Abstract
Objective
To evaluate the validity of 2D video-oculography (VOG) compared with scleral search coils for horizontal AVOR gain estimation in older individuals.
Study Design
Cross-sectional validation study.
Setting
Tertiary care academic medical center.
Patients
Six individuals age 70 and older.
Interventions
Simultaneous eye movement recording with scleral search coil (over right eye) and EyeSeeCam VOG camera (over left eye) during horizontal head impulses.
Main Outcome Measures
Best estimate search coil and VOG horizontal AVOR gain, presence of compensatory saccades using both eye movement recording techniques.
Results
We observed a significant correlation between search coil and VOG best estimate horizontal AVOR gain (r = 0.86, p = 0.0002). We evaluated individual head impulses and found that the shapes of the head movement and eye movement traces from the coil and VOG systems were similar. Specific features of eye movements seen in older individuals, including overt and covert corrective saccades and anticompensatory eye movements, were captured by both the search coil and VOG systems.
Conclusion
These data suggest that VOG is a reasonable proxy for search coil eye movement recording in older subjects to estimate VOR gain and the approximate timing of corrective eye movements. VOG offers advantages over the conventional search coil method; it is portable and easy to use, allowing for quantitative VOR estimation in diverse settings such as a routine office-based practice, at the bedside, and potentially in larger scale population analyses.
Keywords: Aging, Eye movements, Search coils, Vestibulo-ocular reflex, Video-oculography
The head impulse test (HIT) is a well-validated clinical measure of the vestibulo-ocular reflex (VOR) (1). A small-amplitude, high-peak velocity head rotation is delivered by the examiner in a plane that roughly corresponds to a pair of coplanar semicircular canals (right and left horizontal, or right anterior and left posterior [RALP] or left anterior and right posterior [LARP]). Using this technique, the function of each canal can be tested individually, for example, the left horizontal canal can be selectively tested by rotating the head in the plane of the horizontal canals toward the left ear. The normal VOR produces an eye movement nearly equal in velocity and lagging only slightly in time in the opposite direction of head movement so that gaze remains stable. If the function of a particular canal is deficient, the subject generates an insufficient slow-phase eye movement. This leads to image motion on the retina with displacement of the fixation target away from the fovea, requiring a corrective saccade (rapid eye movement) to restore fixation on the target of interest (2). The bedside HIT has been largely a qualitative test, given that methods used to quantify eye movements accurately in response to head impulses, such as the magnetic field search coil technique, have largely been restricted to the research laboratory.
In recent years, several 2D video-oculography (VOG) systems have been developed that have the potential to make quantitative testing of the VOR possible at the bedside and on a more widespread basis. These portable VOG goggle systems consist of high-speed digital cameras that track the pupil and inertial measurement devices that quantify head movements, both mounted on a lightweight glasses frame. A few small studies have demonstrated the validity of horizontal canal VOR gain values obtained with VOG when compared with testing with search coils as the “gold standard” (3,4).
We sought to evaluate the validity of VOG against search coils focusing specifically on older individuals. In previous work, we found a high prevalence of semicircular canal dysfunction in subjects aged 70 years and older using the head impulse dynamic visual acuity (hiDVA) test in each canal plane (5). Limitations of the hiDVA test in older adults include comorbid oculomotor and attentional deficits that may impair performance and reduce the specificity of hiDVA as a measure of semicircular canal function. Validation of the 2D video-oculography horizontal head impulse test in older individuals is an essential step in extending physiologic vestibular testing to the older population.
METHODS
Subjects
We recruited six community-dwelling subjects aged 70 and older (4 male subjects, age range 71–80 yr) to participate in our study. Subjects did not have any instability of the cervical spine or loss of vision that would contraindicate head impulse testing, or any ophthalmologic conditions (e.g., previous corneal abrasion, keratopathy) that would preclude placement of the annular search coil. All subjects gave informed consent to participate, and the study was approved by the Johns Hopkins institutional review board. Two methods were used to record eye movements: 3D magnetic search coils and 2D video-oculography (EyeSeeCam VOG system, Munich, Germany). Recordings with search coils and VOG were made simultaneously during the same manual head impulses, with the search coil placed on the right eye and the camera mounted above the left eye. In these experiments, only horizontal head and eye movements were analyzed.
Experimental Setup
The techniques for search coil calibration and recording in our laboratory have been described previously (6,7). In brief, subjects were seated in the center of a 1 m3 coil frame, and 3D movements of the right eye were recorded using a dual search coil (Chronos Vision GmbH, Berlin, Germany) placed on the sclera. First, the eye was anesthetized with topical proparacaine. Another dual search coil firmly attached to a bite bar sensed head movements. The bar consisted of a Plexiglas plate coated with hardened dental impression compound molded to the subject’s dental occlusion. Eye and bite bar angular position signals were low-pass filtered with an analog, single-pole Butterworth, anti-aliasing filter with a 3-dB bandwidth of 100 Hz and then digitally sampled at 500 Hz. Eye and head position were expressed as rotation vectors and used to derive the angular velocities of the eye and head in head coordinates.
The EyeSeeCam system was used per published specifications, with the camera placed over the left eye (3). The EyeSeeCam system consists of a high-speed digital video camera (220 Hz frame rate), a mirror to reflect the eye image to the camera, and an inertial measurement unit to measure angular head velocity. All components are rigidly mounted onto a lightweight swim goggle frame to minimize slippage of the camera relative to the head, as per manufacturer guidelines. The eye is illuminated with infrared light, and horizontal and vertical eye position is initially calibrated with projected targets from a glasses-mounted laser. The calibrated data were low-pass filtered using a digital Gaussian filter with a bandwidth of 90 Hz.
Experimental Procedure
Subjects were instructed to gaze at a light-emitting diode (LED) located 124 cm directly forward at eye level. The examiner stood in front of the subject (with the subject viewing the target over the examiner’s shoulder) and grasped the head over the mandibular area. The head was kept stationary in a comfortable, “upright” position before each head impulse, placing Reid’s stereotactic line (inferior orbital rim to superior external auditory canal) 7 ± 7 degrees nose-down from the earth-horizontal plane. From this position, the examiner delivered head impulses in the earth-horizontal plane in the direction of the right and left horizontal semicircular canals. The head was moved a small amplitude (5–15 degrees) with high peak velocity (150–250 degrees per second) and acceleration (3,000–4,000 degrees per s2) horizontally, and the direction (leftward or rightward) of the head impulses was randomized to be unpredictable. Ten to 15 head impulses were delivered in both the right and left directions during each run, and 2 to 3 runs (with simultaneous coil and VOG recording) were performed in each patient. Total testing time was approximately 10 to 15 minutes.
Data Analysis
The search coil data were analyzed offline using customized LabVIEW software (8). We discarded data from head impulses during which the eye responses included blinks, saccades, or eye movements that began before the onset of head movement. Specifically for the analysis of head impulse data, an additional low-pass filter of 40 Hz was applied. The onset of each head impulse was calculated by fitting a polynomial curve to horizontal angular head velocity versus time. The point where the magnitude of the fitted curve was greater than 2% of the curve’s peak magnitude (typically, this threshold was 4 degrees per second) was defined as the time of onset.
We used the default settings of EyeSeeCam software, which only accepts horizontal head impulses with peak velocity ≥75 degrees per second and peak acceleration 1000°/s2 or greater in the first 150 ms and with horizontal peak eye velocity greater than vertical peak eye velocity. Given the automatic rejection of head impulses that did not fit these criteria by the EyeSeeCam software, the total number of impulses retained by the search coil and VOG techniques differed by 1 to 2 head impulses. The onset of the head impulse for the EyeSeeCam software is defined as the time when head velocity exceeded 20 degrees per second (9). We also discarded VOG data from head impulses that included blinks, voluntary or quick phase resetting saccades, or eye movements that started before the onset of head movement. Approximately 10% to 15% of head impulses were discarded for these reasons for both techniques.
For both search coil and VOG eye movement recording, we calculated AVOR gain by dividing inverted horizontal eye velocity by horizontal head velocity (10). For the search coil data, our laboratory standard is to estimate a “best value” of AVOR gain for each head impulse by taking the highest instantaneous gain value that occurred in the 30-ms period before peak head velocity (which occurs at around 60 ms). This early window in the course of the head impulse was chosen to capture the eye movement response that is most likely vestibular in origin and to minimize the influence of any catch-up saccades (2,11). For the VOG data, the default gains provided by the EyeSeeCam software include median gain at 40 and 60 ms. To make the search coil and VOG AVOR gain estimates more comparable, we also computed best estimates of VOG AVOR gain by taking the highest gain value between 30 and 60 ms after onset of head movement. Given that simultaneous search coil and VOG recordings were performed at least twice, we calculated intraclass correlation coefficients as a measure of test-retest reliability for both the coil and VOG techniques. Left and right AVOR gains were considered separately and were pooled in subsequent analyses (for a total of 12 observations).
RESULTS
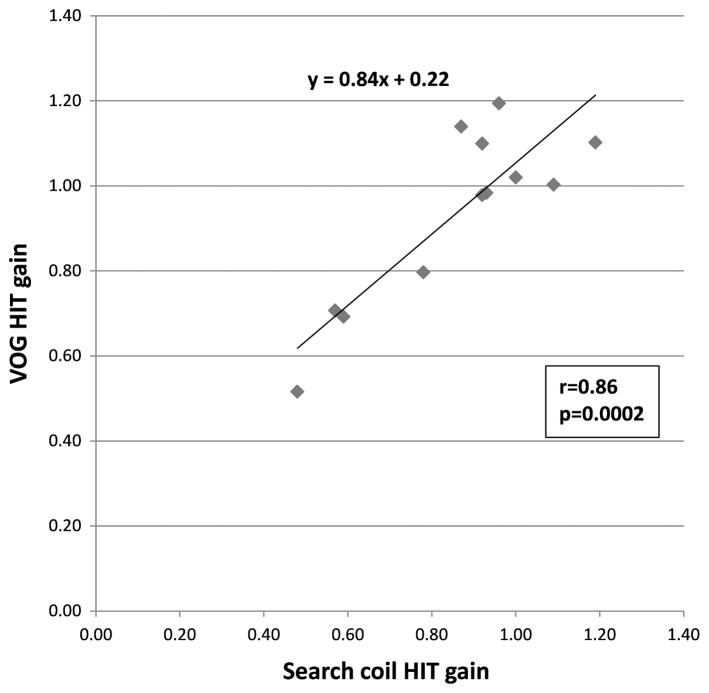
We compared horizontal AVOR gain values computed using the search coil and VOG methods in all subjects. When comparing search coil best estimate AVOR gain with EyeSeeCam default median gains, we observed significant correlations at 40 ms (r = 0.84; p = 0.0006) and 60 ms (r = 0.57; p = 0.05). When comparing search coil best estimate AVOR gain to a computed EyeSeeCam best estimate AVOR gain between 30 and 60 ms, we observed a higher correlation between search coil and VOG data (r = 0.86, p = 0.0002; Fig. 1). Using this latter comparison, a linear regression of the data points gave an equation y = 0.84x + 0.22, where y corresponds to VOG HIT gain and x corresponds to search coil HIT gain (Fig. 1). We observed that several subjects had gain values of greater than 1, using both the search coil and EyeSeeCam methods (Fig. 1). These superunity gains may be due to the prevalent use of magnifying spectacles for presbyopia in older individuals, which have been shown to temporarily increase gain (12).
FIG. 1.
Correlation between search coil and VOG HIT gains. Search coil gains are calculated based on the best estimate of gain in the 30-ms window preceding peak head velocity, whereas VOG gains are calculated based on the best gain between 30 and 60 ms after onset of head movement. Search coil and VOG gains are significantly correlated (r = 0.86; p = 0.0002), and the slope of the regression line is y = 0.84x + 0.22, where x corresponds to search coil gain and y corresponds to VOG gain.
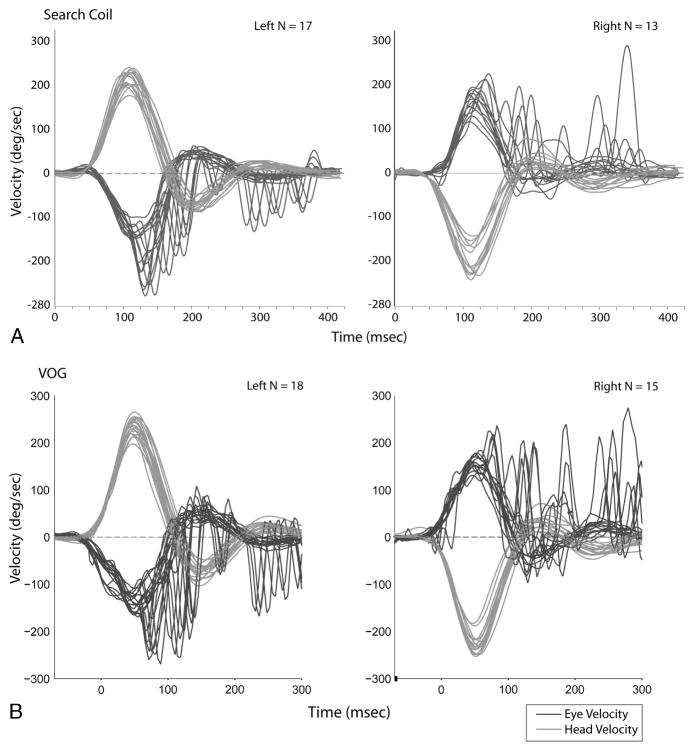
We evaluated the simultaneous search coil and head impulse trace recordings and present 2 illustrative cases (Fig. 2). The first subject had right-sided AVOR gains of 0.78 and 0.80 using search coils and VOG, respectively, and left-sided AVOR gains of 0.59 and 0.69 using search coils and VOG, respectively. She demonstrated prominent covert saccades (occurring during the head impulse) and overt saccades (occurring after the head impulse) for both right and left head impulses, which were captured in both the search coil and VOG recording systems. Although the 2 traces have similar overall profiles, there were differences. Both head and eye velocities are slightly higher in VOG data compared with the coil data, and the overshoot or counter-oscillation in eye velocity occurring after the covert saccade is greater in the VOG data. This difference is most likely due to the software filters used for the search coil signals attenuating the high-frequency signal components more so than for VOG, which would lead to a relatively lower estimated velocity from the coils. A further evaluation of the first 3 consecutive individual eye and head velocity traces in each direction in this subject showed that the shape of the head movement and eye movement traces from the 2 systems were similar (Fig. 3).
FIG. 2.
Head and eye movement traces during head impulse testing on a single individual using search coils (A) versus video-oculography (B). In all panels, head movements are in red, and eye movements are in blue. The x-axis corresponds to time in milliseconds (ms); the axes for search coil and VOG are somewhat offset, given differences in the systems regarding when the first head impulse occurs relative to time 0, that is, the offset does not represent real-time latency differences. Note the covert saccades (occurring during head movement) and overt saccades (occurring after head movement stops) present in both the search coil and VOG data for both right- and leftward head impulses.
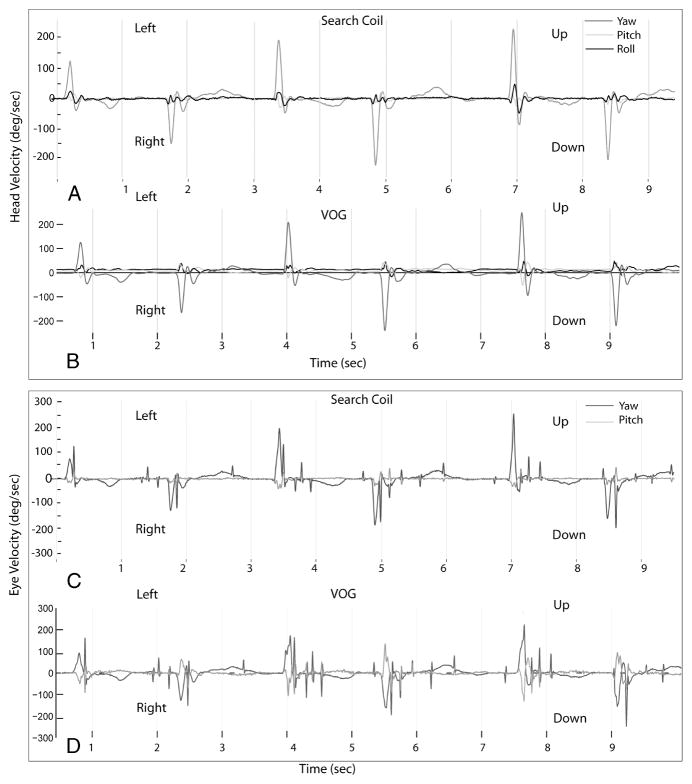
FIG. 3.
Individual head velocity traces in red (A and B) and eye velocity traces in blue (C and D) for the first 6 consecutive head impulses using the search coil (A and C) and VOG (B and D). The x-axis corresponds to time in seconds; the axes for search coil and VOG are somewhat offset, given differences in the systems regarding when the first head impulse occurs relative to time 0, that is, the offset does not represent real-time latency differences. The EyeSeeCam records head movements in 3D (yaw, pitch, roll) and eye movements in 2D (yaw and pitch); these same axes were used for search coil recording also.
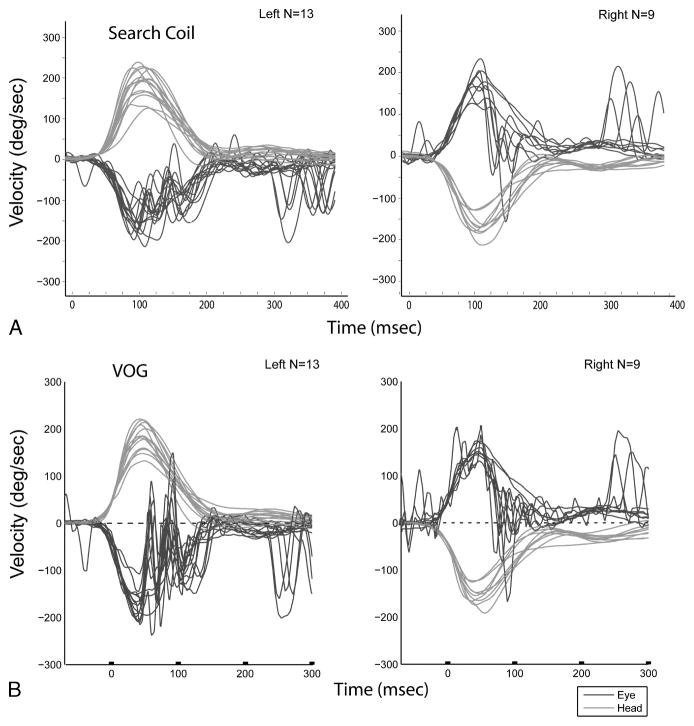
We also present search coil and VOG data from another subject who had right-sided AVOR gains of 1.19 and 1.10 using search coils and VOG, respectively, and left-sided AVOR gains of 0.92 and 0.98 using search coils and VOG, respectively (Fig. 4). The subject demonstrated fairly compensatory AVOR responses initially during the head impulse; however, at a latency of around 80 ms, significant anticompensatory eye movements were observed in both the search coil and VOG recordings. Previous studies have also noted sudden anticompensatory eye movements, or abrupt velocity declines, in older individuals (13,14). Intraclass correlation coefficients (ICCs) were calculated for the search coil and VOG methods as a measure of test-retest reliability of gain estimations. We observed an ICC of 0.896 for the search coil technique, and an ICC of 0.664 to 0.965 for the VOG technique with gains estimated at 60 and 40 ms, respectively.
FIG. 4.
Head and eye movement traces during head impulse testing on a single individual using search coils (A) versus video-oculography (B). In all panels, head movements are in red, and eye movements are in blue. The x-axis corresponds to time in milliseconds (ms); the axes for search coil and VOG are somewhat offset given differences in the systems regarding when the first head impulse occurs relative to time 0, that is, the offset does not represent real-time latency differences. Note the anticompensatory saccade present in both the search coil and VOG data for both right- and left-ward head impulses.
DISCUSSION
These data suggest that VOG is a reasonable and reliable proxy for search coil eye movement recording in older subjects to estimate VOR gain and the approximate timing of corrective eye movements. We observed significant correlations between AVOR gain values using both methods, particularly when the methods of estimating AVOR gain were comparable, although we found significant correlations between coil and VOG AVOR gain estimates at the default EyeSeeCam latencies of 40 and 60 ms as well. Moreover, specific features of eye movements seen in older individuals, including overt and covert corrective saccades and anticompensatory eye movements, were captured by both the search coil and VOG systems. VOG offers advantages over the conventional search coil method. The VOG system is portable and easy to use, allowing for quantitative VOR estimation in diverse settings such as a routine office-based practice, at the bedside, and potentially in larger scale population analyses. In contrast, the search coil technique is invasive, and involves substantial equipment that requires considerable effort and expense to develop and maintain, thereby limiting its broad use. An important limitation of the VOG system is goggle slippage owing to inertia, which could lead to an underestimate of head velocity and consequent overestimation of AVOR gain. Goggle slippage is most pronounced in the first 30 to 40 ms of the head movement; therefore, gain estimations should be made between approximately 30 and 80 ms (after which time nonvestibular mechanisms such as saccades may contribute to the eye movement response) or should be made based on median or mean gain values.
Search coils are considered the gold standard because they provide high-quality 3D data, which can be used to measure the function of all 6 canals, and can be obtained at high sampling frequencies of up to 10 kHz. The search coil technique is thus ideally suited for scientific investigations that require 3D eye and head velocity with high spatial (<0.1 degrees) and temporal (>1,000 Hz) resolution but need small numbers of mobile subjects. Quantitative analysis of fast eye movements such as saccades, for example, may be best performed with the search coil technique, although the frame rate of the EyeSeeCam can be increased to 500 Hz, which could be adequate for saccade analysis. The current VOG technique is ideally suited and can be validly applied for studies that require measurement of horizontal eye velocity with lower spatial and temporal resolution but involve larger numbers and/or less mobile subjects.
Footnotes
E. S. and N. L. have stocks in EyeSeeTec, makers of EyeSeeCam.
The remaining authors have no conflicts of interest to disclose.
References
- 1.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–9. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 2.Crane BT, Demer JL. Human horizontal vestibulo-ocular reflex initiation: effects of acceleration, target distance, and unilateral deafferentation. J Neurophysiol. 1998;80:1151–66. doi: 10.1152/jn.1998.80.3.1151. [DOI] [PubMed] [Google Scholar]
- 3.Bartl K, Lehnen N, Kohlbecher S, Schneider E. Head impulse testing using video-oculography. Ann N Y Acad Sci. 2009;1164:331–3. doi: 10.1111/j.1749-6632.2009.03850.x. [DOI] [PubMed] [Google Scholar]
- 4.MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73:1134–41. doi: 10.1212/WNL.0b013e3181bacf85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agrawal Y, Zuniga MG, Davalos-Bichara M, et al. Decline in semicircular canal and otolith function with age. Otol Neurotol. 2012;33:832–9. doi: 10.1097/MAO.0b013e3182545061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minor LB, Haslwanter T, Straumann D, Zee DS. Hyperventilation-induced nystagmus in patients with vestibular schwannoma. Neurology. 1999;53:2158–68. doi: 10.1212/wnl.53.9.2158. [DOI] [PubMed] [Google Scholar]
- 7.Straumann D, Zee DS. Three-dimensional aspects of eye movements. Curr Opin Neurol. 1995;8:69–71. doi: 10.1097/00019052-199502000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Schubert MC, Migliaccio AA, Della Santina CC. Dynamic visual acuity during passive head thrusts in canal planes. J Assoc Res Otolaryngol. 2006;7:329–38. doi: 10.1007/s10162-006-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glasauer S, von Lindeiner H, Siebold C, Buttner U. Vertical vestibular responses to head impulses are symmetric in downbeat nystagmus. Neurology. 2004;63:621–5. doi: 10.1212/01.wnl.0000135022.14937.a9. [DOI] [PubMed] [Google Scholar]
- 10.Aw ST, Haslwanter T, Halmagyi GM, Curthoys IS, Yavor RA, Todd MJ. Three-dimensional vector analysis of the human vestibuloocular reflex in response to high-acceleration head rotations. I. Responses in normal subjects. J Neurophysiol. 1996;76:4009–20. doi: 10.1152/jn.1996.76.6.4009. [DOI] [PubMed] [Google Scholar]
- 11.Weber KP, Aw ST, Todd MJ, McGarvie LA, Curthoys IS, Halmagyi GM. Horizontal head impulse test detects gentamicin vestibulotoxicity. Neurology. 2009;72:1417–24. doi: 10.1212/WNL.0b013e3181a18652. [DOI] [PubMed] [Google Scholar]
- 12.Crane BT, Demer JL. Effect of adaptation to telescopic spectacles on the initial human horizontal vestibuloocular reflex. J Neurophysiol. 2000;83:38–49. doi: 10.1152/jn.2000.83.1.38. [DOI] [PubMed] [Google Scholar]
- 13.Baloh RW, Enrietto J, Jacobson KM, Lin A. Age-related changes in vestibular function: a longitudinal study. Ann N Y Acad Sci. 2001;942:210–9. doi: 10.1111/j.1749-6632.2001.tb03747.x. [DOI] [PubMed] [Google Scholar]
- 14.Tian JR, Shubayev I, Baloh RW, Demer JL. Impairments in the initial horizontal vestibulo-ocular reflex of older humans. Exp Brain Res. 2001;137:309–22. doi: 10.1007/s002210000671. [DOI] [PubMed] [Google Scholar]