Abstract
Communication between auditory and vocal motor nuclei is essential for vocal learning. In songbirds, the nucleus interfacialis of the nidopallium (NIf) is part of a sensorimotor loop, along with auditory nucleus avalanche (Av) and song system nucleus HVC, that links the auditory and song systems. Most of the auditory information comes through this sensorimotor loop, with the projection from NIf to HVC representing the largest single source of auditory information to the song system. In addition to providing the majority of HVC’s auditory input, NIf is also the primary driver of spontaneous activity and premotor-like bursting during sleep in HVC. Like HVC and RA, two nuclei critical for song learning and production, NIf exhibits behavioral-state dependent auditory responses and strong motor bursts that precede song output. NIf also exhibits extended periods of fast gamma oscillations following vocal production. Based on the converging evidence from studies of physiology and functional connectivity it would be reasonable to expect NIf to play an important role in the learning, maintenance, and production of song. Surprisingly, however, lesions of NIf in adult zebra finches have no effect on song production or maintenance. Only the plastic song produced by juvenile zebra finches during the sensorimotor phase of song learning is affected by NIf lesions. In this review, we carefully examine what is known about NIf at the anatomical, physiological, and behavioral levels. We reexamine conclusions drawn from previous studies in the light of our current understanding of the song system, and establish what can be said with certainty about NIf’s involvement in song learning, maintenance, and production. Finally, we review recent theories of song learning integrating possible roles for NIf within these frameworks and suggest possible parallels between NIf and sensorimotor areas that form part of the neural circuitry for speech processing in humans.
Keywords: Songbird, Song system, Zebra finch, NIf, Vocal learning, Song learning, Learning, Review
1. Introduction
Songbirds offer a tremendous opportunity for studying the sensorimotor integration underlying vocal learning. Like humans, oscine songbirds learn to reproduce conspecific vocalizations during development through a process that requires vocal practice and auditory feedback (Doupe and Kuhl, 2008; Tschida and Mooney, 2012). Songbirds evaluate their vocal performance by using auditory feedback from self-produced vocalizations and use this performance evaluation to adjust motor patterns, gradually shaping vocal output to match a stored template of tutor song (Williams, 2008). Thus, song learning is critically dependent on the action and interaction of the auditory and vocal-motor systems. While a great deal of progress has been made towards understanding how each of these systems functions independently, far less is understood regarding the interactions between them that enable song learning and maintenance. Part of the difficulty in understanding these interactions can be attributed to the nucleus that sits at the interface of the auditory and song systems: the nucleus interfacialis of the nidopallium (NIf). Despite numerous studies designed to elucidate the function of NIf and the importance of its sensorimotor input to the song system, the role of NIf in song learning and production remains unclear.
In songbirds, higher-order auditory processing occurs in the auditory forebrain by a set of highly interconnected structures organized much like auditory cortex in mammals (Jarvis et al., 2005; Wang et al., 2010). These auditory structures include the Field L complex, the avian homologue of primary auditory cortex in mammals, and secondary auditory areas NCM (caudomedial nidopallium) and CM (caudal mesopallium). Motor production of song in oscine songbirds is controlled by a network of discrete sensorimotor nuclei that are collectively referred to as the song system (Nottebohm et al., 1976, 1982). The song system (Fig. 1) consists of two main pathways: the descending motor pathway and the anterior forebrain pathway (AFP). The descending motor pathway is made up of the telencephalic nucleus HVC (used as a proper name) and its efferent target RA (the robust nucleus of the arcopallium), which sends projections to respiratory and vocal motor nuclei in the brainstem (Wild et al., 2000; Wild, 2004). Converging evidence from lesions (Simpson and Vicario, 1990; Aronov et al., 2008), electrical stimulation (Vu et al., 1994), localized cooling (Long and Fee, 2008; Aronov et al., 2011), and single cell recordings (Hahnloser et al., 2002, 2006) indicate that HVC drives the descending motor pathway to shape many of the spectrotemporal features of song. The anterior forebrain pathway consists of a basal ganglia–thalamo-cortical circuit that indirectly links HVC to RA and is critical for song learning (Brainard and Doupe, 2000a). In addition to these two pathways, the song system also contains two recurrent “thalamocortical” pathways that indirectly link RA back to HVC (Schmidt et al., 2004). One of these pathways provides ascending feedback to NIf and HVC from the vocal-respiratory brainstem via thalamic nucleus uvaeformis (Uva) and is critical for normal song production (Striedter and Vu, 1998; Coleman and Vu, 2005; Ashmore et al., 2008; Akutagawa and Konishi, 2010).
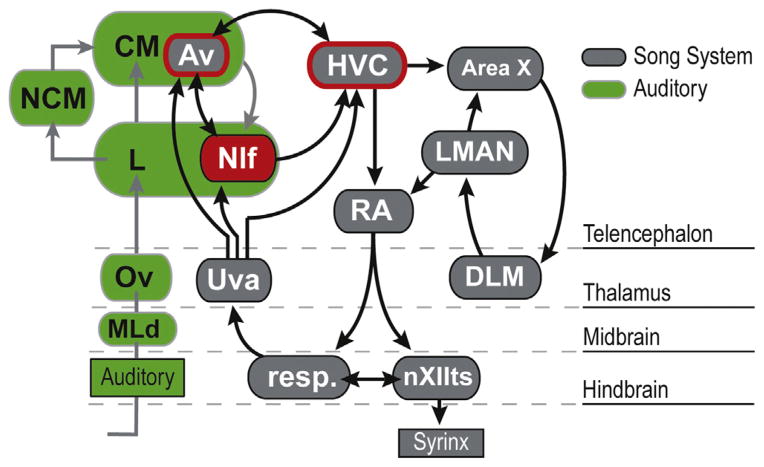
Fig. 1.
Auditory and motor pathways in the avian brain. Input from the auditory pathway (shown in green) reaches NIf from CM, particularly through a reciprocal connection with the Av subdivision of CM. Av also shares a reciprocal connection with HVC. The connections between Av, NIf, and HVC form a sensorimotor loop that links the auditory forebrain and the song system (highlighted in red). The descending motor pathway consists of the projection from HVC to RA and the projections from RA onto brainstem respiratory nuclei (resp.) and the tracheosyringeal portion of the hypoglossal nucleus (nXIIts). Whether the projection from NIf to HVC should also be included in the descending motor pathway is not yet clear (see Section 4). Ascending feedback from brainstem vocal and respiratory centers reaches NIf and HVC via the thalamic nucleus Uva. The anterior forebrain pathway (AFP), which is critical for song learning, consists of Area X, which receives input from HVC, the medial nucleus of the dorsolateral thalamus (DLM), and the lateral magnocellular nucleus of the anterior nidopallium (LMAN), which projects to RA. Abbreviations: L: the Field L complex; MLd: dorsal lateral nucleus of the mesencephalon; NCM: caudal medial nidopallium; Ov: nucleus ovoidalis.
The central location of HVC and its critical importance for song learning and production (Mooney, 2009) have led to numerous studies seeking to understand how activity in HVC influences the activity of other nuclei in the song system. In this review, we focus on NIf, one of the nuclei, which most strongly influences HVC’s own neural activity (Cardin and Schmidt, 2004b; Coleman and Mooney, 2004; Cardin et al., 2005). Like other song system nuclei, NIf exhibits both auditory and vocal-motor activity. Auditory activity in NIf is of particular interest because NIf’s input to HVC is the largest single source of auditory information to the song system (Vates et al., 1996; Cardin and Schmidt, 2004a; Coleman and Mooney, 2004; Cardin et al., 2005; Bauer et al., 2008). In addition, NIf provides nearly all of HVC’s spontaneous excitatory drive (Cardin and Schmidt, 2004a; Cardin et al., 2005), has premotor bursts that precede similar bursts in HVC (McCasland, 1987; Lewandowski and Schmidt, 2011), and drives the replay of premotor-like bursting in HVC during sleep (Hahnloser and Fee, 2007). Given the critical necessity of HVC for song learning and production, NIf is well positioned to have a significant impact on song system function. Paradoxically, while inactivation of NIf affects song production (Naie and Hahnloser, 2011) and learning (Roberts et al., 2012) during the critical sensorimotor learning phase, bilateral lesions of NIf appear to have little effect on the production and auditory-feedback dependent maintenance of song (Cardin et al., 2005; Roy and Mooney, 2009). In this review we discuss these and other findings relevant to NIf and examine what can be said with confidence about NIf’s function in the song system and what awaits additional experimental verification. Finally, we discuss the possible role that NIf may play in song learning and maintenance.
2. Anatomical and physiological characteristics of NIf
2.1. Cytoarchitecture and cellular organization of NIf
NIf lies at the interface of the auditory forebrain and the song system both functionally and physically. NIf is embedded between the auditory forebrain areas L1 and L2a, two major subdivisions of the Field L complex (Fortune and Margoliash, 1992, 1995). It is a small, irregularly shaped nucleus consisting of a thin plate of cells extending dorsocaudally from the dorsal medullary lamina along the anterior surface of L2a (see Fortune and Margoliash, 1992 for a three-dimensional reconstruction of NIf within the Field L complex). Its neurons are distributed without any obvious clustering or orientation, with the exception of neurons near the borders of NIf, which tend to orient along the border (Fortune and Margoliash, 1995). The auditory forebrain contains at least five distinct neural subtypes with one type (the ‘type 5 neuron’) found exclusively in NIf (Fortune and Margoliash, 1992). Type 5 neurons are relatively large and have two distinct types of dendrites: thin dendrites with almost no branching and moderate spine density, and thick dendrites with many branches but an unusual lack of dendritic spines up to their first branch point. Retrograde tracers injected into HVC label primarily type 5 cells in NIf (Fortune and Margoliash, 1995). Some of the type 5 cells labeled along the border of NIf have extensive dendritic arbors extending into the L1 subdivision of Field L (Fortune and Margoliash, 1995); however, it remains unclear whether these dendrites receive input from Field L or its afferents (Vates et al., 1996; Bauer et al., 2008).
2.2. Functional connectivity
2.2.1. Afferent input
Auditory inputs to NIf originate primarily from CM, a secondary auditory area that is strongly innervated by various subdivisions of the Field L complex (Wild et al., 1993; Vates et al., 1996) and shares a reciprocal connection with the secondary auditory area, NCM (Vates et al., 1996). Most of the auditory input from CM to NIf originates from a subdivision of CM, known as nucleus avalanche (Av), that is defined by its strong reciprocal connections with both NIf and HVC (Nottebohm et al., 1982; Akutagawa and Konishi, 2010). NIf also receives a likely somatosensory input from the rostral Wulst (Wild and Williams, 1999). Finally, NIf may receive multisensory (somatosensory, visual and auditory) input from Uva, which also projects directly to HVC (Bischof and Engelage, 1985; Wild, 1994; Coleman et al., 2007; Mendez et al., 2009). Uva’s projections to NIf and HVC arise from two separate and non-overlapping populations of neurons (Akutagawa and Konishi, 2010). Uva plays a critical role in providing feedback from the brainstem respiratory/vocal-motor complex during singing (Okuhata and Nottebohm, 1992; Williams and Vicario, 1993; Schmidt et al., 2004; Ashmore et al., 2005; Coleman and Vu, 2005; Ashmore et al., 2008) and is believed to influence auditory responses driven via NIf and CM (Coleman and Mooney, 2004; Akutagawa and Konishi, 2005; Coleman et al., 2007; Hahnloser et al., 2008).
2.2.2. Efferent projections
NIf has two known efferent targets that link it directly to the song system: Av and HVC. The influence of NIf’s input on activity in Av has yet to be investigated. Much more is known about the excitatory projection from NIf to HVC and the importance of this projection for the learning, maintenance, and production of song is a primary focus of this review. NIf is also reciprocally connected to the hyperpallium accessorium in the rostral Wulst, a somatosensory telencephalic area (Wild and Williams, 1999). A somatosensory role for NIf remains currently underexplored and will not be discussed in this review.
2.3. NIf is the primary source of spontaneous excitatory drive to HVC
Though the details of NIf’s influence on HVC during song are not yet clear, activity in both structures is tightly correlated (Cardin and Schmidt, 2004a) with NIf activity typically preceding HVC activity by less than 2 msec (Coleman and Mooney, 2004). In non-singing anesthetized birds, paired recordings of multiunit activity in NIf and intracellular activity in HVC reveal that spontaneous bursts in NIf precede excitatory postsynaptic potentials (EPSPs) in RA-projecting HVC neurons (HVCRA), HVC interneurons (HVCInt), and Area X-projecting HVC neurons (HVCX), although in the latter cell type, initial EPSPs are followed by inhibition, possibly due to input from HVC interneurons (Coleman and Mooney, 2004). These results suggest that NIf provides a direct short-latency excitatory input to HVC. In support of this conclusion, inactivation of NIf severely attenuates both auditory responses and spontaneous firing in HVC (Cardin and Schmidt, 2004a; Cardin et al., 2005; Roy and Mooney, 2009). Furthermore, injection into NIf of the GABAA-agonist muscimol or the sodium channel blocker lidocaine leads to transient elimination of bursting activity in HVC (Cardin and Schmidt, 2004a). Interestingly, despite the effect on bursts, HVCInt neurons keep spiking after NIf inactivation, suggesting that spontaneous HVCInt activity composed of single spikes is not driven by NIf (Hahnloser et al., 2008).
3. Auditory responses in NIf and HVC
3.1. Auditory inputs to NIf and the song system
In order to utilize auditory feedback to shape and maintain vocal motor patterns, auditory information must reach the song system. To date, two pathways have been identified by which auditory information can reach NIf and the rest of the song system: one involves direct projections from secondary auditory area CM to NIf and HVC, while the other indirectly links the brainstem nucleus LLV (ventral nucleus of the lateral lemniscus) to NIf and HVC via the thalamic nucleus Uva (Vates et al., 1996; Coleman et al., 2007; Shaevitz and Theunissen, 2007; Akutagawa and Konishi, 2010).
The primary auditory input to NIf comes from Av, a subregion of CM (Vates et al., 1996; Akutagawa and Konishi, 2010). Av is distinguished from the rest of CM by its reciprocal connections with both NIf and HVC (Akutagawa and Konishi, 2010). NIf also appears to receive sparse projections from other parts of CM (Vates et al., 1996); however, because Av has been characterized so recently, it is currently difficult to evaluate whether some of the projections between CM and NIf described in previous studies originated from outside the borders of Av. A detailed analysis of auditory tuning in Av neurons has yet to be conducted; however, it has been shown that response selectivity in anesthetized birds for the bird’s own song (BOS) over other auditory stimuli is higher in Av than in other regions of CM (Akutagawa and Konishi, 2010). The second auditory pathway, which links nucleus LLV to NIf and HVC via the intermediary of Uva (Coleman et al., 2007), is less well described and its role is currently unclear, but unlike Av input, LLV input to HVC does not contribute to BOS selectivity in HVC (Coleman et al., 2007; Bauer et al., 2008).
Despite receiving a direct auditory projection from Av, two pieces of evidence indicate that the majority of auditory information reaching HVC comes from NIf. First, simultaneous extracellular recordings from NIf and intracellular recordings in HVC show that stimulus-evoked auditory activity in NIf precedes subthreshold events in all HVC neural subtypes (Coleman and Mooney, 2004). Second, inactivation of NIf severely attenuates both subthreshold and suprathreshold responses to auditory stimuli in HVC (Cardin and Schmidt, 2004a; Coleman and Mooney, 2004; Cardin et al., 2005). Thus, both functional and anatomical studies agree that NIf is the largest single source of auditory information to HVC, and, by extension, the song system. Nevertheless, weak auditory responses can be elicited in HVC following NIf lesions and these disappear when CM is reversibly silenced (Bauer et al., 2008), indicating that HVC still receives some auditory input from CM, specifically Av (Akutagawa and Konishi, 2010). The connections between Av, NIf, and HVC form a loop at the interface of the auditory forebrain and the song system. The exact role of this Av–NIf–HVC loop in song learning and production is currently unknown, but all three nuclei have been implicated in song learning and/or production (Simpson and Vicario, 1990; Mooney, 2009; Lei and Mooney, 2010; Naie and Hahnloser, 2011; Roberts et al., 2012).
3.2. Auditory processing of song stimuli
Neurons in Field L, the primary cortical auditory area, are selective for complex combinations of spectral and temporal features found in natural stimuli, such as conspecific song (Sen et al., 2001; Grace et al., 2003; Theunissen et al., 2004; Nagel and Doupe, 2008; Kim and Doupe, 2011), while CM, which receives input from Field L (Vates et al., 1996; Shaevitz and Theunissen, 2007) tends to be more selective for individual conspecific vocalizations (Gentner and Margoliash, 2003; Theunissen et al., 2004; Akutagawa and Konishi, 2010). CM appears to be the lowest stage of the main auditory pathway at which neurons start showing some specificity for BOS by responding more strongly to BOS than to time-reversed BOS (REVBOS) and conspecific song (Bauer et al., 2008), particularly when recordings are obtained from the Av subdivision of CM (Akutagawa and Konishi, 2010). In contrast to Field L, auditory responses in NIf and HVC are highly dependent on behavioral state and their responses in the awake bird tend to become much more variable in strength and less selective for BOS (Schmidt and Konishi, 1998; Cardin and Schmidt, 2003, 2004a; Raksin et al., 2012).
3.2.1. Sleeping/anesthetized auditory responses
Song system nuclei, including NIf, exhibit selective responses for BOS over other auditory stimuli in sleeping/anesthetized birds (Janata and Margoliash, 1999; Cardin and Schmidt, 2004a; Coleman and Mooney, 2004). Given that NIf receives input from neurons in CM that are both BOS-selective and non-selective, it has been suggested that the increased selectivity for BOS is due to local processing within NIf (Bauer et al., 2008). While auditory responses in HVC are driven primarily by NIf, there are important differences in these two nuclei. First, NIf projection neurons tend to fire at many points throughout a BOS stimulus, while HVC projection neurons exhibit a much sparser firing pattern, with some neurons firing only a single burst of action potentials per song motif (Mooney, 2000; Coleman and Mooney, 2004). Second, while NIf neurons show preferences for BOS, they also respond significantly to non-BOS stimuli, such as the BOS played in reverse (REVBOS) and conspecific song, whereas HVC neurons respond very little to non-BOS stimuli (Janata and Margoliash, 1999; Cardin and Schmidt, 2004a; Coleman and Mooney, 2004; Bauer et al., 2008). It is important to note, however, that multiunit auditory responses in NIf and subthreshold auditory responses in HVC are statistically indistinguishable, indicating that the sparse firing and increased BOS selectivity emerge at the level of HVC, possibly through a simple thresholding mechanism (Coleman and Mooney, 2004).
3.2.2. Awake auditory responses
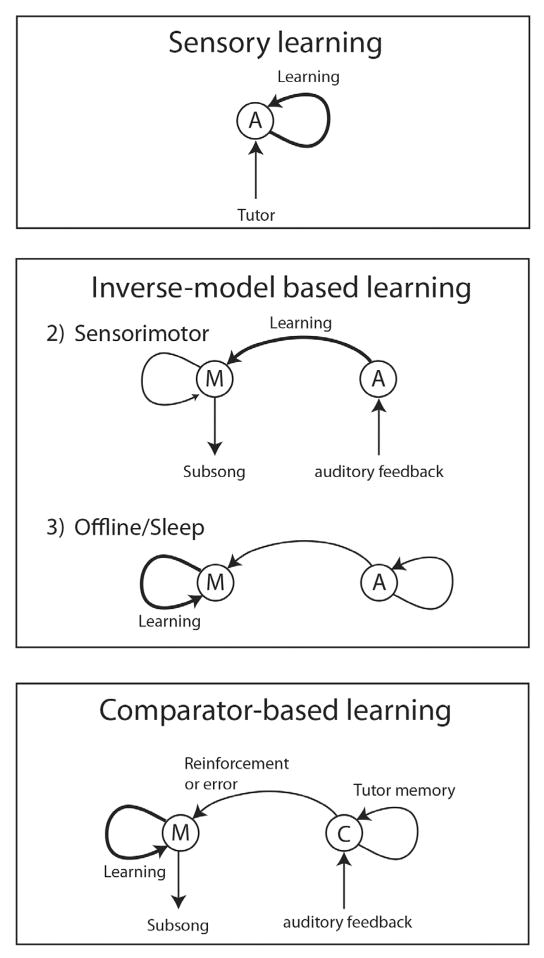
In the awake bird, auditory responses to BOS in both NIf and HVC tend to be much weaker than in the sedated or sleeping bird (Dave et al., 1998; Cardin and Schmidt, 2003, 2004a). Auditory responses are also much more variable, as revealed by strongly fluctuating response strengths measured over time at individual recording sites in NIf and HVC (Cardin and Schmidt, 2003, 2004a). In some cases auditory responses can fluctuate from strong to almost non-existent within minutes (Cardin and Schmidt, 2004a). Such modulation of auditory activity is not observed in Field L and it is currently thought to occur at the level of NIf and HVC (Schmidt and Konishi, 1998; Cardin and Schmidt, 2003; Rauske et al., 2003) through local neuromodulatory influences (see below). The possibility that some degree of modulation might occur in auditory forebrain areas such as CM has never been explicitly investigated; however, auditory responses in the CM of awake birds to both BOS and non-BOS stimuli are reportedly robust (Bauer et al., 2008). Simultaneous recordings in NIf and HVC show that variability in awake auditory responses is strongly correlated between these nuclei (Fig. 2a) (Cardin and Schmidt, 2004a), providing further support for the idea that NIf is the primary driver of auditory responses in HVC in both the sleep and awake states.
Fig. 2.
Norepinephrine and the gating of auditory responses in NIf and HVC. (A) Interleaved air puffs (indicated by asterisks) to the bird’s skin completely suppress auditory responses in NIf (red) and HVC (blue) but have no effect on auditory responses in Field L (green). Auditory responses are measured as response strength with negative numbers signifying a suppression of activity relative to baseline. (B) Localized injection of high norepinephrine (5 mM) into NIf (top panel) causes a complete suppression of auditory responses in HVC evoked by the presentation of BOS. In contrast, injection of low concentrations of NE (0.5 mM) causes an increase in the evoked response as well as a suppression of spontaneous activity, effectively causing a large increase in response strength.
An examination of the receptive fields of putative interneurons in HVC (HVCInt) found that the majority of single units had significant shifts in their receptive fields between the sleeping and awake states (Rauske et al., 2003; Raksin et al., 2012). Generally, neural responses were non-linear and BOS-selective in sleeping birds, but became highly linear in the awake bird, in some cases resembling receptive fields of midbrain auditory neurons (Woolley et al., 2006; Raksin et al., 2012). While a detailed analysis of single-unit auditory receptive field properties has not been performed in NIf, it is likely that such an analysis would show similar trends to those observed in HVC, suggesting that these structures operate under two very different auditory modes depending on the animal’s behavioral state. Functionally, the decrease of BOS selectivity in the NIf and HVC of awake birds affords these structures an increased responsiveness to ethologically relevant stimuli that include conspecific songs and female long calls (Bauer et al., 2008; Raksin et al., 2012; Lewandowski, unpublished data). The degree to which auditory tuning in the awake bird is selective to the BOS might, however, vary with developmental age and even with the species being studied. For example, HVC neurons in juvenile zebra finches show auditory related responses to both tutor song and the bird’s own song during waking states early in development (Nick and Konishi, 2005) and recordings from HVC in Bengalese finches, starlings and several species of sparrows reveal auditory responses that are selective to the BOS in the awake animal (Margoliash and Konishi, 1985; George et al., 2005; Nealen and Schmidt, 2006; Prather et al., 2008; Sakata and Brainard, 2008; Prather et al., 2009).
3.2.3. Auditory responses during singing
Currently it is not known whether NIf neurons are sensitive to auditory input during singing, either in the form of auditory feedback from the song itself or to sounds that occur during singing and cause distortions in the auditory feedback signal. A number of studies recording either from HVC or LMAN (lateral magnocellular nucleus of the anterior nidopallium; a song nucleus that forms part of the anterior forebrain pathway), have failed to find any evidence that neurons in the song system respond to distortions in auditory feedback (Leonardo, 2004; Kozhevnikov and Fee, 2007; Bauer et al., 2008; Prather et al., 2008, but see Sakata and Brainard, 2008), even though such distortions eventually result in the degradation of a bird’s crystallized song (Leonardo and Konishi, 1999; Kozhevnikov and Fee, 2007). Given the lack of auditory responsiveness in these areas during singing, it has been suggested that auditory sensitivity might be gated during singing (Schmidt and Konishi, 1998; Cardin and Schmidt, 2004b; Hahnloser and Ganguly, in press). Several possible mechanisms for such gating have been proposed. While not mutually exclusive, some evidence suggests that gating occurs directly at the level of HVC, either through cholinergic modulation from the basal forebrain (Shea and Margoliash, 2003) or through direct influences from Uva (Akutagawa and Konishi, 2005; Coleman et al., 2007; Hahnloser et al., 2008), while other findings suggests that gating occurs directly at the level of NIf (see below). In contrast to NIf and HVC, sensitivity to distorted auditory feedback has been observed in Field L and CM (Keller and Hahnloser, 2009), consistent with the idea that gating of auditory responses likely occurs for the first time at the level of the song system.
3.3. Auditory responses in NIf are “gated” by norepinephrine
There is direct and indirect evidence that NIf receives several different neuromodulatory inputs. To date these include inputs from the cholinergic (Ryan and Arnold, 1981), noradrenergic (Mello et al., 1998; Castelino and Schmidt, 2010) and possibly dopaminergic (Soha et al., 1996) systems. Given that auditory responses in HVC and NIf are strongly modulated by the animal’s behavioral state (Cardin and Schmidt, 2004a), it has been hypothesized that some of these neuromodulators act directly on NIf to modulate auditory responsiveness. While activation of cholinergic or dopaminergic receptors in NIf only modestly affect auditory responses (Cardin and Schmidt, unpublished data), addition of norepinephrine (or specific noradrenergic receptor agonists) profoundly alters auditory responses (and spontaneous activity) in both NIf and HVC (Fig. 2b) (Cardin and Schmidt, 2004b). Low doses of norepinephrine (NE) applied to NIf (0.5 mM; likely through the activation of α1-receptors) cause an enhancement of the evoked auditory responses recorded in HVC; this is combined with a concomitant decrease in HVC’s spontaneous activity resulting in a dramatic enhancement of the overall auditory signal to noise ratio. In contrast, higher doses of norepinephrine (5 mM; likely mediated through α2-receptors) cause a complete suppression of spontaneous as well as evoked auditory responses in both NIf and HVC (Cardin and Schmidt, 2004b). To test whether norepinephrine is directly involved in mediating behavioral state-dependent changes in auditory responses, Cardin and Schmidt (2004b) infused a cocktail of α1 and α2 adrenergic-receptor antagonists directly into NIf while using gentle arousal from sedation to shift the bird’s behavioral state. In the absence of adrenergic manipulation, brief arousal from sedation causes a near complete suppression of auditory responses in both HVC and NIf but not in field L (Cardin and Schmidt, 2003, 2004a). However, injection of adrenergic-receptor antagonists into NIf completely prevents arousal-induced suppression of BOS responses (Cardin and Schmidt, 2004b), providing direct evidence that norepinephrine mediates arousal-induced suppression in NIf and HVC.
The dose-dependent relationship of norepinephrine on auditory responses, with low doses enhancing responses and higher doses suppressing them, is consistent with its effect in other sensory systems (Aston-Jones and Cohen, 2005). As such it provides a mechanism by which norepinephrine might be able to modulate auditory responses in NIf (and therefore HVC), with small increases in NE enhancing auditory responses and large increases causing a suppression, or “gating”, of auditory responses into the song system. These findings suggest that norepinephrine, possibly in concert with other neuromodulators acting directly on HVC (Shea et al., 2010; Shea and Margoliash, 2010), plays a fundamental role in modulating auditory input into the song system. Whether or not these neuromodulators play a role in directly gating auditory flow during singing is currently unknown.
4. Motor and motor-related neural activity in NIf
4.1. Vocal-motor activity in NIf and the song system
The neural basis of song production has been studied most extensively in HVC and its efferent target RA. Single unit recordings in HVC and RA reveal a sparse motor code underlying the production of song. In the zebra finch, RA-projecting HVC neurons (HVCRA) fire a single burst of action potentials at the same precise time in each song motif (Hahnloser et al., 2002), while projection neurons in RA fire multiple precisely timed bursts during each song motif (Yu and Margoliash, 1996; Leonardo and Fee, 2005). The variability of the timing of each burst across song renditions is remarkably low and typically less than a millisecond in both HVC and RA projection neurons (Chi and Margoliash, 2001; Kozhevnikov and Fee, 2007). Anatomical studies suggest that each HVCRA neuron projects to multiple RA neurons, and that each RA projection neuron receives input from multiple HVCRA neurons (Kittelberger and Mooney, 1999, 2005; Yip et al., 2012). This pattern of connectivity helps explain how the one-burst-per-motif firing pattern of HVCRA neurons gives rise to the multiple precisely timed bursts in RA.
Neither the firing patterns of identified NIf neurons during song, nor the precise connectivity between NIf and HVC neurons have been carefully examined, so the influence of NIf on motor activity in HVC is largely unknown. At the multiunit level, motor activity in NIf, HVC, and RA is broadly similar (Yu and Margoliash, 1996; Schmidt, 2003; Kozhevnikov and Fee, 2007; Lewandowski and Schmidt, 2011). Unlike single unit activity, multiunit activity in all three of these nuclei is characterized by increases in neural activity that precede vocal output and continue throughout song (Fig. 3). Multiunit activity is generally strongest shortly before and during syllable production and weakest during the times corresponding to silent intervals between syllables. Lewandowski and Schmidt (2011) quantified multiunit motor activity in NIf and found that it precedes the onset of song introductory notes by an average 45.7 ± 15.7 ms, which is remarkably similar to the song premotor onset value of 45 ms reported for HVC (Schmidt, 2003; Kozhevnikov and Fee, 2007). Consistent with the idea that activity in NIf during song production is motor and not auditory related, vocalization-related multiunit activity in NIf ends 26.5 ± 13.5 ms before vocal offset, and this cessation of premotor activity is accompanied by a general suppression of neuronal activity in NIf (Fig. 3), with firing rates remaining below baseline levels for an average of 240 ± 204 ms after song offset. This general pattern of premotor activity across many NIf sites is consistent for songs and long calls and agrees with latencies recorded in earlier studies (McCasland, 1987).
Fig. 3.
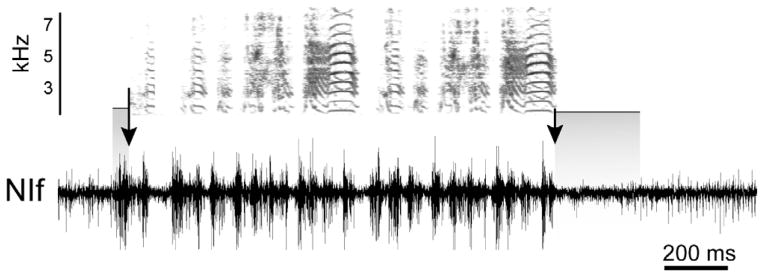
Premotor activity in NIf. Top. Sonogram of a single song with two motifs. Darker colors represent higher power at a given frequency. Bottom. Multiunit activity recorded in NIf during the production of song. Note the increase in neural activity that occurs before the onset of vocal output. On the left, a shaded box highlights this premotor activity for the first introductory note whose onset is indicated by the vertical arrow. The suppression of spontaneous firing following premotor activity can also be seen in this example. On the right, the offset of song is indicated by the vertical arrow and the period of suppressed firing is highlighted with a shaded box.
4.2. NIf and its role in driving premotor-like bursting in HVC in sleeping/sedated birds
Sleep is widely believed to be involved in learning and memory consolidation (Maquet, 2001; Walker et al., 2003; Stickgold, 2005). In humans it positively impacts performance in a number of tasks that include visual discrimination (Mednick et al., 2003), motor learning (Fischer et al., 2002), reaction time (Maquet et al., 2000), and spatial learning (Peigneux et al., 2004). The interaction between exposure to a task and sleep seems to be bi-directional: the exposure to a task affects the activity in the involved brain regions during sleep, and the activity during sleep positively correlates with increases in performance (Huber et al., 2004; Huber et al., 2006). Beneficial roles of sleep for behavioral learning are sometimes are often associated with sleep-related spontaneous neural activity that closely resembles activity that occurred during daytime behavior. In rats for example, hippocampal place cells that fire together during a spatial behavioral task also exhibit an increased tendency to fire together during subsequent slow-wave sleep (Wilson and McNaughton, 1994); and spike sequences of pyramidal cells during wheel running are ‘re-played’ during slow-wave sleep, though at a faster timescale than during behavior (Nadasdy et al., 1999).
In songbirds, some of the spontaneous bursting activity recorded in HVC and RA of sedated or sleeping birds also strongly resembles singing-related premotor activity both in terms of the structure of individual neural bursts and the temporal pattern of burst activity across neural ensembles (Dave and Margoliash, 2000). While it is not known whether the replay of premotor activity patterns during sleep observed in RA (Dave and Margoliash, 2000) and HVC (Chi et al., 2003) also occurs in NIf, functional connectivity studies indicate that NIf is the highest place along the motor pathway (most distant from the muscles) to initiate and drive bursting activity in HVC and RA during sleep (Hahnloser and Fee, 2007). In theory, sleep related bursting activity in NIf and HVC could require input from Uva, the thalamic nucleus that provides ascending sensorimotor projections to both NIf and HVC. However, inactivation of Uva in sleeping birds actually leads to an increase in HVCInt burst rates, indicating that NIf is capable of driving sleep bursts in HVC without input from Uva (Hahnloser et al., 2008). Uva neurons that project to NIf (UvaNIf) and HVC (UvaHVC) form two separate, non-overlapping populations (Akutagawa and Konishi, 2010). While input from UvaHVC neurons actually suppresses bursting activity in HVCInt, bursts from UvaNIf neurons are strongly correlated with subsequent bursting in HVCInt (Hahnloser et al., 2008), indicating that NIf mediates the excitatory Uva drive destined for HVC (Fig. 4). Thus, through its excitatory projection to HVC, NIf plays an important role in driving the premotor-like bursting patterns observed in HVC and RA during sleep.
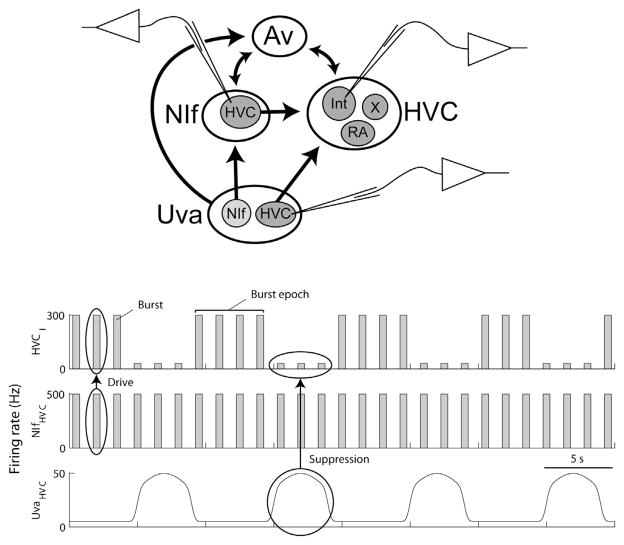
Fig. 4.
Antagonistic influences of NIf and Uva on HVC activity during sleep. Top. Diagram of connections between Uva and the Av–NIf–HVC sensorimotor loop. The populations of neurons whose interactions are outlined in the figure below are indicated by recording electrodes. Bottom. During sleep, HVC interneurons (HVCI) fire high-frequency bursts in brief epochs lasting several seconds. These bursts are driven by bursts in HVC-projecting NIf neurons (NIfHVC) that burst more or less regularly without forming epochs of increased burst rates. Burst epochs in HVCI are also shaped by single spikes (low frequency firing) in UvaHVC neurons: these spikes suppress bursting in HVCI neurons (as opposed to high-frequency bursts in UvaHVC neurons that may have an excitatory influence on HVCI bursting, not shown).
4.3. Potential roles of NIf in the production of song
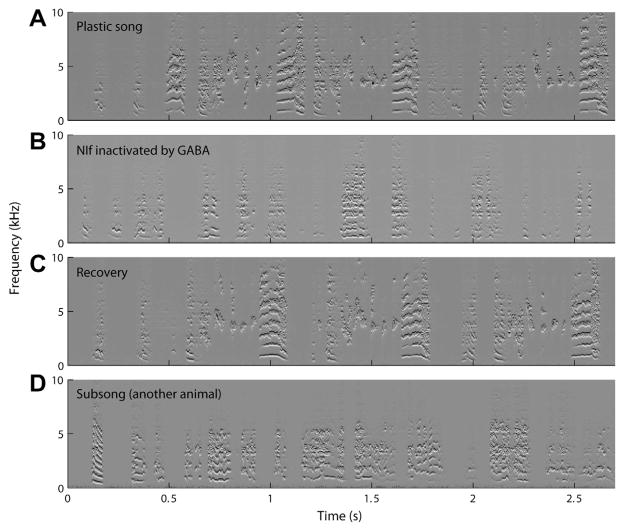
The role of NIf in vocal motor production was investigated by Naie and Hahnloser (2011) using GABA and muscimol mediated reversible inactivation of NIf. The effects of NIf inactivation on song production were examined in zebra finches of three different age groups: young juveniles (36–50 days) singing subsong, older juveniles (51–83 days) singing plastic song, and adults (>100 days). NIf inactivation had no effect on subsong but had a profound effect on plastic song causing it to revert to a state resembling subsong both in terms of spectral sound features and song rhythm (Fig. 5). These findings parallel the effects of HVC lesions in juvenile birds, which do not alter subsong but cause plastic song to revert to the subsong state (Aronov et al., 2008). Because NIf is located upstream of HVC, these results indicate that NIf is critical for driving plastic song production in juveniles.
Fig. 5.
Transient song degradation following bilateral NIf inactivation in a 72 days post-hatch juvenile singing plastic song. (A–C) Injections of GABA into NIf lead to loss of temporal and spectral song structure. (D) Typical subsong (35 days post-hatch, different animal) has similar characteristics as NIf-inactivated songs.
Given the large premotor bursts in NIf that precede song output (Lewandowski and Schmidt, 2011), NIf’s strong excitatory projection to HVC (Coleman and Mooney, 2004), and the effects of NIf lesions on plastic song (Naie and Hahnloser, 2011), it seems reasonable that NIf should play a critical role in the production of crystallized song in adults. However, in contrast to HVC lesions, which in adult birds cause crystallized song to revert to subsong (Aronov et al., 2008), NIf lesions, either temporary (Naie and Hahnloser, 2011) or permanent (Cardin et al., 2005; Roy and Mooney, 2009), cause no significant deterioration in crystallized song. At most, NIf lesions in adult birds cause transient (hours to days) disruptions in syllable sequence stereotypy, manifested as an increased probability of stopping songs in mid-motif and occasional syllable loss or destabilization of harmonic stacks (Cardin et al., 2005; Roy and Mooney, 2009; Naie and Hahnloser, 2011). The decrease in stereotypy may indicate that NIf’s excitatory input helps reinforce the sequence of motor activity in HVC that directs the spectrotemporal features of song; this type of reinforcement would fit with the evidence for direct excitatory input from NIf to HVCRA neurons (Coleman and Mooney, 2004). In the adult bird, the loss of this reinforcing input is presumably compensated for rapidly, as evidenced by the resumption of normal song production within a day following NIf lesions. Theories on NIf’s role in song learning, discussed in Section 5, may help explain why NIf actively shapes the motor output of plastic song in juveniles but only reinforces the production of crystallized song in adults.
In contrast to zebra finches, which sing a highly stereotyped song, lesions of NIf in Bengalese finches do have a profound effect on adult song production. Bengalese finches sing syntactically complex songs composed of a series of phrases, which resemble zebra finch motifs insofar as they consist of a stereotyped sequence of one or more syllables. These phrases are strung together in a pseudorandom order with each phrase having a certain probability of being either repeated or followed by a select number of other phrases (Okanoya, 2004). The effect of NIf lesions on Bengalese finch song can be generalized as a reduction or elimination of low probability phrase-to-phrase transitions, effectively causing songs to become less variable and more deterministic (Hosino and Okanoya, 2000). This suggests that NIf may play a role in controlling higher order syntactic structures in song. In a hierarchical view of the song system, where HVC encodes the structure of motifs/phrases, NIf might influence phrase transitions either by controlling these transitions directly or by injecting neural noise, which might bias phrase transition probabilities. Thus, in Bengalese finches with NIf lesions, songs become less variable because the absence of NIf input causes HVC to follow the more established phrase-to-phrase transitions. In zebra finches, whose songs lack higher order syntactic structures, NIf lesions would not be expected to have significant effects on song production. Further research into the effects of NIf lesions in songbird species with more syntactically complex songs would be particularly informative for the formation of more complete and conclusive theories about NIf’s involvement in the production of adult song.
4.4. Vocalization triggered fast gamma oscillations in NIf
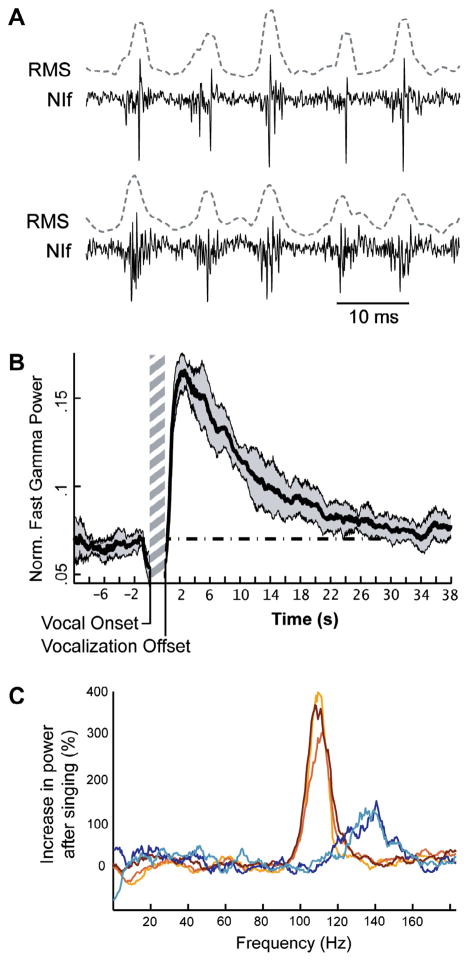
In addition to premotor bursting during vocal production, NIf exhibits a form of vocalization-related neural activity that has yet to be reported in any other avian auditory or song system nucleus. Following the brief suppression of neural activity that occurs after song production (Fig. 3), NIf exhibits strong and coherent oscillatory activity in the fast gamma range (90–150 Hz; Fig. 6) (Lewandowski and Schmidt, 2011). These oscillations are observed both in local field potential (LFP) recordings and from the synchronized spiking activity of neurons in multiunit recordings (Fig. 6a). Similar gamma and fast gamma oscillations are found throughout the mammalian brain (Schoffelen et al., 2005; Siegel et al., 2008), including in human speech processing centers (Giraud and Poeppel, 2012), where their detection is facilitated by the laminated structure of mammalian cortex (Logothetis, 2003). Unlike other instances of event-related gamma oscillations, which rarely outlast the event that triggers them by more than a few seconds (Laurent and Naraghi, 1994; Siegel and Konig, 2003; Brugge et al., 2009; Fukuda et al., 2010), NIf’s gamma oscillations often persist for 30 or more seconds following singing (Fig. 6b), making them about an order of magnitude longer than event-triggered gamma oscillations reported in other systems.
Fig. 6.
Fast gamma oscillations in NIf. (A) Examples of fast gamma oscillations following song from two sites (top and bottom) within the same subject. The strong coherence of neural activity in these multiunit recordings can been seen from the RMS traces (dotted lines) calculated from the neural recordings. Note the similarity of fast gamma oscillation frequencies across different sites in the same subject. (B) Example of the time-course of fast gamma oscillations following song. The average power in the fast gamma band (normalized by the total power across all frequencies to control for broadband changes in oscillation power, see Lewandowski and Schmidt, 2011) before and after song is shown for one exemplar site. Note the strong increase in fast gamma power that begins after song and then gradually returns to baseline values (dotted line) over the course of about 25 s. (C) Distributions of fast gamma power differ across but not within subjects. The average modulation power (% increase in power relative to baseline values for a given frequency) in the 2–4 s following song is shown for five sites across two subjects. Brown-shaded traces with maximal power around 110 Hz are from one subject; Blue-shaded traces with maximal power around 138 Hz are from the second subject. Note the similarity of power distributions for different sites within each subject and the significant differences in power distributions across subjects.
The strength of fast gamma oscillations in NIf is strongly correlated with the amount of premotor activity that precedes them, meaning that longer vocalizations, such as song, are followed by significantly higher magnitude oscillations than shorter vocalizations (e.g. contact calls) (Lewandowski and Schmidt, 2011). These high-magnitude oscillations are strong enough to induce significant phase-locking in a subset of NIf single units. Interestingly, the distribution of power across the fast gamma range is highly stereotyped across sites and song renditions within each subject, but differs significantly across subjects (Fig. 6c) (Lewandowski and Schmidt, 2011). Modeling of oscillatory networks suggests that differences in fast gamma power distributions can be caused by differences in local network configurations (Bartos et al., 2007). It would be interesting to know if the differences in the distribution of fast gamma power between birds in NIf are established developmentally or whether they are shaped by experience, akin to the tutor-song-specific changes in spiking frequency that are observed in RA following first exposure to tutor song (Shank and Margoliash, 2009).
Oscillations that are directly associated with motor actions almost always precede the motor output (Mackay, 1997). The fact that NIf’s oscillations always follow motor output suggests that they are more likely to be involved in functions associated with neural oscillations such as learning and memory (Fell et al., 2001; Axmacher et al., 2006) or facilitating communication between brain regions (Fries, 2005; Schoffelen et al., 2005). Interestingly, a recent study reported fast gamma oscillations during sleep in Area X (Yanagihara and Hessler, 2012), part of the basal ganglia circuitry in birds known to be important for enabling vocal plasticity and facilitating vocal learning (Brainard and Doupe, 2000b; Kao et al., 2005). If fast gamma oscillations are important for vocal learning in songbirds, then the song system would be well placed for becoming an attractive model system for studying the importance of oscillatory activity in motor learning.
5. The role of NIf in song learning and maintenance
5.1. Song learning and maintenance in zebra finches
Song learning is generally divided into several developmental stages. The first is a sensory phase during which juvenile birds hear and create a long-term memory of the tutor song. The second stage of song learning, the sensorimotor phase, begins with the production of subsong, a highly variable vocalization akin to infant babbling (Doupe and Kuhl, 1999; Doupe and Kuhl, 2008; Mooney, 2009). It is believed that the auditory feedback from subsong is used to establish sensorimotor maps of vocal space. At around 45 days post-hatch in the zebra finch, males begin to sing plastic song, a vocalization that is still variable but has more clearly defined syllabic units. With vocal practice and auditory feedback, a bird’s plastic song becomes more structured and stereotyped as it is gradually shaped to match the stored memory of tutor song (Tchernichovski et al., 2001). The final stage of song learning, known as crystallization, occurs at the onset of sexual maturity. At this point the spectrotemporal features of song become fixed, variability is drastically reduced, and, in the case of zebra finches, the subject produces the same highly stereotyped rendition of song for life. Even after song learning has ended, auditory feedback is still required in order to maintain the stereotyped spectrotemporal structure of crystallized song (also called adult song), a process known as song maintenance (Nordeen and Nordeen, 1992; Lombardino and Nottebohm, 2000). Song maintenance is often thought of as an extension of song learning because both processes work to shape vocal output to match an internal representation of the bird’s song (Sober and Brainard, 2009).
Song learning and vocal learning, including speech, are special cases of imitation learning in which the motor system must learn to produce an appropriate sequence of commands to faithfully reproduce a sensory template (usually acquired by sensory exposure to another subject). Models of song learning differ in how they propose auditory feedback is used to facilitate learning. Traditional models involve comparator-based learning circuits (Doya and Sejnowski, 1995; Fiete et al., 2004; Fiete et al., 2007). In these models, vocal performance is evaluated by comparing auditory feedback during singing with a stored template of tutor song or BOS. This auditory-derived performance evaluation signal is then mapped to the underlying vocal motor code and segments of the vocal motor circuitry are reinforced/modified based upon their success/failure to produce the desired auditory feedback. Through many cycles of this comparator-based learning, juveniles gradually shape their vocal output to match the stored template of tutor song. These comparator-based models typically do not ascribe any role to offline mechanisms during sleep (Fee and Goldberg, 2011).
In the inverse model of vocal learning (Fig. 7), auditory feedback during the plastic song phase is not necessarily used to evaluate vocal performance but is instead used to refine the map of auditory/vocal-motor space created during the subsong phase. In this model (Hahnloser and Ganguly, in press), vocal learning occurs when the auditory memory of tutor song is fed through the map of auditory/vocal-motor space to strengthen the motor connections that produce the desired auditory feedback. This process is similar, at least conceptually, to how more prolific vocal learners, such as humans, reproduce vocalizations from memory. As the sensorimotor map of vocal space is refined through auditory feedback, the auditory memory of tutor song is able to modify motor circuitry more accurately. This process can be thought of as the auditory memory of tutor song driving the creation of a motor memory. Because this process does not rely on auditory feedback, the motor memory can in principle be written offline without the bird needing to sing, for example during sleep. Accordingly, inverse-based vocal learning theories may provide a natural framework for describing some of the sleep-related influences on behavioral learning summarized at the beginning of Section 4.2.
Fig. 7.
Sensorimotor learning using inverse models or comparators. Top: common to all song learning models, in the sensory period, the bird stores a sensory memory of tutor song in an auditory area A (represented by the arrow from A to A), for example by virtue of synaptic plasticity among neurons in A. Middle: Inverse-model hypothesis: (1) During the sensorimotor learning phase, birds sing subsongs (using an incomplete motor network represented by arrow pointing from the motor area M to itself) and use auditory feedback to learn an inverse model, a synaptic mapping from auditory features onto neurons in M that generate those features as auditory feedback. (2) During that same sensorimotor learning phase, birds recall the sensory song memory and use the inverse model to transform the sensory memory into an updated motor memory stored in M. This second learning phase does not rely on auditory feedback and so could occur offline, after singing or during sleep. Being the highest area involved in generating sleep bursts, NIf could be part of the network in A that recalls a sensory memory of tutor song. In analogy, the motor area M could stand for HVC. Bottom: Alternatively, according to comparator-based learning hypotheses, auditory feedback is compared against a memory of tutor song in comparator area C. Error or reinforcement signals that result from this comparison are conveyed to motor neurons in order to update synaptic networks in M (see e.g., Fiete et al., 2004, 2007).
In this section, we discuss the evidence for NIf’s involvement in song learning and maintenance and the potential functions that NIf may serve in these processes. NIf’s location at the interface between sensory and motor areas and its role in driving activity in downstream structures during sleep, make it particularly well suited to contribute to offline shaping of motor memories.
5.2. NIf and the maintenance of crystallized song: a critical examination of the evidence
NIf is widely believed to have little to no involvement in song maintenance, a process that is often modeled as an extension of song learning. Here we will examine the reasoning and results of two classes of experiments that led to this conclusion and discuss whether it needs to be reexamined given recent discoveries in the song system.
Prior to the explicit demonstration of a direct anatomical (Bauer et al., 2008; Akutagawa and Konishi, 2010) or functional (Shaevitz and Theunissen, 2007) connection between CM/Av and HVC, it was generally believed that NIf was the only significant source of auditory information to HVC and the song system. Thus, it was presumed that bilateral NIf lesions would effectively ‘deafen’ the song system (Cardin et al., 2005) and cause degradation of crystallized song on the same time-scale (3–4 weeks) as deafening in young adult zebra finches (Nordeen and Nordeen, 1992; Lombardino and Nottebohm, 2000). When experiments found that song remained intact for 8 weeks following NIf lesions, this was taken as evidence that neither NIf, nor auditory input from NIf, was important for the maintenance of song in adult zebra finches (Cardin et al., 2005; Roy and Mooney, 2009). These findings, combined with the lack of motor deficits in song production following NIf lesions, left many researchers questioning whether NIf played any significant role in song system function. We now know, however, that HVC receives direct auditory input from CM/Av, and thus NIf lesions do not effectively ‘deafen’ the song system. What is not clear is how long song can remain intact with reduced, but not eliminated, levels of auditory feedback. To date, song has been tracked for ~2 months following NIf lesions (Cardin et al., 2005; Roy and Mooney, 2009). Consider, however, that even after complete deafening the songs of older adult zebra finches can retain their stereotyped spectrotemporal structure for more than a year (Lombardino and Nottebohm, 2000). Additional studies are needed to determine whether auditory input from CM/Av (and possibly LLV) provides enough information to allow for the perpetual maintenance of song in the absence of NIf, or if these other auditory inputs simply delay the rate at which song decays.
Similar to deafening, denervation of the ts-nerve (ts-nerve cut) causes the eventual degradation of normal adult crystallized song (Roy and Mooney, 2007). Here too, NIf lesions failed to prevent nerve-cut induced song decrystallization (Roy and Mooney, 2009). It has been hypothesized that this decrystallization is the result of song maintenance circuitry attempting to correct for the immediate and permanent spectral distortions of the song auditory-feedback signal caused by the ts-nerve cut. Normal song learning and maintenance are believed to involve the exploration of vocal space followed by the reinforcement of those vocal-motor patterns that successfully reproduce aspects of tutor song/BOS (Fee and Goldberg, 2011). By causing irreparable distortions in vocal output, ts-nerve cuts effectively prevent any vocal-motor patterns from successfully reproducing tutor song/BOS. Thus, any nuclei selectively involved in reinforcing successful vocal-motor patterns could theoretically be removed without significantly altering the manner in which song decrystallizes following ts-nerve cuts. The fact that NIf is not necessary for ts-nerve cut decrystallization is good evidence that NIf is not necessary for the exploration of vocal space in ts-cut birds (Roy and Mooney, 2009), but these results do not preclude NIf’s involvement in other aspects of vocal learning and maintenance.
5.3. Evidence for online and offline song learning
While it is clear that auditory feedback is necessary for vocal learning (Konishi, 1965; Lei and Mooney, 2010), it is not yet clear whether auditory feedback is used to guide modifications to vocal motor circuitry during singing or whether vocal motor circuitry is modified offline at times that are temporally dissociated from singing. Comparator-based vocal learning models usually assume that the processes underlying vocal learning occur online during singing (Fiete et al., 2007; Fee and Goldberg, 2011). Inverse models, on the other hand, do not rely on auditory feedback for the specific transformation of an auditory memory into a motor memory and thus could occur offline, such as during sleep, a period during which changes in song are known to occur (Deregnaucourt et al., 2005; Crandall et al., 2007; Margoliash and Schmidt, 2010). There are three general time windows during which aspects of vocal learning could occur: (1) Online during singing, (2) Offline after singing, and (3) Offline during sleep. We will review the evidence for vocal learning during each of these time windows and discuss how NIf might be involved in vocal learning and maintenance.
5.3.1. Online learning during singing
Vocal learning requires the difficult task of mapping auditory features to the vocal motor code that produced them. The existence of neurons in the auditory forebrain that are sensitive to distortions in auditory feedback during song production suggests that vocal performance evaluation might occur as the bird is singing (Keller and Hahnloser, 2009). While mapping of auditory feedback signals to the vocal motor code could conceivably occur during singing, there is a temporal credit assignment problem that needs to be solved to compensate for the intrinsic delay between the execution of a segment of motor code and the evaluation of the vocal output that was produced. However, recordings in zebra finches at both the single and multiunit level have failed to find evidence for auditory-feedback related information in song motor nuclei during singing (Konishi, 2004; Kozhevnikov and Fee, 2007, but see Sakata and Brainard, 2008). In NIf, the strong suppression of neural activity beginning before the offset of vocal output (Lewandowski and Schmidt, 2011) strongly suggests that auditory feedback, much of which would reach NIf during these periods of inhibition, does not impact activity in NIf during vocal production.
5.3.2. Offline learning after singing in the awake bird
While much offline learning might occur during sleep, it is likely that multiple vocal learning related processes also occur in the awake, non-singing bird. Certainly, plastic song in juveniles changes throughout the day, becoming more stereotyped and presumably more closely matched to an internal template of tutor song (Deregnaucourt et al., 2005). Given the evidence discussed above that vocal performance related information may not reach motor structures during singing, some of the changes in plastic song are likely caused by adjustments to the motor code that are made offline between song bouts.
The recent observation of prolonged periods of fast gamma oscillations in NIf following song bouts (Lewandowski and Schmidt, 2011) suggests a candidate time window during which offline vocal learning processes may occur. In support of this idea, gamma oscillations in the mammalian brain have been linked to numerous functions including memory consolidation (Fell et al., 2001; Axmacher et al., 2006), modulation of communication between brain regions (Buzsaki and Draguhn, 2004; Dan and Poo, 2004; Fries, 2005; Fries et al., 2008), and modulation of sensory processing (Cardin et al., 2009). Fast gamma oscillations in NIf, which induce coherent multiunit bursts at ~8 ms intervals (exact spacing differs between subjects; Fig. 6c), could act as a timing reference signal to facilitate communication in the NIf–Av–HVC sensorimotor loop that links the auditory forebrain and the song system. NIf’s oscillations could also facilitate the process of consolidating an auditory memory of tutor song into a motor memory through synaptic transformations that implement an inverse model. Such consolidation right after singing would have the benefit of being more immediate than sleep-dependent mechanisms. At this time, however, detailed models of how NIf’s fast gamma oscillations could facilitate vocal learning will have to wait until further research determines how these oscillations impact neural activity in other nuclei, particularly Av and HVC, and whether these nuclei also exhibit gamma oscillations.
5.3.3. Offline learning during sleep
The effect of sleep on song learning has been shown both at the behavioral (Deregnaucourt et al., 2005) and neural levels (Shank and Margoliash, 2009; Rauske et al., 2010) at different stages of vocal development. In addition, the replay of premotor activity patterns during sleep observed in song control nuclei such as HVC and RA (Chi et al., 2003; Shank and Margoliash, 2009) suggests a possible neural correlate of sleep-related vocal learning processes. The combined roles of NIf as a driver of both plastic song and premotor-like bursting during sleep point to the possible involvement of NIf in shaping the developing premotor circuitry offline.
A role for sleep-related learning has been suggested in theories of inference learning, such as the wake-sleep algorithm (Hinton et al., 1995), and in theories of imitation learning via inverse models (Fig. 7) (Hahnloser and Ganguly, in press). A primary feature of these latter models is that they represent a direct mapping between the vocal motor neurons that produce a particular acoustic feature and the auditory neurons that respond to that same feature. Briefly, birds would first store an auditory memory of the tutor song during an initial phase of adult song exposure, a memory they then can recall autonomously. In a second stage, they would start with a variable vocal output (vocal babbling) that they use to learn the inverse model that represents the causal mapping between vocal-motor commands and auditory feedback responses. In a third stage, birds would recall the auditory memory of the tutor song and feed it into the inverse model to drive motor neurons, thereby forming a motor memory of the tutor song. This last stage, the transformation of an auditory into a motor memory, would not rely on auditory feedback and could occur during sleep or right after singing.
While experimental support for an inverse model mechanism is still lacking, indirect observations suggest its feasibility. In experiments performed on juvenile birds who have never been exposed to song, Shank and Margoliash (2009) showed that first exposure to song (in a manner that depends on auditory-feedback) causes a dramatic song-specific change in RA activity during sleep that precedes implementation of the actual vocal learning, which only occurs on the day following (i.e. after sleep) tutor exposure. Such a time-line of learning would be predicted by inverse models, which require that a memory of tutor song be formed before sleep-related activation of motor areas can be driven by an auditory memory. Accordingly, sleep-related NIf activity is expected to be dramatically different before and after first exposure to tutor song.
5.4. Potential roles for NIf in song learning
Multiple lines of evidence point to an active role for NIf in song learning. First, inactivation of NIf severely disrupts the production of plastic song in juveniles (Naie and Hahnloser, 2011). However, unlike the vocal motor deficits caused by HVC or RA lesions, the deficits in plastic song following NIf lesions cannot be explained as a general disruption of essential vocal motor circuitry because both subsong and crystallized song can be produced in the absence of NIf (Cardin et al., 2005; Roy and Mooney, 2009; Naie and Hahnloser, 2011). Thus, NIf actively drives the production of plastic song, which is critical for song learning. Second, recent work (Roberts et al., 2012) suggests that NIf also plays a role in either establishing an auditory memory of tutor song or in facilitating communication between auditory areas and song system nuclei (e.g. HVC) during the sensorimotor period of song learning. Specifically, selective inactivation of NIf, either permanently or reversibly, for the duration of a juvenile’s exposure to tutor song, prevents the subject from accurately copying the tutor song. Consistent with this idea, microstimulation in NIf (and HVC) during tutor song presentation also significantly impairs a subject’s ability to copy the tutor song when this perturbation is performed during the sensory phase of learning. In contrast, microstimulation applied to the Field L auditory complex adjacent to NIf does not cause any impairment.
Indirect evidence suggests that NIf may be involved in offline song learning processes. Along with HVC and Av, NIf is part of the sensorimotor loop that links the auditory forebrain and the song system (Akutagawa and Konishi, 2010; Lewandowski and Schmidt, 2011). This loop plays a critical role in enabling auditory information, which is critical for song learning and maintenance, to reach the song system. While the passage of auditory information to the song system appears to be suppressed during singing, the presence of fast gamma oscillations in NIf suggests that a period of increased communication between auditory and vocal motor structures may occur immediately after song production. Given that HVC activation during song can be thought of as divided into discrete windows of 8–10 ms that each represent the sparse activation of a select population of RA-projecting neurons (Leonardo and Fee, 2005) in a synfire-like manner (Fiete et al., 2004; Gibb et al., 2009), it is tempting to speculate that NIf’s fast gamma oscillations, which occur every 7–11 ms, could provide a temporal backbone for integrating traces of vocal motor activity and auditory-feedback (possibly transformed into a vocal performance evaluation signal). Such timing could also be useful for driving the consolidation of an auditory memory into a motor memory proposed by inverse models.
Given that NIf drives premotor-like bursting in HVC neurons during sleep (Hahnloser and Fee, 2007), it is reasonable to suspect that NIf may also influence the replay of vocal motor activity observed in HVC and RA during sleep. The recent observation of bursts of high-gamma oscillations during sleep in the AFP (Yanagihara and Hessler, 2012) suggests that such replay might even be related to the oscillatory activity in NIf following singing. In the inverse model of vocal learning, motor replay would have to access areas like NCM and CM, which have been suggested as sites for the storage of the tutor song memory (Bolhuis and Gahr, 2006; Phan et al., 2006; London and Clayton, 2008). Given that NIf lies at the interface of the auditory and motor system and forms part of the NIf/Av/HVC sensorimotor loop, it is likely to play a critical role in allowing the motor system access the auditory system. It may even play a critical role in the mapping of vocal motor neurons and tutor-selective auditory neurons.
5.5. Potential roles for NIf in song maintenance
The involvement of NIf in the maintenance of adult song is probably the most contentious aspect of NIf’s function. While there is currently no experimental evidence demonstrating that NIf is involved in the maintenance of crystallized song, there are reasons (discussed in Section 5.2) to believe that further investigation may reveal that NIf does in fact play a role in song maintenance. One experiment that could prove particularly informative would be to investigate the necessity of NIf for the recovery of crystallized song following distorted-auditory-feedback (DAF) induced decrystallization (Leonardo and Konishi, 1999). This technique works by using triggered playback of an occluding stimulus (e.g., a noise burst, or another song syllable) to disrupt auditory feedback for a selected portion of a subject’s song. Continuous DAF eventually causes song to decrystallize in a manner similar to deafening (Leonardo and Konishi, 1999; Kozhevnikov and Fee, 2007). What makes this technique interesting, and potentially very powerful, is that once the DAF is stopped, subjects slowly recover normal song over the course of a few months (Leonardo and Konishi, 1999). This process resembles song maintenance, rather than song learning, because it occurs after sexual maturity and subjects recover their own song instead of attempting to achieve a better match of tutor song. If NIf is critically involved in the maintenance of learned vocalizations, then lesions of NIf following DAF-induced song decrystallization should prevent, or at least slow, the recovery of normal song.
6. Conclusions and relevance to speech processing and learning
Song production in birds and speech production in humans share many similarities. At the developmental level, both require auditory feedback for proper acquisition and both progress through similar stages beginning with an early acquisition phase in which speech sounds and song are mapped perceptually. This sensory phase is then followed by a vocal exploratory phase, known in humans and birds respectively as babbling and subsong (Immelmann, 1969; Kuhl, 2004; Doupe and Kuhl, 2008). These early stages then transition to a more intermediate level of processing in which syntactical rules are established (Gardner et al., 2005), eventually leading to the stable production of song or speech (Doupe and Kuhl, 2008).
The requirement for auditory feedback in vocal learning and maintenance in both humans and songbirds has resulted in a similar requirement for both anatomical and functional mappings between auditory areas specialized in processing vocal signals and motor areas responsible for vocal production. This need for an integration of auditory and motor information during speech processing (acquisition, production and perception) has brought about circuit models of the human cortex (Guenther, 1995; Guenther et al., 2006; Hickok et al., 2011) that, by virtue of their rooting in the literature on control theory dealing with internal models, share similarities with circuit models described in the avian song system (Troyer and Doupe, 2000b; Hahnloser and Ganguly, in press).
Internal (forward and inverse) models are powerful tools that the brain may use in its various tasks of learning, monitoring, and evaluating of vocal signals. Given the lack of sensory feedback signals during singing in the vocal control areas of some birds, one possibility is that sensory feedback could act silently to train an inverse song model (Hahnloser and Ganguly, in press). Moreover, sensory feedback signals could be gated off because they may have been replaced by forward predictions of the sensory (auditory and somatosensory) consequences of the generated vocal motor commands (Jordan and Rumelhart, 1992; Troyer and Doupe, 2000a; Hickok et al., 2009), for which there is evidence in higher auditory areas in both mammals (Eliades and Wang, 2008) and birds (Keller and Hahnloser, 2009). Forward models represent an internal estimate of the vocal tract; such models are based on learned associations between issued motor commands and sensory outcomes (Guenther and Ghosh, 2003; Hickok et al., 2011). Once established, forward models could be used during normal vocalizations and presumably be updated by sensory feedback. The main use of forward models is to predict sensory feedback ahead of time and so these models can help to circumvent problems associated with delays of sensory feedback.
In the songbird, NIf fits many of the criteria for serving as an internal model for song because (a) as a hypothetical forward model NIf receives auditory (from CM) and somatosensory input (from Uva), exhibits vocal motor activity, and likely receives corollary motor commands from HVC via its reciprocal input with nucleus Avalanche and (b) as a hypothetical inverse model, NIf provides the main source of auditory input to downstream premotor and motor areas.
Interestingly, NIf shares many features with area Spt (named because of its location in the Sylvian fissure at the parietal–temporal boundary) in the human speech processing circuit. Like NIf, Spt is situated at the center of a network of auditory (superior temporal gyrus) and motor (pars opecularis, premotor cortex) areas (Hickok et al., 2003; Hickok et al., 2009). Area Spt is also involved in speech production, although its exact role is unclear, and it is proposed to act as the internal model of the vocal tract, receiving corollary discharge from the “vocal motor controller” in the motor cortex and sensory input from auditory cortex (Hickok et al., 2011). Although clear differences exist between human speech processing and song control in birds, the general shared computational needs and functional organization between both systems suggest that fundamental principles might be extracted by studying both systems in parallel. Understanding the similarities and differences between NIf and Spt and their respective roles in song and speech processing might be a good place to start. For example, given that Spt responds to distorted auditory feedback (Tourville et al., 2008), it will be worthwhile to probe for similar responses in NIf.
In the songbird, as this review points out, many questions still remain unanswered regarding how auditory to motor transformation occurs during the context of vocal learning and production. NIf is functionally placed, and has many of the neural attributes, to serve a critical function in the learning, maintenance, and production of song, but research into NIf’s role in these processes has yielded more questions than answers. While we know that auditory feedback is necessary for song learning, the mechanisms by which this feedback instructs motor structures such as HVC and RA are not yet understood. Anatomy and auditory neurophysiology suggest that NIf is part of the auditory feedback pathway; however, the gating of auditory responses during singing is not consistent with a conceptual framework in which NIf simply relays auditory inputs to motor structures. It appears likely therefore that most communication between auditory areas and the song system occurs offline, possibly immediately after singing or during sleep, time windows during which NIf could facilitate this communication.
In the zebra finch, NIf’s role in song production and learning appears to be tied mainly to two developmental phases: in a sensorimotor learning phase NIf has a direct online involvement in song production (Naie and Hahnloser, 2011), and in a sensory learning phase NIf is involved in mediation of critical sensory input during song exposure (tutoring) (Roberts et al., 2012). In addition, the effects of NIf lesions on crystallized songs of Bengalese finches suggest that NIf may also play a role in the production of higher order syntactic structures not found in zebra finch song. While it is clear that NIf plays an important role in song learning and production, additional experiments will be necessary to determine the exact nature of NIf’s involvement in these processes. The challenge with studying vocal learning is that it likely involves both online (during singing) and offline (during awake non-singing periods or during sleep) interplay between auditory and motor mechanisms in a slowly developing system. In the end, understanding the mechanism(s) by which NIf facilitates learning will yield fundamental new insights into the neural computations and strategies that underlie sensorimotor learning in general, and the acquisition of learned vocalizations, like human speech, in particular. Conversely, application of some of the conceptual frameworks developed from human speech processing will greatly benefit studies aimed at uncovering the neural mechanisms underlying song production and learning.
References
- Akutagawa E, Konishi M. Connections of thalamic modulatory centers to the vocal control system of the zebra finch. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14086–14091. doi: 10.1073/pnas.0506774102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akutagawa E, Konishi M. New brain pathways found in the vocal control system of a songbird. Journal of Comparative Neurology. 2010;518:3086–3100. doi: 10.1002/cne.22383. [DOI] [PubMed] [Google Scholar]
- Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008;320:630–634. doi: 10.1126/science.1155140. [DOI] [PubMed] [Google Scholar]
- Aronov D, Veit L, Goldberg JH, Fee MS. Two distinct modes of forebrain circuit dynamics underlie temporal patterning in the vocalizations of young songbirds. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31:16353–16368. doi: 10.1523/JNEUROSCI.3009-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore RC, Renk JA, Schmidt MF. Bottom-up activation of the vocal motor forebrain by the respiratory brainstem. Journal of Neuroscience. 2008;28:2613–2623. doi: 10.1523/JNEUROSCI.4547-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore RC, Wild JM, Schmidt MF. Brainstem and forebrain contributions to the generation of learned motor behaviors for song. Journal of Neuroscience. 2005;25:8543–8554. doi: 10.1523/JNEUROSCI.1668-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus–norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernandez G, Elger CE, Fell J. Memory formation by neuronal synchronization. Brain Research Reviews. 2006;52:170–182. doi: 10.1016/j.brainresrev.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature Reviews Neuroscience. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Bauer EE, Coleman MJ, Roberts TF, Roy A, Prather JF, Mooney R. A synaptic basis for auditory–vocal integration in the songbird. Journal of Neuroscience. 2008;28:1509–1522. doi: 10.1523/JNEUROSCI.3838-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof HJ, Engelage J. Flash evoked-responses in a song control nucleus of the zebra finch (Taeniopygia guttata castanotis Gould) Brain Research. 1985;326:370–374. doi: 10.1016/0006-8993(85)90048-4. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nature Reviews Neuroscience. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Auditory feedback in learning and maintenance of vocal behaviour. Nature Reviews Neuroscience. 2000a;1:31–40. doi: 10.1038/35036205. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia–forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000b;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- Brugge JF, Nourski KV, Oya H, Reale RA, Kawasaki H, Steinschneider M, Howard MA., 3rd Coding of repetitive transients by auditory cortex on Heschl’s gyrus. Journal of Neurophysiology. 2009;102:2358–2374. doi: 10.1152/jn.91346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Raksin JN, Schmidt MF. Sensorimotor nucleus NIf is necessary for auditory processing but not vocal motor output in the avian song system. Journal of Neurophysiology. 2005;93:2157–2166. doi: 10.1152/jn.01001.2004. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Song system auditory responses are stable and highly tuned during sedation, rapidly modulated and unselective during wakefulness, and suppressed by arousal. Journal of Neurophysiology. 2003;90:2884–2899. doi: 10.1152/jn.00391.2003. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Auditory responses in multiple sensorimotor song system nuclei are co-modulated by behavioral state. Journal of Neurophysiology. 2004a;91:2148–2163. doi: 10.1152/jn.00918.2003. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Noradrenergic inputs mediate state dependence of auditory responses in the avian song system. Journal of Neuroscience. 2004b;24:7745–7753. doi: 10.1523/JNEUROSCI.1951-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelino CB, Schmidt MF. What birdsong can teach us about the central noradrenergic system. Journal of Chemical Neuroanatomy. 2010;39:96–111. doi: 10.1016/j.jchemneu.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Z, Margoliash D. Temporal precision and temporal drift in brain and behavior of zebra finch song. Neuron. 2001;32:899–910. doi: 10.1016/s0896-6273(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Chi ZY, Rauske PL, Margoliash D. Pattern filtering for detection of neural activity, with examples from HVc activity during sleep in zebra finches. Neural Computation. 2003;15:2307–2337. doi: 10.1162/089976603322362374. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Mooney R. Synaptic transformations underlying highly selective auditory representations of learned birdsong. Journal of Neuroscience. 2004;24:7251–7265. doi: 10.1523/JNEUROSCI.0947-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Roy A, Wild JM, Mooney R. Thalamic gating of auditory responses in telencephalic song control nuclei. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27:10024–10036. doi: 10.1523/JNEUROSCI.2215-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Vu ET. Recovery of impaired songs following unilateral but not bilateral lesions of nucleus uvaeformis of adult zebra finches. Journal of Neurobiology. 2005;63:70–89. doi: 10.1002/neu.20122. [DOI] [PubMed] [Google Scholar]
- Crandall SR, Adam M, Kinnischtzke AK, Nick TA. HVC neural sleep activity increases with development and parallels nightly changes in song behavior. Journal of Neurophysiology. 2007;98:232–240. doi: 10.1152/jn.00128.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Dave A, Yu AC, Margoliash D. Behavioral state modulation of auditory activity in a vocal motor system. Science. 1998;282:2250–2254. doi: 10.1126/science.282.5397.2250. [DOI] [PubMed] [Google Scholar]
- Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- Deregnaucourt S, Mitra PP, Feher O, Pytte C, Tchernichovski O. How sleep affects the developmental learning of bird song. Nature. 2005;433:710–716. doi: 10.1038/nature03275. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annual Review of Neuroscience. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Neuroscience of Birdsong. Cambridge University Press; Cambridge, England: 2008. Birdsong and human speech: common themes and mechanisms; pp. 5–31. [DOI] [PubMed] [Google Scholar]
- Doya K, Sejnowski TJ, editors. A Novel Reinforcement Model of Birdsong Vocalization Learning. MIT Press; 1995. [Google Scholar]
- Eliades SJ, Wang X. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 2008;453:1102–1106. doi: 10.1038/nature06910. [DOI] [PubMed] [Google Scholar]
- Fee MS, Goldberg JH. A hypothesis for basal ganglia-dependent reinforcement learning in the songbird. Neuroscience. 2011;198:152–170. doi: 10.1016/j.neuroscience.2011.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger CE, Fernandez G. Human memory formation is accompanied by rhinal–hippocampal coupling and decoupling. Nature Neuroscience. 2001;4:1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- Fiete IR, Fee MS, Seung HS. Model of birdsong learning based on gradient estimation by dynamic perturbation of neural conductances. Journal of Neurophysiology. 2007;98:2038–2057. doi: 10.1152/jn.01311.2006. [DOI] [PubMed] [Google Scholar]
- Fiete IR, Hahnloser RHR, Fee MS, Seung HS. Temporal sparseness of the premotor drive is important for rapid learning in a neural network model of birdsong. Journal of Neurophysiology. 2004;92:2274–2282. doi: 10.1152/jn.01133.2003. [DOI] [PubMed] [Google Scholar]
- Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proceedings of the Natural Academy of Sciences, USA. 2002;99:11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune ES, Margoliash D. Cytoarchitectonic organization and morphology of cells in the field L complex of male zebra finches (Taeniopygia guttata) Journal of Comparative Neurology. 1992;325:388–404. doi: 10.1002/cne.903250306. [DOI] [PubMed] [Google Scholar]
- Fortune ES, Margoliash D. Parallel pathways and convergence onto HVc and adjacent neostriatum of adult male zebra finches (Taeniopygia guttata) Journal of Comparative Neurology. 1995;360:413–441. doi: 10.1002/cne.903600305. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in Cognitive Science. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28:4823–4835. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Rothermel R, Juhasz C, Nishida M, Sood S, Asano E. Cortical gamma-oscillations modulated by listening and overt repetition of phonemes. NeuroImage. 2010;49:2735–2745. doi: 10.1016/j.neuroimage.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner TJ, Naef F, Nottebohm F. Freedom and rules: the acquisition and reprogramming of a bird’s learned song. Science. 2005;308:1046–1049. doi: 10.1126/science.1108214. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Margoliash D. Neuronal populations and single cells representing learned auditory objects. Nature. 2003;424:669–674. doi: 10.1038/nature01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George I, Cousillas H, Richard JP, Hausberger M. State-dependent hemispheric specialization in the songbird brain. Journal of Comparative Neurology. 2005;488:48–60. doi: 10.1002/cne.20584. [DOI] [PubMed] [Google Scholar]
- Gibb L, Gentner TQ, Abarbanel HD. Inhibition and recurrent excitation in a computational model of sparse bursting in song nucleus HVC. Journal of Neurophysiology. 2009;102:1748–1762. doi: 10.1152/jn.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nature Neuroscience. 2012;15:511–517. doi: 10.1038/nn.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace JA, Amin N, Singh NC, Theunissen FE. Selectivity for conspecific song in the zebra finch auditory forebrain. Journal of Neurophysiology. 2003;89:472–487. doi: 10.1152/jn.00088.2002. [DOI] [PubMed] [Google Scholar]
- Guenther FH. Speech sound acquisition, coarticulation, and rate effects in a neural network model of speech production. Psychological Review. 1995;102:594–621. doi: 10.1037/0033-295x.102.3.594. [DOI] [PubMed] [Google Scholar]
- Guenther FH, Ghosh SS. A model of cortical and cerebellar function in speech. Proceedings of the XVth International Congress of Phonetic Sciences Barcelona: 15th ICPhS Organizing Committee.2003. [Google Scholar]
- Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain and Language. 2006;96:280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard HR, Hahnloser, Ganguly S. Vocal learning with inverse models. In: Quiroga R, Panzeri S, editors. Principles of Neural Coding. CRC Press; in press. [Google Scholar]
- Hahnloser RHR, Fee MS. Sleep-related spike bursts in HVC are driven by the nucleus interface of the nidopallium. Journal of Neurophysiology. 2007;97:423–435. doi: 10.1152/jn.00547.2006. [DOI] [PubMed] [Google Scholar]
- Hahnloser RHR, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- Hahnloser RHR, Kozhevnikov AA, Fee MS. Sleep-related neural activity in a premotor and a basal-ganglia pathway of the songbird. Journal of Neurophysiology. 2006;96:794–812. doi: 10.1152/jn.01064.2005. [DOI] [PubMed] [Google Scholar]
- Hahnloser RHR, Wang CZH, Nager A, Naie K. Spikes and bursts in two types of thalamic projection neurons differentially shape sleep patterns and auditory responses in a songbird. Journal of Neuroscience. 2008;28:5040–5052. doi: 10.1523/JNEUROSCI.5059-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Buchsbaum B, Humphries C, Muftuler T. Auditory–motor interaction revealed by fMRI: speech, music, and working memory in area Spt. Journal of Cognitive Neuroscience. 2003;15:673–682. doi: 10.1162/089892903322307393. [DOI] [PubMed] [Google Scholar]
- Hickok G, Houde J, Rong F. Sensorimotor integration in speech processing: computational basis and neural organization. Neuron. 2011;69:407–422. doi: 10.1016/j.neuron.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Okada K, Serences JT. Area Spt in the human planum temporale supports sensory–motor integration for speech processing. Journal of Neurophysiology. 2009;101:2725–2732. doi: 10.1152/jn.91099.2008. [DOI] [PubMed] [Google Scholar]
- Hinton GE, Dayan P, Frey BJ, Neal RM. The “wake-sleep” algorithm for unsupervised neural networks. Science. 1995;268:1158–1161. doi: 10.1126/science.7761831. [DOI] [PubMed] [Google Scholar]
- Hosino T, Okanoya K. Lesion of a higher-order song nucleus disrupts phrase level complexity in Bengalese finches. NeuroReport. 2000;11:2091–2095. doi: 10.1097/00001756-200007140-00007. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nature Neuroscience. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Immelmann K. Song development in the zebra finch and other estrildid finches 1969 [Google Scholar]
- Janata P, Margoliash D. Gradual emergence of song selectivity in sensorimotor structures of the male zebra finch song system. Journal of Neuroscience. 1999;19:5108–5118. doi: 10.1523/JNEUROSCI.19-12-05108.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis E, Gunturkun O, Bruce L, Csillag A, Karten H, Kuenzel W, Medina L, Paxinos G, Perkel DJ, Shimizu T, Striedter G, Wild JM, Ball GF, Dugas-Ford J, Durand SE, Hough GE, Husband S, Kubikova L, Lee DW, Mello CV, Powers A, Siang C, Smulders TV, Wada K, White SA, Yamamoto K, Yu J, Reiner A, Butler AB Avian Brain Nomenclature C. Avian brains and a new understanding of vertebrate brain evolution. Nature Reviews Neuroscience. 2005;6:151–159. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MI, Rumelhart DE. Forward models: supervised learning with a distal teacher. Cognitive Science. 1992;16:307–354. [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia–forebrain circuit to real-time modulation of song. Nature. 2005;433:638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Keller GB, Hahnloser RH. Neural processing of auditory feedback during vocal practice in a songbird. Nature. 2009;457:187–190. doi: 10.1038/nature07467. [DOI] [PubMed] [Google Scholar]
- Kim G, Doupe A. Organized representation of spectrotemporal features in songbird auditory forebrain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31:16977–16990. doi: 10.1523/JNEUROSCI.2003-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittelberger JM, Mooney R. Lesions of an avian forebrain nucleus that disrupt song development alter synaptic connectivity and transmission in the vocal premotor pathway. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1999;19:9385–9398. doi: 10.1523/JNEUROSCI.19-21-09385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittelberger JM, Mooney R. Acute injections of brain-derived neurotrophic factor in a vocal prernotor nucleus reversibly disrupt adult birdsong stability and trigger syllable deletion. Journal of Neurobiology. 2005;62:406–424. doi: 10.1002/neu.20109. [DOI] [PubMed] [Google Scholar]
- Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Zeitschrift fur Tierpsychologie. 1965;22:770–783. [PubMed] [Google Scholar]
- Konishi M. The role of auditory feedback in birdsong. Annals of the New York Academy of Sciences. 2004;1016:463–475. doi: 10.1196/annals.1298.010. [DOI] [PubMed] [Google Scholar]
- Kozhevnikov AA, Fee MS. Singing-related activity of identified HVC neurons in the zebra finch. Journal of Neurophysiology. 2007;97:4271–4283. doi: 10.1152/jn.00952.2006. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Early language acquisition: cracking the speech code. Nature Reviews Neuroscience. 2004;5:831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- Laurent G, Naraghi M. Odorant-induced oscillations in the mushroom bodies of the locust. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1994;14:2993–3004. doi: 10.1523/JNEUROSCI.14-05-02993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Mooney R. Manipulation of a central auditory representation shapes learned vocal output. Neuron. 2010;65:122–134. doi: 10.1016/j.neuron.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo A. Experimental test of the birdsong error-correction model. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16935–16940. doi: 10.1073/pnas.0407870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo A, Fee MS. Ensemble coding of vocal control in birdsong. Journal of Neuroscience. 2005;25:652–661. doi: 10.1523/JNEUROSCI.3036-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo A, Konishi M. Decrystallization of adult birdsong by perturbation of auditory feedback. Nature. 1999;399:466–470. doi: 10.1038/20933. [DOI] [PubMed] [Google Scholar]
- Lewandowski BC, Schmidt M. Short bouts of vocalization induce longlasting fast gamma oscillations in a sensorimotor nucleus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31:13936–13948. doi: 10.1523/JNEUROSCI.6809-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardino AJ, Nottebohm F. Age at deafening affects the stability of learned song in adult male zebra finches. Journal of Neuroscience. 2000;20:5054–5064. doi: 10.1523/JNEUROSCI.20-13-05054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nature Neuroscience. 2008;11:579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature. 2008;456:189–194. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay WA. Synchronized neuronal oscillations and their role in motor processes. Trends of Cognitive Science. 1997;1:176–183. doi: 10.1016/S1364-6613(97)01059-0. [DOI] [PubMed] [Google Scholar]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C, Aerts J, Del Fiore G, Degueldre C, Meulemans T, Luxen A, Franck G, Van Der Linden M, Smith C, Cleeremans A. Experience-dependent changes in cerebral activation during human REM sleep. Nature Neuroscience. 2000;3:831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- Margoliash D, Konishi M. Auditory representation of autogenous song in the song system of white-crowned sparrows. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:5997–6000. doi: 10.1073/pnas.82.17.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D, Schmidt MF. Sleep, off-line processing, and vocal learning. Brain and Language. 2010;115:45–58. doi: 10.1016/j.bandl.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCasland JS. Neuronal control of bird song production. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1987;7:23–39. doi: 10.1523/JNEUROSCI.07-01-00023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nature Neuroscience. 2003;6:697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- Mello CV, Pinaud R, Ribeiro S. Noradrenergic system of the zebra finch brain: immunicytochemical study of dopamine-b hydroxylase. Journal of Comparative Neurology. 1998;400:207–228. [PubMed] [Google Scholar]
- Mendez JM, Cooper BG, Goller F. Brainstem, thalamic and cortical neurons of the song circuit respond to Vagal nerve stimulation and somatosensory feedback. Society for Neuroscience; Chicago, IL: 2009. p. 196.191/FF156. [Google Scholar]
- Mooney R. Different subthreshold mechanisms underlie song selectivity in identified HVc neurons of the zebra finch. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20:5420–5436. doi: 10.1523/JNEUROSCI.20-14-05420.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. Neural mechanisms for learned birdsong. Learning & Memory. 2009;16:655–669. doi: 10.1101/lm.1065209. [DOI] [PubMed] [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel KI, Doupe AJ. Organizing principles of spectro-temporal encoding in the avian primary auditory area field L. Neuron. 2008;58:938–955. doi: 10.1016/j.neuron.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naie K, Hahnloser RH. Regulation of learned vocal behavior by an auditory motor cortical nucleus in juvenile zebra finches. Journal of Neurophysiology. 2011;106:291–300. doi: 10.1152/jn.01035.2010. [DOI] [PubMed] [Google Scholar]
- Nealen PM, Schmidt MF. Distributed and selective auditory representation of song repertoires in the avian song system. Journal of Neurophysiology. 2006;96:3433–3447. doi: 10.1152/jn.01130.2005. [DOI] [PubMed] [Google Scholar]
- Nick TA, Konishi M. Neural auditory selectivity develops in parallel with song. Journal of Neurobiology. 2005;62:469–481. doi: 10.1002/neu.20115. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behavioural and Neural Biology. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Kelley DB, Paton JA. Connections of vocal control nuclei in the canary telencephalon. Journal of Comparative Neurology. 1982;207:344–357. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. Journal of Comparative Neurology. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Okanoya K. The Bengalese finch – a window on the behavioral neurobiology of birdsong syntax. Behavioral Neurobiology of Birdsong. 2004;1016:724–735. doi: 10.1196/annals.1298.026. [DOI] [PubMed] [Google Scholar]
- Okuhata S, Nottebohm F. Nucleus Uva might be part of a feedback circuit for song processing. Society for Neuroscience Abstracts. 1992;18:527. [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Phillips C, Degueldre C, Del Fiore G, Aerts J, Luxen A, Maquet P. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates longlastingmemories thatmay subserve vocal learning in songbirds. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Nowicki S, Anderson RC, Peters S, Mooney R. Neural correlates of categorical perception in learned vocal communication. Nature Neuroscience. 2009;12:221–228. doi: 10.1038/nn.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory–vocal mirroring in neurons for learned vocal communication. Nature. 2008;451:305–310. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- Raksin JN, Glaze CM, Smith S, Schmidt MF. Linear and nonlinear auditory response properties of interneurons in a high-order avian vocal motor nucleus during wakefulness. Journal of Neurophysiology. 2012;107:2185–2201. doi: 10.1152/jn.01003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauske PL, Chi Z, Dave AS, Margoliash D. Neuronal stability and drift across periods of sleep: premotor activity patterns in a vocal control nucleus of adult zebra finches. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30:2783–2794. doi: 10.1523/JNEUROSCI.3112-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauske PL, Shea SD, Margoliash D. State and neuronal class-dependent reconfiguration in the avian song system. Journal of Neurophysiology. 2003;89:1688–1701. doi: 10.1152/jn.00655.2002. [DOI] [PubMed] [Google Scholar]
- Roberts TF, Gobes SM, Murugan M, Olveczky BP, Mooney R. Motor circuits are required to encode a sensory model for imitative learning. Nature Neuroscience. 2012;15:1454–1459. doi: 10.1038/nn.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Mooney R. Auditory plasticity in a basal ganglia–forebrain pathway during decrystallization of adult birdsong. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27:6374–6387. doi: 10.1523/JNEUROSCI.0894-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Mooney R. Song decrystallization in adult zebra finches does not require the song nucleus NIf. Journal of Neurophysiology. 2009;102:979–991. doi: 10.1152/jn.00293.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SM, Arnold AP. Evidence for cholinergic participation in the control of bird song: acetylcholinesterase distribution and muscarinic receptor autoradiography in the zebra finch brain. Journal of Comparative Neurology. 1981;202:211–219. doi: 10.1002/cne.902020207. [DOI] [PubMed] [Google Scholar]
- Sakata JT, Brainard MS. Online contributions of auditory feedback to neural activity in avian song control circuitry. Journal of Neuroscience. 2008;28:11378–11390. doi: 10.1523/JNEUROSCI.3254-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. Pattern of interhemispheric synchronization in HVc during singing correlates with key transitions in the song pattern. Journal of Neurophysiology. 2003;90:3931–3949. doi: 10.1152/jn.00003.2003. [DOI] [PubMed] [Google Scholar]
- Schmidt MF, Ashmore RC, Vu ET. Bilateral control and interhemispheric coordination in the avian song motor system. Behavioral Neurobiology of Birdsong. 2004;1016:171–186. doi: 10.1196/annals.1298.014. [DOI] [PubMed] [Google Scholar]
- Schmidt MF, Konishi M. Gating of auditory responses in the vocal control system of awake songbirds. Nature Neuroscience. 1998;1:513–518. doi: 10.1038/2232. [DOI] [PubMed] [Google Scholar]
- Schoffelen JM, Oostenveld R, Fries P. Neuronal coherence as a mechanism of effective corticospinal interaction. Science. 2005;308:111–113. doi: 10.1126/science.1107027. [DOI] [PubMed] [Google Scholar]
- Sen K, Theunissen FE, Doupe AJ. Feature analysis of natural sounds in the songbird auditory forebrain. Journal of Neurophysiology. 2001;86:1445–1458. doi: 10.1152/jn.2001.86.3.1445. [DOI] [PubMed] [Google Scholar]
- Shaevitz SS, Theunissen FE. Functional connectivity between auditory areas field L and CLM and song system nucleus HVC in anesthetized zebra finches. Journal of Neurophysiology. 2007;98:2747–2764. doi: 10.1152/jn.00294.2007. [DOI] [PubMed] [Google Scholar]
- Shank SS, Margoliash D. Sleep and sensorimotor integration during early vocal learning in a songbird. Nature. 2009;458:73–77. doi: 10.1038/nature07615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SD, Koch H, Baleckaitis D, Ramirez JM, Margoliash D. Neuron-specific cholinergic modulation of a forebrain song control nucleus. Journal of Neurophysiology. 2010;103:733–745. doi: 10.1152/jn.00803.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SD, Margoliash D. Basal forebrain cholineraic modulation of auditory activity in the zebra finch song system. Neuron. 2003;40:1213–1226. doi: 10.1016/s0896-6273(03)00723-2. [DOI] [PubMed] [Google Scholar]
- Shea SD, Margoliash D. Behavioral state-dependent reconfiguration of song-related network activity and cholinergic systems. Journal of Chemical Neuroanatomy. 2010;39:132–140. doi: 10.1016/j.jchemneu.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron. 2008;60:709–719. doi: 10.1016/j.neuron.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Siegel M, Konig P. A functional gamma-band defined by stimulus-dependent synchronization in area 18 of awake behaving cats. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23:4251–4260. doi: 10.1523/JNEUROSCI.23-10-04251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson HB, Vicario DS. Brain pathways for learned and unlearned vocalizations differ in zebra finches. Journal of Neuroscience. 1990;10:1541–1556. doi: 10.1523/JNEUROSCI.10-05-01541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sober SJ, Brainard MS. Adult birdsong is actively maintained by error correction. Nature Neuroscience. 2009;12:927–931. doi: 10.1038/nn.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soha JA, Shimizu T, Doupe AJ. Development of catecholaminergic innervation in the song system of the male zebra finch. Journal of Neurobiology. 1996;29:473–489. doi: 10.1002/(SICI)1097-4695(199604)29:4<473::AID-NEU5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- Striedter GF, Vu ET. Bilateral feedback projections to the forebrain in the premotor network for singing in zebra finches. Journal of Neurobiology. 1998;34:27–40. [PubMed] [Google Scholar]
- Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science. 2001;291:2564–2569. doi: 10.1126/science.1058522. [DOI] [PubMed] [Google Scholar]
- Theunissen FE, Amin N, Shaevitz SS, Woolley SM, Fremouw T, Hauber ME. Song selectivity in the song system and in the auditory forebrain. Annals of the New York Academy of Sciences. 2004;1016:222–245. doi: 10.1196/annals.1298.023. [DOI] [PubMed] [Google Scholar]
- Tourville JA, Reilly KJ, Guenther FH. Neural mechanisms underlying auditory feedback control of speech. NeuroImage. 2008;39:1429–1443. doi: 10.1016/j.neuroimage.2007.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer TW, Doupe AJ. An associational model of birdsong sensorimotor learning I. Efference copy and the learning of song syllables. Journal of Neurophysiology. 2000a;84:1204–1223. doi: 10.1152/jn.2000.84.3.1204. [DOI] [PubMed] [Google Scholar]
- Troyer TW, Doupe AJ. An associational model of birdsong sensorimotor learning II. Temporal hierarchies and the learning of song sequence. Journal of Neurophysiology. 2000b;84:1224–1239. doi: 10.1152/jn.2000.84.3.1224. [DOI] [PubMed] [Google Scholar]
- Tschida K, Mooney R. The role of auditory feedback in vocal learning and maintenance. Current Opinion in Neurobiology. 2012;22:320–327. doi: 10.1016/j.conb.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephelon and their relation to the song system of adult male zebra finches (Taenopygia guttata) Journal of Comparative Neurology. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Vu ET, Mazurek ME, Kuo YC. Identification of a forebrain motor programming network for the learned song of zebra finches. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1994;14:6924–6934. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- Wang Y, Brzozowska-Prechtl A, Karten HJ. Laminar and columnar auditory cortex in avian brain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12676–12681. doi: 10.1073/pnas.1006645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM. Functional neuroanatomy of the sensorimotor control of singing. Behavioral Neurobiology of Birdsong. 2004;1016:438–462. doi: 10.1196/annals.1298.016. [DOI] [PubMed] [Google Scholar]
- Wild JM, Karten HJ, Frost BJ. Connections of the auditory forebrain in the pigeon (Columba livia) Journal of Comparative Neurology. 1993;337:32–62. doi: 10.1002/cne.903370103. [DOI] [PubMed] [Google Scholar]
- Wild JM, Williams MN. Rostral wulst of passerine birds: II. Intratelencephalic projections to nuclei associated with the auditory and song systems. Journal of Comparative Neurology. 1999;413:520–534. doi: 10.1002/(sici)1096-9861(19991101)413:4<520::aid-cne3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Wild JM, Williams MN, Suthers RA. Neural pathways for bilateral vocal control in songbirds. Journal of Comparative Neurology. 2000;423:413–426. doi: 10.1002/1096-9861(20000731)423:3<413::aid-cne5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Wild MJ. Visual and somatosensory inputs to the avian song system via nucleus uvaeformis (Uva) and a comparison with the projections of a similar thalamic nucleus in a nonsongbird, Columba livia. Journal of Comparative Neurology. 1994;349:512–535. doi: 10.1002/cne.903490403. [DOI] [PubMed] [Google Scholar]
- Williams H. Birdsong and singing behavior. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Cambridge University Press; New York: 2008. pp. 32–49. [Google Scholar]
- Williams H, Vicario DS. Temporal patterning of song production: participation of nucleus uvaeformis of the thalamus. Journal of Neurobiology. 1993;24:903–912. doi: 10.1002/neu.480240704. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Woolley SM, Gill PR, Theunissen FE. Stimulus-dependent auditory tuning results in synchronous population coding of vocalizations in the songbird midbrain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26:2499–2512. doi: 10.1523/JNEUROSCI.3731-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara S, Hessler NA. Phasic basal ganglia activity associated with high-gamma oscillation during sleep in a songbird. Journal of Neurophysiology. 2012;107:424–432. doi: 10.1152/jn.00790.2011. [DOI] [PubMed] [Google Scholar]
- Yip ZC, Miller-Sims VC, Bottjer SW. Morphology of axonal projections from the high vocal center to vocal motor cortex in songbirds. Journal of Comparative Neurology. 2012;520:2742–2756. doi: 10.1002/cne.23084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AC, Margoliash D. Temporal hierarchical control of singing in birds. Science. 1996;273:1871–1875. doi: 10.1126/science.273.5283.1871. [DOI] [PubMed] [Google Scholar]