144 incident cases of Alzheimer’s disease developed over an average follow-up of 4.5 years in the sample of 923 MAP participants. The mean time to AD diagnosis from the date that diet was assessed was 3.8 years (range of 1 to 9, median 3.0). The average MIND diet score for the AD sample was 7.4 (15 possible) and ranged from 2.5 to 12.5. Participants with the lowest scores had lower education, were more likely to be obese and to have diabetes, and reported fewer hours of physical activity and more depressive symptoms. (Table 2) Mean score for the DASH diet was 4.1 (10 possible; range 1.0-8.5) and for the MedDiet, 31.5 (55 (possible; range 18-46). The MIND diet score was correlated with both the MedDiet (r=0.62) and the DASH (r=0.50) diet scores.
Table 2.
Baseline characteristics of 923 MAP participants by tertile of MIND diet score
| MIND DIET SCORE | ||||
|---|---|---|---|---|
|
| ||||
| Baseline Characteristic | Tertile 1 | Tertile 2 | Tertile 3 | |
| MIND diet score | mean (minimum, maximum) |
5.6 (2.5, 6.5) |
7.5 (7.0, 8.0) |
9.6 (8.5, 12.5) |
| Age | mean years | 81.7 | 81.4 | 80.4 |
| Males | percent | 26 | 25 | 22 |
| Education | mean years | 14.3 | 15.1 | 15.6 |
| APOE-ε4 | percent | 21 | 27 | 21 |
| Total Energy Intake | mean calories | 1644 | 1777 | 1792 |
| Cognitive Activity Frequency | mean rating | 3.1 | 3.2 | 3.4 |
| Physical Activity Weekly | mean hours | 2.5 | 3.5 | 4.3 |
| Depressive Symptoms | mean number | 1.3 | 0.9 | 0.9 |
| Body Mass Index | ||||
| percent BMI ≤ 20 | 9 | 5 | 7 | |
| percent BMI ≥30 | 31 | 22 | 24 | |
| Medical Conditions | ||||
| Diabetes | percent | 24 | 21 | 17 |
| Hypertension | percent | 79 | 75 | 72 |
| Hypertensive medication use | percent | 57 | 53 | 53 |
| Myocardial Infarction | percent | 17 | 11 | 16 |
| Stroke | percent | 10 | 6 | 8 |
All variables were age-standardized using 5-year age categories.
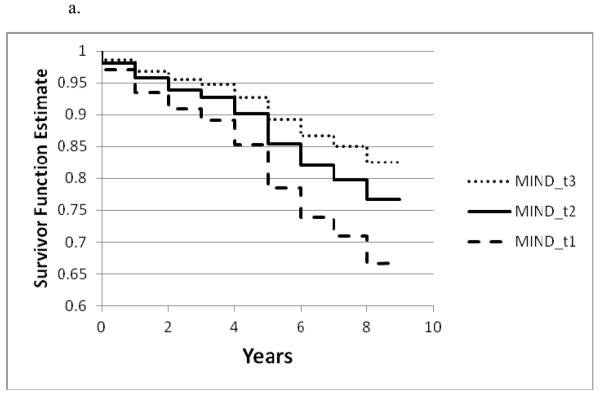
MIND diet score was linearly and statistically significantly associated with lower risk of developing Alzheimer’s disease in the age-adjusted model. (Table 3) In the basic model adjusted for age, sex, education, APOE-ε4, total energy intake, physical activity and participation in cognitively stimulating activities, participants in the top tertile of MIND diet scores (score range: 8.5 – 12.5) had a 53% (HR=0.47; 95% Confidence Interval: 0.29, 0.76) reduction in the rate of developing AD compared with participants in the lowest tertile (score range: 2.5 – 6.5). MAP participants in the middle tertile of MIND diet scores also had a statistically significant 35% reduction in AD rate compared with those in the first tertile (HR=0.65, 95% Confidence Interval: 0.44, 0.98). (Table 3)
Table 3.
Proportional hazards ratios (HR) and 95% confidence intervals (CI) of estimated effects of MIND diet score on time to incident Alzheimer disease in age-adjusted (n=923; 151 AD cases) and basic-adjusted* (n=789; 135 AD cases) models in MAP participants over a mean 4.5 years of follow-up, 2004-2013
| MODEL | Tertile 1 | Tertile 2 | Tertile 3 | P for Linear Trend |
|---|---|---|---|---|
| MIND DIET SCORE | ||||
|
| ||||
| Score Range | 2.5-6.5 | 7-8 | 8.5-12.5 | |
| Age-adjusted | ||||
| HR (95% CI) |
1.0 (referent) |
0.75 (0.52, 1.09) |
0.47 (0.30, 0.73) |
0.0006 |
| Basic-adjusted* | ||||
| HR (95% Confidence Interval) |
1.0 (referent) |
0.65 (0.44, 0.98) |
0.47 (0.29, 0.76) |
0.002 |
| Basic-adjusted + | ||||
| Cardiovascular Conditions | ||||
| HR (95% Confidence Interval) |
1.0 (referent) |
0.64 (0.42, 0.97) |
0.48 (0.29, 0.79) |
0.003 |
|
| ||||
| DASH DIET SCORE | ||||
|
| ||||
| Score Range | 1.0 – 3.5 | 4.0 – 4.5 | 5.0 – 8.5 | |
| Age-adjusted | ||||
| HR (95% CI) |
1.0 (referent) |
0.93 (0.64, 1.36) |
0.56 (0.36, 0.86) |
0.02 |
| Basic-adjusted* | ||||
| HR (95% Confidence Interval) |
1.0 (referent) |
0.98 (0.66, 1.46) |
0.61 (0.38, 0.97) |
0.07 |
| Basic-adjusted + | ||||
| Cardiovascular Conditions | ||||
| HR (95% Confidence Interval) |
1.0 (referent) |
0.98 (0.64, 1.46) |
0.60 (0.37, 0.96) |
0.06 |
|
| ||||
| MEDDIET SCORE | ||||
|
| ||||
| Score Range | 18 – 29 | 30 -34 | 35 - 46 | |
| Age-adjusted | ||||
| HR (95% CI) |
1.0 (referent) |
0.77 (0.54, 1.11) |
0.46 (0.29, 0.74) |
0.001 |
| Basic-adjusted* | ||||
| HR (95% CI) |
1.0 (referent) |
0.81 (0.54, 1.24) |
0.46 (0.27, 0.79) |
0.006 |
| Basic-adjusted + | ||||
| Cardiovascular Conditions | ||||
| HR (95% Confidence Interval) |
1.0 (referent) |
0.81 (0.53, 1.21) |
0.49 (0.29, 0.85) |
0.01 |
Basic-Adjusted model included terms for age, sex, education, APOE-ε4 (any), participation in cognitively stimulating activities, physical activity and total energy intake
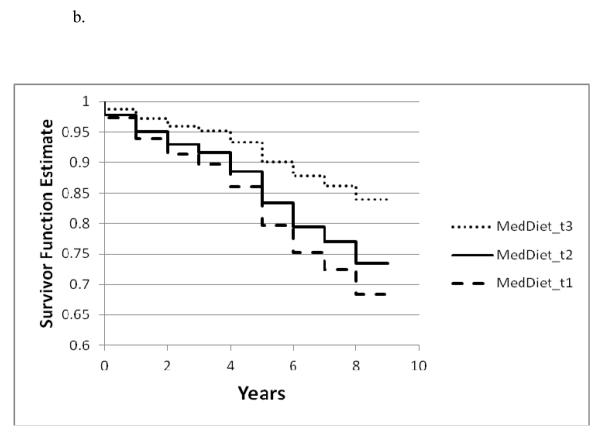
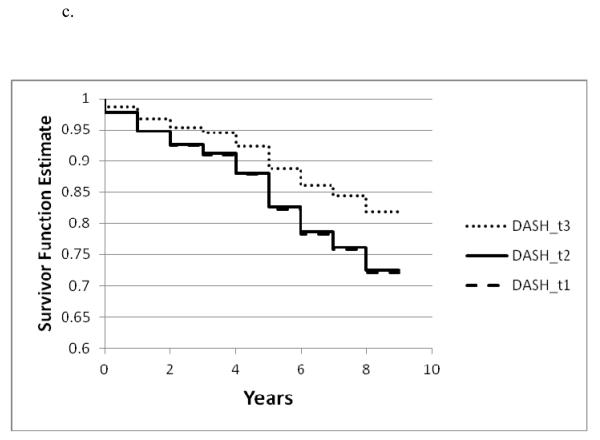
Only the highest tertiles of the DASH and MedDiet diet scores were significantly associated with incident AD compared with the lowest tertile scores. (Table 3) The estimated effects were somewhat higher for the MedDiet diet (54% reduction in AD for tertile 3 vs tertile 1) than for the DASH diet (39% reduction for tertile 3 vs tertile 1) based on the basic-adjusted models. (Table 3 and Figure 1a-1c)
Figure 1a. – 1c.
Survivor function for incident AD by tertile of a. the MIND diet, b. the MedDiet , and c. the DASH diet based on Cox proportional hazards models adjusted for age, sex, education, APOE-ε4 (any), participation in cognitively stimulating activities, physical activity and total energy intake
We investigated whether the MIND diet association could be attributed to diet effects on cardiovascular conditions that have been related to increased risk of AD including diabetes, hypertension, stroke, and myocardial infarction. There was no evidence that the dietary pattern associations with AD were mediated by these conditions, as the hazard ratios from models adjusted for these cardiovascular conditions were very similar to the basic models. (Table 3) Estimates of effects for the DASH and MedDiet diet scores on incident AD did not materially change in analyses of the basic model plus covariates for depressive symptoms and low or high BMI. (Data not shown) However, the effect estimates were modified for the MIND diet score with further adjustment for depression and BMI (tertile 2 HR=0.77 (95% CI: 0.51, 1.17); tertile 3 HR=0.50 (95% CI: 0.30, 0.83); p-value for trend=0.006).
In an attempt to evaluate to what extent the observed effects of the MIND diet on AD could be due to dietary changes in participants with preclinical AD, we reanalyzed the data after eliminating 33 AD cases that were diagnosed under two years of follow-up, but there was no change in the overall results (tertile 2 HR=0.62, p=0.04; tertile 3 HR=0.53, p=0.01). Further elimination of 60 AD cases that were diagnosed within three years of follow-up had minimal impact on the estimated effects (tertile 2 HR=0.63 (p=0.08) and tertile 3 HR=0.53 (p=0.04), although that for the second tertile was only marginally statistically significant.
In further analyses we found no statistical evidence that the association between the MIND diet and incident AD was modified by age, sex, education, physical activity, obesity, low BMI, or histories of stroke, diabetes, or hypertension. Marginally statistically significant interactions were observed for APOE-ε4 (the MIND diet was less protective in ε4 positive participants) and history of myocardial infarction (the MIND diet was more protective in participants with history); p-value for interaction=0.06 for both interactive terms.
DISCUSSION
This prospective study of the MIND diet score provides evidence that greater adherence to the overall dietary pattern may be protective against the development of AD. The estimated effect was a 53% reduction in the rate of AD for persons in the highest tertile of MIND scores and a 35% reduction for the middle tertile of scores compared with the lowest tertile. The estimated effect was independent of other healthy lifestyle behaviors and cardiovascular-related conditions. These data suggest that even modest adherence to the MIND diet score may have substantial benefits for the prevention of AD. By contrast, only the highest concordance to the DASH and MedDiet diets were associated with AD prevention.
The MIND diet pattern was developed á priori to the analyses and independently of the MAP study data. It is a hybrid of basic components from the Mediterranean and DASH diets but with modifications based on comprehensive reviews of the literature on nutrition and the aging brain.17-19 Unlike the Mediterranean and DASH diet scores, the MIND diet specifies frequent weekly consumption of green leafy vegetables in addition to other types of vegetables. Two large U.S. cohort studies reported significantly slower cognitive decline with consumption of 2 or more daily servings of vegetables, with the strongest associations observed for six or more weekly servings of green leafy vegetables.20;21 Further, given that these20;21 and other prospective22-24 studies do not find association between fruits as a general category and cognitive decline, the MIND diet does not specify daily fruit servings as do the DASH and Mediterranean diets. However, the MIND diet has a separate score component for berry consumption to reflect the positive associations reported between intakes of blueberries and strawberries and slower cognitive decline in the Nurses’ Health Study.25 This finding is supported by a number of rodent models showing better memory performance and brain neuroprotection from multiple types of berries.26-29 The MIND diet is more similar to the DASH diet with regard to fish consumption, with an optimal serving of just one meal per week as opposed to 6 meals per week specified by the Mediterranean diet. This level of fish consumption reflects the findings of prospective epidemiological studies that examined its relation to AD prevention.30-32
Whereas, high dietary concordance to the MIND and MedDiet diets were similarly protective against the risk of developing AD, even mild concordance to the MIND diet resulted in a statistically significant AD reduction. In a previous study we observed a stronger inverse association between the MIND diet and cognitive decline than for either the MedDiet or DASH diets.5 This suggests that the MIND diet is not specific to the underlying pathology of AD but perhaps better overall functioning and protection of the brain.
Protective associations with higher DASH diet scores were more modest. This may indicate that the unique recommendations for dairy and low salt in the DASH diet are not of particular relevance for brain health. Whereas the Mediterranean diet pattern has been related to lower risk of incident AD in some1;33 but not all studies34 to date there has not been another prospective study that has investigated the AD relation to the DASH diet.
The study has a number of strengths that lend confidence to the findings. First, selection bias is minimized by the prospective study design whereby community residents free of dementia at the beginning of the study are followed for incident disease. Second, the diagnosis of AD was based on annual neuropsychological testing and structured clinical neurological evaluations by clinicians blinded to the dietary pattern scores. Third, the diet pattern scores were based on a comprehensive semi-quantitative food frequency questionnaire that was validated for use in older community-dwelling Chicago residents. These features reduce the potential for biased and random misclassification of disease status and diet exposures in the analyses. And finally, there was little or no change in the estimates of dietary effects on AD after statistical adjustment for many important risk factors for AD, suggesting that confounding is not a likely explanation for the observed associations.
The primary limitation of the study is that the observational study design precludes interpretation of the findings as cause and effect. Randomized dietary intervention trials would be required to attribute causal effects of the diet patterns to the development of the disease. Another limitation is the reliance on limited information from the food frequency questionnaire to determine consumption of individual food components in the diet scores. For example, the question on berry consumption was based on a single item for strawberries (not other berry types) and the response options ranged from “never” to “2 or more times per week” (not higher frequency of consumption). Similarly, the assessment of olive oil consumption was based on a single item on the type of oil usually used at home. These constricted measures of berry and olive oil consumption do not capture the full upper range of intakes in the population. However, the under-assessment of frequent berry and olive oil consumption is likely to negatively bias the observed AD associations with the MIND diet score—that is, toward the null of no effect. And finally, the relatively short period (3.8 years on average) from diet assessment to disease onset may be capturing diets in individuals who have pre-clinical AD. This raises the possibility that at the time of baseline assessment the incident cases had experienced dietary changes as a result of the disease. We investigated this issue by reanalyzing the data after eliminating cases that occurred within the first 3 years of follow-up and observed little diminishing in the estimated effect of the MIND diet. In addition, in an earlier study of the MAP participants we reported slower cognitive decline with higher MIND scores over up to10 years of follow-up.5
Results of the study suggest that even modest adjustments to the diet may help to reduce one’s risk of developing AD. For example, the MIND diet score specifies just two vegetable servings per day, two berry servings per week, and one fish meal per week. These serving recommendations are much lower than three to four daily servings each for fruits and vegetables specified for a maximum score in the DASH and MedDiet indices and six or more fish meals per week in the MedDiet diet score.
Effective dietary recommendations have far-reaching implications for the public health and the growing burden of dementia in aging populations. A growing literature on the individual foods and nutrients related to brain neuroprotection needs to be considered to specify the food groups and servings that are most likely to protect against brain diseases. Based on the current study, high quality diets such as the Mediterranean and DASH diets can be modified, such as in the MIND diet, to provide better protection against dementia.
Systematic Review
The MIND diet, a hybrid of the cardiovascular Mediterranean and DASH diets, was developed based on an exhaustive review of animal models, laboratory studies and prospective epidemiological studies to identify the nutrients, foods and dietary patterns related to brain health and dementia.
Interpretation
In a previous study the MIND diet was more predictive of slower cognitive decline than either the Mediterranean or DASH diets. In the current study, we examined the relations of these diet patterns to incident Alzheimer’s disease. The MIND and Mediterranean diets had comparable protective relations to AD suggesting that the MIND diet is not specific to the underlying pathology of Alzheimer disease.
Future
These studies indicate that a diet that is specific to brain health is possible but that further diet modifications can improve its role in AD prevention as new information on nutrition and dementia is acquired.
Acknowledgements
The study was funded by grants (R01AG031553 and R01AG17917) from the National Institute on Aging.
Footnotes
Potential Conflicts of Interest The authors have no relevant disclosures of potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns on cognitive decline in older persons. Neurology. 2014 doi: 10.1212/WNL.0000000000000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Martinez-Lapiscina EH, Clavero P, Toledo E, et al. Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. J Neurol Neurosurg Psychiatry. 2013;84:1318–1325. doi: 10.1136/jnnp-2012-304792. [DOI] [PubMed] [Google Scholar]
- (4).Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55:1331–1338. doi: 10.1161/HYPERTENSIONAHA.109.146795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Morris MC, Tangney CC, Wang Y, et al. MIND Diet Score More Predictive than DASH or Mediterranean Diet Scores. Alzheimer’s & Dementia. 2014 [Google Scholar]
- (6).Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush memory and aging project. Curr Alzheimer Res. 2012;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- (8).McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- (9).Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol. 2003;158:1213–1217. doi: 10.1093/aje/kwg290. [DOI] [PubMed] [Google Scholar]
- (10).Folsom AR, Parker ED, Harnack LJ. Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. Am J Hypertens. 2007;20:225–232. doi: 10.1016/j.amjhyper.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med. 2007;44:335–340. doi: 10.1016/j.ypmed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- (12).Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr. 2011;93:601–607. doi: 10.3945/ajcn.110.007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11:400–407. [PubMed] [Google Scholar]
- (14).Buchman AS, Boyle PA, Wilson RS, Bienias JL, Bennett DA. Physical activity and motor decline in older persons. Muscle Nerve. 2007;35:354–362. doi: 10.1002/mus.20702. [DOI] [PubMed] [Google Scholar]
- (15).Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- (16).Bennett DA. Secular trends in stroke incidence and survival, and the occurrence of dementia. Stroke. 2006;37:1144–1145. doi: 10.1161/01.STR.0000219643.43966.0d. [DOI] [PubMed] [Google Scholar]
- (17).Barnes JL, Tian M, Edens NK, Morris MC. Consideration of Nutrient Levels in Studies of Cognitive Decline: A Review. Nutr Rev. 2014 doi: 10.1111/nure.12144. doi: 10.1111/nure.12144. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- (18).Morris MC, Tangney CC. Dietary Fat Composition and Dementia Risk. Neurobiology of Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.03.038. 10.1016/j.neurobiolaging.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Morris MC. Nutritional determinants of cognitive aging and dementia. Proc Nutr Soc. 2012;71:1–13. doi: 10.1017/S0029665111003296. [DOI] [PubMed] [Google Scholar]
- (20).Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67:1370–1376. doi: 10.1212/01.wnl.0000240224.38978.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Kang JH, Ascherio A, Grodstein F. Fruit and vegetable consumption and cognitive decline in aging women. Ann Neurol. 2005;57:713–720. doi: 10.1002/ana.20476. [DOI] [PubMed] [Google Scholar]
- (22).Roberts RO, Geda YE, Cerhan JR, et al. Vegetables, unsaturated fats, moderate alcohol intake, and mild cognitive impairment. Dement Geriatr Cogn Disord. 2010;29:413–423. doi: 10.1159/000305099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Nooyens AC, Bueno-de-Mesquita HB, van Boxtel MP, van Gelder BM, Verhagen H, Verschuren WM. Fruit and vegetable intake and cognitive decline in middle-aged men and women: the Doetinchem Cohort Study. Br J Nutr. 2011;106:752–761. doi: 10.1017/S0007114511001024. [DOI] [PubMed] [Google Scholar]
- (24).Chen X, Huang Y, Cheng HG. Lower intake of vegetables and legumes associated with cognitive decline among illiterate elderly Chinese: a 3-year cohort study. J Nutr Health Aging. 2012;16:549–552. doi: 10.1007/s12603-012-0023-2. [DOI] [PubMed] [Google Scholar]
- (25).Devore EE, Kang JH, Breteler MM, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012;72:135–143. doi: 10.1002/ana.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Joseph JA, Denisova NA, Arendash G, et al. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr Neurosci. 2003;6:153–162. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
- (27).Shukitt-Hale B, Cheng V, Joseph JA. Effects of blackberries on motor and cognitive function in aged rats. Nutr Neurosci. 2009;12:135–140. doi: 10.1179/147683009X423292. [DOI] [PubMed] [Google Scholar]
- (28).Shukitt-Hale B, Lau FC, Carey AN, et al. Blueberry polyphenols attenuate kainic acid-induced decrements in cognition and alter inflammatory gene expression in rat hippocampus. Nutr Neurosci. 2008;11:172–182. doi: 10.1179/147683008X301487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Joseph JA, Shukitt-Hale B, Denisova NA, et al. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Larrieu S, Letenneur L, Helmer C, Dartigues JF, Barberger-Gateau P. Nutritional factors and risk of incident dementia in the PAQUID longitudinal cohort. J Nutr Health Aging. 2004;8:150–154. [PubMed] [Google Scholar]
- (31).Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- (32).Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Annals of Neurology. 1997;42:776–82. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- (33).Tsivgoulis G, Judd S, Letter AJ, et al. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology. 2013;80:1684–1692. doi: 10.1212/WNL.0b013e3182904f69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Feart C, Samieri C, Rondeau V, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]