Abstract
Purpose
A high percentage of paragangliomas express somatostatin receptors that can be utilized for targeted radioisotope therapy. The aim of this study was to describe and discuss the challenges of treating these tumors with 177Lu-[DOTA0,Tyr3]octreotate (DOTATATE) radioisotope therapy using established protocols.
Methods and Results
Three paraganglioma patients were treated with 4–5 cycles of 177Lu-DOTATATE and were evaluated for side effects and response to therapy. Two of the three patients developed severe adverse reactions following their first 177Lu-DOTATATE treatment. One patient developed a catecholamine crisis and tumor lysis syndrome within hours of treatment, requiring intensive care unit (ICU) support, and another developed a catecholamine crisis 3 days after treatment, requiring hospitalization. The treatment protocols at our institution were subsequently modified by increasing the radioisotope infusion time from 15 to 30 min, as recommended in the literature, to 2–4 h and by reducing the administered dose of 177Lu-DOTATATE. Subsequent 177Lu-DOTATATE treatments utilizing the modified protocols were well tolerated, and response to therapy was achieved in all three patients, resulting in significantly improved quality of life.
Conclusion
177Lu-DOTATATE is an exciting new therapeutic option in the management of paragangliomas; however, current treatment protocols described in the literature may need to be modified by lengthening the infusion time and/or lowering the initial treatment dose to prevent or reduce the severity of adverse reactions.
Keywords: 177Lu-DOTATATE, PRRT, Radioiosotope therapy, Paraganglioma, Catecholamine crisis, Tumor lysis syndrome, Neuroendocrine tumor
Introduction
Paragangliomas are rare neuroendocrine tumors that may arise anywhere along the paraganglial system and can be associated with the sympathetic or parasympathetic nervous system. They have a high frequency of hereditary forms with a propensity for multifocal disease and are metastatic in about 10 % of cases. Paragangliomas are known to express high levels of somatostatin receptors (sst), especially subtype sst2, and a new generation of somatostatin analogs such as DOTANOC and DOTATATE has been used successfully in both diagnosis and therapy.
177Lu-DOTATATE is a promising new radioisotope therapy agent that has been used to achieve partial response or stability of disease in up to 80 % of neuroendocrine tumor patients. Its successful use in the treatment of malignant paragangliomas has recently been described in only a few case reports, and severe adverse reactions due to catecholamine crisis or tumor lysis syndrome have not been previously reported in the literature.
The objective of this study was to describe and discuss the challenges of treating paraganglioma patients with currently established 177Lu-DOTATATE protocols and to examine some protocol modifications that may be necessary to reduce the frequency and severity of adverse reactions so that subsequent 177Lu-DOTATATE treatments can be administered to improve patient outcomes.
Methods
Since August 2010, 115 neuroendocrine tumor patients have been treated with 177Lu-DOTATATE radioisotope therapy at our institution, of which 3 were histologically diagnosed with paraganglioma. These three patients are the focus of this case series.
The 177Lu-DOTATATE therapy protocol developed at our institution consists of four induction treatment cycles of 100–200 mCi each, given 8–12 weeks apart, followed by maintenance cycles of 50–100 mCi each, given 6–9 months apart, until evidence of progression of disease or significant toxicity. 177Lu-DOTATATE is given by IV infusion over 2 h, and the patient concurrently receives 1 l of an amino acid solution (2.5 % L-arginine and 2.5 % L-lysine in NS) starting 30 min prior to 177Lu-DOTATATE treatment and given over 2–4 h. The role of the amino acid solution is to prevent renal tubule reabsorption of the radioisotope to prevent the development of renal toxicity. Patients can be treated as inpatients or outpatients. All patients receive whole-body post-177Lu-DOTATATE treatment planar and SPECT/CT imaging at 1–4 days after treatment. Patients also receive a contrast-enhanced diagnostic CT prior to the first 177Lu-DOTATATE treatment and following the fourth 177Lu-DOTATATE treatment.
Results
Patient 1
A 40-year-old female presented with headaches, sweating, hypertension and back pain and was diagnosed with a malignant abdominal paraganglioma originating from the organ of Zuckerkandl, which was resected. Three years later, she developed recurrences in the left frontal bone of the skull and retroperitoneal region, both of which were resected. A 123I-MIBG scan showed additional disease in abdominal and pelvic lymph nodes and bones. She was subsequently treated with nine cycles of Sunitinib, which failed to control her blood pressure (BP) (over 200/100 mmHg), followed by two cycles of 131I-MIBG therapy (200 and 150 mCi, or 7400 and 5550 MBq), which were well tolerated but did not stop the progression of her disease. Her second post-131I-MIBG treatment scan is shown in Fig. 1a. An 111In-octreotide study showed intensely octreotide-avid disease (Fig. 1b), which was also intensely 18F-FDG avid on PET/CT (Fig. 1c), and she was referred for peptide receptor radioisotope therapy (PRRT) with 177Lu-DOTATATE. Her urinary norepinephrine was 30,140 (normal 0–505 nmol/day), urinary metanephrine 22.4 (normal 0.64–5.20 umol/day), urinary normetanephrines 22.0 (normal 0.64–4.20 umol/day), vanillylmandelic acid 440 (normal 9–34.0 umol/day), homovanillic acid 140 (normal 0–82 umol/day), epinephrine <22 (normal 0–125 nmol/day) and 24 h urinary 5-HIAA of 20 (normal 0–43 umol/day).
Fig. 1.
Patient 1. a Second post-131I-MIBG treatment scan. b 111In-octreotide scan. c 18F-FDG PET/CT
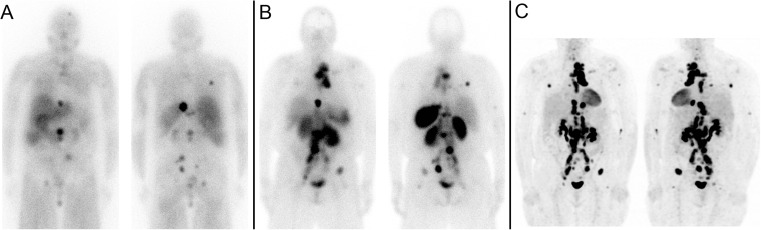
Her first 177Lu-DOTATATE treatment (Fig. 2a) was done as an inpatient with 175 mCi (6475 MBq) and was not well tolerated. Prior to her 177Lu-DOTATATE infusion her blood pressure (BP) was 136/76 mmHg. About 30 min into the infusion of 177Lu-DOTATATE, she developed tachycardia (>100 bpm), profound sweating, shortness of breath and BP around 200/120 mmHg. Her 177Lu-DOTATATE infusion rate was slowed down and given over 4 h instead of the planned 2 h. Her symptoms progressed overnight with worsening lability of blood pressure, flash pulmonary edema, low output failure, acral cyanosis and somnolence, resulting in ICU admission for a catecholamine crisis. Her potassium rose to 6.6 (normal 3.3–4.8 mmol/l), creatinine to 107 from 76 (normal 50–105 umol/l), glucose random to 41.3 from 8.2 (normal 3.3–11.0 mmol/l), phosphorus to 2.01 from 1.26 (normal 0.80–1.45 mmol/l), calcium dropped to 2.33 from 2.54 (normal 2.10–2.60 mmol/l) and LDH rose to 303 from 167 (normal 100–225 U/l), consistent with tumor lysis syndrome in addition to catecholamine crisis. Her troponin I was elevated at 2.06 (normal <0.15 ug/l); however, serial ECGs were normal, and troponin normalized on serial measurements. She was stabilized in the ICU and discharged 6 h later. Within 2 weeks, her paraganglioma symptoms improved significantly with less frequent headaches and flushing as well as resolution of sweating. Her mobility also started to improve.
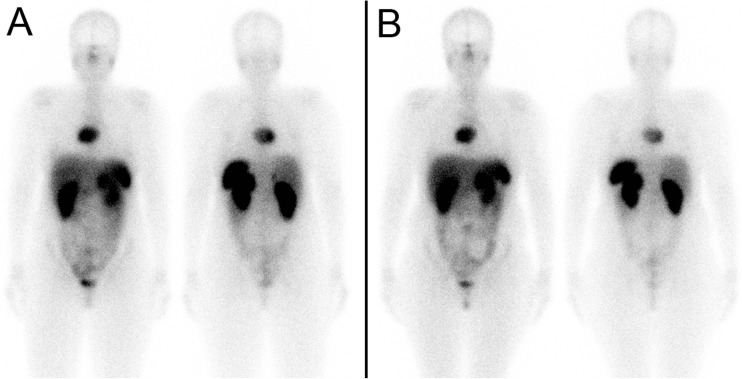
Fig. 2.
Patient 1. a First post 177Lu-DOTATATE treatment scan. b Fifth post 177Lu-DOTATATE treatment scan
Her second treatment was modified by reducing the dose from 175 mCi (6475 MBq) to 125 mCi (4625 MBq), and the infusion was again given over 4 h. The second treatment was much better tolerated, and while she developed intermittent headaches, sweating, palpitations and labile blood pressure during the second 177Lu-DOTATATE infusion, these symptoms resolved shortly after the infusion was completed. The next three treatments were done with a further reduced dose of 50 mCi (1850 MBq), infused over 4 h. These treatments were well tolerated with only occasional spikes in blood pressure, usually toward the end of the 177Lu-DOTATATE infusion, which were treated and controlled with 0.1 mg of clonidine. The patient experienced a dramatic improvement in symptoms and quality of life, and despite being barely mobile with a walker prior to the start of 177Lu-DOTATATE therapy, after five treatments she was able to walk comfortably with a cane. Her chromogranin A decreased from 3200 prior to the first treatment to 550 after the fifth treatment (normal <40 U/l), and post-treatment scans showed mild improvement of metastatic disease (Fig. 2b). Unfortunately, prior to her sixth 177Lu-DOTATATE treatment (14 months after start of therapy), she developed a pathological fracture of the cervical spine and passed away a few months later from sepsis, while hospitalized.
Patient 2
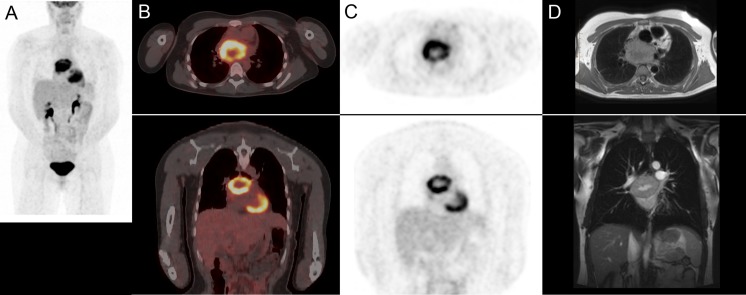
A 29-year-old female was diagnosed with eclampsia during her second trimester with blood pressure of approximately 180/120 mmHg. She had an emergency C-section at 28 weeks because of difficulty controlling her blood pressure and delivered an 890 g male infant. Prior to her pregnancy, she had complained of constant headaches, dizziness, vertigo, sweating and fatigue. Imaging workup revealed a 7.4 × 5.6-cm posterior mediastinal mass between the pulmonary arteries, aorta, SVC and atria, which was pathologically confirmed to be a malignant paraganglioma, later confirmed to be hereditary with a SDH-C mutation c.397C > T, p.R133X. This mass was 18F-FDG positive (Fig. 3a-c), and MRI showed high signal intensity on T1-weighted imaging with a low intensity core, consistent with central necrosis (Fig. 3d). The mass was also 123I-MIBG (Fig. 4a) and 111In-octreotide (Fig. 4b) positive. Although an attempt at surgical resection was made, the tumor was attached to the heart, pulmonary artery and veins and was deemed unresectable. She was referred for PRRT with 177Lu-DOTATATE. Her urinary metanephrines were 41.9 (normal <5.6 umol/day) [13.3 normetanephrine, (normal <2.6), 28.1 3-methoxytyramine (normal <1.3) and 0.6 metanephrine (normal <1.7)]. Norephinephrine total was 3962 (normal <600 nmol/day), dopamine total 12,199 (normal <2600 nmol/day) and epinephrine total 38 (normal <71 nmol/day).
Fig. 3.
Patient 2. a 18F-FDG PET/CT maximum intensity projection (MIP) image; b PET/CT fusion images; c PET; d MRI T1 transaxial and T1 post gadolinium coronal images
Fig. 4.
Patient 2. a 123I-MIBG whole-body scan; b 111In-octreotide scan
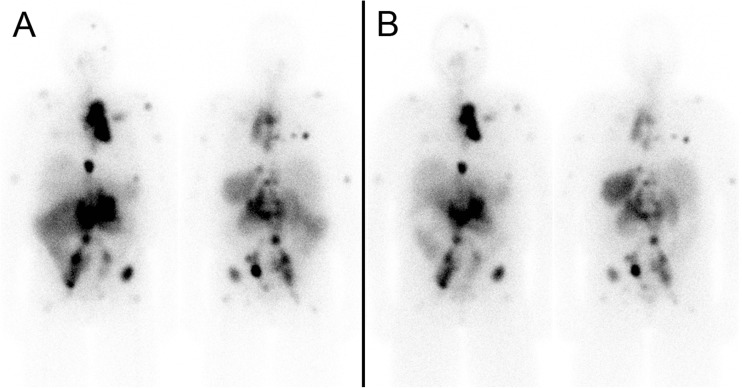
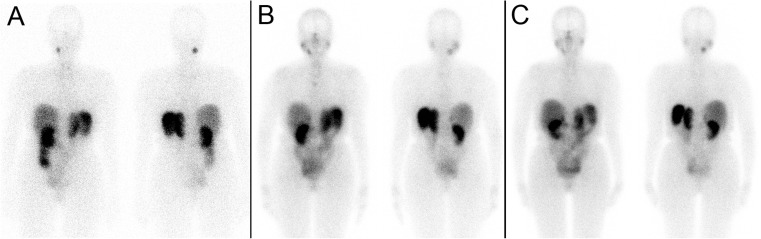
Her first 177Lu-DOTATATE treatment was done as an inpatient with 175 mCi (6475 MBq) (Fig. 5a), initially planned to be given over 2 h. Approximately 45 min into the infusion, she developed flushing and a feeling of warmth, followed by headache, chest pain, diaphoresis and labile BP, and the infusion was slowed down and given over 4 h, which was better tolerated. However, 3 days following the treatment she developed dizziness, shortness of breath and palpitations and was found to have a BP of 72/32 mmHg. Her blood pressure medications were stopped, and she was hospitalized for 2 days while her blood pressure was stabilized.
Fig. 5.
Patient 2. a First post 177Lu-DOTATATE treatment scan. b Fourth post 177Lu-DOTATATE treatment scan
Her next three treatments with 150 mCi (5550 MBq) were given over 4 h and were well tolerated with no significant symptoms. After the four cycles, each given 12 weeks apart (total cumulative dose of 660 mCi or 24.4 GBq), the patient reported dramatically reduced frequency of headaches and complete resolution of diaphoresis and blood pressure instability. After the fourth 177Lu-DOTATATE treatment scan (Fig. 5b), an MRI done 1 year after initiation of 177Lu-DOTATATE therapy showed stable disease with no change in tumor size. Her chromogranin A decreased from 210 prior to the first 177Lu-DOTATATE treatment to 56 following the fourth treatment (normal <40 U/l). She is currently awaiting her fifth 177Lu-DOTATATE treatment.
Patient 3
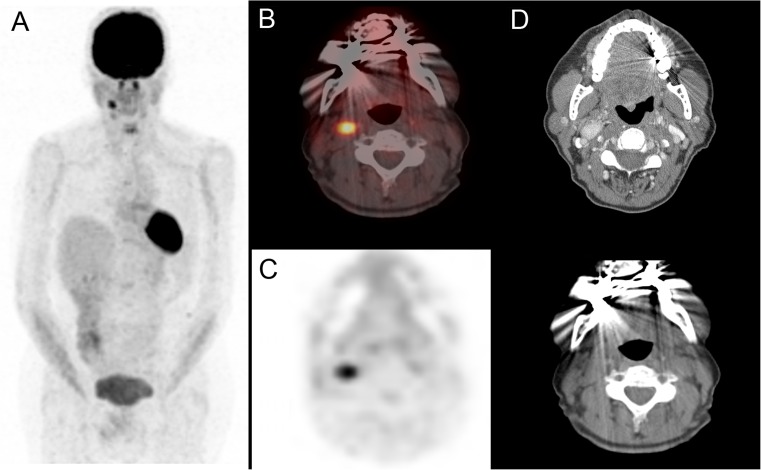
A 41-year-old female was operated on for a presumed uterine fibroid and had an 8-cm mass removed from the pelvis that was histologically confirmed to be a hereditary malignant paraganglioma with an SDH-B mutation in exon 3 (c.286 + 1G > A), (PGL4), in the region of the organ of Zuckerkandl, attached to the right iliac vessels. Twenty-two years later, she developed back pain, and CT showed a destructive L3 lesion with soft tissue extension into the spinal canal. She was surgically managed with partial resection of L3, decompression of the extradural spinal tumor and pedicle screw fixation, followed by radiotherapy (45 Gy in 25 fractions to L3). She was then treated with sunitinib for 42 days, followed by nine cycles of chemotherapy with VCD (vincristine, cyclophosphamide, dacarbazine), but her disease progressed further with new metastases in the right carotid body and thoracic and lumbar spine. An 18F-FDG PET/CT showed an intensely 18F-FDG-avid 2.5-cm right carotid body mass with maximum standardized uptake value (SUVmax) 4.3 (Fig. 6). Her 111In-octreotide scan (Fig. 7a) showed intense uptake in the right carotid body mass and mild uptake in the lumbar spine bone metastases, and she was referred for PRRT with 177Lu-DOTATATE. Prior to PRRT, she complained of severe back pain and fatigue. Her chromogranin A was in the normal range at 36 (normal <40 U/l). Her urinary catecholamines were also in the normal range.
Fig. 6.
Patient 3. a 18F-FDG PET/CT MIP image; b PET/CT fusion image; c PET; d CT images (with and without contrast enhancement)
Fig. 7.
Patient 3. a 111In-Octreotide whole-body scan; b first post-177Lu-DOTATATE treatment scan. c Fifth post 177Lu-DOTATATE treatment scan
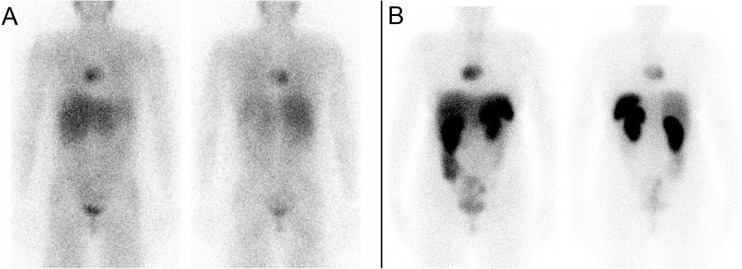
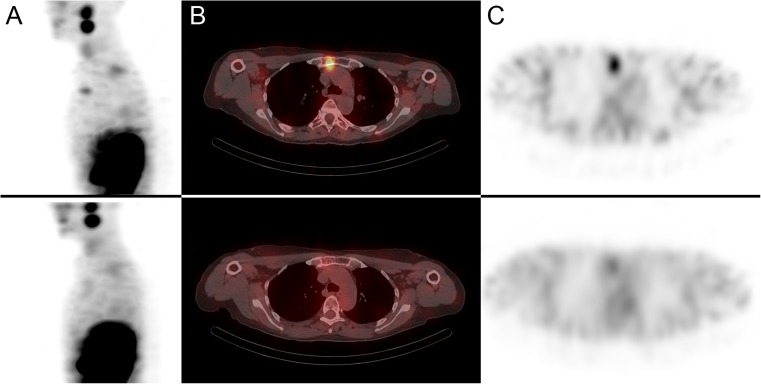
Her first 177Lu-DOTATATE treatment (Fig. 7b) was done as an inpatient and was administered with a cautious dose of 100 mCi (3700 MBq) given over 2 h. The treatment was well tolerated with no significant side effects. Her subsequent four 177Lu-DOTATATE treatment cycles were done with an increased dose of 150 mCi (5550 MBq) given over 2 h, which were all well tolerated. Her symptoms improved significantly, and her back pain and fatigue resolved almost completely. The post fifth 177Lu-DOTATATE treatment scan (Fig. 7c) as well as a follow-up MRI showed an improvement in manubrial (Fig. 8), thoracic and lumbar bone metastases as well as the right carotid body mass, which decreased in size from 2.5 cm to 1.5 cm. She is currently awaiting her sixth 177Lu-DOTATATE treatment cycle.
Fig. 8.
Patient 3. a Sagittal first post-177Lu-DOTATATE treatment scan showing an intensely avid manubrial bone metastasis (top row); sagittal fifth post-177Lu-DOTATATE treatment scan showing excellent treatment response (bottom row). b Transaxial SPECT/CT and c SPECT images
Discussion
Somatostatin (SS) is a polypeptide that is mainly produced by the delta cells of the gastrointestinal tract and by pancreatic islet cells. It exerts inhibitory effects on almost all endocrine and exocrine secretions and acts as an endogenous cell-proliferation inhibitor in various normal and neoplastic tissues. These actions are mediated through specific membrane G-protein-coupled receptors [1]. Five somatostatin receptor subtypes (sst1–sst5) have been identified in humans. Each sst subtype mediates different biological actions of SS via activation of different intracellular systems such as inhibition of adenylate cyclase and reduction of intracellular calcium levels, resulting in inhibition of hormonal secretion [2].
Paragangliomas are rare neuroendocrine tumors that may arise anywhere along the paraganglial system and can be associated with the sympathetic or parasympathetic nervous system. They have a high frequency of hereditary forms (20–30 % have a familial PGL syndrome with germline mutations in genes such as SDH-B, C, D, c-RET, VHL and TMEM127), with a propensity for multifocal disease, and are malignant in about 10 % of cases [3]. Somatostatin receptors are expressed in paragangliomas with the predominant expression of sst2. Mean levels of sst2 mRNA are similar to those observed in GET-NETs; however, some paragangliomas have cytoplasmic localization of sst2 receptor rather than membrane localization, and it has been suggested that this may account for not only the failure of sst2 agonists in controlling catecholamine secretion and tumor proliferation, but also failure of 111In-octreotide imaging to detect some paragangliomas [4–6].
111In-Octreotide is a somatostatin analog with modest sst2 binding that has been reported to have a good sensitivity for head and neck paragangliomas, up to 90 % [7], but a lower sensitivity for detecting paragangliomas overall (74 % reported by Charrier in 2011) with a sensitivity as low as 20 % for abdominal paragangliomas [8]. A new generation of somatostatin analogs with superior sst2 binding has been developed for use with PET/CT imaging including 68Ga-DOTATOC, 68Ga-DOTANOC and 68Ga -DOTATATE (see affinity profiles for sst receptors in Table 1) [9, 10].
Table 1.
Affinity profiles (IC50) of somatostatin receptor subtypes for different somatostatin analogs used in diagnostic imaging and therapy
| sst1 | sst2 | sst3 | sst4 | sst5 | |
|---|---|---|---|---|---|
| Somatostatin-28 | 5.2 | 2.7 | 7.7 | 5.6 | 4.0 |
| 111In-octreotide | >10,000 | 22 | 182 | >1000 | 237 |
| 68Ga-DOTATOC | >10,000 | 2.5 | 613 | >1000 | 73 |
| 68Ga-DOTANOC | >10,000 | 1.9 | 40 | 260 | 7.2 |
| 68Ga-DOTATATE | >10,000 | 0.2 | >1000 | 300 | 377 |
IC50 is expressed in nanomoles (lower values represent higher receptor affinity)
Maurice et al. reported a 68Ga-DOTATATE PET/CT sensitivity of 80 % in the detection of pheochromocytomas and paragangliomas, superior to 123I-MIBG [11], and Naswa reported a sensitivity of 100 % for 68Ga-DOTANOC in 35 patients with pheochromocytoma and paraganglioma, superior to 131I-MIBG [12]. Similar results were obtained by Sharma et al. who reported a sensitivity of 100 % for 68Ga-DOTANOC in 26 head and neck paraganglioma patients [13].
177Lu-DOTATATE has been found to be superior to other radiolabeled somatostatin analogs for use in PRRT because of its high somatostatin receptor subtype 2 binding, superior tumor uptake and lower toxicity profile due to the lower tissue penetration range of the 177Lu radioisotope [9, 14]. PRRT of gastroenteropancreatic neuroendocrine tumors can yield partial response or stability of disease in up to 80 % of patients [15].
The use of 177Lu-DOTATATE in the treatment of pheochromocytoma or paraganglioma patients has been described in a very limited number of patients. Successful treatment has been described in a case of spinal and cranial paraganglioma [16], four patients with non-metastatic neck or mediastinal paragangioma [17], and a carotid body paraganglioma [18]. None of these studies described details of the 177Lu-DOTATATE therapy protocol used or any adverse reactions encountered.
Hormonal crises due to excessive catecholamine secretion in patients with metastatic paraganglioma can lead to hypertension, hypotension, myocardial ischemia, pulmonary edema and shock [19], and such crises have been reported with 131I-MIBG therapy [20–22]. Safford et al. also reported two deaths from myocardial infarction following high dose 131I-MIBG therapy with a dose of 300 mCi (11.1 GBq) [22]. However, a 177Lu-DOTATATE treatment-induced catecholamine crisis in a pheochromocytoma or paraganglioma patient has been described in only one case of malignant pheochromocytoma, in which the patient developed hypotension, excessive sweating and cardiac ischemia 1 day after 177Lu-DOTATATE therapy [23]. The patient was pre-treated with corticosteroids for subsequent treatments; however, the authors did not provide further details. This pre-treatment approach is concerning, as catecholamine crisis induced by exogenous glucocorticoids has recently been described in the literature [24]. Attempts to reduce the patient’s dose or increase the 177Lu-DOTATATE infusion time (177Lu-DOTATATE is given over a 30-min infusion at this institution) were not made in this patient, nor suggested by the authors [25].
Institutions that offer 177Lu-DOTATATE therapy have protocols with varying 177Lu-DOTATATE infusion times ranging from 10 to 15 min [26, 27], to 20 min [28] to 30 min [25, 29]. The protocol developed at our institution involves treating neuroendocrine patients with 177Lu-DOTATATE given over 2 h, which was based on our early observations that longer infusion times decreased the incidence of adverse reactions such as nausea or fatigue. While this is the longest infusion time described in the literature to date, two of our patients still developed a catecholamine crisis even when the infusion time was slowed to 4 h, with one patient requiring ICU support (while also developing tumor lysis syndrome), and another patient requiring short-term hospitalization.
Tumor lysis syndrome due to 177Lu-DOTATATE therapy has not been previously described. It is characterized by massive tumor cell lysis with release of large amounts of potassium, phosphate and uric acid. Deposition of uric acid and calcium phosphate crystals in the renal tubules may lead to acute renal failure. It is extremely rare in solid tumors and is more commonly seen in hematologic malignancies. Management involves clearing the plasma of high potassium, uric acid and phosphorus and prevention of acute renal failure by aggressive intravenous hydration [30–32]. Patient 1 exhibited the classical biochemical markers of tumor lysis syndrome within 12 h of 177Lu-DOTATATE therapy with severely elevated potassium, phosphorus, low calcium and high creatinine levels, which were managed in the intensive care unit setting.
Our experiences suggest that in paraganglioma patients, 177Lu-DOTATATE should be administered over 2 h at least, but preferably over 4 h, and not over 15–30 min as described in the literature. The first 177Lu-DOTATATE treatment should be done as an in-patient, patients should be monitored very closely, and intensive care support should be available if needed. Lower initial 177Lu-DOTATATE treatment doses may also play a role in the design of an optimal treatment protocol. Pre-treatment with glucocorticoids is not recommended as it may potentially increase the risk of a catecholamine crisis occurring. Further research is needed to establish the optimal 177Lu-DOTATATE protocols for treating paraganglioma patients in order to minimize severe adverse reactions.
Conclusion
177Lu-DOTATATE is an exciting new therapeutic agent in the management of paragangliomas; however, severe adverse reactions can occur during and up to several days following treatment. Existing 177Lu-DOTATATE treatment protocols could be modified by lengthening the infusion time of 177Lu-DOTATATE, and/or lowering the initial treatment dose to help prevent adverse reactions or reduce their severity. Such modifications are likely to help paraganglioma patients tolerate 177Lu-DOTATATE treatments better and allow subsequent treatment cycles to maximize the benefits of this therapy and improve treatment outcomes.
Acknowledgments
Conflict of Interest
William Makis, Karey McCann and Alexander JB McEwan declare that they have no conflicts of interest.
Ethical Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study. The manuscript has been read and approved by all the authors, and the requirements for authorship have been met. Each author believes that the manuscript represents honest work.
References
- 1.Reubi JC, Laissue JA. Multiple actions of somatostatin in neoplastic disease. Trends Pharmacol Sci. 1995;16:110–115. doi: 10.1016/S0165-6147(00)88992-0. [DOI] [PubMed] [Google Scholar]
- 2.Reisine T, Bell GI. Molecular biology of somatostatin receptors. Endocr Rev. 1995;16:427–442. doi: 10.1210/edrv-16-4-427. [DOI] [PubMed] [Google Scholar]
- 3.Pacak K, Eisenhofer G, Ahlman H, Bornstein SR, Gimenez-Roqueplo AP, Grossman AB, et al. Pheochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pract Endocrinol Metab. 2007;3:92–102. doi: 10.1038/ncpendmet0396. [DOI] [PubMed] [Google Scholar]
- 4.Saveanu A, Muresan M, de Micco C, Taieb D, Germanetti AL, Sebag F, et al. Expression of somatostatin receptors, dopamine D2 receptors, noradrenaline transporters, and vesicular monoamine transporters in 52 pheochromocytomas and paragangliomas. Endocr Relat Cancer. 2011;18:287–300. doi: 10.1530/ERC-10-0175. [DOI] [PubMed] [Google Scholar]
- 5.Reubi JC, Waser B, Liu Q, Laissue JA, Schonbrunn A. Subcellular distribution of somatostatin sst2A receptors in human tumors of the nervous and neuroendocrine systems: membranous versus intracellular location. J Clin Endocrinol Metab. 2000;85:3882–3891. doi: 10.1210/jcem.85.10.6864. [DOI] [PubMed] [Google Scholar]
- 6.Binderup T, Knigge U, Mellon Mogensen A, Palnaes Hansen C, Kjaer A. Quantitative gene expression of somatostatin receptors and noradrenaline transporter underlying scintigraphic results in patients with neuroendocrine tumors. Neuroendocrinology. 2008;87:223–232. doi: 10.1159/000113128. [DOI] [PubMed] [Google Scholar]
- 7.Koopmans KP, Jager PL, Kema IP, Kerstens MN, Albers F, Dullaart RPF. 111In-octreotide is superior to 123I-metaiodobenzylgianidine for scintigraphic detection of head and neck paragangliomas. J Nucl Med. 2008;49:1232–1237. doi: 10.2967/jnumed.107.047738. [DOI] [PubMed] [Google Scholar]
- 8.Charrier N, Deveze A, Fakhry N, Sebag F, Morange I, Gaborit B, et al. Comparison of [111In]pentetreotide-SPECT and [18F]FDOPA-PET in the localization of extra-adrenal paragangliomas: the case for a patient-tailored use of nuclear imaging modalities. Clin Endocrinol. 2011;74:21–29. doi: 10.1111/j.1365-2265.2010.03893.x. [DOI] [PubMed] [Google Scholar]
- 9.Reubi JC, Schar JC, Waser B, Wenger S, Heppeler A, Schmitt JS, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000;27:273–282. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 10.Wild D, Schmitt JS, Ginj M, Mäcke HR, Bernard BF, Krenning E, et al. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur J Nucl Med Mol Imaging. 2003;30:1338–1347. doi: 10.1007/s00259-003-1255-5. [DOI] [PubMed] [Google Scholar]
- 11.Maurice JB, Troke R, Win Z, Ramachandran R, Al-Nahhas A, Naji M, et al. A Comparison of the performance of 68Ga-DOTATATE PET/CT and 123I-MIBG SPECT in the diagnosis and follow-up of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2012;39:1266–1270. doi: 10.1007/s00259-012-2119-7. [DOI] [PubMed] [Google Scholar]
- 12.Naswa N, Sharma P, Nazar AH, Agarwal KK, Kumar R, Ammini AC, et al. Prospective evaluation of 68Ga-DOTA-NOC PET-CT in phaeochromocytoma and paraganglioma: preliminary results from a single centre study. Eur Radiol. 2012;22:710–719. doi: 10.1007/s00330-011-2289-x. [DOI] [PubMed] [Google Scholar]
- 13.Sharma P, Thakar A, Suman S, Dhull VS, Singh H, Naswa N, et al. 68Ga-DOTANOC PET/CT for baseline evaluation of patients with head and neck paraganglioma. J Nucl Med. 2013;54:841–847. doi: 10.2967/jnumed.112.115485. [DOI] [PubMed] [Google Scholar]
- 14.Kwekkeboom DJ, de Herder WW, van Eijck CHJ, Kam BL, van Essen M, Teunissen JJM, et al. Peptide receptor radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med. 2010;40:78–88. doi: 10.1053/j.semnuclmed.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Campana D, Capurso G, Partelli S, Nori F, Panzuto F, Tamburrino D, et al. Radiolabelled somatostatin analogue treatment in gastroenteropancreatic neuroendocrine tumours: factors associated with response and suggestions for therapeutic sequence. Eur J Nucl Med Mol Imaging. 2013;40:1197–1205. doi: 10.1007/s00259-013-2402-2. [DOI] [PubMed] [Google Scholar]
- 16.Cecchin D, Schiavi F, Fanti S, Favero M, Manara R, Fassina A, et al. Peptide receptor radionuclide therapy in a case of multiple spinal canal and cranial paragangliomas. J Clin Oncol. 2011;29:e171–e174. doi: 10.1200/JCO.2010.31.7131. [DOI] [PubMed] [Google Scholar]
- 17.Zovato S, Kumanova A, Dematte S, Sansovini M, Bodei L, di Sarra D, et al. Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE in individuals with neck or mediastinal paraganglioma (PGL) Horm Metab Res. 2012;44:411–414. doi: 10.1055/s-0032-1311637. [DOI] [PubMed] [Google Scholar]
- 18.Gupta SK, Singla S, Karunanithi S, Damle N, Bal C. Peptide receptor radionuclide therapy with 177Lu-DOTATATE in a case of recurrent carotid body paraganglioma with spinal metastases. Clin Nucl Med. 2014;39:440–441. doi: 10.1097/RLU.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 19.Kizer JR, Koniaris LS, Edelman JD, St. John Sutton MG. Pheochromocytoma crisis, cardiomyopathy, and hemodynamic collapse. Chest. 2000;118:1221–1223. doi: 10.1378/chest.118.4.1221. [DOI] [PubMed] [Google Scholar]
- 20.Troncone L, Rufini V, Montemaggi P, Danza FM, Lasorella A, Mastrangelo R. The diagnostic and therapeutic utility of radioiodinated metaiodobenzylguanidine (MIBG) Eur J Nucl Med. 1990;16:325–335. doi: 10.1007/BF00842788. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki M, Iwaoka T, Yamauchi J, Tokunaga H, Naomi S, Inoue J, et al. A case of Sipple’s syndrome with malignant pheochromocytoma treated with 131I-metaiodobenzyl guanidine and a combined chemotherapy with cyclophosphamide, vincristine and dacarbazine. Endocr J. 1994;41:155–160. doi: 10.1507/endocrj.41.155. [DOI] [PubMed] [Google Scholar]
- 22.Safford SD, Coleman RE, Gockerman JP, Moore J, Feldman JM, Leight GS, et al. Iodine-131 metaiodobenzylguanidine is an effective treatment for malignant pheochromocytoma and paraganglioma. Surgery. 2003;134:956–962. doi: 10.1016/S0039-6060(03)00426-4. [DOI] [PubMed] [Google Scholar]
- 23.de Keizer B, van Aken MO, Feelders RA, de Herder WW, Kam BLR, van Essen M, et al. Hormonal crises following receptor radionuclide therapy with the radiolabeled somatostatin analogue [177Lu-DOTA0, Tyr3]octreotate. Eur J Nucl Med Mol Imaging. 2008;35:749–755. doi: 10.1007/s00259-007-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosas AL, Kasperlik-Zaluska AA, Papierska L, Bass BL, Pacak K, Eisenhofer G. Pheochromocytoma crisis induced by glucocorticoids: a report of four cases and review of the literature. Eur J Endocrinol. 2008;158:423–429. doi: 10.1530/EJE-07-0778. [DOI] [PubMed] [Google Scholar]
- 25.Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 26.Werhmann C, Senftleben S, Zachert C, Muller D, Baum RP. Results of individual patient dosimetry in peptide receptor radionuclide therapy with 177Lu DOTA-TATE and 177Lu DOTA-NOC. Cancer Biother Radiopharm. 2007;22:406–416. doi: 10.1089/cbr.2006.325. [DOI] [PubMed] [Google Scholar]
- 27.Hubble D, Kong G, Michael M, Johnson V, Ramdave S, Hicks RJ. 177Lu-octreotate, alone or with radiosensitizing chemotherapy, is safe in neuroendocrine tumour patients previously treated with high-activity 111In-octreotide. Eur J Nucl Med Mol Imaging. 2010;37:1869–1875. doi: 10.1007/s00259-010-1483-4. [DOI] [PubMed] [Google Scholar]
- 28.Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38:2125–2135. doi: 10.1007/s00259-011-1902-1. [DOI] [PubMed] [Google Scholar]
- 29.Sandstrom M, Garske U, Granberg D, Sundin A, Lundqvist H. Individualized dosimetry in patients undergoing therapy with 177Lu-DOTA-D-Phe1-Tyr3-octreotate. Eur J Nucl Med Mol Imaging. 2010;37:212–225. doi: 10.1007/s00259-009-1216-8. [DOI] [PubMed] [Google Scholar]
- 30.Davidson MB, Thakkar S, Hix JK, Bhandarkar ND, Wong A, Schreiber MJ. Pathophysiology, clinical consequences, and treatment of tumor lysis syndrome. Am J Med. 2004;116:546–554. doi: 10.1016/j.amjmed.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 31.Mott FE, Esana A, Chakmakjian C, Herrington JD. Tumor lysis syndrome in solid tumors. Support Cancer Ther. 2005;2:188–191. doi: 10.3816/SCT.2005.n.012. [DOI] [PubMed] [Google Scholar]
- 32.Mughal TI, Ejaz AA, Foringer JR, Coiffier B. An integrated clinical approach for the identification, prevention and treatment of tumor lysis syndrome. Cancer Treat Rev. 2010;36:164–176. doi: 10.1016/j.ctrv.2009.11.001. [DOI] [PubMed] [Google Scholar]