Abstract
Background
Pulmonary fibrosis is characterized by excessive accumulation of collagen and α-smooth muscle actin (aSMA) in the lung. The key molecules that promote these phenotypes are of clinical interest.
Objectives
TSLP has been found at high levels in patients with asthma and idiopathic pulmonary fibrosis, and TSLP has been proposed as a primary driver of lung fibrotic disease. We asked whether TNFSF14 (aka LIGHT) controls TSLP production to initiate fibrosis.
Methods
Expression of TSLP and initiation of pulmonary fibrosis induced by bleomycin were assessed in mice deficient in LIGHT. The ability of recombinant LIGHT, given intratracheally to naïve mice, to promote TSLP and fibrosis was also determined.
Results
Genetic deletion of LIGHT abolished lung TSLP expression driven by bleomycin, accompanied by near-complete absence of accumulation of lung collagen and aSMA. Furthermore, recombinant LIGHT administered in vivo induced lung expression of TSLP in the absence of other inflammatory stimuli, and strikingly reproduced the primary features of bleomycin-driven disease in a TSLP-dependent manner. Blockade of LIGHT binding to either of its receptors, HVEM and LTβR, inhibited clinical symptoms of pulmonary fibrosis, and correspondingly both receptors were found on human bronchial epithelial cells, a primary source of TSLP. Moreover, LIGHT induced TSLP directly in human bronchial epithelial cells and synergized with IL-13 and TGF-β in vivo to promote TSLP in the lungs and drive fibrosis.
Conclusions
These results show that LIGHT is a profibrogenic cytokine that may be a key driver of TSLP production during the initiation and development of lung fibrotic disease.
Keywords: Pulmonary fibrosis, asthma, IPF, bronchial epithelial cell, TNFSF14, LIGHT, TSLP, HVEM
INTRODUCTION
Deregulated cellular and fibrotic activity in the lungs is characteristic of several inflammatory diseases including idiopathic pulmonary fibrosis (IPF), systemic sclerosis (SSc), asthma, and chronic obstructive pulmonary disease (COPD) (1–4). These features are thought to be primarily the result of a repair response to epithelial injury. When uncontrolled, they can lead to serious complications and sometimes death because of substantial deposition of extracellular matrix proteins (ECM) that essentially replace normal tissue with scar tissue (1–4). Pulmonary fibrosis can affect middle-aged and older adults and this is one of the most severe rheumatologic conditions since many patients live only 3 to 5 years post-diagnosis and die of respiratory failure. Current treatments involve immunotherapy with corticosteroids (5), but there is a great need to define new targets for fibrosis intervention in general.
Fibrotic diseases involve infiltration of T cells as well as B cells, macrophages, eosinophils, and granulocytes to the fibrotic foci (2, 6), followed by the deposition of collagen, and production of other ECM proteins that may contribute in several ways to the overall fibrotic phenotype (7). In addition, accumulation of myofibroblast cells with smooth muscle characteristics, or hyperplasia of smooth muscle cells, is also seen in many responses that can further impede normal tissue elasticity and function. A number of factors may contribute to fibrosis including TGF-β that can directly promote production of ECM proteins by epithelial cells and fibroblasts and IL-13 that may activate macrophages to produce TGF-β as well as directly stimulate myofibroblasts, smooth muscle cells, and epithelial cells (3, 4). However, whether there are primary driver molecules that control inflammation and are also responsible for production of pro-fibrotic factors such as TGF-β and IL-13 has been unclear. Thymic Stromal LymphoPoietin (TSLP) has been suggested to be a master-switch that initiates allergic inflammation in the lung and other tissues, including in asthmatics (8). More recently, TSLP has also been implicated in controlling fibrotic activity in several model systems, including strongly contributing to asthma-like remodeling in the lungs. Indeed human bronchial epithelial cells from asthmatic airways and lung tissue from patients with IPF express high levels of TSLP (8–11). Furthermore, the forced expression of TSLP in lung epithelial cells of transgenic mice results in spontaneous asthma- and IPF-like inflammation in the lungs and significant fibrosis (8, 12). Although TSLP expression might be induced directly by allergens or epithelial damage, it remains a possibility that other molecules can be upstream of TSLP and represent primary drivers of this molecule to orchestrate the fibrotic process. Furthermore, similar mechanisms may operate in both allergic asthma as well as diseases such as IPF.
Previous work from our laboratory showed that the cytokine TNF superfamily protein 14 (TNFSF14), known as LIGHT and CD258, strongly contributed to lung tissue remodeling in mice chronically challenged with allergen in models of severe asthma (13). Strikingly, we now show that a LIGHT-deficiency almost completely abrogated fibrotic activity in the lungs in another model induced by the epithelium-damaging antibiotic bleomycin, that produces symptoms of fibrosis reminiscent of those seen in the lungs in several diseases (14). Most interestingly, induction of LIGHT preceded that of TSLP, and LIGHT was required for TSLP expression. Importantly, injection of recombinant LIGHT into naïve mice promoted TSLP, and phenocopied the deposition of collagen and accumulation of alpha smooth muscle actin that was induced by bleomycin. Moreover, TSLP receptor activity was required for LIGHT to drive collagen and alpha smooth muscle actin in the lung. In accordance, both receptors for LIGHT, LTβR and HVEM, were constitutively expressed on human bronchial epithelial cells and LIGHT directly promoted TSLP expression by these cells. Furthermore, LIGHT induced, and acted with, IL-13 and TGF-β, to drive maximal TSLP expression and fibrotic features in vivo. LIGHT may then be an attractive target for delaying the onset, or halting the progression, of fibrotic activity in IPF as well as other pulmonary diseases.
Materials and Methods
Mice
Six- to 8-week-old female WT, LIGHT−/− (originating from Dr. K. Pfeffer (15)), HVEM−/− (originating from Dr. K. Pfeffer (16)) or TSLPR−/− mice (originating from Dr. S. Ziegler (17)) were either purchased (Jackson Laboratories) or bred in house on the C57BL/6 background. Six to 8-week old female WT BALBc or IL4Rα−/− mice on the BALBc background were purchased from Jackson Laboratories. All experiments were in compliance with the regulations of the La Jolla Institute for Allergy and Immunology Animal Care Committee in accordance with guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care.
Experimental protocols
For bleomycin experiments, mice were challenged with intratracheal bleomycin 0.2U/mouse (Sigma) and monitored for lung inflammation and fibrosis after 7 or 14 days. For LIGHT-induced experiments, mice were given 10 μg of recombinant mouse LIGHT (1794-LT/CF, R&D Systems) or PBS on days 1 and 2 and sacrificed on day 3. For the neutralization of LIGHT-LTβR interactions, mice were administered 200 μg of anti-LTβR mAb (LLTB2) (18) given i.v. one day prior to injection of bleomycin or rLIGHT and every other day thereafter until the end of the experiment. The anti-LTβR mAb (LLTB2) specifically blocks the binding of LIGHT to LTβR, but does not disrupt interactions between LTβR and its other functional ligand LTα1β2 (18). For the neutralization of TGF-β signaling, mice were administered 300 μg of anti-TGF-β mAb (1D11, BioXCell) given i.p. one day prior to injection of rLIGHT and every other day thereafter until the end of the experiment.
Western Blot
Bronchoalveolar lavage (BAL) was performed by intratracheal insertion of catheter and lavaging with 0.8–0.9 ml of PBS. Soluble LIGHT production was assessed in the BAL by western blot analysis using anti-LIGHT mAb (clone MAB1794, R&D).
Quantitative real-time PCR
Lung samples were harvested at various times and total RNA was isolated using TRIzol reagent (Invitrogen). Single-strand cDNA was prepared by reverse transcribing 5 μg of total RNA using Transcriptor First Strand cDNA kit (Roche). For quantitation of LIGHT, Collagen, alpha Smooth Muscle Actin (aSMA), TGF-β, IL13, and TSLP, cDNA samples were amplified in IQ SYRB Green Supermix (Bio-Rad Laboratories) using the following primer pairs: LIGHT: forward, 5′-ACA GCC TTC AGT GTT TGT GGT G-3′; reverse, 5′-TCC GGT GGT TCT GTT CCA G-3′, Collagen: forward, 5′-GAG CCC TCG CTT CCG TAC TC-3′; reverse, 5′-TGT TCC CTA CTC AGC CGT CTG T-3′, aSMA: forward, 5′-TCT CTA TGC TAA CAA CGT CCT GTCA-3′; reverse, 5′-CCA CCG ATC CAG ACA GAG TAC TT-3′, TGF-β: forward, 5′-CCC TAT ATT TGG AGC CTG GA-3′; reverse, 5′-GGA AGC TTC GGG ATT TAT GG-3′, IL13: 5′-CTT GCC TTG GTG GTC TCG-3′, reverse, 5′-CGT TGC ACA GGG GAG TCT-3′, muTSLP: forward, 5′-TCG AGG ACT GTG AGA GCA AGC CAG-3′; reverse, 5′-CTG GAG ATT GCA TGA AGG AAT ACC-3′, huTSLP: forward, 5′-TAT GAG TGG GAC CAA AAG TAC CG-3′; reverse, 5′-GGG ATT GAA GGT TAG GCT CTG G-3′. All samples were run in triplicate, and the mean values were used for quantification.
Collagen measurement
Formalin (4% in PBS) fixed lung sections were stained with Masson’s Trichrome to evaluate collagen using an image analysis system (Image-Pro Plus; Media Cybernetics) (19). For lung sections, over 50 images per section were acquired (on 10x objective) on a Nikon 80i microscope and stitched on Photoshop to reconstitute the full lung. A defined area of interest (AOI) was created and quantified for blue color corresponding to collagen using Image-Pro Plus. AOIs were used throughout the lung sections to average the amount of collagen per section. The Trichrome ratio was calculated by dividing the average AOI value for the experimental condition with the average AOI value for control mice injected with PBS (e.g. AVG (AOI BLEO)/AVG (AOI PBS)).
Immunohistochemistry
Lung samples were de-paraffinized by sequential placement in xylene and ethanol. To evaluate alpha smooth muscle actin in the peribronchial areas, lung sections were treated with Fc block diluted in 10% Donkey serum (in PBS) and stained with rabbit polyclonal antibody to alpha smooth muscle actin (clone 1A4, ab7817-abcam) at 1:200 concentration. Rhodamine Red-X AffiniPure Donkey anti-rabbit (Jackson Immunoresearch, 711295152) was used at a 1:500 concentration to provide the fluorescent signal. Smooth muscle thickness was then evaluated in the peribronchial area using Image-Pro Plus from multiple random bronchi throughout the lungs. For TSLP, lung sections were stained with Goat polyclonal antibody to TSLP (clone L18, Santa Cruz Biotechnology). Donkey anti-goat IgG-FITC (sc-2024, Santa Cruz Bioteachnology) used at 1:500 concentrations provided the fluorescent signals. Slides were mounted and read on a Nikon 80i microscope and analyzed by LSM-Image software.
In vitro assays
Bronchoepithelial alveolar Sputum cells (BEAS-2B) (Lonza) were stimulated with 100ng/ml of recombinant human LIGHT (R&D, 664-LI/CF) for 72h in Epilife media (Life technologies). TSLP was then measured by immunostain using anti-TSLP mAb (clone AF1398, from R&D) and qPCR analyses. LIGHT receptors were visualized on BEAS-2B using anti-LTβR and anti-HVEM in parallel with isotype control antibodies (Biolegend). For TGF-β neutralization, 30 μg/ml of anti-TGF-β mAb (1D11) or its Isotype control were added into culture.
Statistical analyses
Statistical analysis was performed using GraphPad Prism software. A nonparametric t test or Mann-Whitney test was used where indicated. A P value < 0.05 was considered statistically significant. |, p < 0.05; ||, p < 0.01, |||, p < 0.005; ||||, p < 0.001; n/s not significant.
Results
TNFSF14/LIGHT is upregulated in a model of bleomycin-induced IPF
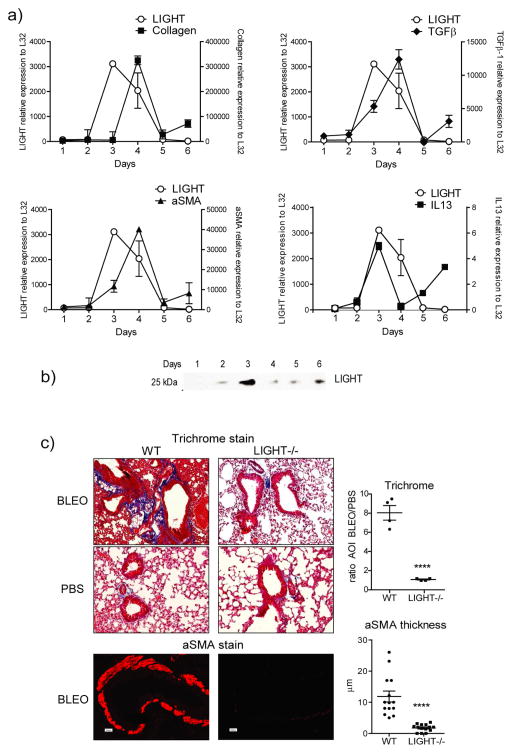
We assessed the expression of LIGHT in the lungs in a fibrotic model induced by intratracheal instillation of bleomycin. Mice displayed scruffy fur, kyphosis, and lack of activity after exposure to bleomycin. Supporting the hypothesis that LIGHT is an early catalyst of fibrotic disease, expression of transcripts for LIGHT were readily detected as early as 3 days post-instillation in the lungs and preceded the peak expression of mRNA for the major fibrotic features, collagen and alpha smooth muscle actin (Fig. 1a). In this model, deposition of collagen protein and accumulation of alpha smooth muscle actin positive cells starts around day 5 and is sustained thereafter. LIGHT mRNA also preceded or coincided with upregulation of TGF-β and IL-13 mRNA, two other factors implicated in promoting fibrotic activity (Fig. 1a). Importantly, soluble LIGHT protein was detected in bronchoalveolar lavage from bleomycin-treated mice, correlating with the mRNA expression profile for LIGHT transcripts in the lungs, with peak accumulation seen at day 3 (Fig. 1b). This data suggested that LIGHT might be a contributing factor to fibrosis in this model.
Figure 1. LIGHT is induced by bleomycin and LIGHT-deficient mice exhibit decreased lung fibrosis.
(a–b) Wild type (WT) mice were sensitized with bleomycin intratracheally (i.t.). (a) Kinetics of expression of mRNA for LIGHT, alpha smooth muscle actin (aSMA), TGF-β, and IL-13, assessed by qPCR analysis of lung samples. mRNA expression calculated relative to L32. Values are mean ± SEM of 2 mice per time point. Selected time points were repeated in 3 additional experiments. (b) Soluble LIGHT was assessed in the bronchoalveolar lavage by Western Blot. Data are representative of 3 experiments. (c) WT and LIGHT−/− mice were sensitized with bleomycin or PBS intratracheally. Mice were sacrificed on day 7. Fibrotic phenotypes were assessed by analyzing collagen deposition (trichrome stain) and peribronchial aSMA accumulation in lung sections. Representative sections are shown (left). Quantitation was performed as in materials and methods (right). Trichrome data show mean ratio of area of interest (AOI) values over PBS of 4 individual mice per condition. aSMA data show values of 14 random bronchi scored from 4 mice per condition. Data are representative of 6 experiments.
TNFSF14/LIGHT-deficiency decreases lung fibrosis
To test whether LIGHT was required to initiate lung fibrotic disease, WT and LIGHT−/− mice were monitored for collagen deposition with Masson’s trichrome stain in lung sections, and alpha smooth muscle actin accumulation was assessed in the lung by immunohistochemistry. Quite strikingly, bleomycin-treated LIGHT−/− mice failed to accumulate significant amounts of collagen and alpha smooth muscle actin in their lungs (Fig. 1c). Collagen accumulation in WT mice, based on quantitation of trichrome-stained areas, was 8-fold higher than in LIGHT−/− mice, while alpha smooth muscle actin accumulation around the bronchioles was dramatically absent in LIGHT−/− mice and almost at the level seen in naïve animals (Fig. 1c). These results clearly demonstrate that the absence of LIGHT protects mice from developing pulmonary fibrosis driven by bleomycin.
Soluble TNFSF14/LIGHT induces lung fibrosis similar to bleomycin
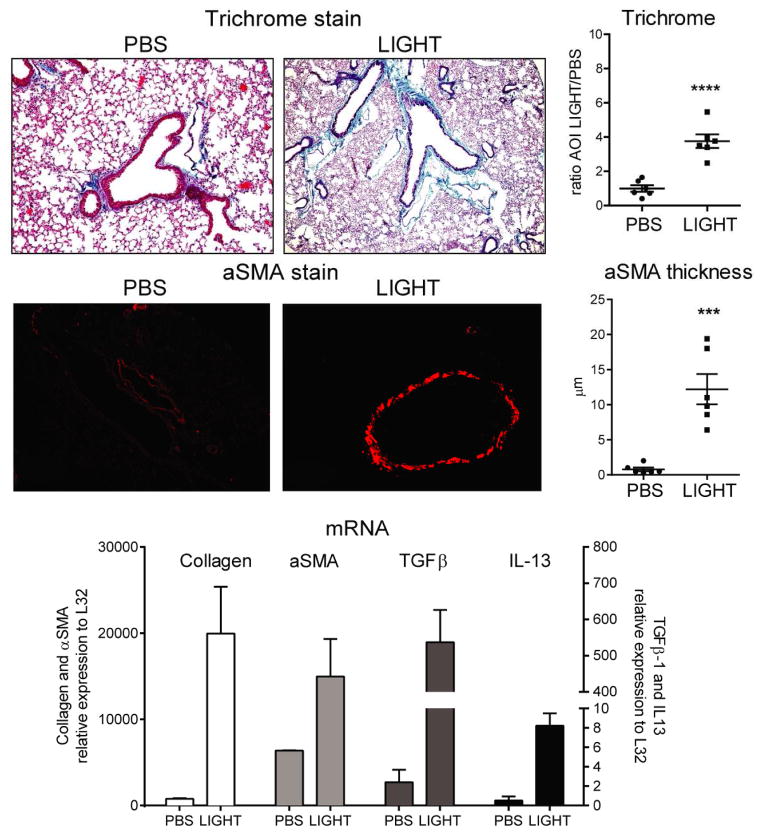
Because a deficiency of LIGHT almost completely abolished fibrotic activity after bleomycin treatment, we asked whether LIGHT was able to drive these features in the absence of other inflammatory stimuli. For this purpose, we injected naïve mice with 10 μg of recombinant murine LIGHT or PBS intratracheally on two consecutive days. LIGHT induced significant lung collagen deposition by day 3, albeit to a lesser extent than that observed with bleomycin (~2-fold difference, Fig. 2 vs. Fig. 1). Impressively, alpha smooth muscle actin accumulation around the bronchioles was 12 times higher in LIGHT-treated mice compared to PBS controls and quite similar to that induced by bleomycin (Fig. 2). Moreover, qPCR analysis of lungs of LIGHT-treated mice confirmed strong up-regulation of collagen and alpha smooth muscle actin transcripts, along with TGF-β and IL-13 transcripts (Fig. 2). These results strongly emphasize the pro-fibrotic activity that LIGHT can exert without other extrinsic stimuli.
Figure 2. Recombinant LIGHT induces lung fibrosis.
WT mice were injected with 10 μg of soluble rmLIGHT administered intratracheally on days 1 and 2, and sacrificed on day 3. Fibrotic phenotypes were scored after analyzing trichrome stained and aSMA stained lung sections. Trichrome data show mean ratio of AOI values over PBS of 6 individual mice per condition. aSMA data show mean values of random bronchi scored from 6 mice per condition. qPCR analysis was performed on lung samples and mRNA expression of LIGHT, collagen, IL-13 and TGF-β calculated relative to L32. mRNA values are mean ± SEM of 3 mice per condition. Data are representative of 6 experiments.
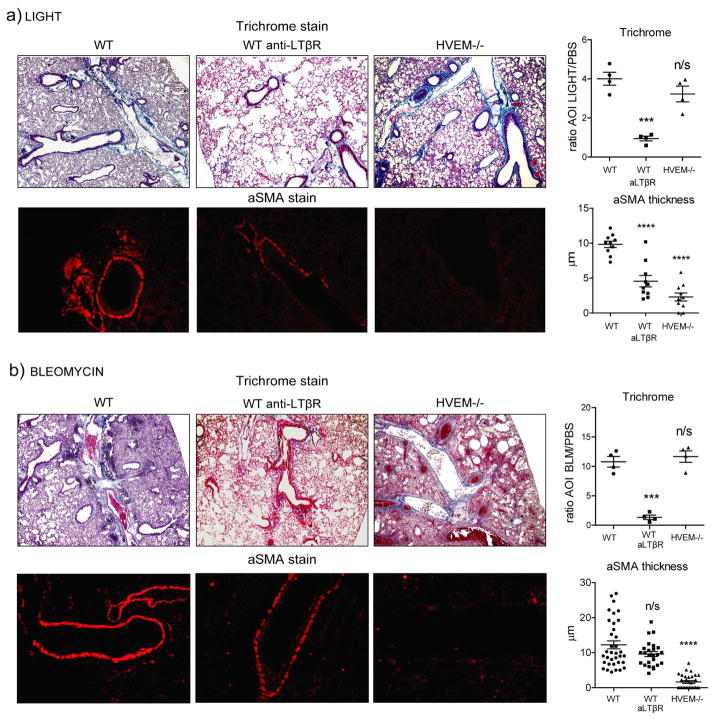
TNFSF14/LIGHT utilizes both LTβ R and HVEM to drive lung fibrosis
LIGHT has two receptors in the TNFR superfamily, LTβR/TNFRSF3 and HVEM/TNFRSF14. In order to determine whether LIGHT drove pulmonary fibrosis through one or both of its receptors, we assessed responses in HVEM−/− mice as well as WT mice treated with an antibody that selectively neutralizes LIGHT-LTβR interactions. The results were similar in mice treated with bleomycin and those treated with recombinant LIGHT alone (Fig. 3). Interestingly, collagen accumulation as quantified by trichrome staining in the lung was strongly reduced when LIGHT-LTβR interaction was neutralized, whereas in the absence of HVEM interaction this response was still induced to normal levels. However, alpha smooth muscle actin expression was strongly inhibited in the lungs without LIGHT-HVEM interactions (Fig. 3). The latter still accumulated when LIGHT-LTβR interactions were blocked, although with bleomycin there was a trend to reduced expression, and with recombinant LIGHT there was a statistically significant reduction (Fig. 3). Why blocking LTβR reduced alpha smooth muscle actin to a greater extent with LIGHT compared to bleomycin is not clear. LTβR is generally constitutive on cells that express it, whereas HVEM can be highly upregulated. HVEM may then have been more highly expressed due to the inflammatory effect of bleomycin, making LTβR dispensable for induction of alpha smooth muscle actin. Overall, these results show that both receptors can participate in fibrosis driven by LIGHT, although there may be differential contributions potentially depending on the target cell types within the tissue and the inflammatory environment.
Figure 3. HVEM and LTβR participate in promoting lung fibrotic activity induced by bleomycin and recombinant LIGHT.
WT mice were compared to mice treated with 200 μg neutralizing anti-LTβR and to HVEM−/− mice. (a) Mice were treated with 10μg rmLIGHT intratracheally on days 1 and 2 before sacrificing on day 3. (b) Mice were treated with bleomycin intratracheally and sacrificed on day 7. Fibrotic phenotypes were assessed as before in the lungs. Trichrome data show mean ratio of AOI values over PBS of 4 individual mice per condition. aSMA data show values of 10 (a) to 35 (b) random bronchi scored from 4 mice per condition. Results are representative of 3 experiments performed.
TNFSF14/LIGHT regulates TSLP in vivo to promote pulmonary disease
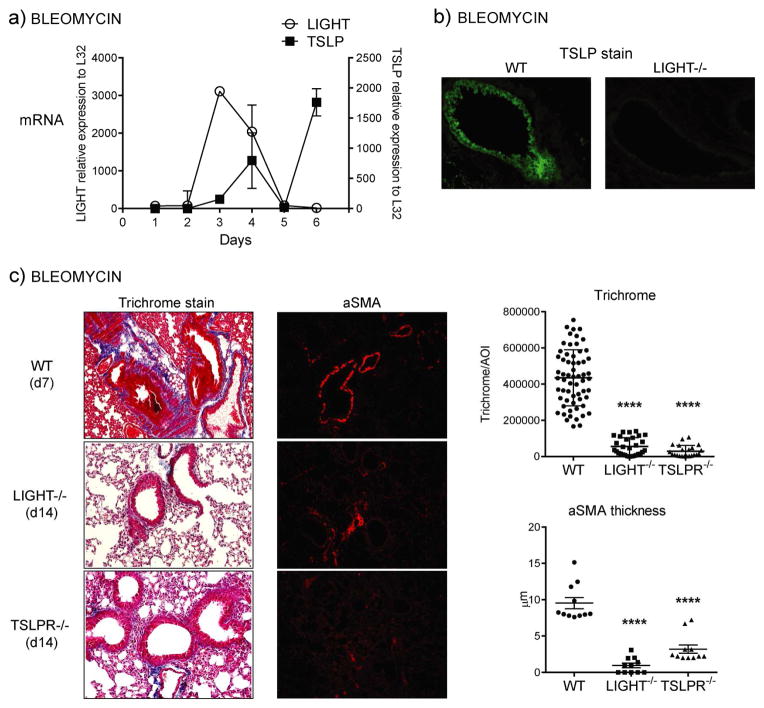
As we found LIGHT was active in both the model here and previously in allergen models of asthma (13), we asked whether it might control TSLP, a molecule thought to be common to asthma and IPF and important for initiation of the pulmonary profibrotic response (8, 9). Most interestingly, we observed that TSLP was induced in the lungs of bleomycin-challenged animals after the peak of LIGHT expression (Fig. 4a). TSLP was largely found in bronchial epithelial cells and to a lesser extent in alveolar epithelial cells and some other unidentified cells. Suggesting a direct relationship between the molecules, LIGHT-deficient mice displayed decreased expression of TSLP in the lung in bronchial epithelium, and other cells, after bleomycin treatment, as visualized by immunohistochemistry of lung sections (Fig. 4b). Moreover, TSLPR−/− mice to a large extent mimicked the phenotype of LIGHT−/− mice in being resistant to accumulating collagen and alpha smooth muscle actin in the lung induced by bleomycin, although some induction of alpha smooth muscle actin was still visualized in the absence of TSLP activity (Fig. 4c).
Figure 4. TSLP expression in response to bleomycin is dependent on LIGHT.
(a) Kinetics of LIGHT and TSLP mRNA expression by qPCR in lung samples of WT mice treated i.t. with bleomycin. (b) Lungs from WT and LIGHT−/− mice sensitized i.t. with bleomycin were assessed for TSLP expression on day 7 by IHC (green stain). (c) WT, LIGHT−/− and TSLPR−/− mice were compared for lung fibrotic phenotypes after sensitization i.t. with bleomycin. Trichrome and aSMA data show values of between 30–60 random AOI and 11 random bronchi, respectively, scored from 3 mice per group. Results are representative of 3 experiments.
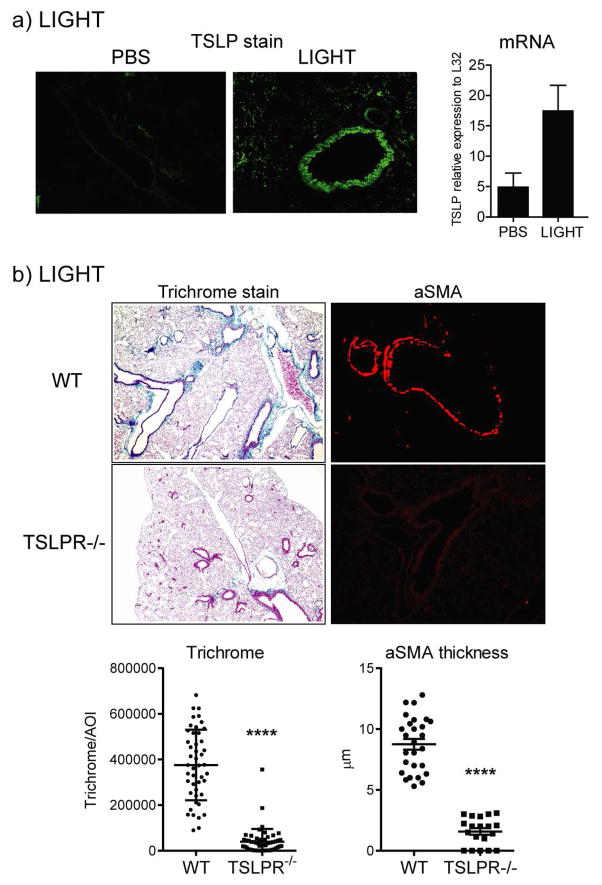
We then injected recombinant LIGHT intratracheally to determine if it could replicate the action of bleomycin and promote TSLP expression in vivo in naïve WT mice. This led to the up-regulation of TSLP mRNA transcripts in the lungs over 3 days, and induction of TSLP protein was easily visualized by immunohistochemistry, again largely expressed in bronchial epithelial cells, but also in alveolar epithelial cells and other cells (Fig. 5a). Most importantly, recombinant LIGHT failed to induce strong collagen deposition and alpha smooth muscle actin accumulation in the lungs of TSLPR−/− mice (Fig. 5b).
Figure 5. Recombinant LIGHT induces TSLP expression in vivo to promote lung fibrosis.
(a) TSLP expression was measured in the lungs by IHC or PCR 24h after the last LIGHT injection in WT mice treated with 10μg of rmLIGHT or PBS given i.t. on days 1 and 2. (b) WT and TSLPR−/− mice were treated with rmLIGHT as above and fibrotic activity in the lungs assessed on day 3. Trichrome and aSMA data show values of between 25–40 random AOI and 25 random bronchi, respectively, scored from 4 mice per group. Results are representative of 3 experiments.
TNFSF14/LIGHT induction of lung fibrosis is partially dependent on IL-13 signaling
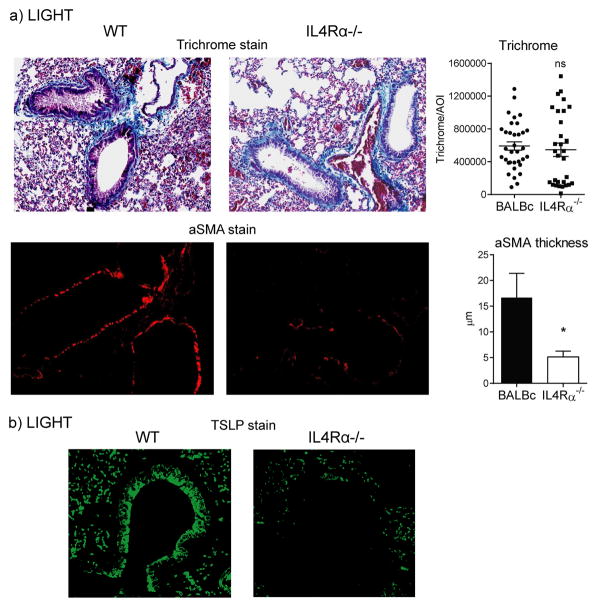
In an allergen-driven severe asthma model, previous work from our laboratory demonstrated that LIGHT participated in regulating the expression of IL-13 (13). As induction of LIGHT mRNA was seen with IL-13 mRNA in mice injected with bleomycin, and recombinant LIGHT also upregulated IL-13 mRNA, we tested whether IL-13 might play a role in the fibrotic activity of LIGHT by using IL4Rα−/− mice in which IL-13 signaling as well as IL-4 signaling is disrupted. While collagen accumulation was equally upregulated in IL4Rα−/− mice injected with recombinant LIGHT, alpha smooth muscle actin deposition in the lung was significantly impaired (Fig. 6a). Interestingly, we observed a reduction in TSLP expression by bronchial epithelial cells, while TSLP was still visualized in alveolar epithelial cells and other cells (Fig. 6b). This implies that IL-13 promoted by LIGHT participates in upregulating TSLP as well as contributes to deposition of alpha smooth muscle actin.
Figure 6. LIGHT-induced lung fibrosis is partially dependent on IL-13 signaling.
WT and IL-4Rα−/− mice were injected i.t. with rmLIGHT as before. Lung accumulation of collagen and aSMA (a), as well as TSLP expression by IHC (b), were assessed at day 3. Trichrome and aSMA data represent scores of between 30–40 random AOI and 30 random bronchi, respectively, from 3 individual mice per group.
TGF-β synergizes with TNFSF14/LIGHT to promote TSLP expression and lung fibrosis
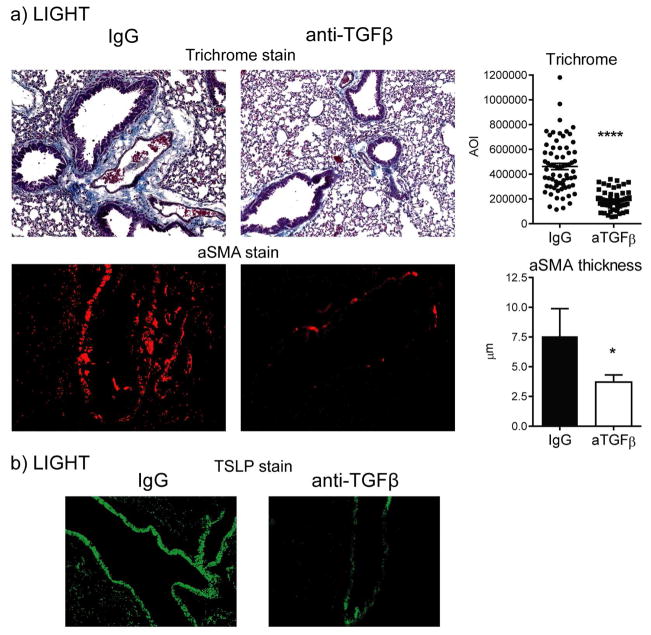
We also previously suggested that LIGHT could promote TGF-β expression by lung macrophages in models of allergen-induced asthma (13). We then tested whether neutralization of TGF-β would affect maximal expression of TSLP and fibrosis induced by LIGHT. We observed a significant decrease in alpha smooth muscle actin and collagen deposition in the lungs of mice injected with anti-TGF-β, although not to baseline levels (Fig. 7a). Correlating with this, we found that TSLP expression was also reduced, albeit to a lesser extent in bronchial epithelial cells than in the other cells capable of expressing TSLP (Fig. 7b). Thus, TGF-β upregulated by LIGHT also contributes to TSLP expression and is central to the fibrotic activity of LIGHT.
Figure 7. TGF-β participates in lung fibrosis induced by LIGHT.
WT mice were treated with anti-TGF-β or control IgG and injected i.t. with rmLIGHT as before. Lung accumulation of collagen and aSMA (a), as well as TSLP expression by IHC (b), were assessed at day 3. Trichrome and aSMA data represent scores of between 50–70 random AOI and 30 random bronchi, respectively, from 3 individual mice per group.
TNFSF14/LIGHT promotes TSLP in human bronchial epithelial cells in a TGF-β-independent manner
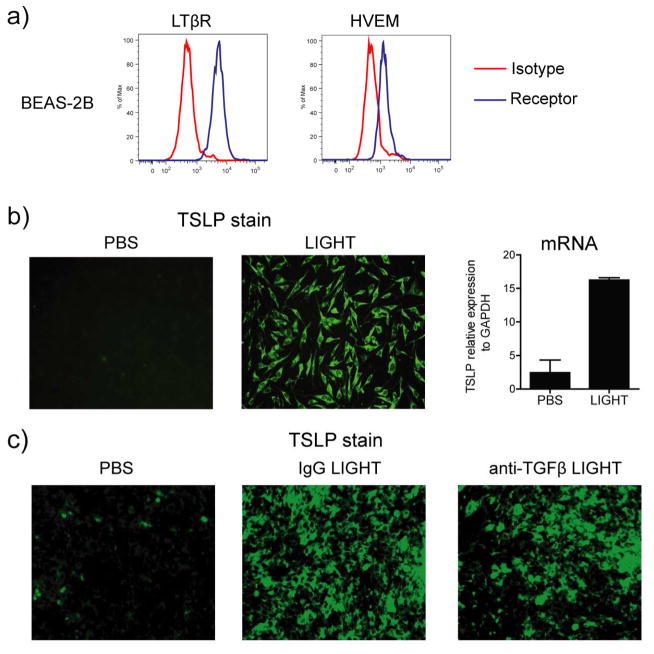
As the primary sources of TSLP in the mouse lung appeared to be bronchial epithelial cells, and we wished to extend these results to clinically applicable cell types, we first tested whether human bronchial epithelial cells expressed the receptors for LIGHT. Interestingly, both LTβR and HVEM were constitutively expressed on these cells (Fig. 8a). Most significantly, recombinant human LIGHT strongly induced the expression of mRNA and protein for TSLP in the absence of any other extrinsic stimulus (Fig. 8b). As bronchial epithelial cells are capable of producing TGF-β, and TGF-β participated in maximal expression of TSLP in vivo (Fig. 7), we blocked this molecule. However, we did not see any significant decrease in TSLP expression by these cells (Fig. 8c), suggesting that LIGHT can exert a direct effect on epithelial cells to promote TSLP expression.
Figure 8. LIGHT directly induces TSLP expression in human bronchial epithelial cells in a TGF-β-independent manner.
(a) Human bronchial epithelial cells (BEAS-2B) were assessed for LTβR and HVEM expression by flow cytometry. Isotype control in red. (b and c) BEAS-2B cells were stimulated in vitro with soluble rhLIGHT for 72h, in the absence (b) or presence of anti-TGF-β or control IgG (c). TSLP expression was evaluated by IHC and mRNA levels by qPCR. PCR values are mean ± SEM of triplicate samples per condition. Data are representative of 2–4 experiments.
Taken together, these results substantiate the idea that LIGHT can act as a primary driver of TSLP expression in cells of the lung by both direct and indirect mechanisms and may represent a master regulator of factors that result in pro-fibrotic activities in several pulmonary diseases.
Discussion
Fibrosis, tissue remodeling, and deregulated cellular activity is a particular concern in numerous persistent inflammatory states including those seen in asthma, GvHD, IPF, and some autoimmune diseases such as systemic sclerosis, RA, Crohn’s disease, and lupus. Therefore, defining the molecules that are fibrotic factors or might drive activity of other fibrotic factors could prove useful for future therapeutic approaches. Here, we show that LIGHT is induced early during the course of fibrotic activity initiated by epithelial damage from the antibiotic bleomycin and that is reminiscent of that seen in humans with idiopathic pulmonary fibrosis. Importantly, the expression of LIGHT preceded that of another fibrotic factor TSLP that has been suggested to be a master regulator itself, and a deficiency in LIGHT almost completely abrogated TSLP induction, fibrosis, and tissue remodeling in the lung. Our data emphasize the novel role of LIGHT as a central modulator of the initiation of lung fibrotic disease. They additionally suggest it may represent a marker for early disease progression and that blocking LIGHT activity could represent an effective therapy in interstitial lung disease.
Strikingly, the injection of recombinant murine LIGHT alone by intratracheal instillation resulted in similar fibrotic features to those driven by bleomycin, implying that LIGHT was not simply a feature of fibrosis in this scenario but was a major driver of fibrotic activity. The extent of lung fibrosis was less pronounced in mice injected with LIGHT compared to bleomycin-treated mice. However, other factors made within the lung environment most likely additionally contribute to fibrosis by synergizing with LIGHT. Most importantly, LIGHT injection up-regulated three major pro-fibrotic factors, TGF-β, IL-13, and TSLP. Previous work from our laboratory found that LIGHT could promote TGF-β expression in lung macrophages and IL-13 expression in lung eosinophils associated with tissue remodeling in the lungs driven by chronic administration of allergen (13). However, the identification of TSLP as a downstream product of LIGHT activity and a major mediator of the pro-fibrotic effects of LIGHT dramatically highlight the central and novel role of LIGHT in perpetuating the clinical manifestations of pulmonary fibrosis. TSLP was suggested to be central to lung inflammation some time ago, and indeed, several lines of evidence support a critical role of TSLP signaling in the generation of Th2 responses (8). Most significantly, transgenic mice where TSLP was over-expressed in bronchial epithelium exhibited tissue remodeling in the lungs (12), suggesting this molecule may be a master regulator of the fibrotic process. In this paper we additionally contribute to this notion by showing that bleomycin-induced lung fibrosis is also dependent on TSLP. However, our finding that LIGHT was induced before TSLP in the model here with bleomycin, that recombinant LIGHT alone upregulated TSLP in the lungs and in vitro in human bronchial epithelial cells, and that TSLP was required for several of the major fibrotic features downstream of LIGHT, places LIGHT as another primary driver of disease.
Whether LIGHT will always contribute to TSLP expression in lung inflammatory responses remains to be determined. Also, whether TSLP is always required for fibrosis in the lungs under different inflammatory conditions is another question that will be answered in future studies. We previously utilized a severe asthma model with house dust mite extract to show LIGHT was active in driving lung collagen and alpha smooth muscle actin accumulation (13). However, it is not known if TSLP is required for the response in the model we previously utilized. Chen et al (20) found that blocking TSLP inhibited all of the hallmarks of lung inflammation, including peribronchial collagen accumulation, in a model with house dust mite that had some similarities to the one we used. However, a separate report found that TSLP was not important for house dust mite driven lung inflammation where a different immunization protocol was employed (21). This suggests that there may be variability in the use of these molecules that will be important to understand. Recent positive clinical data from a study of anti-TSLP antibody in allergic asthmatics (22) does, however, suggest that anything that can impact TSLP expression may be of interest for future therapy of lung disease.
Central to LIGHT playing a driver role in fibrotic activity is obviously that its receptors, HVEM and LTβR, are available. We previously highlighted the potential significance of HVEM and LTβR on eosinophils and macrophages, respectively, in contributing to lung tissue remodeling in our allergen studies, with LIGHT promoting IL-13 and TGF-β production from these cell types (13). Both HVEM and LTβR can additionally be expressed on other lymphoid cells such as dendritic cells. However, our finding here that both receptors are constitutively expressed on bronchial epithelial cells may be of primary significance to the potential activities of these cells and to development or perpetuation of the fibrotic response. As epithelial cells are arguably one of the main sources of TSLP, this is likely to explain at least in part why LIGHT may be such a prominent contributor to the development of the lung fibrotic phenotype. Interestingly, blocking IL-13 signaling in vivo reduced LIGHT-driven TSLP expression by bronchial epithelial cells, but did not strongly affect TSLP production by other lung cells, which likely included alveolar epithelial cells, dendritic cells, and fibroblasts. This led to a partial reduction of the fibrotic activity as visualized by decreased alpha smooth muscle actin accumulation, and may have been explained by IL-13 both contributing to maximal TSLP expression as well as having a direct effect on smooth muscle cells that can express IL4Rα on their surface (23). Previous studies have also suggested that IL-13 may be a factor regulating TSLP expression. Miyata et al found that intranasal treatment of mice with IL-13 upregulated TSLP in nasal epithelial cells, and blockade of TSLP reduced IL-13-induced eosinophilia in the nasal mucosa (24). Additionally, IL-13 transgenic mice displayed enhanced levels of TSLP, and TSLP participated in the exacerbated allergen-driven lung inflammatory responses displayed by these mice (25, 26). Thus, the ability of LIGHT to both directly promote TSLP and also promote IL-13 production to further enhance TSLP expression appear to explain much of its strong fibrotic activity.
In addition, TGF-β neutralization in vivo also revealed that TGF-β was important for the overall fibrotic action of LIGHT. However, anti-TGF-β had a slightly different effect than was seen in IL-4Rα−/− mice in that LIGHT-driven TSLP expression by bronchial epithelial cells was only partially reduced, but TSLP in the other lung cells was largely absent. This translated to a similar decrease in alpha smooth muscle actin as seen in IL-4Rα−/− mice, but collagen accumulation was additionally inhibited in the lungs. The latter is in line with many results that have previously shown a central role for TGF-β in directly promoting ECM proteins from epithelial cells or fibroblasts (1–4). Nevertheless, we found that LIGHT-induction of TSLP expression by bronchial epithelial cells in vitro was TGF-β-independent. Taken together these data suggest that LIGHT can drive fibrosis in two ways, directly by promoting TSLP expression by bronchial epithelial cells, and indirectly by inducing IL-13 and TGF-β that also feed into the TSLP axis as well as have profibrotic effects on lung epithelial cells, myofibroblasts, or mature smooth muscle cells.
In conclusion, LIGHT is a major directive protein in the development of pulmonary fibrotic activity, in part via its ability to act on epithelial cells in the lung through binding to its constitutively expressed receptors LTβR and HVEM. Most importantly, LIGHT can directly and indirectly regulate TSLP, enhancing its expression by bronchial epithelial cells and other lung resident cells. This is likely to subsequently engage other molecules downstream of TSLPR and/or result in direct signals from the TSLPR that may synergize with signals from the receptors for LIGHT. A fully human LIGHT-specific antibody (SAR252067) that competitively inhibits the binding of LIGHT to LTβR and HVEM is currently in phase II clinical trials for inflammatory bowel disease (27), but our data reinforce the notion that LIGHT may be as attractive a target for the therapy of lung fibrotic disease. It does however remain important to understand when LIGHT is made in alternate disease indications. Some published data reported that LIGHT was upregulated in the sputum of asthmatic patients (28) nevertheless more expression analysis is warranted in asthma as well as patients with IPF and interstitial lung disease in general.
Key Messages.
Bleomycin driven TSLP production and fibrosis are dependent on TNFSF14/LIGHT.
Intratracheal administration of TNFSF14/LIGHT induces TSLP in the lungs.
TNFSF14/LIGHT upregulates TSLP in human bronchial epithelial cells.
Acknowledgments
We thank Klaus Pfeffer and Steve Ziegler for originally making available mice deficient in LIGHT, HVEM, and TSLPR. This work was supported by NIH grants AI070535 and AI100905 to M.C. This is manuscript number # 1765 from the La Jolla Institute for Allergy and Immunology.
Abbreviations used
- TNFSF
Tumor necrosis factor superfamily protein
- LIGHT
homologous to Lymphotoxins, exhibits Inducible expression, competes with HSV Glycoprotein D for HVEM, a receptor expressed by T lymphocytes
- TSLP
thymic stromal lymphopoietin
- BAL
Bronchoalveolar lavage
- I.T
intratracheal
- IPF
idiopathic pulmonary fibrosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thannickal VJ, Toews GB, White eS, Lynch P, Jr, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 2.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009 Jan 7;2(2):103–21. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenbloom J, Castro SV, Jimenez SA. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med. 2010 Feb 2;152(3):159–66. doi: 10.7326/0003-4819-152-3-201002020-00007. [Research Support, Non-U.S. Gov’t Review] [DOI] [PubMed] [Google Scholar]
- 4.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012 Jul;18(7):1028–40. doi: 10.1038/nm.2807. [Research Support, N.I.H., Intramural Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boin F, Wigley F. Connective tissue diseases: Immunosuppressive therapy in SSc: what is the target? Nat Rev Rheumatol. 2009 Jul;5(7):357–8. doi: 10.1038/nrrheum.2009.108. [DOI] [PubMed] [Google Scholar]
- 6.Huaux F, Liu T, McGarry B, Ullenbruch M, Xing Z, Phan SH. Eosinophils and T lymphocytes possess distinct roles in bleomycin-induced lung injury and fibrosis. J Immunol. 2003 Nov 15;171(10):5470–81. doi: 10.4049/jimmunol.171.10.5470. [DOI] [PubMed] [Google Scholar]
- 7.Izuhara K, Arima K, Ohta S, Suzuki S, Inamitsu M, Yamamoto KI. Periostin in Allergic Inflammation. Allergol Int. 2014 Mar 25; doi: 10.2332/allergolint.13-RAI-0663. [DOI] [PubMed] [Google Scholar]
- 8.Comeau MR, Ziegler SF. The influence of TSLP on the allergic response. Mucosal immunology. 2010 Mar;3(2):138–47. doi: 10.1038/mi.2009.134. [Review] [DOI] [PubMed] [Google Scholar]
- 9.Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. Journal of Immunology. 2005 Jun 15;174(12):8183–90. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 10.Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. Journal of Immunology. 2008 Aug 15;181(4):2790–8. doi: 10.4049/jimmunol.181.4.2790. [Comparative Study Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 11.Datta A, Alexander R, Sulikowski MG, Nicholson AG, Maher TM, Scotton CJ, et al. Evidence for a functional thymic stromal lymphopoietin signaling axis in fibrotic lung disease. Journal of Immunology. 2013 Nov 1;191(9):4867–79. doi: 10.4049/jimmunol.1300588. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo J, Omori M, Gyarmati D, Zhou B, Aye T, Brewer A, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J Exp Med. 2005 Aug 15;202(4):541–9. doi: 10.1084/jem.20041503. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doherty T, Soroosh P, Khorram N, Fukuyama S, Rosenthal P, Cho JY, et al. The tumor necrosis factor family member light is a target for asthmatic airway remodeling. Nat Med. 2011;17(5):596–603. doi: 10.1038/nm.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40(3):362–82. doi: 10.1016/j.biocel.2007.08.011. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U, Pfeffer K. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med. 2002 Jun 17;195(12):1613–24. doi: 10.1084/jem.20020215. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Subudhi SK, Anders RA, Lo J, Sun Y, Blink S, et al. The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. J Clin Invest. 2005 Mar;115(3):711–7. doi: 10.1172/JCI200522982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpino N, Thierfelder W, Chang M, Saris C, Turner S, Ziegler S, et al. Absence of an Essential Role for Thymic Stromal Lymphopoietin Receptor in Murine B-Cell Development. Molecular and Cellular Biology. 2004;24(6):2584–92. doi: 10.1128/MCB.24.6.2584-2592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anand S, Wang P, Yoshimura K, Choi IH, Hilliard A, Chen YH, et al. Essential role of TNF family molecule LIGHT as a cytokine in the pathogenesis of hepatitis. J Clin Invest. 2006 Apr;116(4):1045–51. doi: 10.1172/JCI27083. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, et al. Inhibition of airway remodeling in IL-5-deficient mice. Journal of Clinical Investigation. 2004 Feb;113(4):551–60. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen ZG, Zhang TT, Li HT, Chen FH, Zou XL, Ji JZ, et al. Neutralization of TSLP inhibits airway remodeling in a murine model of allergic asthma induced by chronic exposure to house dust mite. PLoS One. 2013;8(1):e51268. doi: 10.1371/journal.pone.0051268. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013 Jan;131(1):187–200. e1–8. doi: 10.1016/j.jaci.2012.08.002. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 22.Gauvreau GM, O’Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014 May 29;370(22):2102–10. doi: 10.1056/NEJMoa1402895. [Multicenter Study Randomized Controlled Trial Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 23.Kirstein F, Horsnell WG, Kuperman DA, Huang X, Erle DJ, Lopata AL, et al. Expression of IL-4 receptor alpha on smooth muscle cells is not necessary for development of experimental allergic asthma. J Allergy Clin Immunol. 2010 Aug;126(2):347–54. doi: 10.1016/j.jaci.2010.04.028. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyata M, Nakamura Y, Shimokawa N, Ohnuma Y, Katoh R, Matsuoka S, et al. Thymic stromal lymphopoietin is a critical mediator of IL-13-driven allergic inflammation. Eur J Immunol. 2009 Nov;39(11):3078–83. doi: 10.1002/eji.200939302. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 25.Oh MH, Oh SY, Yu J, Myers AC, Leonard WJ, Liu YJ, et al. IL-13 induces skin fibrosis in atopic dermatitis by thymic stromal lymphopoietin. Journal of Immunology. 2011 Jun 15;186(12):7232–42. doi: 10.4049/jimmunol.1100504. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Z, Oh MH, Yu J, Liu YJ, Zheng T. The Role of TSLP in IL-13-induced atopic march. Sci Rep. 2011;1:23. doi: 10.1038/srep00023. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013 Feb;12(2):147–68. doi: 10.1038/nrd3930. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hastie A, Moore W, Meyers D, Vestal P, Li H, Peters S, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allerg Clin Immunol. 2009;125(5):1028–36. doi: 10.1016/j.jaci.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]