Abstract
This wide-ranging review presents an overview of the respiratory-vocal system in songbirds, which are the only other vertebrate group known to display a degree of respiratory control during song rivalling that of humans during speech; this despite the fact that the peripheral components of both the respiratory and vocal systems differ substantially in the two groups. We first provide a brief description of these peripheral components in songbirds (lungs, air sacs and respiratory muscles, vocal organ (syrinx), upper vocal tract) and then proceed to a review of the organization of central respiratory-related neurons in the spinal cord and brainstem, the latter having an organization fundamentally similar to that of the ventral respiratory group of mammals. The second half of the review describes the nature of the motor commands generated in a specialized “cortical” song control circuit and how these might engage brainstem respiratory networks to shape the temporal structure of song. We also discuss a bilaterally projecting “respiratory-thalamic” pathway that links the respiratory system to “cortical” song control nuclei. This necessary pathway for song originates in the brainstem’s primary inspiratory center and is hypothesized to play a vital role in synchronizing song motor commands both within and across hemispheres.
Keywords: song system, breathing, avian, brainstem, songbirds, vocalization, singing, neural, basal ganglia, medulla, sparse code
1 INTRODUCTION
In mammals, there is a wealth of information on the anatomical and functional organization of the central respiratory system (Bianchi and Gestreau, 2009; Feldman, 2011). Much of this information originates from the 1970s and 1980s when groups of respiratory-related neurons, now collectively called the ventral respiratory group (VRG), were identified electrophysiologically and anatomically at different locations in the ventral medulla (Feldman, 1986; Holstege and Kuypers, 1982; Merrill, 1970; Smith et al., 1989). Despite the now numerous and detailed studies of the physiological characteristics, anatomical connectedness, and functional organization of respiratory-related neurons, studies of the respiratory system more often than not have been undertaken in the absence of cortical input. While such an approach is advantageous in defining the microcircuitry underlying respiratory pattern generation, it misses the opportunity to study the respiratory system in a more integrative manner and how it might become reconfigured by higher brainstem and cortical inputs, e.g., during vocal production (Davis and Zhang, 1996; Davis et al., 1996; Holstege, 1989; Jürgens, 1994, 2002; Subramanian et al., 1994). Furthermore, a “reduced preparation” approach also misses possible opportunities to study non-respiratory roles for these circuits.
In birds, which are the most vocal group of animals other than humans and a few other primates, research into respiration and vocalization has had a history almost the reverse of that in mammals. Thus, although there have been numerous studies of avian respiration and its vagal control, these were, until the 1990s, almost entirely carried out in the periphery (Gleeson and Molony, 1989); whereas studies of the central control of vocalization began in the 1970s, when the “song system” of songbirds was discovered in canaries (Nottebohm et al., 1976) and “the Minnesota group” began the study of the neural basis of social communication in fowl (Phillips and Peek, 1975). These studies, however, were carried out in the virtual absence of any firm knowledge of the organization of a central respiratory system in birds and, in fact, the discovery of a mammalian-like distribution of respiratory-related neurons in the avian brainstem was not made until the 1990s (Reinke and Wild, 1997, 1998; Wild, 1993a, 1994b). But even these relatively late studies were carried out in the context of the control of vocal production, rather than as attempts to investigate the makeup of the avian central respiratory system. No one, to our knowledge, has or ever has had a grant to study the neural basis of the central control of avian respiration, other than in the context of vocalization! Because of the defined nature of the forebrain circuitry that innervates the respiratory hindbrain (Vicario, 1993; Wild, 1993a,b), songbirds offer a unique perspective for studying the respiratory system within the context of vocal production (Ashmore et al., 2005; Mendez et al., 2012; Sturdy et al., 2003). Moreover, with the discovery of an ascending pathway from the respiratory hindbrain to the origin of a thalamocortical projection that is part of the song system (Ashmore et al., 2005, 2008; Reinke and Wild, 1998; Striedter and Vu, 1998), songbirds offer the opportunity to study possible nonrespiratory roles for classically defined respiratory circuitry.
The following parts of this review are intended to acquaint the reader with enough basic information about the avian respiratory-vocal system (as we prefer to call it) to understand and hopefully appreciate the contributions our feathered friends have made and can continue to make to the problems of respiratory-vocal integration and coordination. We are of the firm belief that comparative studies can do nothing other than reveal, to the ultimate benefit of a common understanding, similarities and differences in modes of neural and functional organization within different classes of animal.
Readers interested in acquainting themselves with background material on avian respiration and vocalization should consult some of the excellent reviews in the 4th volume of Form and Function in Birds (King and McLelland, 1989) and the two volume work on Bird Respiration (Seller, 1987a,b). More recent accounts of the avian respiratory-vocal motor and sensorimotor system may be found in Goller and Cooper (2008), Suthers (1997), Suthers and Zollinger (2008), and Suthers et al. (1999).
2 PERIPHERAL MECHANICS OF BREATHING IN (SONG)BIRDS
2.1 LUNGS
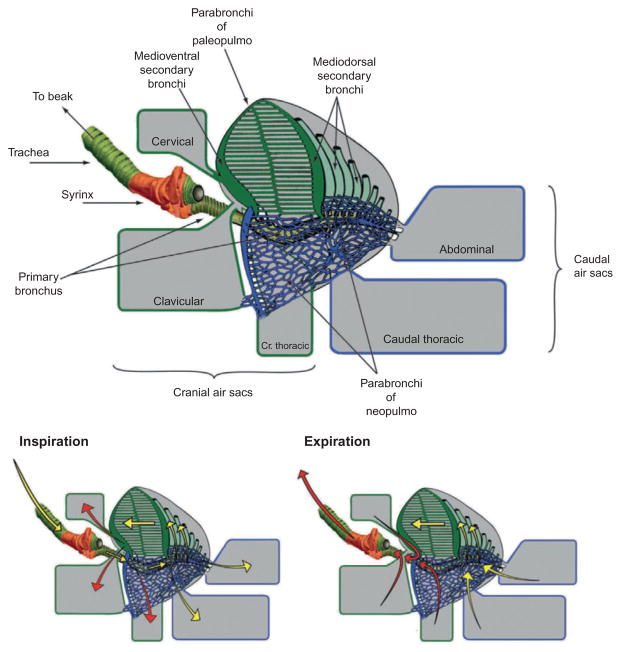
The most obvious difference between the respiratory system of birds and mammals is that, in birds, the sites of gas exchange and ventilation are separated into lungs and air sacs, respectively. In birds, the lungs are dorsally located in the thoracic cavity and deeply indented by the vertebral parts of the ribs, to which they are attached. In different groups of birds, the lungs are composed either totally of a paleopulmo (penguins) or of a paleopulmo, and a more or less elaborate neopulmo (e.g., passerines). Songbirds belong to the latter category, having an extensively elaborated neopulmo. Each lung is composed of a primary bronchus, four groups of secondary bronchi, and many parabronchi, which are the tubes (~0.5 mm diameter) through which gas exchange takes place (Fig. 1).
FIGURE 1.
Airflow in the lung and air sacs of a typical passerine bird. (Top) Anatomy of the avian respiratory apparatus. The trachea splits into the left and right primary bronchus (only the left bronchus is shown here) at the level of the syrinx (shaded in orange; grey in the print version). The primary bronchusthen splits further into secondary bronchi which split further into either theparabronchi of the paleopulmo (green; dark grey in the print version) or neopulmo (blue; dark grey in the print version). The bronchi of the lung are directly connected to a system of air sacs. According to their bronchial connections, air sacs are divided into two primary groups, cranial air sacs (cervical, clavicular, and cranial thoracic) and caudal air sacs (caudal thoracic and abdominal). (Bottom) Pattern of airflow during the respiratory cycle. During inspiration (left), oxygenated air (yellow arrows; white in the print version) flows into the caudal air sacs as well as the paleopulmo and neopulmo (not shown). Unoxygenated air (red arrow; dark grey in the print version) flows into the cranial air sacs after having passed through the lungs. During expiration (right), oxygenated air from the caudal air sacs flows into the lung, while unoxygenated air from the cranial air sacs get pushed out the trachea. This system allows for continuous flow of oxygenated air through the lungs during both inspiration and expiration. Airflow in the paleopulmo occurs in the same direction during both inspiration and expiration (large arrow). Airflow in the neopulmo is believed to be bidirectional during both respiratory phases.
This figure is adapted from Duncker (1972) and Düring et al. (2013).
Air is pumped through the paleopulmo parabronchi in the same direction, from caudal to cranial, during both inspiration and expiration, but in both directions in the neopulmo (see Scheid and Piiper, 1989). That the unidirectional flow through the lung is not likely to be the result of air sac ventilation is indicated by the fact that American alligators and monitor lizards also have unidirectional flow through their lungs, but do not have air sacs (Farmer and Sanders, 2010; Schachner et al., 2013). There is little expansion of the avian lung during breathing; if anything the lung increases in size slightly during expiration rather than inspiration, this increase having been likened to a sponge filling with water. In birds, there is no equivalent of the muscular diaphragm of mammals; thoracic and abdominal cavities are, therefore, not separated thereby. The absence of a diaphragm is presumably correlated with the absence of a phrenic nucleus, but this is not actually known.
The lungs receive an extensive innervation from vagal and sympathetic branches, via the pulmonary plexus. Respiratory afferents are present in the vagus, the pulmonary branch of which projects, via the nodose ganglion, to the lateral parasolitary nucleus (lPs) of nucleus tractus solitarius (nTS) (Katz and Karten, 1983) where it makes GABAergic synapses on postsynaptic neurons (Fortin et al., 1994). Vagal afferents are of at least two types, mechanosensory and chemosensory (see Gleeson and Molony, 1989). The former are slowly adapting responses to inflation of the respiratory system, insensitive to hypoxia or hypercapnia. They fire throughout inspiration, but the specific location of these receptors is unknown; indeed, they could lie anywhere in the thoracoabdominal cavity, including the air sacs (Kubke et al., 2004). Their contribution to the control of respiratory function is unclear. Considered much more significant in avian respiration are the vagally innervated pulmonary chemoreceptors known as intrapulmonary CO2-sensitive receptors (IPCs), which are not sensitive to airway distension, hyperoxia, or hypoxia. A great deal of work has been devoted to these receptors (reviewed in Gleeson and Molony, 1989), although, like the mechanoreceptors, they have not been identified anatomically, despite their physiologically recorded presence in the parabronchi of the paleopulmo. They are very sensitive to both static and dynamic changes in PCO2 and increase their discharge frequency as the PCO2 of intrapulmonary gas is decreased. However, some receptors have a peak discharge frequency during inspiration, some during expiration and some during both phases of the respiratory cycle, presumably correlated with the time during the respiratory cycle at which the receptor is exposed to fresh air. In general, IPCs inhibit inspiration, but with respect to respiratory timing, they do not seem to act like mammalian stretch receptors (i.e., are not the equivalent of a Hering-Breuer stretch receptor), since their increased activity inhibits inspiratory or expiratory flow generated by breathing movements. It has been proposed, therefore, that they act in a negative feedback manner to control the flow of air through the lungs on a moment by moment basis (Gleeson and Molony, 1989).
2.2 AIR SACS AND RESPIRATORY MUSCLES
The air sacs (through which no significant gas exchange takes place) together with the respiratory muscles form the bellows for ventilating the lungs. Lungs are attached to air sacs by many ostia, these attachments being either direct, i.e., air sac attached to a primary or secondary bronchus, or indirect, i.e., air sac attached to one or many parabronchi. The air sacs are thin-walled, transparent, inflatable membranes enclosing anatomically complex spaces throughout the cervical, and thoracoabdominal compartments. Air sac diverticulae may extend into the bones. Air sacs are divided into two groups: a cranial group consisting of the cervical, clavicular (aka interclavicular) and cranial thoracic sacs, and a caudal group consisting of the caudal thoracic and abdominal sacs (Fig. 1). Together, they make up about 20% of the body’s volume. All air sacs are paired structures, except for the clavicular sac, which surrounds the syrinx and thus allows the interchange of air between the interconnected air sacs on each side of the body. At rest pressure, differences between the different air sacs are therefore small. If the clavicular sac is punctured, the bird can no longer vocalize, which has been thought to imply that a pressure balance within and without the syrinx is necessary for vocal production. During singing, abdominal muscles (which are the primary determinant of air sac pressure) do not show EMG amplitude difference on the right versus the left side despite changes in resistance on the two sides of the syrinx, the bird’s vocal organ (Goller and Suthers, 1999). Nevertheless, pressure differences between anterior and posterior sacs are possible during vocalization. During cooing in doves, for instance, a significant pressure difference has been recorded between the clavicular and caudal thoracic sacs, which could imply a measure of independent control of pressure in different air sacs, with possible implications for vocalization (Beckers et al., 2003; see also Mackleprang and Goller, 2013).
An important difference between the ventilation of birds and mammals is that, in birds, both inspiration and expiration are active processes, even during quiet breathing (Fedde et al., 1964). Expansion and contraction of the thoracoabdominal cavity is effected by the respiratory muscles, assisted initially by elastic forces (Scheid and Piiper, 1989). The attachments, composition, biochemical characteristics, actions, and innervation of these muscles have been described and summarized by Fedde (1987). The abdominal and most of the internal intercostal muscles are expiratory, while the scalenes, costal levators and most of the external intercostal muscles are inspiratory. A complete list for the chicken is provided by Fedde (1987). In lightly anesthetized chickens, Fedde et al. (1964) found that the activity of inspiratory and expiratory muscles overlapped into the succeeding respiratory phase, thereby bringing about a smooth transition between the two phases. In contrast, during singing in zebra finches, Wild et al. (1998) found no overlap of inspiratory and expiratory muscle activity. Whether the activity of expiratory-related and inspiratory-related premotor neurons in the brainstem overlap each other to any extent is not known, since they have not been recorded simultaneously (McLean et al., 2013; Reinke and Wild, 1997, 1998; Wild, 1994b).
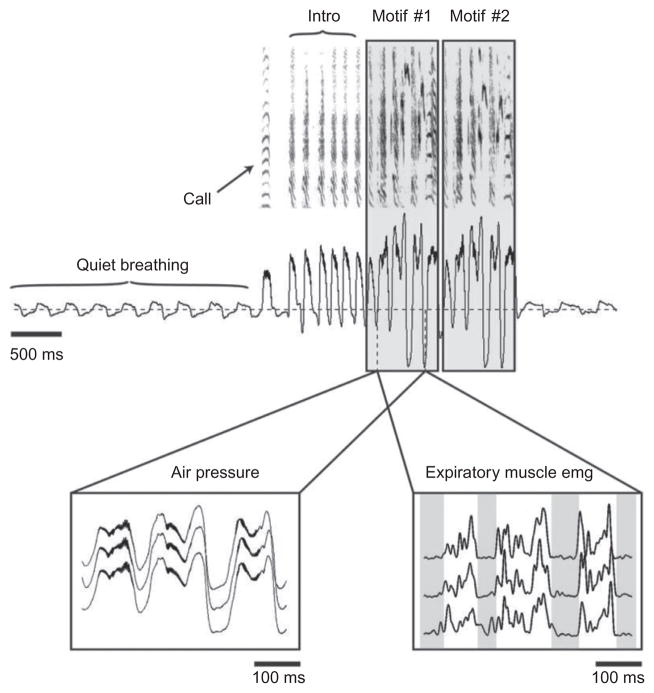
Most but not all vocalizations occur during expiration (Beckers et al., 2003; Gaunt et al., 1982; Goller and Daley, 2001; Leadbeater et al., 2005); most song syllables in bird song are in the range of tens of milliseconds duration; and most syllables, even part syllables, e.g., in canary song, are accompanied by a separate expiratory pulse (EP) (pulsatile expiration; Hartley and Suthers, 1990). When the syllable repetition rate exceeds the ability of the expiratory muscles to follow, such as in fast trilling in canaries or cardinals, then many syllables may be produced during the positive air sac pressure of a single extended expiration (Hartley and Suthers, 1990; Suthers and Zollinger, 2004). Between and within songbird species the duration of EPs varies significantly for different syllables, but, within individual birds, it is remarkably similar for production of the same syllable. Furthermore, EPs may be dynamically modulated to produce acoustic effects characteristic for the species. This is especially apparent in songbirds such as the zebra finch whose song is highly stereotyped (Franz and Goller, 2002), where the precise pattern of air sac pressure modulation can be observed even when syringeal resistance is controlled for by pinning the lateral labia, suggesting that such modulation arises in large part from modulation of the expiratory drive by the respiratory system (Goller and Cooper, 2004, 2008; Fig. 2).
FIGURE 2.
Modulation of the respiratory pattern and underlying expiratory musculature during singing in songbirds. Amplitude and temporal pattern of respiratory pressure (horizontal line indicates ambient pressure) during vocalization (illustrated as spectrogram in top panel) change markedly from that during normal, silent respiration. A typical zebra finch song bout (shown here) typically consists of one or more motifs preceded by a variable number of introductory notes (intro), with each motif being made up of a stereotyped sequence of syllables. In this example, the song bout contains two motifs and is also preceded by a short distance call (call). Each of the song syllables is associated with a highly stereotyped expiratory pressure pulse (left inset) and expiratory muscle activity (right inset). (Left inset) Air sac pressure measurement for three consecutive syllables measured from three different motifs. (Right inset) EMG recordings from the abdominal expiratory muscles from the same three syllable sequences.
This figure was generated based on recordings kindly provided by F. Goller.
Between song syllables or phrases, songbirds usually take a minibreath of about 30 ms duration, effected by inspiratory muscles (Hartley, 1990; Wild et al., 1998). The volume of air inspired in these minibreaths is sufficient to replace the air lost during the preceding syllable, thereby preventing the bird from running out of air during the song (Hartley and Suthers, 1989). Minibreaths are abandoned, however, during high syllable repetition rates such as in trilling. Also during singing, as compared with quiet breathing, there is a dramatic increase in air sac pressure, up to 50 times in brown thrashers, for instance.
Higher levels of air sac pressure are generally accompanied by increased amplitude of abdominal muscle EMG and intensity of sound. When air sac pressure is experimentally increased during singing, e.g., by a short pulse of air injected into the cranial thoracic air sac, there is a compensatory decrease in the amplitude of the EMG of abdominal muscles throughout the remainder of the sung syllable (Suthers et al., 2002). But when air sac volume, and therefore the volume of air, is experimentally reduced by injections of inert dental medium into the air sacs, breathing frequency increases during quiet respiration. However, during singing, although there is a reduction in pressure, flow, and sound amplitude, there is no compensatory increase in EMG activity (Plummer and Goller, 2008). Together, these two experiments could suggest some sort of stretch or pressure receptor in expiratory muscles or other structures in the thoracoabdominal cavity that respond to expansion of the air sacs and bring about a reflex reduction of expiratory effort. Presumably, when air sac volume is reduced, such receptors do not fire and no reduction EMG amplitude occurs. However, in the only published study of a respiratory muscle in birds (DeWet et al., 1971), very few muscle spindles were found in m. transversus abdominis of chickens. Neuroepithelial body-like receptors have been identified in the air sacs of zebra finches, but their adequate stimuli are unknown (Kubke et al., 2004).
2.3 SYRINX
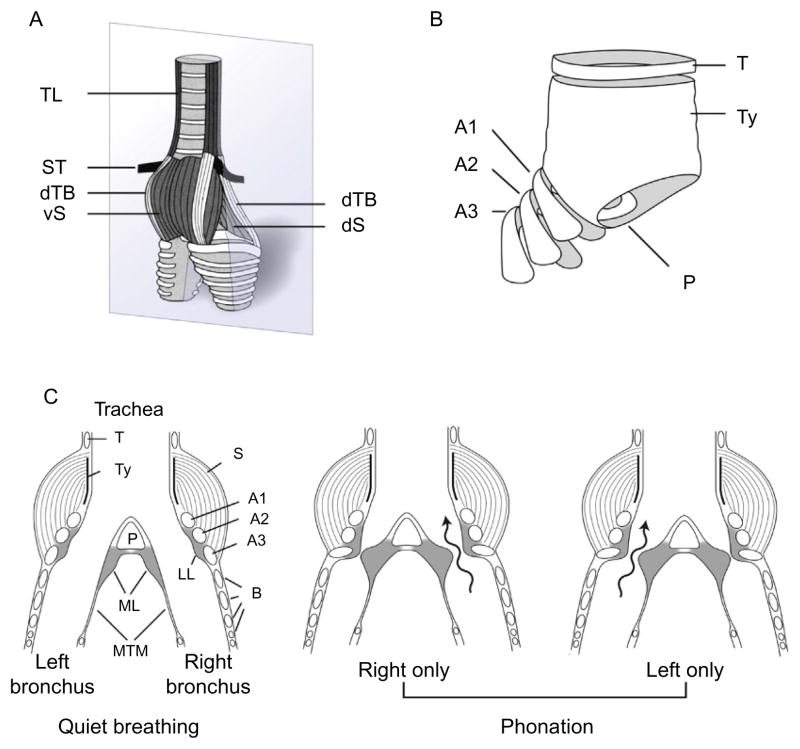
The syrinx, rather than the larynx, is the bird’s vocal organ (Düring et al., 2013). It is a mechano-muscular valve situated deep in the thoracic cavity at the junction of trachea and primary bronchi (Figs. 1 and 3). Like the mammalian larynx, it modulates pressure in the airways caudal to it and regulates air flow available to the upper respiratory-vocal tract during normal respiration and during song (Brackenbury, 1980). Because of its position at the caudal end of the trachea, however, it does not provide protection of the airways such as the larynx provides. This can be a potentially life-threatening situation for birds, such as parrots, that develop aspergillus, a plug of which can fall down the trachea and block the airway. Depending on the species, the avian syrinx may have only two pairs of extrinsic muscles, as in non-songbirds (e.g., ducks, pigeons), or two pairs of extrinsic muscles and four to six pairs of intrinsic muscles, as in songbirds (Düring et al., 2013). Unlike the mammalian larynx, however, the syrinx of songbirds is bipartite, with a separate sound source on each side that can be controlled independently by the brain and the innervation supplied unilaterally to the paired syringeal muscles by descending branches of the hypoglossal nerve (NXIIts).
FIGURE 3.
Anatomy of the songbird syrinx and phonatory mechanism. (A) Ventrolateral external view of a thrasher syrinx depicting syringeal muscles. (B) Cartilage components of the syrinx include three tracheo-bronchial semi-rings (A1–A3) as well as the tympanum (Ty), which contains at its caudal end the pessulus (P), an important attachment site for the medial labium (ML). (C) Schematic ventral view of the songbird syrinx in quiet respiratory (left panel) and phonatory (center and right panels) configurations. During vocalization, the medial (ML) and lateral labia (LL) are set into vibration when they are adducted into the expiratory air stream. In preparation for phonation, the syrinx moves rostral. Contraction of the ipsilateral dorsal syringeal muscles (dS and dTB) rotates the bronchial cartilages into the syringeal lumen, moving the lateral and medial labia into the expiratory air stream where they are set into vibration to produce sound. Phonation may be bilateral (not shown) or unilateral. Unilateral phonation is achieved by closing one side of the syrinx through full adduction of the labium on that side, so that sound (wavy arrows) is only generated on the partially open contralateral right (centre panel) or left (right panel) side. Abbreviations: B, bronchus; ICM, membrane of the interclavicular air sac; T, trachea; M, syringeal muscle; ML, medial labium; LL, lateral labium; MTM, medial tympaniform membrane; P, pessulus; TL, m. tracheolateralis; ST, m. sternotrachealis; vS, m. syringealis ventralis; vTB, m. tracheobronchialis ventralis; dTB, m. tracheobronchialis dorsalis; dS, m. syringealis dorsalis; T, tracheal cartilage; Ty, A1–A3, B, bronchial cartilages; P, pessulus.
(A) and (B) Modified from Riede and Goller (2010). Figure legend is modified from Suthers et al. (1999).
Various theories of syringeal function were common until recently. Phonation was usually thought to be the result of vibration of the medial tympaniform membranes (MTM, see Fig. 3), but the work of Goller and Larsen (1997b) has shown that the medial and lateral labia vibrate when they are adducted into the expiratory air stream, thus resembling the apposing actions and vibration of the laryngeal vocal folds in humans; the important difference being that the songbird can do this on each side of the syrinx independently. In fact, some songbirds are known to sing two completely harmonically unrelated notes simultaneously, one on each side (the two-voice theory of song production). The functions of the syrinx during song are complex and it is impossible to do justice to its abilities here. It is to Rod Suthers, Franz Goller and their colleagues that we owe our current conception of the workings of songbird syrinx during singing (Goller and Larsen, 1997a,b; Goller and Suthers, 1996a,b; Larsen and Goller, 2002; Suthers, 1990; Suthers et al., 1999). Advances have been due to the ability to measure subsyringeal pressure and airflow in the bronchus of each side, combined with electromyographic activity of individual muscles of the syrinx, during singing in several species of songbird (e.g., brown thrasher, northern cardinal, cowbird, Australian magpie).
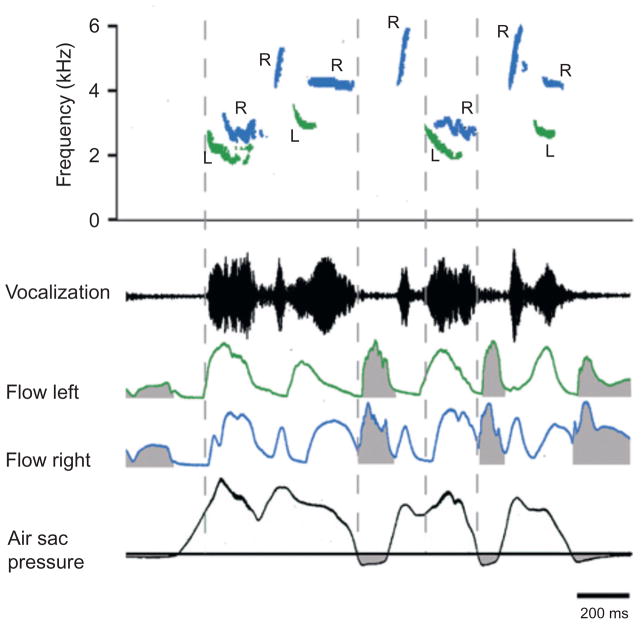
Endoscopic observation of the effects of direct electrical stimulation of syringeal muscles in anesthetized songbirds has provided a greater level of understanding regarding the details of syringeal function. Figure 3 shows the anatomy and proposed phonatory function of the tracheobronchial-type syrinx of a brown thrasher. The four pairs of intrinsic muscles are arranged on either the ventral or dorsal sides of the syrinx. Ventral muscles are generally concerned with the control of fundamental frequency, although one also brings about abduction of the lateral labia. Dorsal muscle activation brings about at least partial labial adduction, thereby reducing glottal aperture, apposing the labia and generating sound on one or both sides. Some species of songbird use one side of the syrinx more than the other for sound production (i.e., are peripherally lateralized for song production, e.g., canaries) but each of the few species thus far measured switch back and forth between sides during song and some even during the production of single syllables (Fig. 4). Cardinals, for instance, use the right side for the higher parts of a frequency modulated sweep and the left for the lower frequency parts, with some overlap in the middle of the sweep when both sides of the syrinx are used. Curiously, no muscle spindles have been found in songbird syringeal muscles, although some afferents are present in the tracheosyringeal nerve (Bottjer and Arnold, 1982). These project centrally via an anastomosis with the vagus to the lateral parasolitary subnucleus of nTS (Wild, 2004a), to the spinal trigeminal nucleus and possibly also to the principal trigeminal nucleus. Peripherally, they may innervate some form of airway receptor.
FIGURE 4.
Example of a song from the brown thrasher illustrating the switching between left and right syrinx showing syllables with two-voice and single-voice components. Contributions from the right syrinx are shown in blue; not distinguishable in print, those from the left syrinx in green, and inspiratory minibreaths are shaded in grey. Airflow measurements are measured by placing a thermistor probe in the primary bronchus.
This figure is modified from figure 4 in Suthers et al. (1994).
During the expiratory phase of quiet breathing when songbirds are not singing, syringeal muscles abduct the labia and tympaniform membranes (Gaunt and Gaunt, 1977; Goller and Suthers, 1996a,b), otherwise the bird wheezes with every expiration and, in fact, this is exactly what happens if the ventral syringeal muscles are individually denervated or if the syringeal nerves are cut (Riede et al., 2010; Vicario, 1991b). Consistent with these observations, motoneurons in the tracheosyringeal component of the twelfth cranial nerve nucleus (XIIts) in the medulla, which innervates the syrinx, have a normal expiratory-related rhythmical discharge. However, no inhibition has been observed concomitant with the end of expiration (Sturdy et al., 2003), despite the fact that the inspiratory-related premotor nucleus parambigualis (PAm) in the more rostral medulla sends projections to nXIIts, as well as to spinal motoneurons innervating inspiratory muscles (Reinke and Wild, 1998).
2.4 UPPER VOCAL TRACT
There has been a long debate about whether suprasyringeal components of the vocal tract can modify sound generated by the syrinx. The current consensus view is that it can and does, but what mechanisms are involved is still under active experimentation. The long trachea of birds could, in theory, modify filter properties by changing its length during singing, but this possibility has been experimentally ruled out, at least for zebra finches (Daley and Goller, 2004). At the upper end of the trachea the larynx might also be assumed to be involved in vocalization, but the evidence for this is indirect: apparent projections from the song system onto the dendrites of laryngeal motoneurons (Wild, 1993b). During normal quiet breathing the glottis can be observed to widen during inspiration and constrict slightly during expiration, to limit the amount of air lost at the end of inspiration (White and Chubb, 1967; J.M. Wild unpublished observations in pigeons), perhaps consistent with the post-inspiratory phase of breathing in mammals. As an extension of the trachea, as it were, the beak could also influence filter properties of the upper vocal tract by opening or closing during song production, thereby changing the length of the upper vocal tract. There is good evidence that beak aperture is correlated with sound amplitude and to some extent with sound frequency, with larger gapes being correlated with more intense sound and higher notes (Westneat et al., 1993), but the relationship between gape size and sound frequency is non-linear and complicated. Beak gape, and even tongue and laryngeal movements, may be more related to the control of the size and shape of the oropharyngeal-esophageal cavity, the volume of which in cardinals has been shown to be inversely related to sound frequency (Goller and Cooper, 2008; Riede et al., 2006, 2013; Suthers and Zollinger, 2008). The close relationship of the neural control of upper vocal tract structures to that of the syrinx and expiration has been studied anatomically by Wild and Krützfeldt (2012).
2.5 CHEMORECEPTORS
The carotid body in birds seems to play a similar role as that in mammals, with increases in PO2 in arterial blood leading to a reduction in the discharge of vagal fibers and then a decrease in the minute volume of ventilation. The reader is referred to Gleeson and Molony (1989) for further details.
3 CENTRAL ORGANIZATION OF RESPIRATORY-RELATED NEURONS
3.1 RESPIRATORY MUSCLE MOTONEURONS
The location and dendritic morphology of some respiratory muscle motoneurons have been described for pigeons, a songbird (zebra finch) and a parrot (budgerigar) (Reinke and Wild, 1997, 1998; Wild, 1994b). Motoneurons innervating the inspiratory muscles M. scalenus caudalis, M. levatores costarum, and Mm. intercostalis externi extend from the lowest brachial level, where they are located at the ventral edge of the medial motor column, throughout upper thoracic levels, where they are located at the tip of the ventral horn, there being no lateral motor column present at these levels. Motoneurons innervating abdominal expiratory muscles extend from lower thoracic levels into upper lumbosacral levels and are located at the tip of the ventral horn, as for inspiratory muscle motoneurons. All these motoneuron somata have a similar morphology and two sets of dendrites. One set extends medially as far as the midline, some axons appearing to terminate in the gray matter near the central canal and some in the white matter of the ventral funiculus. Another set extends dorsolaterally, mainly into the lateral funiculus. These latter motoneurons are targeted by axons descending from the brainstem nucleus retroambigualis (RAm), which travel at the edge of the lateral funiculus until they reach the appropriate spinal level. These axons then turn ventromedially to “run down” the dorsolaterally extending dendrites, on which they appear to terminate en passant and then on the motoneuron cell bodies. The medially extending dendrites appear to receive fewer RAm axons, which cross diagonally through the gray matter towards their targets, but whether they receive other respiratory premotor inputs is unknown.
3.2 ORGANIZATION OF RESPIRATORY-RELATED NEURONS IN THE BRAINSTEM
The first demonstration of respiratory premotor neurons in birds was made in pigeons and songbirds (Wild, 1993a). Rhythmical, expiratory-related (ER) discharges of single units were recorded ventrolaterally in the caudal medulla and uppermost spinal cord of birds anesthetized with ketamine and xylazine. NMDA receptors were, therefore, blocked, resulting in extended expiratory periods (Wild, 1994b). The location of ER sites was very similar to a nucleus, subsequently named “retroambigualis” (RAm), whose neurons were retrogradely labeled from injections of tracer into the lower thoracic spinal cord. This nucleus extends diagonally throughout the dorsal central medulla, from the tracheosyringeal motor nucleus (CN XIIts) medially almost to the ventrolateral periphery of the brainstem. Rostrally, it does not extend as far as the obex. Injections of tracers into RAm anterogradely label fibers and terminations in proximity to motoneurons retrogradely labeled from abdominal expiratory muscles in the lower thoracic and upper lumbar spinal cord ventral horn (Wild, 1993a, 1994b).
Systematic searches of respiratory-related neurons throughout the brainstem of pigeons, songbirds and budgerigars (Reinke and Wild, 1997, 1998) revealed inspiratory-related (IR) discharges of single units in similar mediolateral locations to those in RAm, but more rostrally, from caudal to the obex through levels rostral to the obex. In other words, IR units formed a rostral extension of ER units throughout the ventrolateral medulla, straddling the obex. Since the IR units were located in proximity to motoneurons innervating the larynx, they were collectively called nucleus Parambigualis (PAm). Note that the larynx in birds is innervated solely by glossopharyngeal nerve, not by the vagus, as in mammals. Injections of tracers at IR sites anterogradely labeled fibers and terminations in proximity to motoneurons in the lower brachial and upper thoracic spinal cord that were retrogradely labeled from inspiratory muscles. Injections of tracers into the spinal ventral horn at these levels retrogradely labeled neurons throughout PAm, as well as in RAm via fibers of passage. The physiology of neurons in and adjacent to PAm is discussed in more detail below.
Rostral to the level of the rostral pole of the hypoglossal nucleus and extending to the level of the cochlear nuclei—i.e., rostral to PAm—ER units were again encountered, but these were confined ventrolaterally in the brainstem to a round nucleus (in cross section) that was named for its position as RVL (rostroventrolateral medulla).
As noted by Reinke and Wild (1997, 1998), the distribution of respiratory premotor neurons in birds closely resembles that of the VRG in mammals and appears to be similarly functionally organized, at least at a basic level (Fig. 5). It has been specifically suggested that RAm is directly comparable to nucleus retroambiguus (NRA; also named the caudal VRG (cVRG) in the mammalian literature (Ellenberger and Feldman, 1990; Holstege and Kuypers, 1982; Merrill, 1970)), even though its mediolateral extent is greater than that of NRA (Wild, 1993a, 1994b). It should be noted, however, that, like NRA, RAm is not just a respiratory premotor nucleus, but rather is functionally diverse. For instance, like NRA (VanderHorst and Holstege, 1997), it has premotor neurons that project upon motoneurons at lumbosacral spinal levels that innervate muscles involved in reproductive behavior (Wild and Balthazart, 2013).
FIGURE 5.
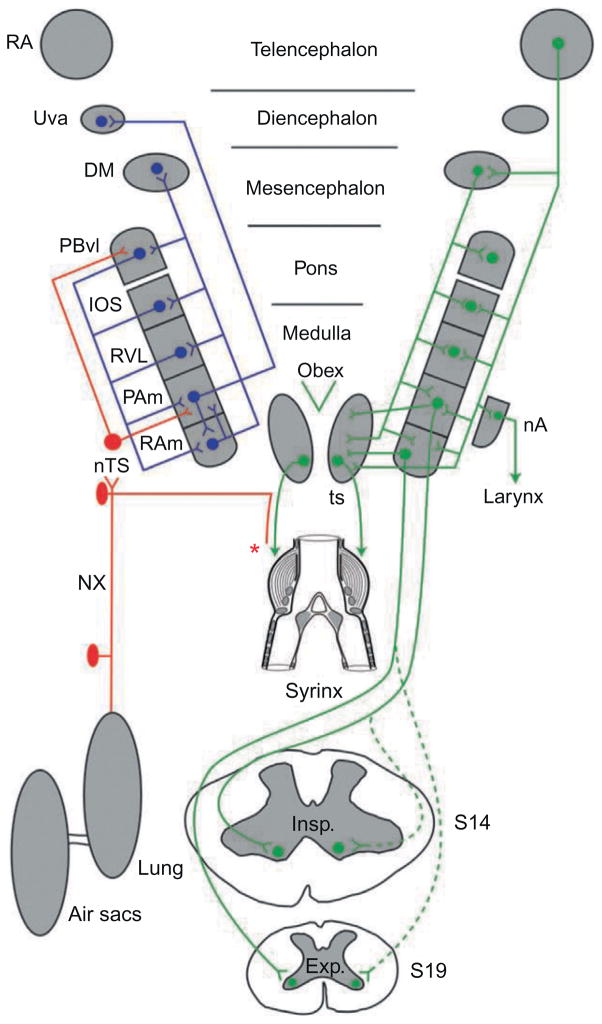
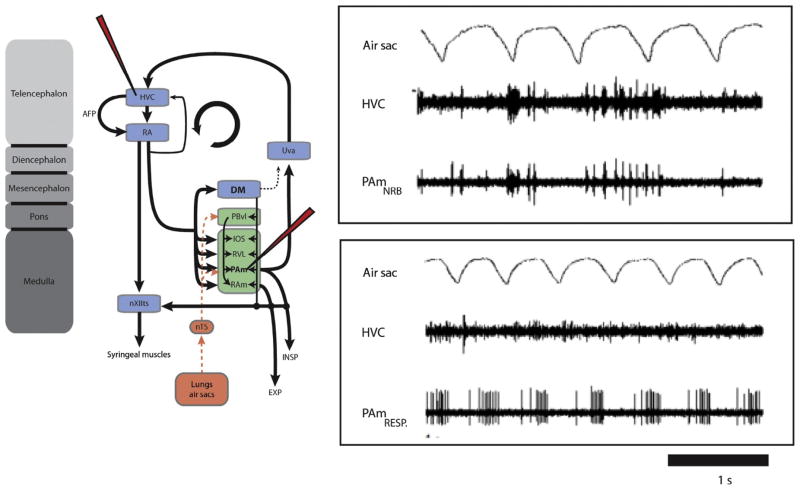
Nuclei and some of the interconnections of the respiratory-vocal system in a songbird. Abbreviations from top down: RA, robust nucleus of the arcopallium; Uva, nucleus uvaeformis; DM, dorsomedial nucleus of the intercollicular complex; PBvl, ventrolateral part of the parabrachial nucleus; IOS, nucleus infra-olivarus superior; RVL, ventrolateral nucleus of the rostral medulla; PAm, nucleus parambigualis; nTS, nucleus of the solitary tract; nA, nucleus ambiguus; RAm, nucleus retroambigualis; XIIts, tracheosyringeal part of the hypoglossal nucleus; ts, tracheosyringeal nerve; NX, cranial nerve X (vagus); Insp, inspiratory motoneurons at the level of spinal cord segment 14 (lower brachial); Exp, expiratory motoneurons at the level of spinal cord segment 19 (lower thoracic). Expiratory and inspiratory motoneurons receive predominantly contralateral projections from RAm and PAm, respectively—indicated by solid lines. Dots represent cell bodies and inverted arrowheads terminations. The two sides are symmetrical, of course, but for clarity the projections of RA and DM to all the nuclei of the ventrolateral pons and medulla, as well as to XIIts, are shown on the right, and the cascade of descending projections is shown on the left, as are the ascending recurrent pathways from RAm and Pam, these being predominantly ipsilateral. Note the sensory input from the lungs, air sacs and syrinx that reaches nTS, and hence PAm and Uva. The specific origin/type of syringeal afferents (illustrated by the asterisk) is presently unknown.
Figure modified from Wild (2008).
3.3 RESPIRATORY-VOCAL CIRCUITRY
A thoroughgoing account of vocalization in birds, as in mammals, requires a complete account of the neurochemical events characteristic of the sequences of expiration and inspiration attendant on calling in non-songbirds or singing in songbirds. Such an account is not yet possible at this time, but some basic facts are known. The basic wiring diagram of the respiratory-vocal system is summarily depicted in Fig. 5.
Vagal afferents from the lungs mediating information from intrapulmonary CO2 receptors (IPCs) make GABAergic synapses on postsynaptic neurons in the lateral parasolitary nucleus (lPs) of nTS (Fortin et al., 1994; Katz and Karten, 1983; Wild, 2008). lPs then projects upon PAm and, more rostrally, upon the ventrolateral parabrachial nucleus (PBvl), a suggested homologue of the Kölliker-Fuse nucleus of mammals (Wild and Arends, 1987; Wild et al., 1990). In turn, PBvl provides a dense terminal field to the vocal motor nucleus (XIIts) and to PAm and RAm (Reinke and Wild, 1998; Wild et al., 2009). The physiology of PBvl neurons has not been explored in relation to either quiet breathing or vocalization and a role for PBvl in singing has not been established. In songbirds, as for the Kölliker-Fuse nucleus in mammals (Dutschman and Herbert, 2006; Dutschman et al., 2009), it may be involved in the control of respiratory phase and perhaps with the control of minibreaths.
Because most vocalizations are emitted during expiration, RAm is the key nucleus. It is composed of several, separate groups of neurons having different targets: (1) bulbospinal neurons that target expiratory motoneurons in the thoracic and upper lumbar spinal cord, predominantly contralaterally; (2) bulbospinal neurons that target cloacal motoneurons in the sacral spinal cord; (3) premotor neurons that target XIIts; (4) a group with unidentified targets that project their axons into the vagus nerve; (5) neurons with predominantly ipsilateral ascending projections to other nuclei of the respiratory-vocal system, including PAm, RVL, nucleus infra-olivarus superior (IOS, which may be similar to the mammalian retrofacial nucleus), PBvl, and the dorsomedial nucleus (DM) of the intercollicular complex (ICo) (Kubke et al., 2005; Wild, 1994b; Wild et al., 1993, 2009). RAm on one side projects strongly to RAm on the other side. The XIIts-projecting group of RAm neurons is divided into excitatory and inhibitory subgroups, the latter being glycinergic. XIIts motoneurons have an expiratory-related discharge during quiet breathing, which seems likely to be driven by RAm. However, since XIIts motoneurons do not appear to be inhibited during inspiration, the glycinergic input to RAm may be more related to shaping XIIts neuron discharge during singing (Sturdy et al., 2003). Putative interconnections and interactions between the different RAm-neuron types have not been defined, but those between the bulbospinal and XIIts-projecting neurons are suspected of mediating respiratory-vocal integration and coordination (Sturdy et al., 2003).
DM is likely equivalent to lateral and possibly dorsolateral parts of the periaqueductal gray (PAG) of mammals (Holstege, 1989; Kingsbury et al., 2012; Wild and Balthazart, 2013) and, when electrically stimulated, will drive vocalizations accompanied by appropriate respiratory patterning in both songbirds and non-songbirds (Brown, 1965; Potash, 1970; Wild, 1997; Wild and Balthazart, 2013). DM is present in all birds, but since non-songbirds do not learn their vocalizations as songbirds do, they do not have forebrain song control nuclei, making DM in these species (e.g., ducks, chicken, pigeons, etc.) the highest respiratory-vocal nucleus in the neuraxis.
3.4 PHYSIOLOGICAL PROPERTIES OF HINDBRAIN RESPIRATORY NEURONS IN SONGBIRDS
The original description of respiratory areas in the avian ventrolateral medulla used extracellular neural recordings to confirm that firing patterns occurred during the appropriate portion of the respiratory cycle, in combination with connectional studies of bulbospinal projections (Wild, 1993a). However, an exhaustive characterization of activation patterns relative to the breathing cycle has not been performed until recently (McLean et al., 2013). Given the overall similarities between the anatomical organization of the mammalian and avian respiratory system, such a characterization is likely to further define the similarities and differences between birds and mammals.
In mammals, the respiratory hindbrain contains a column of respiratory-related neurons in the ventrolateral medulla that is collectively known as the ventral respiratory column (VRC) (Feldman, 1986; Smith et al., 2009), although it is now known that neurons in this region are also involved in several other motor activities (Holstege, 2014). This column extends all the way from the caudalmost portion of the facial nucleus (VII) to the spinomedullary junction and contains three areas that are fundamental to the generation and shaping of the respiratory rhythm. These areas, known as the Bötzinger complex (BötC), the preBötzinger complex (preBötC), and rostral ventral respiratory group (rVRG), lie adjacent to each other in rostrocaudal order. In addition to their anatomical location and projection pattern, each of these three respiratory areas can be characterized by the firing pattern of its neurons relative to the breathing cycle.
Of the six general classes of cells observed in the respiratory brainstem (Bianchi et al., 1995; Richter, 1982), the BötC contains primarily neurons that are active during the expiratory phase of breathing, either in the early phase of expiration (Post-I neurons) or in the later phase of expiration (E-augment neurons). In contrast to BötC, the preBötC, whose neurons are under strong control of the PAG (Subramanian and Holstege, 2013), has been identified as the primary pacemaker for driving rhythmic inspiratory activity (Smith et al., 1991). The preBötC contains primarily neurons that are active during the inspiratory phase, either early during inspiration (Early-I neurons) or immediately prior to and during the inspiratory phase (pre-I/I neurons). The rVRG, which typically contains inspiratory neurons that project down the spinal cord, tends to contain mostly neurons whose firing activity increases throughout the inspiratory phase. These neurons are known as I-ramp or I-augment neurons (Bianchi et al., 1995; Feldman et al., 1985; Guyenet et al., 2002).
Recording from the area in the ventrolateral medulla of zebra finches previously defined as PAm, McLean et al. (2013) recorded discharge patterns that were strikingly similar to those reported in the mammalian inspiratory areas, pre-BötC and rVRG (Fig. 6). The majority of neurons recorded in PAm were either directly inspiratory (48%) or occurred immediately prior to inspiration (19%). While the primary class of neuron was clearly inspiratory related, other cell classes, but in much smaller number, could also be found in this area. This observation is actually consistent with what has been observed in mammals, where areas that are primarily inspiratory also contain neurons whose discharge pattern occurs during the expiratory phase (Connelly et al., 1992).
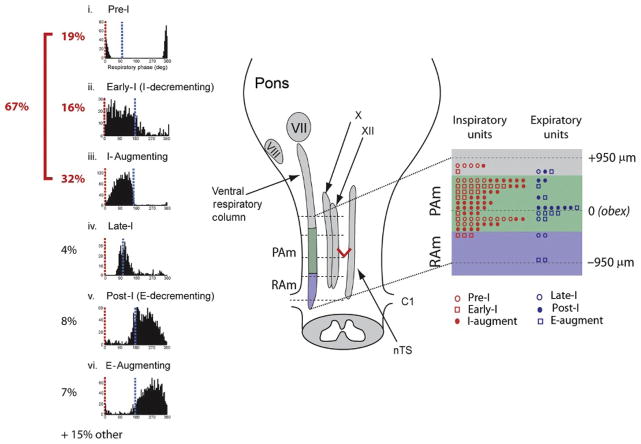
FIGURE 6.
Summary of cell types and their spatial localization in PAm. The left most panel shows the phase histograms of PAm respiratory-related neurons aligned vertically. Respiratory cycles are aligned to the onset of the inspiratory phase (red; 0° (light grey in the print version)) to show the respective contribution of different cell types throughout the respiratory cycle. The time of occurrence of the transition from inspiration to expiration (blue; grey in the print version) could vary within the respiratory cycle. (i) Pre-I phase histogram. (ii) Early-I phase histogram. (iii) I-Augment phase histogram. (iv) Late-I phase histogram. (v) Post-I phase histogram. (vi) E-Augment phase histogram. The schematic on the right illustrates the ventral respiratory column and other brainstem nuclei together with the rostrocaudal distribution of recorded respiratory-related cells with respect to the obex. The rostrocaudal location and cell type is schematically represented in an enlargement of the portion of the VRC spanning the obex (red V) (dark grey in print) on the right. VII, facial nucleus; VIII, vestibulocochlear nuclei; X, nucleus ambiguus; XII, hypoglossal nucleus; nTS, nucleus of the Tractus solitarius.
Modified from figure 7 in Mclean et al. (2013).
Of interest to the possible homologies between avian and mammalian respiratory circuits, about a third (32%) of the neurons in PAm were classified as I-augment (also known as ramp-I), which is one of the defining cells of rVRG. These cells project, like rVRG cells, to spinal motoneurons controlling inspiration (Reinke and Wild, 1997, 1998). Interestingly, PAm also contained a significant number of cells that could be characterized as either Early-I (I Decrementing; 16%; see also Reinke and Wild, 1997) or pre-I (19%), cell types that are characteristic of the preBötC, which is typically associated with inspiratory drive. Although there was no obvious topographic distribution of cell classes in PAm, early-I neurons were more likely to be recorded in the more rostral part of PAm, suggesting that it might be the avian equivalent of preBötC. Further support for this suggestion is evidenced by the fact that both PAm and the preBötC send projections to the hypoglossal nucleus, whereas the rVRG does not (Borgmann et al., 2011). As an extension of this homology, it may be that RVL, which lies directly rostral to PAm, is equivalent to the mammalian BötC rather than the preBötC, as previously suspected (Reinke and Wild, 1997). While a rigorous characterization of RVL neurons has not been performed, preliminary results suggest that they are primarily expiratory related (see Section 3.2).
In addition to the classically defined respiratory neurons, PAm also contained a population of neurons the activity of which was not locked to the respiratory rhythm but was instead correlated with activity in “cortical” song control nuclei, which make up the so-called “song system.” This specialized circuit is described in more detail in the next section and provides both drive to the vocal-respiratory system during song and, somewhat paradoxically, receives input from the respiratory system via a specialized “brainstem-thalamic” pathway.
4 LINKING THE “SONG SYSTEM” TO THE VOCAL-RESPIRATORY HINDBRAIN
4.1 FUNCTIONAL ORGANIZATION OF THE SONG MOTOR PATHWAY
In songbirds, the entire ventral respiratory column (IOS, RVL, PAm and RAm) receives strong projections from nucleus RA (robust nucleus of the Arcopallium). In addition to its projections to the respiratory brainstem, RA also sends projections to nucleus ambiguus (which innervates the larynx), nucleus DM in the midbrain, and XIIts, the last containing motoneurons that innervate the syringeal vocal musculature (Fig. 7). Interestingly, the only respiratory structure not to receive a direct input from RA is the PBvl, which projects to all of the structures that make up the VRG and receives a major input from nTS (see above). RA is necessary for song production (Ashmore et al., 2005, 2008) and forms part of the “song system’s” descending motor pathway. While the homologous status of arcopallial nuclei is controversial, RA’s projection patterns, physiology and expression of genes selective for layer 5 of motor cortex (Vicario, 1991a; Wild, 1993b; Leonardo and Fee, 2005; Sober et al., 2008; Dugas-Ford et al., 2012), suggest that it might actually be functionally equivalent to layer 5 of mammalian motor cortex, which supplies major descending projections to subtelencephalic targets (Zeier and Karten, 1971; but see Martinez-Garcia et al., 2007 for a different interpretation). Although birds do not possess a layered neocortex (but see Wang et al., 2010), their pallial organization is functionally similar to that of mammals (Butler et al., 2011; Reiner et al., 2004). It is therefore reasonable to think of the song system as a model for cortical modulation of medullary respiratory circuits.
FIGURE 7.
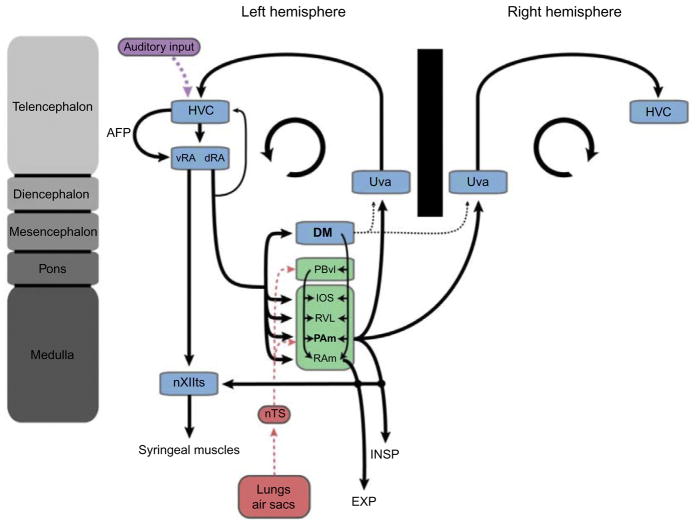
Schematic representation of the avian song system and its relationship to the respiratory system. This figure highlights the bilateral nature of the projections from PAm to Uva and HVC. Details of several important circuits necessary in song learning and perception (e.g., the anterior forebrain pathway (AFP) and the ascending auditory pathway) have been omitted for simplicity.
The projections from RA to its various output structures are mostly ipsilateral but this pattern varies across species. In some songbirds, such as the canary, projections are primarily ipsilateral but there is a significant contralateral component (Wild et al., 2000). This is in contrast to the zebra finch, whose projections are almost exclusively ipsilateral. Such a purely ipsilateral projection pattern implies that motoneurons innervating the syrinx must receive song motor commands exclusively from the ipsilateral RA. Because songs are always produced in a seamless fashion, no matter what side of the syrinx is used (Suthers, 1990), motor commands between left and right RA must therefore be coordinated to a remarkable degree. How exactly such coordination is achieved is presently unclear, especially given that there exist no direct connections between the left and right RA, although there are abundant direct connections between left and right RAm (Wild et al., 2009). There is also, however, indirect evidence that the inspiratory brainstem might play a critical role in coordinating vocal motor activity across hemispheres (see next section).
In most schematic representations of the song circuit, RA is shown as a single, anatomically distinct, nucleus containing two functionally different sets of projections, one innervating motoneurons of the syrinx (nXIIts) and another innervating vocal-respiratory control areas at several rostrocaudal levels of the brainstem. Because these two sets of projections originate from anatomically distinct regions (Vicario, 1991a; Wild, 1993b), RA can be thought of as two separate functional parts interconnected by a dense network of interneurons (Spiro et al., 1999). The ventral two thirds of the nucleus (vRA) projects to XIIts and contains a myotopic map of the different syringeal muscles (Sober et al., 2008; Vicario, 1991a) and is therefore well placed to control the acoustic features of song. The dorsal third of RA (dRA), by contrast, projects almost exclusively to respiratory targets. These include the above-mentioned brainstem respiratory areas as well as DM (Roberts et al., 2008; Vicario, 1991a), whose suggested mammalian homologue, the lateral PAG, is thought to act as a behavioral modulator of breathing (Subramanian and Holstege, 2010; Subramanian et al., 2008). The dorsal part of RA is therefore well placed for controlling the temporal features of song. Interestingly, the same RA neurons that project to the respiratory areas also send collaterals back to HVC (used as a proper name), a “cortical” premotor nucleus critical for song production (see below) (Fig. 7). The function of this feedback projection is not known, but an efference copy is possible (Roberts et al., 2008).
RA is necessary for normal song production (Ashmore et al., 2005, 2008) and is the final common telencephalic output of the “song system.” It is HVC, however, from which RA receives its primary motor input that appears to control many of the moment-to-moment commands that specify the acoustic and temporal characteristics of individual syllables in the song (Amador et al., 2013; Hahnloser et al., 2002; Long and Fee, 2008; Yu and Margoliash, 1996). HVC lies at the intersection of the motor pathway, the auditory system and a specialized basal ganglia loop, known as the anterior forebrain pathway that indirectly links HVC to RA. In addition to its role in sensorimotor aspects of song learning (Mooney, 2009), HVC is critical for song production because lesions (Aronov et al., 2008; Nottebohm et al., 1976) and brief electrical perturbations (Ashmore et al., 2005; Vu et al., 1994, 1998; Wang et al., 2008) severely disrupt song. HVC receives a major input from a small nucleus (nucleus Uvaeformis) in the caudolateral thalamus, which is bilaterally innervated by a population of neurons in the respiratory-related nucleus (PAm) in the ventrolateral medulla (Reinke and Wild, 1998; Striedter and Vu, 1998). This “respiratory-thalamic” pathway is thought to provide important timing information to HVC, and therefore the rest of the song system. The significance of this pathway and its role in singing is discussed below.
4.2 A SPARSE NEURAL CODE FOR SONG
During normal eupneic breathing, measured either during quiet wakefulness (R.C. Ashmore, unp. obs.) or anesthesia (Ashmore et al., 2008), RA does not show any rhythmic activity in phase with the respiratory rhythm. Instead RA fires at relatively high tonic rates (8–25 Hz) that are thought to be necessary for providing general muscle tone to the syringeal musculature (Leonardo and Fee, 2005; Long and Fee, 2008). During sleep or anesthesia, tonic firing in RA is sporadically interrupted by bursts of activity that are driven by HVC (Hahnloser et al., 2002). In contrast to the general lack of modulated activity during normal breathing, RA and its input structure, HVC, show dramatic changes in activation when the bird sings, with an overall increase in neural activity that is highly modulated in its firing frequency.
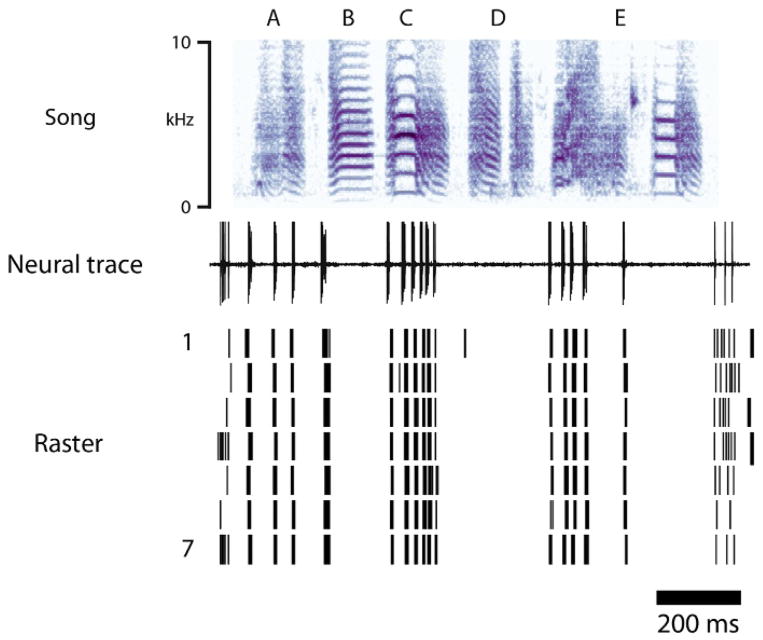
In zebra finches, HVC activity increases dramatically prior to the onset of individual syllables in the song by a relatively fixed latency of 45 ms, consistent with its premotor role in song production (Kozhevnikov and Fee, 2007; Schmidt, 2003). While it was initially assumed that all neurons in HVC were active during the production of all syllables in a song, a landmark study by Hahnloser et al. (2002) showed that individual premotor neurons in HVC are actually only active for a very short and a very specific period of time. Using antidromic identification to record specifically from HVC neurons that project to RA (HVCRA neurons), the authors showed that these neurons only produce a single burst of action potentials (lasting ~6 ms) within a given song motif (the stereotyped sequence of syllables in a zebra finch song that is repeated multiple times within a song bout, see Fig. 2). The precision of each burst is remarkable, occurring at exactly the same time in the song and exhibiting a temporal jitter across renditions that is <1 ms (Fig. 8). Recording from a number of these neurons, they and others (Fee et al., 2004; Fiete et al., 2004; Hahnloser et al., 2002) proposed that individual HVCRA neurons are briefly active during different, but specific, time windows and that song is therefore “tiled” by these sequentially active neurons. In this scheme, small groups of co-active neurons (neurons A), would drive the next small group of neurons (neurons B), and song would therefore be coded by the sequential activation of small populations of neurons (A→B→C→…). This domino-like activation chain is in many computational models also referred to as a synfire chain of sparsely active neurons, and has been suggested, on theoretical grounds, to be optimized for vocal production and learning (Fiete et al., 2004, 2007; Gibb et al., 2009b; Jin, 2009; however, see Amador et al. (2013) for an alternative interpretation).
FIGURE 8.
A sparse motor code for song. Example recording from a neuron in RA during the production of a song motif. Top trace represents the spectrogram of a song motif with syllables labeled A–E. Middle and bottom traces show respectively a single unit recording while the bird is singing the motif and a raster plot showing spike timing during seven renditions of the motif. Notice the near perfect alignment of spikes across multiple renditions (1 through 7) of the song motif.
This figure is based on data generously provided to the authors by Kyler J. Brown and Dan Margoliash.
The sparse code of one burst per song motif in HVC translates in RA to an equally precise neural code. RA activity is presumably driven on a moment-to-moment basis by bursting inputs from HVC (Fee et al., 2004; Hahnloser et al., 2002), but here neurons produce multiple bursts of action potentials throughout a single song motif with a degree of precision and across rendition variability that rivals that observed in HVC (Chi and Margoliash, 2001; Leonardo and Fee, 2005; Olveczky et al., 2011) (Fig. 8). It is this neural output that is presumably imposed onto the syringeal motoneurons and the respiratory brainstem. Whether RA neurons projecting to the respiratory system and syringeal motoneurons will display the same type of temporal pattern is not known. Interestingly, these precise bursts recorded during singing appear to get “replayed” in RA during sleep, suggesting that some form of offline song replay might occur in the song system (Dave and Margoliash, 2000; Dave et al., 1998; Margoliash and Schmidt, 2010).
4.3 HVC AND ITS CONTROL OF RESPIRATORY TIMING DURING SONG
Song production, like any other behavior, occurs at many different time scales, from the millisecond level needed to coordinate the syringeal muscles and produce the fine acoustic details of a note, to the much longer time scales that determine the overall duration of syllables and their sequence in the song. While anatomically distinct compartments within the “song system” network are likely to contribute in distinct ways to the overall timing of song, HVC appears to play a unique role.
Using local bilateral cooling of HVC (by placing miniature Peltier devices on the dura above left and right HVC) as a method for slowing down the internal dynamics within the nucleus, Long and Fee (2008) showed that song tempo could be dramatically slowed down by as much as 45%. Remarkably, song lengthening was linearly correlated with HVC cooling, showing only slight alteration in acoustic structure. In fact, “cooled” songs were almost identical to songs artificially stretched with Photoshop! In other words, cooling HVC caused a nearly linear increase in song duration by stretching notes, syllables, and silent gaps. In a follow-up experiment measuring air sac pressure to gain more precise timing information regarding syllable onset and offset, and therefore gap duration (Andalman et al., 2011), HVC cooling was shown to stretch both EPs (which make up syllables, Hartley, 1990) and inspiratory pulses (IPs; which make up the gaps, Hartley and Suthers, 1989). Interestingly, many of the measured IPs did not stretch nearly as much as the EPs, with HVC cooling having little effect on the early part of the IP, stretching instead primarily the second half of the IP as it transitions to the next EP. These findings support the idea that gap duration is controlled in part by circuits downstream of HVC. Given that brief electrical stimulation in PAm is able to disrupt song temporal structure (Ashmore et al., 2005), it is tempting to suggest that intrinsic circuitry in the respiratory brainstem might play a significant role in controlling gap duration. Although speculative, the pulmonary input from the lateral parasolitary subnucleus to PBvl that might relay information from IPCs could perhaps be influential in this context (Wild, 2004a).
Further support for a fundamental role of HVC in controlling vocal-respiratory patterns comes from work on birds in the early phases of vocal learning. At this stage, juveniles have not yet learned to link syllables with individual EPs and silent gaps with IPs. Instead, they produce multiple syllables separated by silent gaps within a single long EP, a pattern that is never observed in older birds. HVC lesions in young birds that have just mastered the one-to-one relationship between EPs and syllables causes then to relapse into the more immature vocal patterns that lack vocal-respiratory coordination (Veit et al., 2011). Together, these finding suggest not only that HVC plays a fundamental role in controlling the overall timing of song but that it also plays a critical role in establishing the coordination between vocal muscular control and breathing.
4.4 THE SONG SYSTEM PROVIDES DIRECT DRIVE TO THE RESPIRATORY SYSTEM
Brief electrical stimulation in either HVC or RA causes a very short latency (~20 ms) increase in respiratory drive, exemplified by a change in air sac pressure on top of the normal respiratory pressure pattern (Ashmore et al., 2005; Mendez et al., 2012). Interestingly, the size (and sign) of the pressure change is strongly dependent on the phase of respiration, suggesting that stimulation does not simply cause additive drive but rather engages the respiratory circuits in a more complex manner. Therefore, while cortical song circuits can clearly influence respiratory output, it is still unclear how song temporal structure is controlled. Is it determined directly by HVC, which imposes a precise motor code that simply drives respiratory circuits to produce a song specific pattern? Or, alternatively, does HVC provide a motor drive that enables respiratory networks to transform the precisely timed inputs it receives from RA into song appropriate respiratory patterns? In the first scenario, HVC and the song system would appear to control much of the timing of song, whereas in the second case, a much more significant role would be attributed to respiratory circuit dynamics for controlling key aspects of the song temporal structure.
Based on a relatively straightforward model showing that a basic oscillatory input can produce complex respiratory patterns that resemble the normal respiratory patterns observed during song (Trevisan et al., 2006), Mendez et al. (2012) went out to directly test the effect of stimulating RA on respiratory pattern dynamics. Using air sac pressure to measure respiratory patterns, they showed, using simple stimulation patterns in RA, that they could create a 1:1 entrainment of the respiratory rhythm when the stimulus frequency was closely matched to the respiratory rhythm. More interesting, however, when they “forced” the system to respond to frequencies greater than the respiratory rhythm, they observed respiratory rhythms that converged onto stable (and theoretically predicted) subharmonic patterns (1:2, 1:3, 2:3, etc.) of the stimulus frequency. In other words, the respiratory system reached new stable entrainment regimes that could be directly predicted by the input frequency. In some cases, when stimulus strength was increased, the expiratory and inspiratory pressure pulses showed dramatic increases in amplitude that were similar in size to those normally observed during singing and whose pattern approximated the complex subharmonic temporal patterns that occur in normal song. Interestingly, these effects on the respiratory patterns were strongly state dependent, occurring only in awake and not in anesthetized animals.
Although this experimental paradigm of stimulating cortical areas during quiet respiration is not the same as that which occurs during normal singing, it suggests that the integration of cortical input by the respiratory networks might play a crucial role in shaping song temporal patterns. In the end, therefore, it is likely that song temporal patterns are generated in multiple parts of the song system. As such, HVC could still generate the moment-to-moment information (Hahnloser et al., 2002) that is transformed in RA into song motor commands that drive the vocal musculature of the syrinx to determine the key elemental gestures in the song (Amador et al. 2013). In addition, however, by sending a precise temporal code to the respiratory brainstem, via RA, HVC could provide the “drive” that causes respiratory circuits to generate their own temporal pattern.
The possibility that the respiratory system might play a key role in determining song temporal features does not necessarily contradict the findings observed following HVC cooling. During cooling, motor commands generated in HVC would be slowed and the input to the network would therefore be maintained for a longer period of time consequently causing the respiratory system to simply transform this input into a longer EP. Assuming that the respiratory system is an active participant in the transformation of motor commands, cooling HVC further might change the frequency and amplitude of the cortical input signal to a degree sufficient to place the respiratory network into a different parameter space and causing it to transform the input signal into a different EP altogether. Recent experiments performed in the canary suggest that this is exactly what happens (Goldin et al., 2013).
5 SONG PRODUCTION AND THE “RESPIRATORY-THALAMO-CORTICAL” PATHWAY
5.1 THE “RESPIRATORY-THALAMIC” PATHWAY IS NECESSARY FOR SONG
In addition to its bulbospinal projections, the inspiratory-related respiratory nucleus PAm also sends, via separate neurons (Wild, 2004b), strong bilateral projections to the small thalamic nucleus Uva (Reinke and Wild, 1998; Striedter and Vu, 1998). This projection is of particular interest in the context of song production because Uva provides, again via separate groups of neurons (Akutagawa and Konishi, 2005; Hahnloser et al., 2008), both a direct and an indirect projection to HVC (Coleman and Vu, 2005; Striedter and Vu, 1998; Williams and Vicario, 1993). The indirect projection is via the intermediary of NIf (nucleus interfacialis) and Av (nucleus Avalanche) (Akutagawa and Konishi, 2010), two sensorimotor structures that play a key role in vocal motor learning and project directly onto HVC (Lewandowski et al., 2013).
The thalamic nucleus Uva is a compact, grape-shaped nucleus lying in the caudolateral dorsal thalamus, dorsal to the most lateral aspect of the posterior commissure. Unlike the majority of the nuclei in the song system, Uva is not sexually dimorphic. It has a core of densely packed neurons and a dorsal “horn” of less densely packed, CRF-positive neurons (Williams et al., 1989). Its afferents arise from a variety of sources in addition to PAm, e.g., the dorsal column nuclei (Wild, 1994a), the ventral nucleus of the lateral lemniscus (Coleman et al., 2007), the medial habenular nucleus (Akutagawa and Konishi, 2005), and possibly DM (Striedter and Vu, 1998). The PAm projection to Uva specifically and densely targets the core of each side, with projections to the contralateral side crossing predominantly in the underlying posterior commissure (Wild, 1994b). Each core nucleus then projects ipsilaterally directly to HVC. The somatosensory and auditory inputs preferentially target the dorsal “horn,” especially laterally, which then projects indirectly to HVC, via NIf. Uva in songbirds has a corresponding nucleus in non-songbirds, called caudal DLP (cDLP, dorsolateral posterior nucleus, caudal par; Wild, 1994a), which has some similar connectivity to that in songbirds. It has been likened to the suprageniculate nucleus of the posterior thalamic group in mammals (Gamlin and Cohen, 1986).
Because PAm receives a direct projection from RA, the pathway between PAm and Uva, which we call here “respiratory-thalamic,” provides a direct functional link between the respiratory brainstem and HVC (Ashmore et al., 2008), and completes a loop in the song system (Fig. 7). This pathway is necessary for normal song production because both bilateral and unilateral lesions of Uva cause immediate (and long lasting) song deficits (Coleman and Vu, 2005; M.F. Schmidt, unp. obs.). Bilateral lesions cause severe acoustic degradation which appear to result primarily in aborted song attempts of acoustically distorted vocalizations that are difficult to categorize but possibly represent attempted syllables. Interestingly, birds with unilateral Uva lesions, which show similar immediate deficits as bilateral lesioned birds, eventually recover their song. The mechanism of this recovery is not understood but might involve compensation through an alternate pathway that also links RA indirectly back to HVC (RA→DMP (thalamus)→mMAN→HVC) but which in itself is not necessary for song production (Coleman and Vu, 2005; Foster and Bottjer, 2001; Williams et al., 2012).
The exact function of the “respiratory-thalamic” pathway is not known, but indirect evidence suggests that it might be involved in contributing to the overall temporal structure of song. In addition to evidence that Uva lesions cause an inability to transition past the first syllable of a motif, application of brief (<7 ms) electrical stimuli to PAm, which is at the origin of this pathway, during singing causes an immediate (within 30 ms) truncation of the ongoing syllable and a resetting of the overall song pattern (Ashmore et al., 2005). The similarity in behavioral consequences following PAm stimulation to those observed in HVC or RA (Ashmore et al., 2005; Vu et al., 1994, 1998; Wang et al., 2008) has led to the suggestion that PAm forms an integral part of the song pattern generating circuit (Ashmore et al., 2005; Schmidt and Ashmore, 2008). This is in contrast to nucleus XIIts, which also receives robust input from RA, but which innervates the tracheosyringeal musculature. Stimulation of this nucleus only causes a temporary acoustic distortion without any effect on song temporal structure (Ashmore et al., 2005). Taken together, these findings argue against a simple top-down control by a hierarchically organized motor pathway. Instead, it suggests that song is controlled by a recurrent loop in which the “respiratory-thalamic” pathway plays an important role in determining song acoustic and temporal structure.
5.2 A ROLE FOR THE RESPIRATORY SYSTEM IN HEMISPHERIC COORDINATION
In birds, there exists neither a corpus callosum nor any other commissural system that directly connects left and right HVC or any other forebrain song control nucleus (Wild, 1994b). By virtue of its bilateral projections to left and right Uva, PAm is functionally connected to HVC in each hemisphere (Ashmore et al., 2008) and might, therefore, play a key role in coordinating premotor activity between hemispheres. Precise coordination is critical because HVC drives syringeal muscles in a primarily ipsilateral fashion and motor commands need to be in near perfect register for both halves of the syrinx to produce a coordinated output. This is especially critical for songbird species that produce songs that are seamless, even though they can alternate between the left and right halves of the syrinx at switch rates that can exceed 40 Hz (Allan and Suthers, 1994).
Support for cross-hemispheric coordination comes from simultaneous neural recording from small neural populations in left and right HVC of singing birds. In contrast to single HVCRA neurons, which fire only a single burst per song motif, measurements of population activity in left and right HVC show nearly identical overall increases and decreases in firing rate during song (Schmidt, 2003; Yu and Margoliash, 1996). Cross-correlation analyses reveal that activity is highly synchronized between sides at syllable onset and in many cases also at note onset (Schmidt, 2003). Interestingly, while HVC activity is generally less robust during the “inspiratory” silent intervals, these gaps often contain a short burst of activity that is tightly synchronized across hemispheres. These results suggest that premotor activity in HVC is synchronized at key transitions in the song by a common input, which, given the anatomical layout, is likely to come from Uva. Support for this interpretation come from preliminary findings showing that unilateral Uva lesions, in addition to their effect on song, also cause a desynchronization of HVC activity across hemispheres (Coleman and Vu, 2000).
5.3 NEURAL PROPERTIES OF THE RESPIRATORY-THALAMIC PATHWAY
The existence of discrete periods of synchronous activity in HVC across hemispheres suggests that the “respiratory-thalamic” pathway sends precisely timed signals to HVC to help synchronize local circuit dynamics both within HVC and across hemispheres. If the hypothesis is correct that Uva sends precisely timed signals that synchronize HVC circuits, one would expect that song-related activity in Uva precedes premotor activity in HVC and that it be precisely time locked to the onset of syllables, notes and possibly even the synchronized bursts that occur during the inter-syllable gap. While the relationship between Uva and HVC activity has not been measured, preliminary findings suggest that Uva activity mostly precedes syllable onset (Aronov and Fee, 2008).
How timing signals recorded in Uva might arise from the circuitry in PAm is not known. PAm contains all of the classes of respiratory neurons found in its mammalian homologue, preBötC. One possibility, therefore, is that the respiratory circuitry within PAm becomes reconfigured in such a way during singing that it contributes to the generation of time-locked premotor activity in Uva. An additional possibility is that neurons that project directly to Uva might be specialized for relaying timing information to Uva. While antidromically identified recordings of these neurons have not been performed, PAm contains an unusual class of neurons that might well represent Uva-projecting neurons. These neurons, coined nonrespiratory bursting (NRB) neurons by Ashmore et al. (2008), do not show any significant phase locking to the respiratory pattern, but display instead intermittent bursting activity. Paired recordings from PAm and HVC (or RA), reveal that these NRB neurons, while not coupled to the respiratory rhythm, are strongly correlated in their bursting activity with HVC and RA in a way that is partially dependent on the existence of a functionally intact “respiratory-thalamic” pathway (Fig. 9). NRB neurons seem to become recruited during artificially evoked vocalization (by electrically stimulation of DM) and are likely physically intermixed with the more classic respiratory neurons in PAm, because both can be recorded simultaneously from the same electrode tip (Ashmore et al., 2008). Interestingly, the strongest argument that NRB neurons might form part of a functional respiratory network might come from the preliminary observation that their firing pattern might operate under two different modes, switching from an intermittent bursting rhythm to a normal respiratory rhythm when the animal’s behavioral state transitions from sedation to wakefulness (R. Ashmore, Pers. Comm.).
FIGURE 9.
Spontaneous activity of nonrespiratory bursting (NRB) neurons in PAm is correlated with bursting in the contralateral HVC. A, Simultaneous recordings of two single units in the left PAm while maintaining the same unit in the contralateral HVC. For the top pair, the recording electrode was placed closer to a site showing the characteristic nonrespiratory bursting pattern. Bursts in these neurons occur at approximately the same time as bursts (and subsequent pauses) in HVC. In the bottom pair, a respiratory neuron recorded several minutes after the unit on the left shows a complete lack of correlated activity with the same HVC neuron.
5.4 INTEGRATING THE RESPIRATORY SYSTEM WITH SONG CONTROL
The existence of a recurrent loop linking the respiratory brainstem back to “cortical” vocal control nuclei and the ability to synchronize hemispheres at least once per syllable offers an interesting perspective on the role of the respiratory system in vocal production. Not only is the respiratory system involved in transforming vocal motor commands into respiratory gestures (EPs or IPs), but it is also well placed to play a fundamental role in shaping overall song temporal pattern and syllable sequencing.
During the production of a given song syllable, one might imagine that motor commands generated by syllable-specific local synfire chains in HVC send a precisely timed code that, after being transformed in ventral RA, activates syringeal motoneurons in ways that determine the acoustic characteristics of that syllable. This same code from HVC would also be sent, via dorsal RA, to the respiratory brainstem where intrinsic dynamics within vocal-respiratory networks would transform this code into a syllable-specific respiratory pattern. How this transformation occurs is not known, but an interesting question for future work is how much of the characteristics of the respiratory pattern are determined by the precise timing of the input (from dorsal RA) and how much by the dynamical properties of the respiratory system.
Because songs are not made up of single syllables, a key unanswered question is how syllable sequences are generated. Here, too the respiratory system might play a critical role. Following the production of a given syllable, the switch from EP to inspiratory minibreath might activate intrinsic dynamics within PAm that could lead to the eventual initiation of the next syllable through precisely generated timing signals that are transmitted by the “respiratory-thalamic” pathway to HVC, where they could activate a different local synfire chain that would code for the next syllable (Gibb et al., 2009a). Interrupting this signal, either by stimulation in PAm or by a lesion of Uva, would presumably prevent this transition to the next syllable, which is in fact what is observed experimentally (Ashmore et al., 2005; Coleman and Vu, 2005). Because PAm receives a strong input from the lateral parasolitary nucleus (lPs) of nTS, it is conceivable that the transition from a minibreath to the generation of a new timing signal would be strongly influenced by vagal inputs that relay to PAm, via nTS, afferent signals regarding the internal state of the respiratory periphery (lungs, air sacs). This possibility has only recently been explored in songbirds (Mendez et al., 2010) and a general role for sensory input into vocal control is generally under appreciated. In cats, however, the general role of respiratory input within the context of vocalization, and the specific importance of pulmonary feedback for the ability to vocalize, was explored and demonstrated over 15 years ago (Davis and Zhang, 1996; Davis et al., 1996; Nakazawa et al., 1997).
The above scenario for syllable transitions might of course also be true for note transitions, but it is more difficult to imagine how these would occur because notes are not separated by inspiratory minibreaths. Nevertheless, it is conceivable that the “respiratory-thalamic” pathway sends timing signals to activate local synfire chains in HVC more than just at the onset of each new syllable. In fact, if the synchronous activation of left and right HVC is any measure of the frequency of timing signals sent by the “respiratory-thalamic” pathway, it is likely that local synfire chains in HVC are activated quite frequently throughout the song (Schmidt, 2003). Interestingly, the relatively frequent refresh rate that would activate local circuits in HVC and ensure synchronization of left and right HVC might actually do more than simply ensure that motor output is well synchronized. Recent evidence in zebra finches using either local cooling (Long and Fee, 2008) or brief electrical stimulation in HVC (Wang et al., 2008) suggests that the source of the motor commands that specify song might actually alternate between hemispheres multiple times during song (Schmidt, 2008). In fact in some cases, it appears that the switch rate between left and right HVC might at times be as high as 20 Hz. If the “respiratory-thalamic” pathway is responsible for orchestrating the switching between sides, it is conceivable that this pathway carries more than just timing information but also instructions that specify the side that sends the motor command.
6 CONCLUDING REMARKS
The goal of this review was to highlight the differences and similarities between the respiratory system in (song)birds and mammals, and to highlight the state of our knowledge regarding how “cortical” circuits engage the respiratory system during vocal production, an area that has received surprisingly little attention in mammals. In addition to highlighting top-down control of the respiratory system, this review also highlights the intriguing possibility that brainstem respiratory circuits might play roles beyond respiratory control. This work suggests important future directions for work in both songbirds and mammals.
Songbird: (a) Record from respiratory brainstem and respiratory-thalamic pathway in singing birds, (b) determine the relative contributions of the song system and internal circuit dynamics of the respiratory system to the determination of the song temporal pattern, (c) inhibit activity (e.g., using optogenetics) of discrete populations of respiratory neurons and ask how shutting down these populations impacts vocal activity (acoustic and air sac pressure), (d) record and manipulate activity in thalamus-projecting PAm neurons and test their role in song timing and syllable sequencing.
Mammals: (a) Need to develop mammalian models (perhaps bats) to study respiratory-vocal coupling and (b) investigate the possibility of having bottom-up influence of the respiratory brainstem on cortical activity. The idea of having a respiratory-thalamic projection might seem unusual given the focus of studying respiratory brainstem circuits only within the context of an isolated pattern generator for respiratory rhythm production. Older literature, however, suggests that respiratory activity can be measured in a number of higher order structures that include thalamus and amygdala (Chen et al., 1992; Vibert et al., 1979).
Acknowledgments
The authors would like to thank Drs. Judy McLean, Franz Goller, and Gert Holstege for carefully reading the manuscript and offering insightful comments. They would also like to thank Drs. Franz Goller and Dan Margoliash for providing original data to create some of the figures. This work was supported by grants NIH R01DC6102 to M. F. S. and NIH 2 R01 NS029467-16A to Dr. R.A. Suthers (PI) and J.M. Wild (Contractual collaborator) and by a Human Frontiers Science Program project grant to Drs. A. Doupe, J.M. Wild, and W.S. Bialek.
References
- Akutagawa E, Konishi M. Connections of thalamic modulatory centers to the vocal control system of the zebra finch. Proc Natl Acad Sci USA. 2005;102:14086–14091. doi: 10.1073/pnas.0506774102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akutagawa E, Konishi M. New brain pathways found in the vocal control system of a songbird. J Comp Neurol. 2010;518:3086–3100. doi: 10.1002/cne.22383. [DOI] [PubMed] [Google Scholar]
- Allan SE, Suthers RA. Lateralization and motor stereotypy of song production in the brown-headed cowbird. J Neurobiol. 1994;25:1154–1166. doi: 10.1002/neu.480250910. [DOI] [PubMed] [Google Scholar]
- Amador A, Perl YS, Mindlin GB, Margliash D. Elemental gestures are encoded by song premotor cortical neurons. Nature. 2013;495:59–64. doi: 10.1038/nature11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andalman AS, Foerster JN, Fee MS. Control of vocal and respiratory patterns in birdsong: dissection of forebrain and brainstem mechanisms using temperature. PLoS One. 2011;6:e25461. doi: 10.1371/journal.pone.0025461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov D, Fee MS. Recordings of antidromically identified projection neurons in motor thalamic nucleus uvaeformis (Uva) of the songbird. Society for Neuroscience; San Diego, CA: 2008. [Google Scholar]
- Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008;320:630–634. doi: 10.1126/science.1155140. [DOI] [PubMed] [Google Scholar]
- Ashmore RC, Wild JM, Schmidt MF. Brainstem and forebrain contributions to the generation of learned motor behaviors for song. J Neurosci. 2005;25:8543–8554. doi: 10.1523/JNEUROSCI.1668-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore RC, Renk JA, Schmidt MF. Bottom-up activation of the vocal motor forebrain by the respiratory brainstem. J Neurosci. 2008;28:2613–2623. doi: 10.1523/JNEUROSCI.4547-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers GJL, Suthers RA, ten Cate C. Mechanisms of frequency and amplitude modulation in ring dove song. J Exp Biol. 2003;206:1833–1843. doi: 10.1242/jeb.00364. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Gestreau C. The brainstem respiratory network: an overview of a half century of research. Respir Physiol Neurobiol. 2009;168:4–12. doi: 10.1016/j.resp.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavitsaubie M, Champagnat J. Central control of breathing in mammals—neuronal circuitry, membrane-properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Borgmann A, Abdala A, Zhang R, Rybak IA, Paton J, Smith J. Spiking behavior and membrane potential trajectories of pre-BotC and hypoglossal neurons recorded from the rat in situ. FASEB J. 2011;25:1074.11. [Google Scholar]
- Bottjer SW, Arnold AP. Afferrent neurons in the hypoglossal nerve of the zebra finch (Poephila guttata): localization with horseradish peroxidase. J Comp Neurol. 1982;210:190–197. doi: 10.1002/cne.902100209. [DOI] [PubMed] [Google Scholar]
- Brackenbury J. Respiration and production of sound by birds. Biol Rev. 1980;55:363–378. [Google Scholar]
- Brown JL. Vocalization evoked from the optic lobe of a songbird. Science. 1965;149:1002–1003. doi: 10.1126/science.149.3687.1002. [DOI] [PubMed] [Google Scholar]
- Butler AB, Reiner A, Karten H. Evolution of the amniote pallium and the origins of mammalian neocortex. Ann NY Acad Sci. 2011;1225:14–27. doi: 10.1111/j.1749-6632.2011.06006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Eldridge FL, Wagner PG. Respiratory-associated thalamic activity is related to level of respiratory drive. Respir Physiol. 1992;90:99–113. doi: 10.1016/0034-5687(92)90137-l. [DOI] [PubMed] [Google Scholar]
- Chi Z, Margoliash D. Temporal precision and temporal drift in brain and behavior of zebra finch song. Neuron. 2001;32:899–910. doi: 10.1016/s0896-6273(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Vu ET. Neural activity in HVc of adult zebra finches during the song recovery following unilateral lesions of nucleus uvaformis. Soc Neurosci Abstr. 2000;26:2031. [Google Scholar]
- Coleman MJ, Vu ET. Recovery of impaired songs following unilateral but not bilateral lesions of nucleus uvaeformis of adult zebra finches. J Neurobiol. 2005;63:70–89. doi: 10.1002/neu.20122. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Roy A, Wild JM, Mooney R. Thalamic gating of auditory responses in telencephalic song control nuclei. J Neurosci. 2007;27:10024–10036. doi: 10.1523/JNEUROSCI.2215-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly CA, Dobbins EG, Feldman JL. Pre-Botzinger complex in cats—respiratory neuronal discharge patterns. Brain Res. 1992;590:337–340. doi: 10.1016/0006-8993(92)91118-x. [DOI] [PubMed] [Google Scholar]
- Daley M, Goller F. Tracheal length changes during zebra finch song and their possible role in upper vocal tract filtering. J Neurobiol. 2004;59:319–330. doi: 10.1002/neu.10332. [DOI] [PubMed] [Google Scholar]
- Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- Dave A, Yu AC, Margoliash D. Behavioral state modulation of auditory activity in a vocal motor system. Science. 1998;282:2250–2254. doi: 10.1126/science.282.5397.2250. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Zhang SP. Midbrain and medullary regulation of respiration and vocalization. Prog Brain Res. 1996;107:315–325. doi: 10.1016/s0079-6123(08)61873-7. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Zhang SP, Winkworth A, Bandler R. Neural control of vocalization: respiratory and emotional influences. J Voice. 1996;10:23–38. doi: 10.1016/s0892-1997(96)80016-6. [DOI] [PubMed] [Google Scholar]
- DeWet PD, Farrell PR, Fedde MR. Number and morphology of muscle spindles in the transversus abdominis muscle in the chicken. Poult Sci. 1971;50:1349–1357. doi: 10.3382/ps.0501349. [DOI] [PubMed] [Google Scholar]
- Dugas-Ford J, Rowell JJ, Ragsdale CW. Cell-type homologies and the origins of the neocortex. Proc Natl Acad Sci USA. 2012;109:16974–16979. doi: 10.1073/pnas.1204773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncker HR. Structure of Avian lungs. Respir Physiol. 1972;14:44–63. doi: 10.1016/0034-5687(72)90016-3. [DOI] [PubMed] [Google Scholar]
- Düring DN, Ziegler A, Thompson CK, Ziegler A, Faber C, Müller J, Scharff C, Elemans CPH. The songbird syrinx morphome: a three-dimensional, high-resolution, interactive morphological map of the zebra finch vocal organ. BMC Biol. 2013;11:1. doi: 10.1186/1741-7007-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschman M, Herbert H. The Kölliker-Fuse nucleus gates the post-inspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci. 2006;24:1071–1084. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- Dutschman M, Moerschel M, Rybak IA, Dick TE. Learning to breathe: control of the inspiratory-expiratory phase transition shifts from sensory- to central-dominated during postnatal development in rats. J Physiol. 2009;587 (20):4931–4948. doi: 10.1113/jphysiol.2009.174599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Subnuclear organization of the lateral tegmental field of the rat. I: nucleus ambiguus and ventral respiratory group. J Comp Neurol. 1990;294:202–211. doi: 10.1002/cne.902940205. [DOI] [PubMed] [Google Scholar]
- Farmer CG, Sanders K. Unidirectional airflow in the lungs of alligators. Science. 2010;327:338–340. doi: 10.1126/science.1180219. [DOI] [PubMed] [Google Scholar]
- Fedde MR. Respiratory muscles. In: Seller TJ, editor. Bird Respiration. CRC Press; Boca Raton, FL: 1987. pp. 3–37. [Google Scholar]
- Fedde MR, Burger RE, Kitchell RL. Electromyographic studies of the effects of bodily position and anaesthesia on the activity of the respiratory muscles of the domestic cock. Poult Sci. 1964;43:839–846. [Google Scholar]
- Fee MS, Kozhevnikov AA, Hahnloser RHR. Neural mechanisms of vocal sequence generation in the songbird. In: Zeigler HP, Marler P, editors. Behavioral Neurobiology of Birdsong. New York Academy of Sciences; New York: 2004. pp. 153–170. [DOI] [PubMed] [Google Scholar]
- Feldman JL. Neurophysiology of breathing in mammals. In: Bloom FE, editor. Handbook of Physiology; Section I: The Nervous System. Intrinsic Regulatory Systems of the Brain. IV. American Physiological Society; Bethesda, MD: 1986. pp. 463–524. [Google Scholar]
- Feldman JL. Looking forward to breathing. Prog Brain Res. 2011;188:213–218. doi: 10.1016/B978-0-444-53825-3.00019-X. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Loewy AD, Speck DF. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat—an autoradiographic study. J Neurosci. 1985;5:1993–2000. doi: 10.1523/JNEUROSCI.05-08-01993.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiete IR, Hahnloser RHR, Fee MS, Seung HS. Temporal sparseness of the premotor drive is important for rapid learning in a neural network model of birdsong. J Neurophysiol. 2004;92:2274–2282. doi: 10.1152/jn.01133.2003. [DOI] [PubMed] [Google Scholar]
- Fiete IR, Fee MS, Seung HS. Model of birdsong learning based on gradient estimation by dynamic perturbation of neural conductances. J Neurophysiol. 2007;98:2038–2057. doi: 10.1152/jn.01311.2006. [DOI] [PubMed] [Google Scholar]
- Fortin G, Foutz AS, Champagnat J. Respiratory rhythm generation in chick hindbrain: effects of MK-801 and vagotomy. Neuroreport. 1994;5:1137–1140. doi: 10.1097/00001756-199405000-00029. [DOI] [PubMed] [Google Scholar]
- Foster EF, Bottjer SW. Lesions of a telencephalic nucleus in male zebra finches: influences on vocal behavior in juveniles and adults. J Neurobiol. 2001;46:142–165. doi: 10.1002/1097-4695(20010205)46:2<142::aid-neu60>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Franz M, Goller F. Respiratory units of motor production and song imitation in the zebra finch. J Neurobiol. 2002;51:129–141. doi: 10.1002/neu.10043. [DOI] [PubMed] [Google Scholar]
- Gamlin PDR, Cohen DH. A second ascending visual pathway from the optic tectum to the telencephalon in the pigeon (Columba livia) J Comp Neurol. 1986;250:296–310. doi: 10.1002/cne.902500304. [DOI] [PubMed] [Google Scholar]
- Gaunt AS, Gaunt SLL. Mechanics of the syrinx in Gallus gallus. II Electromyographic studies of ad libitum vocalizations. J Morphol. 1977;152:1–20. doi: 10.1002/jmor.1051520102. [DOI] [PubMed] [Google Scholar]
- Gaunt AS, Gaunt SLL, Casey RM. Syringeal mechanics reassessed: evidence from Streptoplia. Auk. 1982;99:474–494. [Google Scholar]
- Gibb L, Gentner TQ, Abarbanel HD. Brain stem feedback in a computational model of birdsong sequencing. J Neurophysiol. 2009a;102:1763–1778. doi: 10.1152/jn.91154.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb L, Gentner TQ, Abarbanel HD. Inhibition and recurrent excitation in a computational model of sparse bursting in song nucleus HVC. J Neurophysiol. 2009b;102:1748–1762. doi: 10.1152/jn.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M, Molony V. Control of breathing. In: King AS, McLelland J, editors. Form and Function in Birds. Academic Press; London: 1989. pp. 439–484. [Google Scholar]
- Goldin MA, Alonso LM, Alliende JA, Goller F, Mindlin GB. Temperature induced syllable breaking unveils nonlinearly interacting timescales in birdsong motor pathway. PLoS One. 2013;8:e67814. doi: 10.1371/journal.pone.0067814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goller F, Cooper BG. Peripheral motor dynamics of song production in the zebra finch. In: Zeigler HP, Marler P, editors. Behavioral Neurobiology of Birdsong. New York Academy of Sciences; New York: 2004. pp. 130–152. [DOI] [PubMed] [Google Scholar]
- Goller F, Cooper BG. Peripheral mechanisms of sensorimotor integration during singing. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Cambridge University Press; New York: 2008. pp. 99–114. [Google Scholar]
- Goller F, Daley MA. Novel motor gestures for phonation during inspiration enhance the acoustic complexity of birdsong. Proc R Soc Lond B Biol Sci. 2001;268:2301–2305. doi: 10.1098/rspb.2001.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goller F, Larsen ON. A new mechanism of sound generation in songbirds. Proc Natl Acad Sci USA. 1997a;94:14787–14791. doi: 10.1073/pnas.94.26.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goller F, Larsen ON. In situ biomechanics of the syrinx and sound generation in pigeons. J Exp Biol. 1997b;200:2165–2176. doi: 10.1242/jeb.200.16.2165. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles in controlling the phonology of bird song. J Neurophysiol. 1996a;76:287–300. doi: 10.1152/jn.1996.76.1.287. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles in gating airflow and sound production in singing brown thrashers. J Neurophysiol. 1996b;75:867–876. doi: 10.1152/jn.1996.75.2.867. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Bilaterally symmetrical respiratory activity during lateralized birdsong. J Neurobiol. 1999;41:513–523. doi: 10.1002/(sici)1097-4695(199912)41:4<513::aid-neu7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci. 2002;22:3806–3816. doi: 10.1523/JNEUROSCI.22-09-03806.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnloser RHR, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- Hahnloser RHR, Wang CZH, Nager A, Naie K. Spikes and bursts in two types of thalamic projection neurons differentially shape sleep patterns and auditory responses in a songbird. J Neurosci. 2008;28:5040–5052. doi: 10.1523/JNEUROSCI.5059-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley RS. Expiratory muscle activity during song production in the canary. Respir Physiol. 1990;81:177–188. doi: 10.1016/0034-5687(90)90044-y. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Suthers RA. Airflow and pressure during canary song: evidence for minibreaths. J Comp Physiol A. 1989;165:15–26. [Google Scholar]
- Hartley RS, Suthers RA. Lateralization of syringeal function during song production in the canary. J Neurobiol. 1990;21:1236–1248. doi: 10.1002/neu.480210808. [DOI] [PubMed] [Google Scholar]
- Holstege G. Anatomical study of the final common pathway for vocalization in the cat. J Comp Neurol. 1989;284:242–252. doi: 10.1002/cne.902840208. [DOI] [PubMed] [Google Scholar]
- Holstege G. The periaqueductal gray controls brainstem emotional motor systems including respiration. Prog Brain Res. 2014;209:379–405. doi: 10.1016/B978-0-444-63274-6.00020-5. [DOI] [PubMed] [Google Scholar]
- Holstege G, Kuypers HG. The anatomy of brain stem pathways to the spinal cord in cat. A labeled amino acid tracing study. Prog Brain Res. 1982;57:145–175. doi: 10.1016/S0079-6123(08)64128-X. [DOI] [PubMed] [Google Scholar]
- Jin DJ. Generating variable birdsong syllable sequences with branching chain networks in avian premotor nucleus HVC. Phys Rev E. 2009;80:051902. doi: 10.1103/PhysRevE.80.051902. [DOI] [PubMed] [Google Scholar]
- Jürgens U. The role of the periaqueductal grey in vocal behavior. Behav Brain Res. 1994;62:107–117. doi: 10.1016/0166-4328(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Jürgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Katz DM, Karten HJ. Visceral representation within the nucleus of the tractus solitarius in the pigeon, Columba livia. J Comp Neurol. 1983;218:42–73. doi: 10.1002/cne.902180104. [DOI] [PubMed] [Google Scholar]
- King AS, McLelland J, editors. Form and Function in Birds. Academic Press; London: 1989. [Google Scholar]
- Kingsbury MA, Kelly AM, Schrock SE, Goodson JL. Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLoS One. 2012;6:e20720. doi: 10.1371/journal.pone.0020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhevnikov AA, Fee MS. Singing-related activity of identified HVC neurons in the zebra finch. J Neurophysiol. 2007;97:4271–4283. doi: 10.1152/jn.00952.2006. [DOI] [PubMed] [Google Scholar]
- Kubke MF, Ross JM, Wild JM. Vagal innervation of the air sacs in a songbird, Taenopygia guttata. J Anat. 2004;204:283–292. doi: 10.1111/j.0021-8782.2004.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubke MF, Yazaki-Sugiyama Y, Mooney R, Wild JM. Physiology of neuronal subtypes in the respiratory-vocal integration nucleus retroamigualis of the male zebra finch. J Neurophysiol. 2005;94:2379–2390. doi: 10.1152/jn.00257.2005. [DOI] [PubMed] [Google Scholar]
- Larsen ON, Goller F. Direct observation of syringeal muscle function in songbirds and a parrot. J Exp Biol. 2002;205:25–35. doi: 10.1242/jeb.205.1.25. [DOI] [PubMed] [Google Scholar]
- Leadbeater E, Goller F, Riebel K. Unusual phonation, covarying song characteristics and song preferences in female zebra finches. Anim Behav. 2005;70:909–919. [Google Scholar]
- Leonardo A, Fee MS. Ensemble coding of vocal control in birdsong. J Neurosci. 2005;25:652–661. doi: 10.1523/JNEUROSCI.3036-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski BC, Alexei A, Hahnloser R, Schmidt MF. At the interface of the auditory and vocal motor systems: NIf and its role in vocal processing, production and learning. J Physiol. 2013;107:178–192. doi: 10.1016/j.jphysparis.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature. 2008;456:189–194. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackleprang R, Goller F. Ventilation patterns of the songbird lung/air sac system during different behaviors. J Exp Biol. 2013;216:3611–3619. doi: 10.1242/jeb.087197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D, Schmidt MF. Sleep, off-line processing, and vocal learning. Brain Lang. 2010;115:45–58. doi: 10.1016/j.bandl.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Garcia F, Novejarque A, Lanuza E. Evolution of the amygdala in vertebrates. In: Kaas JH, Bullock TH, editors. Evolution of Nervous Systems: a Comprehensive Reference. Non-Mammalian Vertebrates. Vol. 2. Academic Press-Elsevier; Amsterdam: 2007. pp. 255–334. [Google Scholar]
- McLean J, Bricault S, Schmidt MF. Characterization of respiratory neurons in the rostral ventrolateral medulla, an area critical for vocal production in songbirds. J Neurophysiol. 2013;109:948–957. doi: 10.1152/jn.00595.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez JM, Dall’Asen AG, Goller F. Disrupting vagal feedback affects birdsong motor control. J Exp Biol. 2010;213:4193–4204. doi: 10.1242/jeb.045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez JM, Mindlin GB, Goller F. Interaction between telencephalic signals and respiratory dynamics in songbirds. J Neurophysiol. 2012;107:2971–2983. doi: 10.1152/jn.00646.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill EG. The lateral respiratory neurones of the medulla: their associations with nucleus amhiguus, nucleus retroambigualis, the spinal accessory nucleus and the spinal cord. Brain Res. 1970;24:11–28. doi: 10.1016/0006-8993(70)90271-4. [DOI] [PubMed] [Google Scholar]
- Mooney R. Neural mechanisms for learned birdsong. Learn Mem. 2009;16:655–669. doi: 10.1101/lm.1065209. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Shiba K, Satoh I, Yoshida K, Nakajima Y, Konno A. Role of pulmonary afferent inputs in vocal on-switch in the cat. J Neurosci Res. 1997;29:49–54. doi: 10.1016/s0168-0102(97)00071-0. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Olveczky BP, Otchy TM, Goldberg JH, Aronov D, Fee MS. Changes in the neural control of a complex motor sequence during learning. J Neurophysiol. 2011;106:386–397. doi: 10.1152/jn.00018.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R, Peek F. Brain organization and neuromuscular control of vocalization in birds. In: Wright P, Caryl P, Vowles D, editors. Hormones and Behaviour in Higher Vertebrates. Elsevier; Amsterdam: 1975. pp. 243–274. [Google Scholar]
- Plummer EM, Goller F. Singing with reduced air sac volume causes uniform decrease in the airflow and sound amplitude in the zebra finch. J Exp Biol. 2008;21:66–78. doi: 10.1242/jeb.011908. [DOI] [PubMed] [Google Scholar]
- Potash LM. Vocalizations elicited by brain stimulation in Coturnix coturnix Japonica. Behavior. 1970;36:149–167. doi: 10.1163/156853970x00286. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Mello CV, Jarvis ED. Songbirds and the revised avian brain nomenclature. Ann NY Acad Sci. 2004;1016:77–108. doi: 10.1196/annals.1298.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke H, Wild JM. Distribution and connections of inspiratory premotor neurons in the brainstem of the pigeon (Columba livia) J Comp Neurol. 1997;379:347–362. [PubMed] [Google Scholar]
- Reinke H, Wild JM. Identification and connections of inspiratory premotor neurons in songbirds and budgerigar. J Comp Neurol. 1998;391:147–163. [PubMed] [Google Scholar]
- Richter DW. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- Riede T, Goller F. Peripheral mechanisms for vocal production in birds—differences and similarities to human speech and singing. Brain Lang. 2010;115:69–80. doi: 10.1016/j.bandl.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Suthers RA, Fletcher NH, Blevins WE. Songbirds tune their vocal tract to the fundamental frequency of their song. Proc Natl Acad Sci USA. 2006;103:5543–5548. doi: 10.1073/pnas.0601262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Fisher JH, Goller F. Sexual dimorphism of the zebra finch syrinx indicates adaptation for high fundamental frequencies in males. PLoS One. 2010;5:e11368. doi: 10.1371/journal.pone.0011368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Schilling N, Goller F. The acoustic effect of vocal tract adjustments in zebra finches. J Comp Physiol A. 2013;199:57–69. doi: 10.1007/s00359-012-0768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TF, Klein ME, Kubke MF, Wild JM, Mooney R. Telencephalic neurons monosynaptically link brainstem and forebrain premotor networks necessary for song. J Neurosci. 2008;28:3479–3489. doi: 10.1523/JNEUROSCI.0177-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachner ER, Cieri RL, Butler JP, Farner CG. Unidirectional pulmonary airflow patterns in the savannah monitor lizard. Nature. 2013;506:367–370. doi: 10.1038/nature12871. http://dx.doi.org/10.1038/nature12871. [DOI] [PubMed] [Google Scholar]
- Scheid P, Piiper J. Respiratory mechanics and air flow in birds. In: King AS, McLelland J, editors. Form and Function in Birds. Academic Press; London: 1989. pp. 369–391. [Google Scholar]
- Schmidt M. Pattern of interhemispheric synchronization in HVc during singing correlates with key transitions in the song pattern. J Neurophysiol. 2003;90:3931–3949. doi: 10.1152/jn.00003.2003. [DOI] [PubMed] [Google Scholar]
- Schmidt MF. Using both sides of your brain: the case for rapid interhemispheric switching. PLoS Biol. 2008;6:2089–2093. doi: 10.1371/journal.pbio.0060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MF, Ashmore RC. Integrating breathing and singing: forebrain and brainstem mechanisms. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Cambridge University Press; New York: 2008. pp. 115–135. [Google Scholar]
- Seller TJ, editor. Bird Respiration. CRC Press; Boca Raton, FL: 1987a. [Google Scholar]
- Seller TJ, editor. Bird Respiration. CRC Press; Boca Raton, FL: 1987b. [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger H, Ballanyi K, Richter DW, Feldman JL. Pre-Boetzinger complex: a brain stem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala APL, Rybak IA, Paton JFR. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B. 2009;364:2577–2587. doi: 10.1098/rstb.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sober SJ, Wohlgemuth MJ, Brainard MS. Central contributions to acoustic variation in birdsong. J Neurosci. 2008;28:10370–10379. doi: 10.1523/JNEUROSCI.2448-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro JE, Dalva MB, Mooney R. Long-range inhibition within the zebra finch song nucleus ra can coordinate the firing of multiple projection neurons. J Neurophysiol. 1999;81:3007–3020. doi: 10.1152/jn.1999.81.6.3007. [DOI] [PubMed] [Google Scholar]
- Striedter GF, Vu ET. Bilateral feedback projections to the forebrain in the premotor network for singing in zebra finches. J Neurobiol. 1998;34:27–40. [PubMed] [Google Scholar]
- Sturdy CB, Wild JM, Mooney R. Respiratory and telencephalic modulation of vocal motor neurons in the zebra finch. J Neurosci. 2003;23:1072–1086. doi: 10.1523/JNEUROSCI.23-03-01072.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH, Holstege G. Periaqueductal gray control of breathing. Adv Exp Med Biol. 2010;669:353–358. doi: 10.1007/978-1-4419-5692-7_72. [DOI] [PubMed] [Google Scholar]
- Subramanian HH, Holstege G. Stimulation of the midbrain periaqueductal gray modulates preinspiratory neurons in the ventrolateral medulla in the rat in vivo. J Comp Neurol. 2013;521:3083–3098. doi: 10.1002/cne.23334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH, Balnave RJ, Chow CM. Behavioural control of breathing in mammals: role of the midbrain periaqueductal gray. In: Champagnat J, Denavit-Saubié M, Fortin G, Foutz AS, Thoby-Bisson M, editors. Post-Genomic Perspectives in Modeling and Control of Breathing. Kluwer Academic/Plenum Publishers; 1994. pp. 135–141. [Google Scholar]
- Subramanian HH, Balnave RJ, Holstege G. The midbrain periaqueductal gray control of respiration. J Neurosci. 2008;28:12274–12283. doi: 10.1523/JNEUROSCI.4168-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthers RA. Contributions to birdsong from the left and right sides of the intact syrinx. Nature. 1990;347:473–477. [Google Scholar]
- Suthers RA. Peripheral control and lateralization of song. J Neurobiol. 1997;33:632–652. [PubMed] [Google Scholar]
- Suthers RA, Zollinger SA. Producing song—the vocal apparatus. In: Zeigler HP, Marler P, editors. Behavioral Neurobiology of Birdsong. New York Academy of Sciences; New York: 2004. pp. 109–129. [DOI] [PubMed] [Google Scholar]
- Suthers RA, Zollinger SA. From brain to song: the vocal organ and vocal tract. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Cambridge University Press; New York: 2008. pp. 78–98. [Google Scholar]
- Suthers RA, Goller F, Hartley RS. Motor dynamics of song production by mimic thrushes. J Neurobiol. 1994;25:917–936. doi: 10.1002/neu.480250803. [DOI] [PubMed] [Google Scholar]
- Suthers RA, Goller F, Pytte C. The neuromuscular control of birdsong. Philos Trans R Soc Lond B Biol Sci. 1999;354:927–939. doi: 10.1098/rstb.1999.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthers RA, Goller F, Wild JM. Somatosensory feedback modulates the respiratory motor program of crystallized birdsong. Proc Natl Acad Sci USA. 2002;99:5680–5685. doi: 10.1073/pnas.042103199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan MA, Mindlin GB, Goller F. Nonlinear model predicts diverse respiratory patterns of birdsong. Phys Rev Lett. 2006;96:0508103. doi: 10.1103/PhysRevLett.96.058103. [DOI] [PubMed] [Google Scholar]
- VanderHorst VG, Holstege G. Estrogen induces axonal outgrowth in the nucleus retroambiguus-lumbosacral motoneuronal pathway in the adult female cat. J Neurosci. 1997;17:1122–1136. doi: 10.1523/JNEUROSCI.17-03-01122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit L, Aronov D, Fee MS. Learning to breathe and sing: development of respiratory-vocal coordination in young songbirds. J Neurophysiol. 2011;106:1747–1765. doi: 10.1152/jn.00247.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert JF, Caille D, Bertrand F, Gromysz H, Hugelin A. Ascending projection from the respiratory centre to mesencephalon and diencephalon. Neurosci Lett. 1979;11:29–33. doi: 10.1016/0304-3940(79)90051-x. [DOI] [PubMed] [Google Scholar]
- Vicario DS. Organization of the zebra finch song control system: II. Functional organization of outputs from nucleus robustus archistriatalis. J Comp Neurol. 1991a;309:486–494. doi: 10.1002/cne.903090405. [DOI] [PubMed] [Google Scholar]
- Vicario DS. Contributions of syringeal muscles to respiration and vocalization in the zebra finch. J Neurobiol. 1991b;22:63–73. doi: 10.1002/neu.480220107. [DOI] [PubMed] [Google Scholar]
- Vicario DS. A new brain stem pathway for vocal control in the zebra finch song system. Neuroreport. 1993;4:983–986. doi: 10.1097/00001756-199307000-00037. [DOI] [PubMed] [Google Scholar]
- Vu ET, Mazurek ME, Kuo YC. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci. 1994;14:6924–6934. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu ET, Schmidt MF, Mazurek ME. Interhemispheric coordination of premotor neural activity during singing in adult zebra finches. J Neurosci. 1998;18:9088–9098. doi: 10.1523/JNEUROSCI.18-21-09088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZH, Herbst JA, Keller GB, Hahnloser RHR. Rapid interhemispheric switching during vocal production in a songbird. PLoS Biol. 2008;6:2154–2162. doi: 10.1371/journal.pbio.0060250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Brzozowska-Prechtl A, Karten HJ. Laminar and columnar auditory cortex in avian brain. Proc Natl Acad Sci USA. 2010;107:12676–12681. doi: 10.1073/pnas.1006645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westneat MW, Long J, Hoese JH, Nowicki S. Kinematics of birdsong: functional correlation of cranial movements and acoustic features in sparrows. J Exp Biol. 1993;182:147–171. doi: 10.1242/jeb.182.1.147. [DOI] [PubMed] [Google Scholar]
- White SS, Chubb JC. The muscles and movements of the larynx of Gallus domesticus. J Anat. 1967;102:575. [Google Scholar]
- Wild JM. The avian nucleus retroambigualis: a nucleus for breathing, singing and calling. Brain Res. 1993a;606:319–324. doi: 10.1016/0006-8993(93)91001-9. [DOI] [PubMed] [Google Scholar]
- Wild JM. Descending projections of the songbird nucleus robustus archistriatalis. J Comp Neurol. 1993b;338:225–241. doi: 10.1002/cne.903380207. [DOI] [PubMed] [Google Scholar]
- Wild JM. Visual and somatosensory inputs to the avian song system via nucleus uvaeformis (Uva) and a comparison with the projections of a similar thalamic nucleus in a nonsongbird, Columba livia. J Comp Neurol. 1994a;349:512–535. doi: 10.1002/cne.903490403. [DOI] [PubMed] [Google Scholar]
- Wild JM. The auditory-vocal-respiratory axis. Brain Behav Evol. 1994b;44:192–209. doi: 10.1159/000113577. [DOI] [PubMed] [Google Scholar]
- Wild JM. Neural pathways for the control of birdsong production. J Neurobiol. 1997;33:653–670. doi: 10.1002/(sici)1097-4695(19971105)33:5<653::aid-neu11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Wild JM. Pulmonary and syringeal afferent input to the avian song system: a possible role in the temporal control of vocalization. Int. Congr. Neuroethology; Nyborg, Denmark. 2004a. [Google Scholar]
- Wild JM. Functional neuroanatomy of the sensorimotor control of singing. In: Zeigler HP, Marler P, editors. Behavioral Neurobiology of Birdsong. New York Academy of Sciences; New York: 2004b. pp. 438–462. [DOI] [PubMed] [Google Scholar]
- Wild JM. Birdsong: anatomical foundations and central mechanisms of sensorimotor integration. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Cambridge University Press; New York: 2008. pp. 136–152. [Google Scholar]
- Wild JM, Arends JJA. A respiratory-vocal pathway in the brainstem of the pigeon. Brain Res. 1987;407:191–194. doi: 10.1016/0006-8993(87)91237-6. [DOI] [PubMed] [Google Scholar]
- Wild JM, Balthazart J. Neural pathways for the control of reproductive behaviour in male Japanese quail. J Comp Neurol. 2013;521:2067–2087. doi: 10.1002/cne.23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM, Krützfeldt NOE. Trigeminal and telencephalic projections to jaw and other upper vocal tract premotor neurons in songbirds: sensorimotor circuitry for beak movements during singing. J Comp Neurol. 2012;520:590–605. doi: 10.1002/cne.22752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM, Arends JJA, Zeigler HP. Projections of the parabrachial nucleus in the pigeon (Columba livia) J Comp Neurol. 1990;293:499–523. doi: 10.1002/cne.902930402. [DOI] [PubMed] [Google Scholar]
- Wild JM, Karten HJ, Frost BJ. Connections of the auditory forebrain in the pigeon (Columba livia) J Comp Neurol. 1993;337:32–62. doi: 10.1002/cne.903370103. [DOI] [PubMed] [Google Scholar]
- Wild JM, Goller F, Suthers RA. Inspiratory muscle activity during bird song. J Neurobiol. 1998;36:441–453. doi: 10.1002/(sici)1097-4695(19980905)36:3<441::aid-neu11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Wild JM, Williams MN, Suthers RA. Neural pathways for bilateral vocal control in songbirds. J Comp Neurol. 2000;423:413–426. doi: 10.1002/1096-9861(20000731)423:3<413::aid-cne5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Wild JM, Kubke MF, Mooney R. Avian nucleus retroambigualis: cell types and projections to other respiratory-vocal nuclei in the brain of the zebra finch (Taeniopygia guttata) J Comp Neurol. 2009;512:768–783. doi: 10.1002/cne.21932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H, Vicario DS. Temporal patterning of song production: participation of nucleus uvaeformis of the thalamus. J Neurobiol. 1993;24:903–912. doi: 10.1002/neu.480240704. [DOI] [PubMed] [Google Scholar]
- Williams H, Ball GF, Faris PL. Evidence for subdivisions within Uvaeformis, an avian song nucleus. Soc. Neurosci. Meeting; 1989. p. 618. [Google Scholar]
- Williams SM, Nast A, Coleman MJ. Characterization of synaptically connected nuclei in a potential sensorimotor feedback pathway in the zebra finch song system. PLoS One. 2012;7:e32178. doi: 10.1371/journal.pone.0032178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AC, Margoliash D. Temporal hierarchical control of singing in birds. Science. 1996;273:1871–1875. doi: 10.1126/science.273.5283.1871. [DOI] [PubMed] [Google Scholar]
- Zeier H, Karten HJ. The archistriatum of the pigeon: organization of afferent and efferent connections. Brain Res. 1971;31:313–326. doi: 10.1016/0006-8993(71)90185-5. [DOI] [PubMed] [Google Scholar]