SUMMARY
PHRF1 functions as an essential component of the TGF-β tumor suppressor pathway by triggering degradation of the homeodomain transcription factor TGIF. This leads to redistribution of cPML into the cytoplasm, where it coordinates phosphorylation and activation of Smad2 by the TGF-β receptor. In acute promyelocytic leukemia (APL), acquisition of PML-RARα is known to impede critical aspects of TGF-β signaling, including myeloid differentiation. Although these defects are thought to rely on suppression of cPML activity, the mechanisms underlying this phenomenon remain enigmatic. Here, we find that an abnormal function of PML-RARα is to interfere with TGIF breakdown, presumably by competing with PHRF1 for binding to TGIF, culminating in cPML sequestration and inactivation. Enforcing PHRF1 activity is sufficient to restore TGF-β cytostatic signaling in human blasts and suppress APL formation in a mouse model of APL, providing proof-of-concept data that suppression of PHRF1 activity by PML-RARα represents a critical determinant in APL pathogenesis.
INTRODUCTION
The PML tumor suppressor plays an important role in constraining both hematological and non-hematological malignancies, yet much remains to be learned about how it is regulated or how it might be inactivated during tumor progression (de The et al., 2012; Dos Santos et al., 2013). In the vast majority of APL patients, PML is fused to RARα, engendering an oncogenic fusion protein PML-RARα capable of initiating acute leukemia by suppressing differentiation along the myeloid lineage (Grignani et al., 1993; Scaglioni and Pandolfi, 2007). In transgenic mice, ectopic expression of PML-RARα in the myeloid lineage causes leukemia with features of APL, underscoring unequivocally the causal role for PML-RARα acquisition in APL development (Brown et al., 1997). Functionally, PML-RAR α was initially thought to act as a transcriptional repressor to antagonize myeloid differentiation and promote APL-initiating cell self-renewal. However, there is accumulating evidence that PML-RARα can also interfere with the ability of the PML isoforms encoded by the intact remaining allele to elicit a variety of tumor suppressive functions, such as growth arrest and terminal differentiation (de The and Chen, 2010; Licht, 2006; Salomoni and Pandolfi, 2002; Scaglioni and Pandolfi, 2007). For instance, PML-RARα has been shown to antagonize cPML activity that is instrumental to integration of the transforming growth factor beta (TGF-β) tumor suppressor program (Lin et al., 2004). Yet, the molecular mechanisms by which PML-RARα disables cPML function in TGF-β signaling remain to be elucidated.
TGF-β signaling is initiated by the formation of a complex consisting of two types of transmembrane Ser/Thr kinase receptor, TβRI and TβRII (Massague, 2008). TGF-β binding to TβRII induces recruitment and phosphorylation of TβRI, which in turn phosphorylates Smad2 and Smad3 (Smad2/3), a process facilitated by the adaptor protein SARA (Massague, 2008). The role of cPML in TGF-β signaling is to bridge together Smad2/3 and SARA and bring that complex within the proximity of TβRI (Lin et al., 2004; Seo et al., 2006). Phosphorylation of Smad2/3 induces association with Smad4 and translocation of the complexes to the nucleus, where they regulate expression of TGF-β target genes (Massague, 2008).
The phosphorylation of Smad2/3 can be limited from the nucleus by TGIF (TG interacting factor), which belongs to the TALE family of homeodomain proteins. Mechanistically, TGIF interacts with and interferes with the nucleocytoplasmic transit of cPML, thereby precluding assembly of the cPML/SARA complex and concomitant phosphorylation of Smad2/3 (Ettahar et al., 2013; Faresse et al., 2008; Lin et al., 2004; Seo et al., 2006). Besides cPML, TGIF has also been shown to interact with retinoic acid receptor alpha (RARα) and repress its transcriptional activity (Bartholin et al., 2006).
Collectively, these observations underscored an ability of TGIF to associate with both cPML and RARα, raising the question of whether there is any functional interplay between TGIF and PML-RARα. In our efforts to probe this possibility, we found that acquisition of PML-RARα in human APL blasts caused abnormal TGIF accrual, ultimately culminating in cPML inactivation and suppression of TGF-β signaling. We went on to investigate the underlying mechanisms, focusing our attention on PHRF1, a TGIF ubiquitin ligase recently identified in our laboratory as an essential component of the TGF-β signaling pathway (Ettahar et al., 2013). Remarkably, we found that PML-RARα associated with and engaged TGIF in a physical complex that compromises its interaction with PHRF1, leading to excessive TGIF accumulation. Such a mechanism seems to play an important role in APL progression, since restoration of PHRF1 activity was sufficient to trigger myeloid differentiation in human blasts and restrain APL progression in a mouse model of APL. Therefore, our findings that expression of PML-RARα impinges on PHRF1 function shed new mechanistic insights into the etiology of APL, likely paving the way for therapeutic breakthroughs to curb this life-threatening disease.
RESULTS AND DISCUSSION
PML-RARα Blocks PHRF1-Induced TGIF Degradation
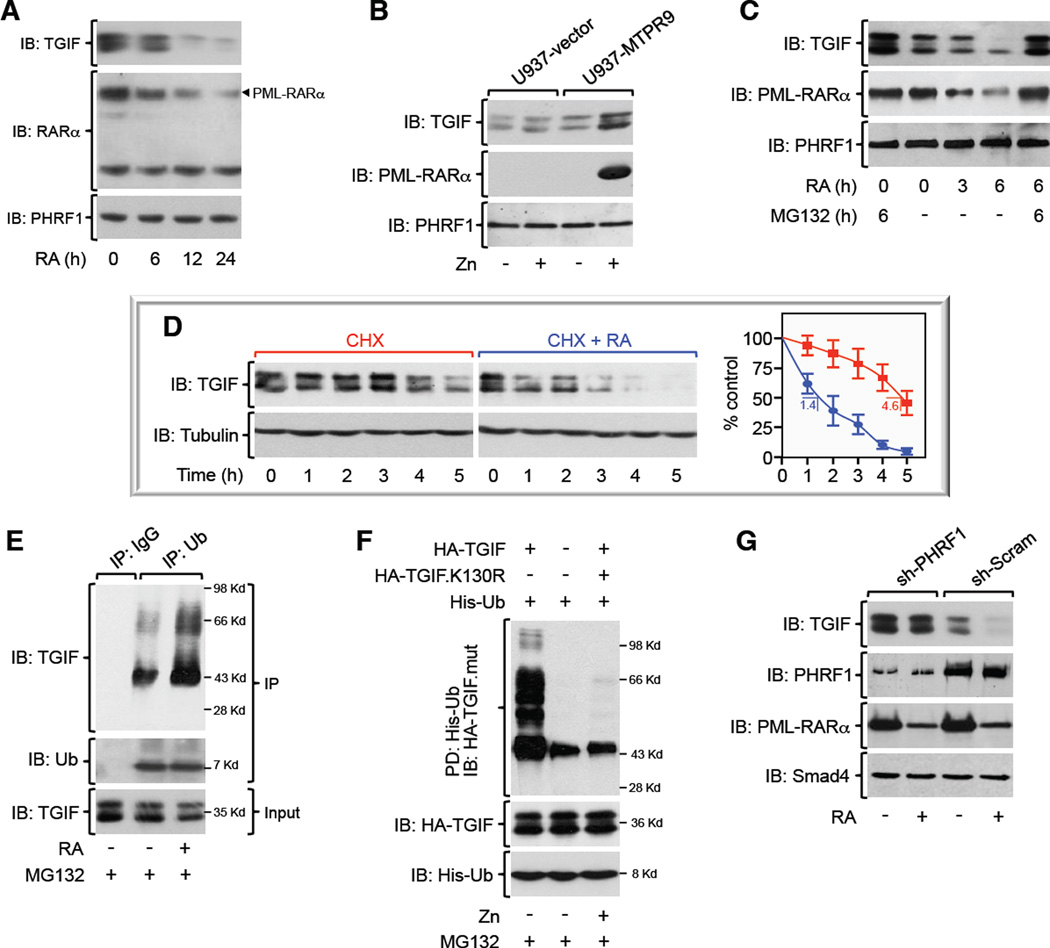
PML-RARα has been shown to hamper TGF-β-induced growth arrest and myeloid cell differentiation (Grignani et al., 1993; Lin et al., 2004), but the molecular mechanisms underlying this phenomenon remain elusive. Our published data that TGIF restricts TGF-β signaling by antagonizing cPML prompted us to explore whether PML-RARα could disrupt the interplay between TGIF and cPML, in turn endowing leukemia cells with the capability to evade TGF-β cytostatic signaling (Ettahar et al., 2013; Faresse et al., 2008; Seo et al., 2006). We initially tested this possibility by employing NB4 blasts, which derive from an APL patient and exhibit the particularity to undergo proteasomal degradation in response to retinoic acid (RA) (Lanotte et al., 1991; Lin et al., 2004). Quite surprisingly, RA stimulation caused a marked decrease in the expression levels of the TGIF protein (Figure 1A). Under these experimental conditions, RA stimulation did not cause any significant change in the expression of PHRF1 (Figure 1A), an E3 ubiquitin ligase that governs TGIF degradation (Ettahar et al., 2013). Likewise, there were no significant changes in the expression of other major components of the TGF-β signaling pathway, including TβRI, TβRII, SARA, cPML, Smad2, Smad3, and Smad4 (Figures 1G, 3A–C, S3A, and data not shown). As another specificity control, RA stimulation was void of any effect on the abundance of the TGIF protein in the human Burkitt’s lymphoma cell line BL41 bearing constitutively active c-Myc, the human leukemia cell line K562 harboring the oncogenic fusion protein Bcr-Abl, or mouse embryonic fibroblasts (MEFs) (Figure S1A), indicating that RA induced decreased TGIF expression by triggering PML-RARα degradation. In further support to the notion that PML-RARα stabilizes TGIF, NB4 cells display higher TGIF expression compared to K562 or BL41 cells (Figure S1A). It is also noteworthy that depletion of RARα or depletion/deletion of PML failed to decrease TGIF expression (Figures S1A–C), highlighting an acquired ability of PML-RARα to engender a gain-of-function that cannot be fulfilled by either protein alone. We independently validated this finding by demonstrating increased TGIF abundance following induction of PML-RARα expression in the human hematopoietic precursor cells, U937-MTPR9 (Figure 1B), which carry PML-RARα under the control of a zinc-inducible promoter (Grignani et al., 1993). Here again, expression of PML-RARα had no effect on the expression of PHRF1 or any other components of TGF-β signaling described earlier (Figures 1B, S1E, S4B, and data not shown). Collectively, these findings revealed an ability of PML-RARα to trigger aberrant TGIF accrual, providing an initial hint for how PML-RARα disrupts TGF-β signaling in APL blasts.
Figure 1. PML-RARα Expression Stabilizes TGIF. See also Figure S1.
(A, B) Expression of TGIF, PML-RARα, or PHRF1 in NB4 blasts exposed to RA for various times (A), or U937-MTPR9 cells treated with Zn for 24 hr (B) was analyzed by immunoblotting.
(C) NB4 cells were incubated with RA and/or MG132 for the indicated times and analyzed by immunoblotting.
(D) NB4 blasts were incubated with cycloheximide (CHX) alone or together with RA for various times before being analyzed by immunoblotting. The half-life of TGIF is indicated.
(E) NB4 blasts were treated with RA for 24 hr before being incubated with MG132 for the last 6 hr. Endogenous TGIF polyubiquitination was analyzed by coimmunoprecipitation.
(F) Transfected U937-MTPR9 cells were treated with Zn for 24 hr before being incubated with MG132 for the last 6 hr. Lysates were pulled-down (PD) with nickel-sepharose and immunoblotted with anti-HA.
(G) NB4 blasts expressing sh-Scram or sh-PHRF1 were treated with RA for 24 hr and analyzed by immunoblotting.
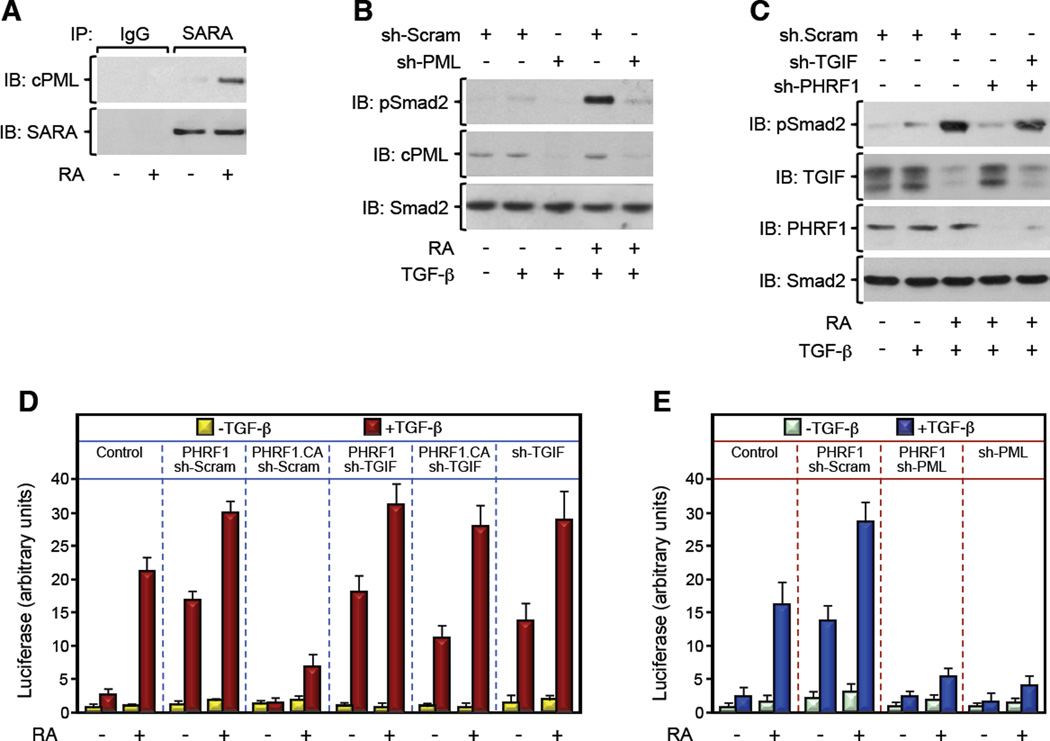
Figure 3. PML-RARα Suppresses TGF-β Signaling by Disrupting PHRF1-Driven TGIF Degradation. See also Figure S3.
(A) NB4 blasts were treated with RA for 24 hr, and the association of endogenous cPML and SARA was analyzed by coimmunoprecipitation.
(B, C) NB4 blasts expressing the indicated combinations of sh-RNAs were cultured with RA for 24 hr. Then, cells were treated with TGF-β for 30 min and phosphorylation of Smad2 was analyzed by immunoblotting using anti-phospho-Smad2 (pSmad2) antibody.
(D, E) NB4 blasts were transfected with ARE3-Lux together with FAST1 and the indicated combination of expression vectors and treated with RA for 24 hr before being treated with TGF-β for the last 16 hr. Luciferase activity was measured and data are expressed as mean ± SD (n = 3).
To investigate whether PML-RARα increases expression of the TGIF protein by preventing its degradation, and if so, whether this process occurs via PHRF1, we undertook the following approaches. First, we challenged NB4 blasts with RA in the presence or absence of MG132, and found that blocking the proteasome machinery was effective in preventing PML-RARα degradation and the associated decline in TGIF abundance (Figure 1C). Second, decreasing expression of PML-RARα in NB4 blasts by RA treatment, or conversely, inducing expression of PML-RARα in U937-MTPR9 cells by Zn treatment, had little or no effect on TGIF mRNA expression (Figure S1D). Third, expression of PML-RARα increased expression of the TGIF protein from a TGIF transgene driven by a constitutive CMV promoter (Figure S1E), further suggesting that PML-RARα fosters TGIF abundance by a transcriptional-independent mechanism. Fourth, we analyzed the half-life of the TGIF protein in cycloheximide (CHX)-chase experiments, and found that RA stimulation accelerated the turnover of the TGIF protein (Figure 1D). Fifth, we conducted comparative analyses using wild-type TGIF and a PHRF1-resistant mutant, TGIF.K130R, and the result underscored an inability of PML-RARα to further sustain expression of TGIF.K130R, as compared to the wild-type counterpart (Figure S1E). Sixth, stimulation of NB4 blasts with RA resulted in increased polyubiquitination of endogenous TGIF (Figure 1E). Seventh, exposure of U937-MTPR9 cells to Zn decreased TGIF polyubiquitination to an extent similar to that observed for the PHRF1-resistant mutant, TGIF.K130R (Figure 1F). Finally, depleting PHRF1 in NB4 blasts prevented RA-induced TGIF decay, despite the strong decrease in the expression level of PML-RARα (Figure 1G). Taken together, these data strongly suggest that expression of PML-RARα interferes with PHRF1-induced TGIF polyubiquitination and degradation by the proteasome.
PML-RARα Competes with PHRF1 for binding to TGIF
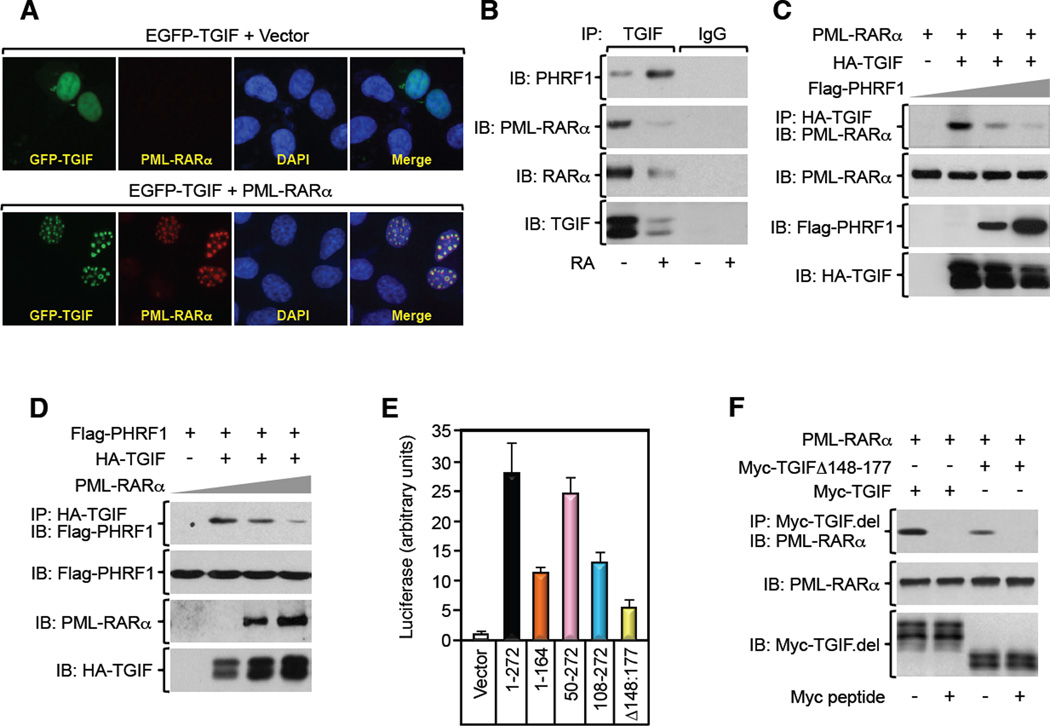
Previous studies from our and other laboratories have shown that TGIF can interact with both cPML and RARα in many cell systems (Bartholin et al., 2006; Faresse et al., 2008; Seo et al., 2006). Coimmunoprecipitation experiments confirmed the interaction of TGIF with cPML or RARα as well as the disruption of the TGIF/RARα complex by RA (Figures 2B, S2B,C,E–H). As PML-RARα retains large segments of both proteins (de The et al., 1991), we considered the possibility that PML-RARα may associate with and divert TGIF from PHRF1, thereby causing increased TGIF abundance. In fact, we found that expression of PML-RARα caused redistribution of TGIF into nuclear microspeckles, providing an initial indication that TGIF interacts with PML-RARα (Figure 2A). Subsequent coimmunoprecipitation studies revealed a robust interaction between endogenous PML-RARα and TGIF in NB4 blasts, and this decreased upon RA stimulation (Figure 2B). Strikingly, concomitant with the decrease in the PML-RARα/TGIF interaction, the PHRF1/TGIF interaction was increased, despite the strong decrease in the total pool of TGIF (Figure 2B), suggesting that PML-RARα and PHRF1 might compete for binding to TGIF. We also performed coimmunoprecipitation experiments using U937-MTPR9 cells, and found that inducing PML-RARα expression by Zn stimulation was effective in promoting assembly of the PML-RARα/TGIF complex, likely occurring at the expense of the PHRF1/TGIF complex (Figure S2A). Further evidence that PML-RARα and PHRF1 competitively share TGIF was obtained by showing that PML-RARα was able to displace the PHRF1/TGIF interaction in a dose-response manner, and vice versa (Figures 2C,D). This effect is specific to PML-RARα, as overexpression of PML or RARα separately did not affect the expression levels of TGIF, or its association with PHRF1 (Figures S2B,C). In our attempt to unravel the basis of these competitive interactions, we conduced an in depth analysis of the TGIF/PML-RARα interaction by means of coimmunoprecipitation and mammalian two-hybrid assays. We found that PML-RARα displayed affinity for two distinct regions in TGIF (Figures 2E,F, S2D), suggesting that association of PML-RARα with TGIF might mask the PHRF1 binding site in TGIF. In further support to this notion, PML interacted with the N-terminus of TGIF, whereas RARα interacted with the C-terminus of TGIF (Figures S2E–H). Interestingly, disrupting the PHRF1-binding domain (TGIFΔ148–177) in TGIF (Ettahar et al., 2013) caused a significant decrease in the TGIF/PML-RARα interaction (Figures 2E,F), supporting the hypothesis that PML-RARα and PHRF1 compete for the same domain in TGIF. Together, these results provide strong evidence that PML-RARα and PHRF1 form mutually exclusive complexes with TGIF.
Figure 2. PML-RARα Competes with PHRF1 for Binding to TGIF. See also Figure S2.
(A) PML−/− MEFs were transfected with pEGFP-TGIF in the absence or presence of PML-RARα and immunostained with anti-PML and DAPI.
(B) NB4 blasts were treated with RA for 24 hr, and the association of TGIF with PML-RAR α or PHRF1 was analyzed by coimmunoprecipitation.
(C, D) U937 cells were transfected with the indicated combination of PML-RARα, HA-TGIF, and Flag-PHRF1, and the association of HA-TGIF with PML-RARα or Flag-PHRF1 was analyzed by coimmunoprecipitation.
(E) U937 cells were transfected with pG5E1b-Luc together with Gal4-PML-RARα and VP16-TGIF deletion mutants. Luciferase activity was measured and data are expressed as mean ± SD of triplicates.
(F) The interaction of PML-RARα with TGIF deletion mutants in transfected U937 cells was analyzed by coimmunoprecipitation.
Restoration of PHRF1 Activity in APL Blasts Rescues TGF-β Signaling
The results outlined so far revealed an acquired ability of PML-RARα to implement excessive TGIF accumulation, presumably by disrupting its interaction with PHRF1. In so doing, PML-RARα could conceivably antagonize cPML function in TGF-β signaling. Several lines of evidence support this theory. First, treatment of NB4 cells with RA increased the interaction between endogenous cPML and SARA (Figure 3A), which is known to take place in the cytoplasm (Lin et al., 2004; Seo et al., 2006). A similar effect was observed when the interaction of cPML with TβRI was examined (Figure S3A). Second, exposure of NB4 blasts to RA elicited a marked increase in TGF-β-induced Smad2 phosphorylation, but failed to elicit any response in NB4 cells depleted for cPML (Figure 3B), which is in consonance with previous observations that PML-RARα blocks TGF-β signaling by antagonizing cPML (Lin et al., 2004). Third, depleting PHRF1 in NB4 blasts blunted RA-induced Smad2 phosphorylation (Figure 3C), directly linking PHRF1 to the PML-RARα/cPML/Smad2 axis. Finally, depleting TGIF restored RA-induced Smad2 phosphorylation in cells depleted for PHRF1 (Figure 3C), reinforcing the hypothesis that PHRF1 influences the PML-RARα/cPML/Smad2 axis by enforcing TGIF degradation. Taken together with our earlier protein-protein interaction analyses, these findings strongly suggest that PML-RARα engages TGIF in a complex that compromises its association with PHRF1, a mechanistic model that likely explains why expression of PML-RARα triggers excessive TGIF accumulation in blasts, in turn setting an attenuated TGF-β/Smad signaling.
To validate this scenario, we extended our analysis to the TGF-β transcriptional program, using the ARE3-Lux reporter, a surrogate readout of Smad2 transcriptional activity. As shown in Figure 3D, enforced expression of PHRF1 increased the sensitivity of NB4 blasts to TGF-β-induced transcription even in the absence of RA stimulation. This increase likely depends on TGIF degradation, as enforced expression of a catalytic inactive mutant of PHRF1, PHRF1.CA, exerted a dominant-negative effect that was completely reversed in cells depleted for TGIF (Figure 3D). Likewise, the PHRF1 rescue effect was also dependent on cPML, since its depletion was sufficient to render again NB4 blasts overexpressing PHRF1 unresponsive to TGF-β (Figure 3E). These observations were independently validated using a Smad3-specific reporter (Figures S3B,C). Based on these findings, it is tempting to suggest that the blockade of TGF-β signaling in APL blasts harboring PML-RARα might stem from abnormal TGIF accrual and the resulting cPML inactivation rather than other mechanisms.
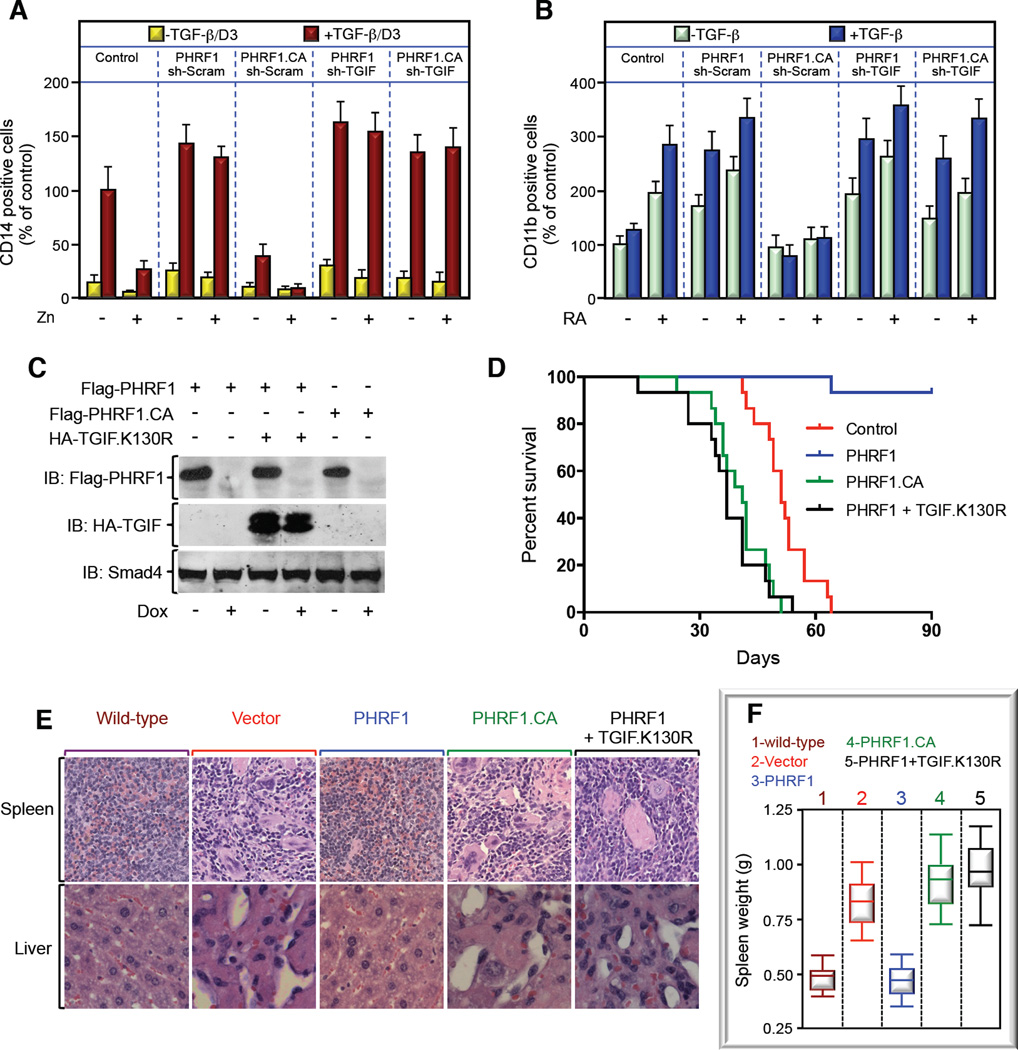
To further challenge this scenario, we interrogated the ability of PML-RARα to confer resistance to TGF-β-induced myeloid differentiation, a prominent hallmark of APL development (Grignani et al., 1993). To this end, we used U937-MTPR9 cells, which undergo massive monocytic differentiation in response to TGF-β signaling in the absence of PML-RARα expression (Grignani et al., 1993). Congruent with the literature, inducing PML-RARα in U937-MTPR9 cells resulted in a potent decrease in TGF-β-induced myeloid differentiation (Figures 4A, S4A). Strikingly, overexpression of PHRF1 blunted this PML-RARα inhibitory effect, whereas overexpression of PHRF1.CA elicited the opposite response (Figures 4A, S4A). Of note, simultaneous TGIF depletion was sufficient to restore TGF-β responsiveness in PHRF1.CA-expressing cells (Figures 4A, S4A), demonstrating that PHRF1.CA exerts its dominant inhibitory effect via stabilization of endogenous TGIF. Finally, depleting cPML disrupted the ability of PHRF1 to restore TGF-β-induced myeloid differentiation (Figures S4B,4C). Similar results were obtained in experiments investigating the granulocytic differentiation of NB4 cells in response to RA and TGF-β stimulation (Figure 4B). Taken together, these results provide strong evidence that disruption of PHRF1 function contributes to resistance of APL blasts to TGF-β signaling.
Figure 4. PML-RARα Drives APL by Impeding PHRF1-Driven TGIF Degradation. See also Figure S4.
(A) U937-MTPR9 cells stably expressing the indicated expression vectors were cultured with Zn for 16 hr before being treated with TGF-β plus vitamin D3 (TGF-β/D3) for 5 days. Cell differentiation was evaluated by determining the number of positive CD14 cells.
(B) NB4 cells were transiently transduced with the indicated combinations of lentiviruses and cultured with or without RA and TGF-β for 5 days. Cell differentiation was evaluated by determining the number of positive CD11b cells.
(C, D) MRP8 blasts were stably transfected with Dox-repressible Flag-PHRF1 or Flag-PHRF1.CA alone or together with HA-TGIF.K130R. Expression of PHRF1 or TGIF was determined by direct immunoblotting (C). MRP8 stable cell lines were transplanted to FVB mice, and survival was recorded in a Kaplan-Meier graph (D).
(E, F) Representative H&E staining pictures of liver and spleen (E) or weights of spleen (F) of mice scarified in (D).
Restoration of PHRF1 Activity Suppresses APL Formation in vivo
Besides APL, TGF-β cytostatic signaling is disrupted in many types of human leukemia, suggesting the existence of diverse mechanisms that disable the TGF-β tumor suppressive function in human hematological malignancies (Blank and Karlsson, 2011; Dong and Blobe, 2006; Lin et al., 2004). To investigate whether restoration of TGF-β signaling in APL blasts could influence APL progression in vivo, we conducted transplantation experiments using APL blasts from hMRP8-PML/RARα mice, which harbor constitutive expression of PML-RARα in the hematopoietic lineage (Brown et al., 1997). In comparison to the native MRP8-PML/RARα transgenic mice, MRP8-transplanted mice develop APL with similar features, but with very short latency, thus enabling rapid generation of APL-bearing mice. Initial biochemical experiments showed that exposure of MRP8 cells to RA induced TGIF degradation, and this was accompanied with increased association of TGIF with PHRF1 and concomitant disassembly of the TGIF/PML-RARα complex (Figures S4D,E). Next, we generated MRP8 blasts stably expressing wild-type PHRF1 or PHRF1.CA under the control of a doxycycline (Dox)-repressible promoter (Figure 4C), with the aim to circumvent the PHRF1-dependent myeloid differentiation until transplantation into recipient mice that were maintained under Dox-free conditions, thus enabling expression of PHRF1 or PHRF1.CA. During the 90 days observation period, all 15 mice transplanted with control or MRP8-PHRF1.CA blasts developed fatal APL, whereas 14 out of 15 mice transplanted with MRP8-PHRF1 remained healthy and free of tumors (Figure 4D). Of note, the body of the mouse that died unexpectedly (without any apparent complication) was severely decomposed, precluding any analysis to assess for the presence of MRP8-PHRF1 blasts. Interestingly, we noticed that expression of PHRF1.CA resulted in a slight but significant decrease in mice survival when compared to mice receiving MRP8-vector blasts (Figure 4D), which is in good agreement with our earlier observations that PHRF1.CA functions as a dominant negative mutant. To substantiate these observations, we carried histopathological experiments to assess infiltration of immature myeloid cells into the liver or spleen, a process known to impinge on their integrity (Omidvar et al., 2013). In contrast to mice transplanted with MRP8-vector or MRP8-PHRF1.CA cells, we were unable to see any alterations in the liver or spleen of mice transplanted with MRP8-PHRF1 cells (Figure 4E). These findings were independently validated by demonstrating the absence of exogenous PHRF1 expression in the spleen of MRP8-PHRF1 mice as well as increased weight of the spleen of MRP8-vector or MRP8-PHRF1.CA mice compared to MRP8-PHRF1 mice (Figures 4F, S4F). To directly demonstrate that this PHRF1 tumor suppressive function is mediated via TGIF degradation, we performed similar in vivo transplantation experiments using MRP8-PHRF1 cells expressing the PHRF1-resistant mutant, TGIF.K130R, surmising that preventing TGIF degradation would be sufficient to oppose PHRF1-driven APL suppression. In fact, expression of TGIF.K130R in MRP8-PHRF1 blasts restored APL formation in mice (Figures 4C–F, S4H). In an alternative complementary approach, depleting TGIF blocked both RA-induced granulocytic differentiation of MRP8 cells in vitro and APL formation by these cells in vivo (Figures S4G–H). Together, these findings suggest that disruption of PHRF1-driven TGIF degradation by PML-RARα is instrumental in the pathogenesis of APL.
Concluding Remarks
Acquisition of the PML-RARα oncogene represents by far the most common basis of inactivation of the PML tumor suppressor in APL. Despite the tremendous progress made in understanding the etiology of APL, the molecular mechanisms by which PML-RARα drives leukemogenesis remain unclear. Therefore, our findings that acquisition of PML-RARα impinges on the PHRF1 tumor suppressor network not only holds tantalizing promises for unraveling mechanistic paradigms of APL pathogenesis, but also raise the provocative concept that the PML-RARα/TGIF/PHRF1 module could be an important target for elaborating innovative therapeutic strategies to combat APL malignancies that develop resistant to conventional drugs, such as retinoic acid. For instance, identifying drugs that impede the interaction between PML-RARα and TGIF could restore the ability of PHRF1 to trigger TGIF degradation, ultimately leading to APL blast differentiation and attendant leukemia regression despite the resistance of PML-RARα to retinoic acid.
EXPERIMENTAL PROCEDURES
Cell Culture
HEK293T, K562, MEFs, and NB4 cells were cultured in DMEM supplemented with 10 % fetal calf serum (FCS). U937-MTPR9 and MRP8 cells were maintained in RPMI supplemented with 10 % FCS. Stable cell lines were maintained in media containing G418 (500 µg/ml), puromycin (10 µg/ml), and/or Zeocin (500 µg/ml).
Immunoprecipitation and Immunoblotting
Cell lysates were prepared using TNMG buffer as previously described (Demange et al., 2009). They were then incubated with antibody for 2 hr, followed by adsorption to sepharose-coupled protein G for 1 hr. Immune-complexes were separated by SDS-PAGE and analyzed by immunoblotting. In CHX-chase experiments, the expression level of TGIF was determined by scanning laser densitometry.
Myeloid Differentiation Assay
Myeloid differentiation was examined as previously described (Grignani et al., 1993). Briefly, cells were incubated with anti-CD14 or anti-CD11b antibody for 60 min at 4°C. Then, cells were incubated for 60 min at 4°C with fluorescein-labeled secondary antibody and analyzed by FACS using a FACS Caliber (Becton–Dickinson).
Transplantation Experiments
MRP8 cells from hMRP8-PML/RARα mice were described previously (Omidvar et al., 2013). Leukemia was propagated by intravenous injection of blasts (106 viable MRP8 cells) into 6 weeks-old syngeneic FVB mice. Animal handling was done according to the guidelines of institutional animal care committee.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Di Croce L, Pandolfi PP, and Kogan SC for providing reagents. We thank Dr. Maher J for its critical reading of the manuscript. This work was supported by INSERM, CNRS, Association pour la Recherche sur le Cancer, Ligue Comité de Paris, and NIH R01-AR059070 to AA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
CP, M-ZZ, and AA conceived and designed the experiments. CP and M-ZZ performed the in vitro experiments with the assistance of AE, LL, and OF and the in vivo experiments with the assistance of SK, OM, and GT. CP, M-ZZ, and AA wrote the paper with the help of GT and LL
The authors declare they have no conflict of interest.
REFERENCES
- Bartholin L, Powers SE, Melhuish TA, Lasse S, Weinstein M, Wotton D. TGIF inhibits retinoid signaling. Mol Cell Biol. 2006;26:990–1001. doi: 10.1128/MCB.26.3.990-1001.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank U, Karlsson S. The role of Smad signaling in hematopoiesis and translational hematology. Leukemia. 2011;25:1379–1388. doi: 10.1038/leu.2011.95. [DOI] [PubMed] [Google Scholar]
- Brown D, Kogan S, Lagasse E, Weissman I, Alcalay M, Pelicci PG, Atwater S, Bishop JM. A PMLRARα transgene initiates murine acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 1997;94:2551–2556. doi: 10.1073/pnas.94.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de The H, Chen Z. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat Rev Cancer. 2010;10:775–783. doi: 10.1038/nrc2943. [DOI] [PubMed] [Google Scholar]
- de The H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A. The PML-RARα fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- de The H, Le Bras M, Lallemand-Breitenbach V. The cell biology of disease: Acute promyelocytic leukemia, arsenic, and PML bodies. J Cell Biol. 2012;198:11–21. doi: 10.1083/jcb.201112044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demange C, Ferrand N, Prunier C, Bourgeade MF, Atfi A. A model of partnership co-opted by the homeodomain protein TGIF and the Itch/AIP4 ubiquitin ligase for effective execution of TNF-α cytotoxicity. Mol Cell. 2009;36:1073–1085. doi: 10.1016/j.molcel.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dong M, Blobe GC. Role of transforming growth factor-beta in hematologic malignancies. Blood. 2006;107:4589–4596. doi: 10.1182/blood-2005-10-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos GA, Kats L, Pandolfi PP. Synergy against PML-RARα: targeting transcription, proteolysis, differentiation, and self-renewal in acute promyelocytic leukemia. J Exp Med. 2013;210:2793–2802. doi: 10.1084/jem.20131121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettahar A, Ferrigno O, Zhang MZ, Ohnishi M, Ferrand N, Prunier C, Levy L, Bourgeade MF, Bieche I, Romero DG, et al. Identification of PHRF1 as a tumor suppressor that promotes the TGF-β cytostatic program through selective release of TGIF-driven PML inactivation. Cell Rep. 2013;4:530–541. doi: 10.1016/j.celrep.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Faresse N, Colland F, Ferrand N, Prunier C, Bourgeade MF, Atfi A. Identification of PCTA, a TGIF antagonist that promotes PML function in TGF-β signalling. EMBO J. 2008;27:1804–1815. doi: 10.1038/emboj.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignani F, Ferrucci PF, Testa U, Talamo G, Fagioli M, Alcalay M, Mencarelli A, Peschle C, Nicoletti I, et al. The acute promyelocytic leukemia-specific PML-RARα fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell. 1993;74:423–431. doi: 10.1016/0092-8674(93)80044-f. [DOI] [PubMed] [Google Scholar]
- Lanotte M, Martin-Thouvenin V, Najman S, Balerini P, Valensi F, Berger R. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3) Blood. 1991;77:1080–1086. [PubMed] [Google Scholar]
- Licht JD. Reconstructing a disease: What essential features of the retinoic acid receptor fusion oncoproteins generate acute promyelocytic leukemia? Cancer Cell. 2006;9:73–74. doi: 10.1016/j.ccr.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Lin HK, Bergmann S, Pandolfi PP. Cytoplasmic PML function in TGF-β signalling. Nature. 2004;431:205–211. doi: 10.1038/nature02783. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFβ in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidvar N, Maunakea ML, Jones L, Sevcikova S, Yin B, Himmel KL, Tennant TR, Le Beau MM, Largaespada DA, Kogan SC. PML-RARα co-operates with Sox4 in acute myeloid leukemia development in mice. Haematologica. 2013;98:424–427. doi: 10.3324/haematol.2011.057067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomoni P, Pandolfi PP. The role of PML in tumor suppression. Cell. 2002;108:165–170. doi: 10.1016/s0092-8674(02)00626-8. [DOI] [PubMed] [Google Scholar]
- Scaglioni PP, Pandolfi PP. The theory of APL revisited. Curr Top Microbiol Immunol. 2007;313:85–100. doi: 10.1007/978-3-540-34594-7_6. [DOI] [PubMed] [Google Scholar]
- Seo SR, Ferrand N, Faresse N, Prunier C, Abecassis L, Pessah M, Bourgeade MF, Atfi A. Nuclear retention of the tumor suppressor cPML by the homeodomain protein TGIF restricts TGF-β signaling. Mol Cell. 2006;23:547–559. doi: 10.1016/j.molcel.2006.06.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.