Abstract
Background
Rotavirus is the most common cause of severe dehydrating gastroenteritis in developing countries. Safe, effective, and affordable rotavirus vaccines are needed for developing countries.
Methods
In a double-blind placebo controlled multicentre trial, 6799 infants aged 6 to 7 weeks were randomised to receive three doses of an oral human-bovine natural reassortant vaccine (116E) or placebo at ages 6, 10, and 14 weeks. Primary outcome was severe (≥11 on the Vesikari scale) rotavirus gastroenteritis. Efficacy outcomes and adverse events were ascertained through active surveillance.
Findings
At analyses, the median age was 17·2 months; over 96% subjects received all three doses of the vaccine/placebo and ~1% were lost to follow up. 4532 and 2267 subjects were randomly assigned to receive vaccine and placebo, respectively. The per protocol analyses included 4354 subjects in the vaccine and 2187 subjects in the placebo group. 71 events of severe rotavirus gastroenteritis were reported in 4752 person years among the vaccinees compared to 76 events in 2360 person years in the placebo recipients; vaccine efficacy against severe rotavirus gastroenteritis was 53·6% (95% CI 35·0–66·9; P<0·001) and 56·4% (95% CI 36·6–70·1; P <0·001) in the first year of life. The number of infants needed to be immunized to prevent one severe rotavirus gastroenteritis episode was 55 (95% CI 37–97). The incidence of severe rotavirus gastroenteritis/100 person years was 1·5 in vaccine and 3·2 in placebo group and an incidence rate ratio of 0·46 (95% CI 0·33–0·65). The absolute rate reduction for severe rotavirus gastroenteritis was 1·7 (95% CI 2·5–0·9). Efficacy against severe gastroenteritis of any aetiology was 18·6% (95% CI 1·9–32·3); it was 24·1% (95% CI 5·8–38·7) in the first year of life. The prevalence of immediate, solicited, and serious adverse events were similar in both groups. There were six cases of intussusception amongst 4532 vaccinees and two amongst 2267 placebo recipients (P=0·73). All intussusception cases occurred after the third dose. Among vaccine and placebo recipients, the minimum interval between dosing and intussusception was 112 and 36 days, respectively.
Interpretation
The monovalent human-bovine (116E) rotavirus vaccine is effective and well-tolerated in Indian infants.
INTRODUCTION
Rotavirus is the leading cause of severe gastroenteritis among children in developing countries.1 Two oral, live attenuated rotavirus vaccines, RotaTeq (Merck) and Rotarix (GlaxoSmithKline Biologicals), are currently available.2 The World Health Organisation (WHO) recommends universal introduction of safe and efficacious rotavirus vaccines in national immunisation programs.2
The protective efficacy of these vaccines is high in affluent, but declines substantially in middle and low income countries.3–11 Even with moderate clinical efficacy in developing countries, the impact on incidence of moderate to severe disease and hospitalisations for gastroenteritis, and related mortality is relatively higher and of significant public health importance.2,7–9,12
Many countries including India have not introduced rotavirus vaccines in their immunisation programs and where introduced with GAVI support, there are sustainability challenges at current vaccine prices when external support is no longer available. In developing countries, the availability of affordable and efficacious rotavirus vaccines is critical.
The 116E rotavirus strain, characterised under the Indo-US Vaccine Action Program13 is a naturally occurring reassortant strain G9P[11], containing one bovine rotavirus gene P[11] and 10 human rotavirus genes. 116E readily infected hospital born neonates in Delhi, India and was considered well adapted to the neonatal gut and naturally attenuated, since the neonatal infection was asymptomatic.14,15 The candidate strain was adapted to grow in Vero-cells and found to be safe and immunogenic.16 We report results of a multicenter phase III trial evaluating efficacy of the vaccine against severe rotavirus gastroenteritis (RVGE) and tolerability in low resource urban and rural settings in India.
METHODS
Study design and participants
This double blind placebo controlled trial evaluated the efficacy of 116E vaccine against severe RVGE and tolerability when administered as a three dose series at 6, 10, and 14 weeks of age. Trial sites were Delhi (urban), Pune (rural), and Vellore (60% urban, 40% rural) in India. Based on routine surveillance at the sites, the female literacy rates were 74·3% (Delhi), 61% (Pune), and 75·3% (Vellore). The infant mortality rates were 39·8 (Delhi), 37·5 (Pune), and 30·4 (Vellore) per thousand live births.17 The first subject was recruited in March 2011. This was an event driven trial and the findings reported are up to the data cut-off date of November 5, 2012.
Infants between 6 to 7 weeks were eligible for enrolment, if the parents consented for participation and had no plans to move out of the study area during the next 24 months. Infants were excluded if they had received a rotavirus vaccine, had documented immunodeficiency or chronic gastroenteritis or any other condition adjudged by the investigator as an exclusion criteria. Presence of any illness requiring hospital referral and diarrhoea on the day of enrolment was a temporary exclusion.
Vaccine
0·5 ml of the 116E vaccine (ROTAVAC®, Bharat Biotech International Limited, India) contained not less than 105 Fluorescent Focus Unit. The placebo was identical in content, packaging, and appearance to the vaccine but did not contain the virus. The vaccine/placebo was stored at −20°C±5 °C; buffer was stored at room temperature. Vaccine/placebo was administered 5 to 10 minutes after administration of 2·5 mL of citrate bicarbonate buffer. Other childhood vaccines (Diphtheria-wPertussis-Tetanus, Haemophilus influenzae b, Hepatitis B, and Oral Polio Vaccine) were given concurrently. Mothers were not given any specific instructions regarding breastfeeding around the time of vaccination.
Ethical considerations
Ethical and administrative clearances were obtained from the three sites, the Department of Biotechnology (India), and Western Institutional Review Board (USA). Written informed consent was taken from parents. The study was conducted in compliance with the protocol, good clinical practices, and national regulatory and ethical guidelines.18,19 A data safety monitoring board (DSMB) periodically reviewed study data. Monitoring and pharmacovigilance was conducted by Quintiles. Independent consultants conducted laboratory and site audits.
Randomisation and masking
Infants were randomly assigned in a 2:1 (vaccine:placebo) ratio to receive three doses of vaccine/ placebo at 6 to 7 weeks, ≥10 weeks, and ≥14 weeks of age.
Randomisation was performed by Cenduit, LLC, Germany, with stratification by site, and a block size of 12. Three letter codes were used to maintain the ratio of 2:1 for vaccine and placebo, two letters (X and O) were assigned to the vaccine and one to the placebo (J). The test article team was independent and based in a separate room with restricted access; they did not interact with other study teams at the site. The test article team obtained the letter code (for vaccine/placebo) through the Interactive Voice Response System (or Interactive Web Response System). The letter code on the vaccine/placebo vial was masked with the subject identification number before sending the vial to the clinical coordinator administering the test article to the enrolled infant.
Ascertainment of efficacy and safety outcomes
Subjects were observed at the study clinic for at least 30 minutes following the vaccine/placebo administration for immediate adverse events (IAE).
In the first one third of enrolled subjects, solicited adverse events (fever, vomiting, diarrhoea, cough, runny nose, irritability, rash) and any other adverse events (AE) reported by families were documented daily for a period of 14 days after each dose.
All subjects were contacted weekly at home by trained field workers to identify gastroenteritis, signs and symptoms of suspected intussusception, hospitalisations, and other illnesses.
Gastroenteritis was defined as passage of ≥3 looser-than-normal or watery stools in a 24 hour period with or without vomiting. Subjects with gastroenteritis were provided packets of oral rehydration salts solution and zinc tablets.20 Subjects with dehydration or other illnesses requiring hospitalization were sent to designated hospitals by study physicians. Gastroenteritis episode characteristics were documented for each day through home or hospital visits, and a stool specimen was collected up to 7 days after the last day of gastroenteritis.
Mothers were given mobile telephones with the study team contact numbers, digital thermometers, and participant booklets. Families were instructed to call, or bring their child to the study clinic for gastroenteritis, other illnesses, or presence of signs and symptoms of suspected intussusception; study physicians were available round the clock. Costs of medical care (including transportation) for outpatient visits and hospitalisations were covered by the study. Independent paediatricians served as safety advisors at each site and reviewed safety data periodically.
Subjects with one or more signs or symptoms of suspected intussusception (abdominal distension, abdominal lump, ≥3 vomiting episodes in an hour, and blood in stools) were examined by a paediatrician, referred to a paediatric surgeon, and hospitalised, as necessary. An Adjudication Committee comprising of a paediatric surgeon, paediatrician, and a radiologist reviewed all investigator-diagnosed cases of intussusception to provide the final diagnosis using Brighton Criteria Level 1.21
The subjects’ families, study teams, paediatricians in referral hospitals, laboratory staff, and committee members were all blinded to the treatment allocation. Enrolled subjects were identified only by a unique subject identification number.
Immunogenicity and viral shedding subset
A subset of 150 subjects at each site constituted the immunogenicity and viral shedding subgroup. In these subjects, 2 ml blood was drawn at baseline and 28 days after the third dose of the vaccine/ placebo to estimate serum anti-rotavirus IgA and stool specimens were obtained prior to and on days 3 and 7 after each dose for shedding of vaccine virus.
Analyses of specimens
Rotavirus was detected in stools using a commercial enzyme immunoassay (Premier™ Rotaclone; Meridian Bioscience, USA). Rotaclone positive stools were analyzed for G (VP7) and P (VP4) genotypes by multiplex polymerase chain reaction (PCR).22,23 VP6 gene detection assay by PCR was done for specimens that could not be genotyped.24 The PCR assay was not designed to differentiate vaccine G9P[11] from wild G9P[11].
Serum anti-rotavirus IgA was determined by an enzyme-linked immunosorbent assay (ELISA) with a standard curve method.25 Seroconversion was defined as a 4-fold rise in titre from paired serum samples.
Sample size
With an assumed vaccine efficacy of 60%, an attack rate of 2·6% over 1·5 years, 20% dropout rate, and 89% power, a total of 6800 subjects were required to accrue 85 cases of severe RVGE, the primary endpoint, for a ratio of vaccine to placebo of 2:1. To allow for a conclusion that the vaccine was efficacious, the lower bound of the 95% confidence interval (CI) had to be ≥20%.
Statistical analyses
Analyses were done by Quintiles using SAS® Version 9·2. Efficacy analyses were performed on the per-protocol (PP) and intent-to-treat (ITT) populations. The PP population was considered the primary population and included all subjects who received the same treatment at all three doses of vaccine/placebo within the prescribed windows and had episodes occurring more than 14 days after the third dose. The ITT population included all subjects who received at least one dose of vaccine/placebo and included episodes occurring after the first dose.
Vesikari scores were computed for each episode during analyses.26 An episode of gastroenteritis was considered as a new event based on resolution of diarrhoea for ≥7 days from time of end of the previous episode.. For each outcome, only the first event was counted for each subject. The follow up period for each event was calculated as time to occurrence of the event; the date of dropout or until the data cut-off date.
Vaccine efficacy was defined as 100 × (1 − [nv/Fv]/[np/Fp]) person time incidence rate where nv and np were the number of subjects with at least one case in the vaccine/placebo groups; Fv and Fp were the total length of follow up in the relevant treatment group. The numbers of cases in the vaccine and placebo groups were assumed to follow Poisson distributions with respective parameters λvFv and λpFp. Given the total number of cases n, the number in the vaccine group follows a binomial distribution with n trials and probability parameter λvFv /(λvFv+λpFp). P-values and confidence intervals for vaccine efficacy were computed using exact binomial methods27
The proportion of subjects with AEs were compared between groups using Fisher’s Exact Test and the results presented as odds ratios, 95% CI, and P values. All events were coded using Medical Dictionary for Regulatory Activities (MedDRA) Version 15·0.
The results presented are for the PP population, unless otherwise stated.
Data access
The data presented in the manuscript is available to all primary authors with the exception of listings of individual subject allocation, the site teams remain blinded because the study was ongoing. All primary authors were responsible for the decision to submit the manuscript, coordinated by the corresponding author.
Role of funding sources
The Department of Biotechnology, and Biotechnology Industry Research Assistance Council, Government of India, New Delhi, India; the Bill & Melinda Gates Foundation (#52714) to PATH, USA; Research Council of Norway; Department for International Development, United Kingdom; National Institutes of Health, Bethesda, USA; Bharat Biotech International Limited, Hyderabad, India provided funding. The funders had no role in the conduct of the trial and data collection.
RESULTS
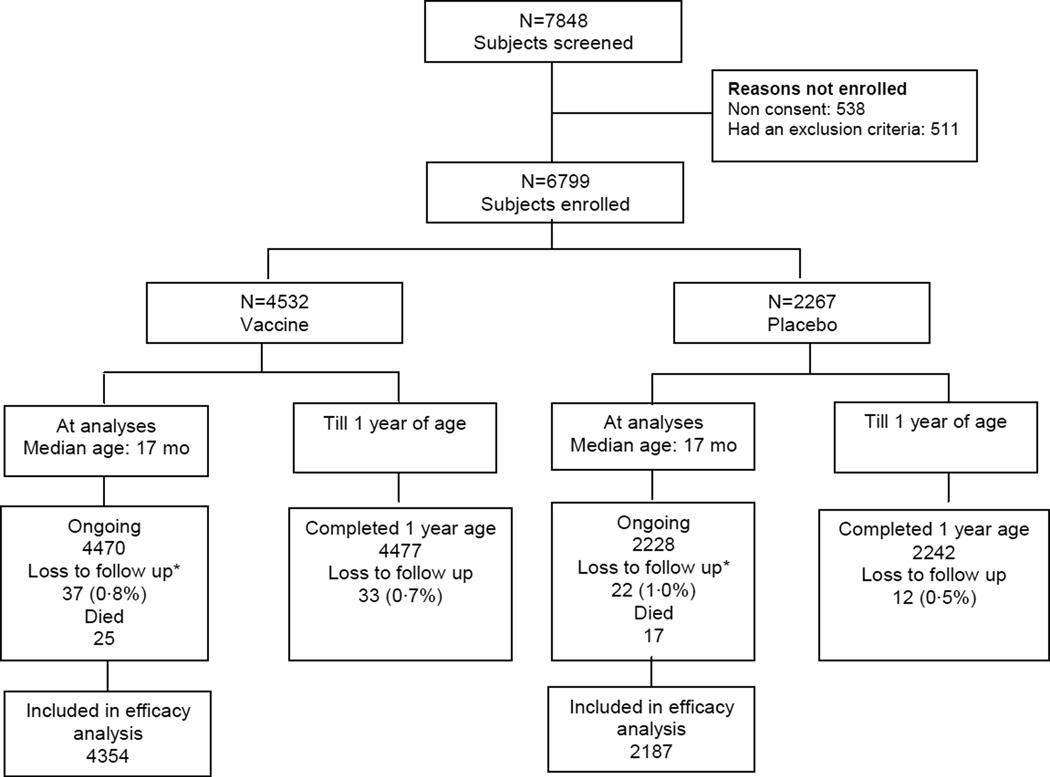
A total of 7848 subjects were screened and 6799 enrolled; 3799 in Delhi and 1500 each in Pune and Vellore. Of these 4532 received vaccine and 2267 placebo. At analysis, the median age of subjects was 17·2 (range 13·4 to 21·7) months, all subjects had reached one year of age, and loss to follow up was around 1% (Figure 1). The total follow up time for the PP population was 4752 years in the vaccine and 2360 years in the placebo groups. Compliance with dosing (96·3% of subjects) was high and administration close to the recommended age (Table 1).
Figure 1.
Flowchart of subject disposition
*Reasons for loss to follow up in vaccinees/placebo recipients: Lost to follow up 12/12; family refused further participation 25/10
Table 1.
Baseline characteristics and compliance to administration of vaccine/placebo in enrolled subjects
| Number of subjects | Vaccine 4532 |
Placebo 2267 |
|---|---|---|
| Delhi | 2532 | 1267 |
| Pune | 1000 | 500 |
| Vellore | 1000 | 500 |
| Age in weeks at dosing, Mean (SD) | ||
| Dose 1 | 6·8 (0·6) | 6·8 (0·6) |
| Dose 2 | 11·7 (2·4) | 11·7 (2·4) |
| Dose 3 | 16·3 (2·8) | 16·4 (2·8) |
| Gender, n (%) | ||
| Males | 2330 (51·4) | 1157 (51·0) |
| Subjects who received each dose, n (%) | ||
| Dose 1 | 4532 (100·0) | 2267 (100·0) |
| Dose 2 | 4409 (97·3) | 2221 (98·0) |
| Dose 3 | 4356 (96·1) | 2190 (96·6) |
Following the accumulation of the target number of primary endpoint cases, the independent biostatistics team of Quintiles, South Africa analysed and provided results to the DSMB for review. The DSMB concluded that the primary hypothesis had been satisfied and advised unblinding of data and that subjects be followed until all reached 2 years of age to obtain data for safety and efficacy in the second year of life. Site teams, who had any contact with subjects, remain blinded to individual subject allocation.
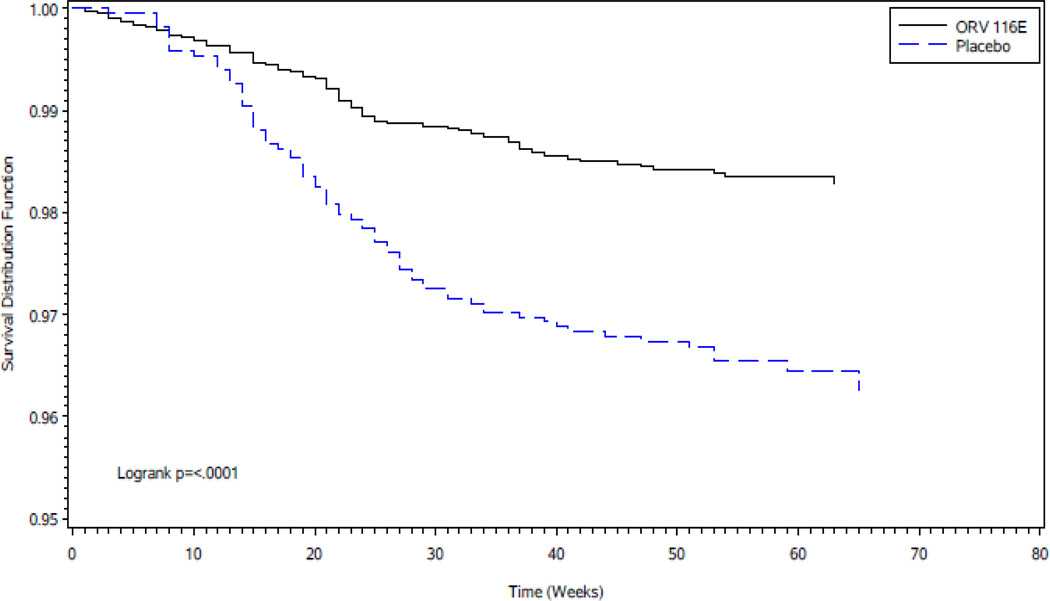
Vaccine efficacy for severe RVGE was 53·6% (95% CI 35·0–66·9; P<0·001). The lower confidence limit exceeded the pre-specified criterion of 20%. Survival curves in the vaccine group compared to the placebo group showed a significantly increased cumulative proportion of subjects without severe RVGE (Figure 2). The site specific analyses were performed and a Forest Plot with vaccine efficacy by site is provided in the Supplementary Appendix Figure S1. There was no statistically significant interaction of treatment group by site on vaccine efficacy (P=0.29). Efficacy during the first year of life against severe RVGE was 56·4% (Table 2). Efficacy against RVGE of any severity and other secondary outcomes is shown in Table 2. The number of infants needed to be immunized to prevent one severe RVGE episode was 55 (95% CI 37–97) and for RVGE of any severity 31 (95% CI 21– 54).28
Figure 2.
Kaplan Meier Survival Curves for severe RVGE in vaccine and placebo groups in the per protocol population
Time 0 represents 15 days following receipt of third dose of vaccine/placebo
Table 2.
Efficacy of the vaccine in the prevention of gastroenteritis in the per protocol population
| Endpoints | n | VE | 95% CI | P value | |
|---|---|---|---|---|---|
| Vaccine N=4354 |
Placebo N= 2187 |
||||
| Severe RVGE | |||||
| Overall* | 71 | 76 | 53·6 | 35·0–66·9 | <0·001 |
| At 1 year of age | 56 | 64 | 56·4 | 36·6–70·1 | <0·001 |
| Severe RVGE requiring hospitalisation or supervised rehydration therapy | |||||
| Overall* | 71 | 76 | 53·6 | 35·0–66·9 | <0·001 |
| At 1 year of age | 56 | 64 | 56·4 | 36·6–70 | <0·001 |
| Very severe RVGE | |||||
| Overall* | 10 | 11 | 54·4 | −18·3–82·6 | 0·11 |
| At 1 year of age | 9 | 9 | 49·8 | −42·6–82·4 | 0·22 |
| RVGE of any severity | |||||
| Overall* | 287 | 216 | 34·6 | 21·6–45·3 | <0·001 |
| At 1 year of age | 226 | 171 | 34·6 | 19·7–46·6 | <0·001 |
| RVGE of any severity requiring hospitalisation or supervised rehydration therapy | |||||
| Overall* | 277 | 201 | 32·0 | 18·0–43·5 | <0·001 |
| At 1 year of age | 218 | 161 | 32·9 | 17·2–45·5 | <0·001 |
| Severe gastroenteritis of any aetiology | |||||
| Overall* | 308 | 188 | 18·6 | 1·9–32·3 | 0·03 |
| At 1 year of age | 221 | 145 | 24·1 | 5·8–38·7 | 0·01 |
Median age was 17·2 months at the time of analyses
Severe gastroenteritis: episodes with a Vesikari score ≥11
Severe RVGE: episodes with Vesikari score ≥11 and presence of rotavirus (Rotaclone positive and VP6 or VP4 and VP7 positive by RT-PCR) strains. Includes all RVGE except where G9P[11] was isolated
Very severe gastroenteritis: episodes with Vesikari score ≥16
Hospitalisation: inpatient admission for at least 6 hours in a treatment facility or hospital
Supervised rehydration therapy: administration of oral rehydration salts solution or intravenous fluids
The incidence of severe RVGE per 100 person years was 1·5 in vaccine and 3·2 in placebo group and an incidence rate ratio of 0·46 (95% CI 0·33–0·65). The absolute rate reduction for severe RVGE was 1·7 (95% CI 2·5–0·9).
Efficacy against severe gastroenteritis of any aetiology was 18·6% and in the first year of life it was 24·1% (Table 2).
The efficacy estimates by ITT were similar, the protection against severe RVGE was 54·7% (95% CI 37·2–67·3; P<0·001). Protection against very severe RVGE was 61·5% (95% CI 5·1–84·9; P=0·04; data provided in Supplementary Appendix Table S1). The most common (83%) RV genotypes identified in the 147 primary cases of severe RVGE were G2P[4] (33%), G1P[8] (31%), G12P[6] (14%), and G12P[8] (5%). Although the trial was not powered to evaluate efficacy against individual rotavirus genotypes, a post-hoc analyses was performed on the PP population. The confidence intervals of the genotype specific results are consistent with the overall protective efficacy (Table 3). The statistical test for interaction between vaccine efficacy and genotype of the infecting strains was not significant (P=0·19). In the PP population, G9P[11] was not detected in any rotavirus associated gastroenteritis cases. In the PP population, G9P[11] was not detected in any rotavirus associated gastroenteritis cases.Seroconversion to the vaccine was observed in the immunogenicity subset 4 weeks after the third dose; 39·9% in the vaccine (288 pairs) and 18·4% in the placebo (136 pairs) groups (OR 2·95, 95% CI 1·77–5·05), suggesting that wild type rotavirus infections were common during the immunisation period. In post-hoc analyses, three-fold increase was detected in 46·9% of the vaccinees and 19·1% of the placebo recipients (OR 3·73, 95% CI 2·25–6·32). Of the 306 vaccine recipients in the subset, G9P[11] was shed in 12·2% after dose 1, and 2·0% after dose 2 and 1·3% after dose 3.
Table 3.
Protective efficacy of the vaccine against severe gastroenteritis caused by different RV genotypes
| n (%) Vaccine N= 4354 |
n (%) Placebo N= 2187 |
VE (%) | 95% CI | |
|---|---|---|---|---|
| All | 71 (1·6) | 76 (3·5) | 53·6 | 35·0–66·9 |
| G2P[4] | 21 (0·5) | 27 (1·2) | 60·9 | 29·1–79·2 |
| G1P[8] | 25 (0·6) | 19 (0·9) | 31·3 | −29·9–63·8 |
| G12P[6] | 8 (0·2) | 13 (0·6) | 69·1 | 20·5–89·0 |
| G12P[8] | 3 (0·1) | 5 (0·2) | 69·9 | −53·1–95·4 |
| Others* | 14 (0·3) | 12 (0·5) | 41·4 | 37·2–67·3 |
Includes all genotypes causing 7 cases or less (G9P[4], G9P[8], G1P[4], G1P[6], G2P[6], G1P[0], G0P[0], G12P[11])
Differences in efficacy by genotype were not statistically significant
Adverse events
Nineteen of 4531 vaccinees and 8 of 2265 placebo recipients (P=0·84) reported an IAE; these were vomiting or spitting up of the oral contents; one placebo recipient had a rash. Due to their temporality, most events were labelled as related; all were mild and none resulted in hospitalisation or death. In the 14-day period post dosing, AEs were ascertained in 1530 vaccinees and 768 placebo recipients. Using MedDRA coding, the common AEs following the three doses in the vaccine and placebo groups were general disorders and administration site conditions 84·9% vs 85·5% (P=0·71), respiratory thoracic and mediastinal disorders 55·2% vs 56·1% (P=0·69), gastrointestinal disorders 33% vs 29·4% (P=0·09), skin and subcutaneous tissue disorders 8·7% vs 10·3% (P=0·22), infection and infestations 6·9% vs 7·6% (P=0·55), and metabolism and nutrition disorders 5·2% vs 5·5% (P=0·77). Analyses for solicited AEs showed similar prevalence of fever, vomiting, diarrhoea, cough, runny nose, irritability, and rash (P ≥0·3 for all comparisons).
G9P[11] genotype was identified in 22 cases of gastroenteritis, 20 after the first and 2 after the second dose; an approximate rate of one gastroenteritis event in 600 doses for any dose and around one in 200 for the first dose. By Vesikari score, all were classified as mild or moderate.26 It is pertinent to note that we did not examine stools for other enteropathogens.
Serious adverse events coded using MedDRA are shown in Table 4. One case of urticaria in the vaccine group and one each of acute gastroenteritis and suspected sepsis in the placebo group were labelled as related to the product based on temporality of occurrence. Expectedly in this age group, lower respiratory tract infections and gastroenteritis were the most common causes for hospitalisation. Of the 25 (0·6%) deaths in 4532 vaccinees and 17 (0·8%) in 2267 placebo recipients (P=0·33); none was considered related to the vaccine.
Table 4.
Serious adverse events coded by MedDRA system organ classification and preferred terms
| Vaccine N=4531 n (%) |
Placebo N=2265 n (%) |
P value | |
|---|---|---|---|
| Children who had an SAE | 925 (20·3) | 499 (22·0) | 0·13 |
| Infections and infestations | 777 (17·1) | 418 (18·5) | 0·19 |
| Lower respiratory tract infection | 261 (5·8) | 124 (5·5) | 0·66 |
| Gastroenteritis | 221 (4·9) | 109 (4·8) | 0·95 |
| Gastroenteritis Rotavirus | 77 (1·7) | 69 (3·0) | <0·001 |
| Bronchopneumonia | 76 (1·7) | 38 (1·7) | 1·00 |
| Pneumonia | 60 (1·3) | 21 (0·9) | 0·19 |
| Respiratory, thoracic and mediastinal disorders | 129 (2·8) | 71 (3·1) | 0·54 |
| Wheezing | 106 (2·3) | 54 (2·4) | 0·93 |
| General disorders and administration site conditions | 72 (1·6) | 33 (1·5) | 0·75 |
| Pyrexia | 62 (1·4) | 29 (1·3) | 0·82 |
Using Brighton Diagnostic Criteria Level 1, the intussusception committee confirmed 6 cases of intussusception in 4532 vaccinees (0·13%) and 2 in 2267 (0·09%) placebo recipients (P=0·73). All events occurred post dose 3. Among vaccine and placebo recipients, the minimum interval between dosing and intussusception was 112 and 36 days, respectively.
DISCUSSION
Results of this study provide a high degree of confidence in the efficacy of the 116E rotavirus vaccine and the study satisfied the primary efficacy hypothesis. Moreover, vaccine efficacy in the first year of life, when rotavirus disease burden is highest, was 56·4%. 116E protected against rotavirus gastroenteritis of varying degrees of severity, with protection generally increasing with clinical severity. Importantly, 116E also reduced severe gastroenteritis of any aetiology, reflecting the importance of rotavirus as a cause of severe gastroenteritis in infants in India. ITT analyses strongly supported the PP analyses.
Although comparisons across rotavirus vaccine studies are difficult due to differing populations, protocols, attack rates, and study procedures, the point estimate of efficacy (56·4%) against severe RVGE during the first year of life for 116E vaccine is comparable to that seen for RotaTeq® and Rotarix® when evaluated in developing countries. In a combined analysis of two independent double-blind, placebo-controlled multicentre Phase III efficacy trials conducted in Africa (Ghana, Kenya, Mali)8 and Asia (Bangladesh, Vietnam)9, efficacy of RotaTeq against severe RVGE during the first year of life was 58·9% (95% CI 40·0–72·3).29 In a double-blind, placebo-controlled multicenter Phase III efficacy trial conducted in Africa (South Africa, Malawi), efficacy of Rotarix against severe RVGE during the first year of life was 61·2% (95% CI 44·0–73·2).7
For RotaTeq and Rotarix, the efficacy in the second year of life has been generally lower compared to the first year of life.30 Complete data on the efficacy of 116E in the second year of life are not yet available.
Immune responses to vaccination as measured by the pre-specified criteria of a four-fold increase above baseline in serum anti-rotavirus IgA were observed in 39·9% of the vaccine recipients. This rate was lower than the 89·7% observed in the phase Ib/IIa trial. There were a number of differences in study population and study conduct between these two studies that may explain the differences in immune responses. In the Phase Ib/IIa trial, the eligibility criteria were more stringent, the study population was healthier and those severely malnourished were excluded, the rotavirus vaccine was not administered concomitantly with childhood vaccines, it is known that co-administration of OPV interferes with the immunogenicity of rotavirus vaccines.31–33 Further, the age at first vaccination was slightly higher in the Phase Ib/IIa (8 weeks) as compared to the Phase III trial (6–7 weeks) and maternally-derived serum anti-rotavirus IgG are known to block rotavirus replication. Also, breast feeding was restricted for 30 minutes prior to and post dosing in the Phase Ib/IIa but not in the phase III trial; this may have an impact on the “take” of rotavirus vaccines. Variability in serum anti-rotavirus IgA immune response rates across different populations have been reported for other rotavirus vaccines too.34,35
116E was well tolerated when administered with other childhood vaccines. Analyses of immediate adverse events, solicited, and unsolicited adverse events in the 14-day post vaccination window, serious adverse events, deaths, and cases of intussusception showed no unfavourable imbalances among recipients of 116E. A thorough evaluation of risk of intussusception will await phase IV surveillance studies. 116E may cause gastroenteritis, but if so, only rarely and mostly of mild severity.
These findings are of interest for several reasons. Firstly, the rotavirus strain (G9P[11]) that forms the basis of the 116E rotavirus vaccine is an unusual strain and rarely causes clinical disease in India or elsewhere.1,16,36 That 116E provided heterotypic protection across a broad array of commonly circulating rotavirus genotypes in India argues strongly that 116E will provide protection throughout India and in other regions of the world.
Secondly, 116E is the first rotavirus vaccine to demonstrate protective efficacy in India, a country comprising a quarter of the entire global mortality due to rotavirus gastroenteritis.1 116E protected against severe RVGE requiring hospitalisation or supervised rehydration therapy, an important observation in a country where healthcare is difficult to access and most expenses are out-of-pocket. In general, live oral vaccines have performed less well when administered to infants in developing countries. An example is oral polio vaccine which requires a large number of doses to be administered to infants in India in order to achieve immunity.37 The underlying basis for suboptimal performance of oral live-attenuated vaccines in India and elsewhere in developing countries has not been delineated and is probably multifactorial including factors such as passive transfer of large concentrations of maternal antibody, poor nutritional status, breastfeeding practices, and frequent exposure to multiple enteric pathogens.
Thirdly, despite the modest efficacy of 116E vaccine in India, the number of severe RVGE cases and deaths averted by vaccine are likely to be higher than in the developed world because of the significantly higher incidence of severe RVGE.1,2 In fact, despite the modest efficacy of all rotavirus vaccines in developing countries, the WHO recommends introduction of rotavirus vaccination as a part of the national immunisation programs in these populations because of the high disease burden. Lastly, the development of 116E rotavirus vaccine represents a paradigm shift in new vaccine development and serves as an example of how developing countries can develop these powerful tools and address endogenous infectious diseases of high burden without relying exclusively upon multinational pharmaceutical companies. 116E was developed by an Indian company with substantial technical and financial support from a unique government-led public-private partnership. 116E will first be targeted to Indian infants, but later to infants in other developing countries. Government, bilateral, and non-government organisation push funding and technical support substantially de-risked the project for the manufacturer resulting in a favourable price commitment for the public sector at the time of product launch (<US$ 1·00/dose). Finally, this successful product development validates the concept that new vaccines and other health commodities can be developed through socially committed collaborative efforts with effective government participation, and engagement of small to medium size enterprises resulting in substantially lower investment. In this regard, the vaccine is a product of a path setting model for developing health technologies at prices that ensure wider access in places where these are needed most.
Supplementary Material
RESEARCH IN CONTEXT.
Systematic review
The Cochrane Collaboration performed a systematic review in Year 2012, of the rotavirus vaccines and their efficacy. This review evaluated a monovalent rotavirus vaccine (RV1; Rotarix, GlaxoSmithKline Biologicals) and a pentavalent rotavirus vaccine (RV5; RotaTeq, Merck & Co, Inc). In the first two years of life, RV1 prevented more than 80% of severe cases of rotavirus gastroenteritis in low mortality countries and at least 40% of severe rotavirus gastroenteritis in high mortality countries. In the first two years of life, RV 5 reduced severe cases of rotavirus gastroenteritis by more than 80% in low mortality countries, and by 40 to 57% in high mortality countries. Severe cases of gastroenteritis from all causes were reduced by 73% to 96% in low mortality countries, and 15% in high mortality countries, after vaccination with RV5.
Interpretation
The findings of the efficacy of 116E from the current trial based on median follow up till 17 months of age are consistent with those for RV1 and RV5 in high mortality countries with a protective efficacy of 53·6% against severe rotavirus gastroenteritis and 18·6% against severe gastroenteritis of any aetiology. Complete data regarding efficacy in the second year of life and post marketing surveillance to evaluate the risk of rare side effects including intussusception are pending. Gastroenteritis is more common and severe in developing countries so even moderately efficacious vaccines would have substantial vaccine attributable reduction in severe rotavirus gastroenteritis in such populations.
ACKNOWLEGMENTS
Subjects and families who willingly participated in the trial
Local governments for the support extended to the study team.
Paediatricians in referral hospitals who provided care to enrolled subjects.
Data management, project management, medical monitoring, and pharmacovigilance teams at Quintiles, India; the Clinical Data Operations – Biostatistics team at Quintiles, South Africa and United Kingdom.
Dr. Jean-Michel Andrieux, ANTHA Clinical Quality Consulting, France for quality assurance audits at the three sites and the central investigation laboratory, and Ms. Monica McNeal, Cincinnati Children’s Hospital Medical Centre, USA for the laboratory audits.
Dr. VK Paul and the neonatal unit at All India Institute of Medical Sciences, New Delhi, India National Institute of Allergy and Infectious Diseases (NIAID) at National Institutes of Health (NIH), USA, and Centers for Diseases Control, USA.
Dr. VM Katoch, Director General, Indian Council of Medical Research, India.
Dr. K VijayRaghavan, Secretary, Department of Biotechnology, Government of India.
Dr. NK Ganguly, Former Director General, Indian Council of Medical Research, India.
Centre for International Health, University of Bergen, Norway.
Dr. Krishna Ella for sustained support to this innovation and mentorship.
Committees and departments of the Government of India; Ministry of Health and Family Welfare; and Ministry of Science and Technology, Government of India for their guidance and encouragement.
Funding: Department of Biotechnology, and Biotechnology Industry Research Assistance Council, Government of India, New Delhi, India; Grant from the Bill & Melinda Gates Foundation (#52714) to PATH, USA; Research Council of Norway, Department for International Development, United Kingdom; National Institutes of Health, Bethesda, USA; Bharat Biotech International Limited, Hyderabad, India
†. INDIA ROTAVIRUS VACCINE GROUP
Krishna M Ella, Bharat Biotech International Limited, Andhra Pradesh, India (Sponsor); Madhu Mahesh, Centre for Health Research and Development, Society for Applied Studies, New Delhi, India (Study Coordinator): Farhana Afzal Rafiqi, Centre for Health Research and Development, Society for Applied Studies, New Delhi, India (Study Coordinator); Girish Dayma, KEM Hospital Research Centre, Pune, Maharashtra, India (Data Manager); Anand Pandit, KEM Hospital Research Centre, Pune, Maharashtra, India (Sub Investigator); Anuradha Bose, Christian Medical College, Vellore, Tamil Nadu, India (Sub Investigator); Vinohar Balraj, Christian Medical College, Vellore, Tamil Nadu, India (Sub Investigator); Deepak More, Translational Health Science and Technology, Gurgaon, Haryana, India (Laboratory Manager); Pankaj Ghatbandhe, Translational Health Science and Technology, Gurgaon, Haryana, India (Laboratory); Taranjeet Kaur, Translational Health Science and Technology, Gurgaon, Haryana, India (Laboratory); Sharan Basava, Translational Health Science and Technology, Gurgaon, Haryana, India (Laboratory); Sudhir Babji, Wellcome Laboratory, Christian Medical College, Vellore, Tamil Nadu, India (Laboratory); Amit Mohindru, PATH, India (Coordination Unit); Manju Bagdwal, Centre for Health Research and Development, Society for Applied Studies, New Delhi, India (Coordination Unit); Elizabeth Horigan, Ex-National Institutes of Health, USA (Vaccine Development Committee); Margaret Wecker, Advancing Rotavirus Vaccines Development Project, PATH, USA (Management Committee); Jorge Flores, Clinical and Regulatory Affairs, Vaccine Development Global Program, PATH, USA (Management Committee); David Alli, Administration and Finance, Vaccine Development Global Program, PATH, USA (Management Committee); Kårstein Måseide, Research Council of Norway, Norway (Management Committee); Unni Hirdman Rørslett, Research Council of Norway, Norway (Management Committee); Tor A Strand, University of Bergen, Norway (Management Committee); Balaji K. Ananth, PATH, India (Management Committee)
Data Safety Monitoring Board: Mathuram Santosham, Johns Hopkins University, USA; Olivier Fontaine, World Health Organization, France; Lawrence Moulton, Johns Hopkins University, USA; Satinder Aneja, Kalawati Saran Children’s Hospital, New Delhi, India; Nik Zarifah Nik Hussain Reed, Ex-World Health Organization, Singapore
Intussusception Case Adjudication Committee: Dr. Veereshwar Bhatnagar, Professor, Department of Pediatric Surgery, All India Institute of Medical Sciences, New Delhi, India; Dr Arun Kumar Gupta, Professor & Head, Department of Radiodiagnosis, All India Institute of Medical Sciences, New Delhi, India; Dr. Madhulika Kabra, Professor, Genetics Unit, Department of Pediatrics, All India Institute of Medical Sciences, New Delhi, India
Safety Advisors: Siddharth Ramji, Professor and Head, Department of Neonatology, Maulana Azad Medical College, New Delhi, India; Amita Sapru, Associate Research Consultant, Department of Pediatrics, KEM Hospital Research Centre, Pune, Maharashtra, India; Prabhakar Moses, Child Health Unit III, Department of Child Health, Christian Medical College, Vellore, Tamil Nadu, India
Sponsor: Bharat Biotech International Limited, Genome Valley, Andhra Pradesh, India
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: Clinical Trial Registry-India (CTRI/2010/091/000102); Clinicaltrials.gov (NCT01305109)
CONTRIBUTORS
NB, JB and MKB prepared the manuscript and all authors reviewed and approved. TRC, AB, JJ, NG, AK, GK, SSR, SJ, JM, AA, HS, VA for design of protocol, trial implementation strategy and conduct. NB, KA and ST contributed to the design of protocol, trial implementation strategy, oversight of trial conduct and data analyses. JB, MKB, GT, RG, HBG, GC, TSR contributed to the trial design and interpretation of data and laboratory guidance. KM, GVJAH, SP for product development. MP, RK contributed to data analyses. SV for analyses of specimens.
I declare that I have full access to all the data in the study and final responsibility for the decision to submit for publication.
CONFLICT OF INTEREST
KM, GVJAH and SP are employees of Bharat Biotech International Limited. Other authors have no conflict of interest.
REFERENCES
- 1.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Rotavirus vaccines: WHO Position Paper January 2013. Wkly Epidemiol Rec. 2013;88:49–64. [PubMed] [Google Scholar]
- 3.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 5.Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomized, double-blind controlled study. Lancet. 2007;370:1757–1763. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- 6.Linhares AC, Velázquez FR, Pérez-Schael I, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–1189. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 7.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhoea in African infants. N Engl J Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 8.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomized, double blind, placebo-controlled trial. Lancet. 2010;376:606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 9.Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomized, double blind, placebo controlled trial. Lancet. 2010;376:615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 10.Sow SO, Tapia M, Haidara FC, et al. Efficacy of the oral pentavalent rotavirus vaccine in Mali. Vaccine. 2012;30:A71–A78. doi: 10.1016/j.vaccine.2011.11.094. [DOI] [PubMed] [Google Scholar]
- 11.Armah GE, Kapikian AZ, Vesikari T, et al. Efficacy, immunogenicity, and safety of two doses of a tetravalent rotavirus vaccine RRV-TV in Ghana with the first dose administered during the neonatal period. J Infect Dis. 2013;208:423–431. doi: 10.1093/infdis/jit174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, et al. Effect of rotavirus vaccination on death from childhood diarrhoea in Mexico. N Engl J Med. 2010;362:299–305. doi: 10.1056/NEJMoa0905211. [DOI] [PubMed] [Google Scholar]
- 13.National Institutes of Health. Background: Indo US Vaccine Action Program. [accessed July 4, 2013]; http://www.niaid.nih.gov/about/organization/dmid/indo/Pages/background.aspx.
- 14.Bhan MK, Lew JF, Sazawal S, Das BK, Gentsch JR, Glass RI. Protection conferred by neonatal rotavirus infection against subsequent rotavirus diarrhoea. J Infect Dis. 1993;168:282–287. doi: 10.1093/infdis/168.2.282. [DOI] [PubMed] [Google Scholar]
- 15.Bhandari N, Sharma P, Glass RI, et al. Safety and immunogenicity of two live attenuated human rotavirus vaccine candidates, 116E and I321, in infants: Results of a randomized controlled trial. Vaccine. 2006;24:5817–5823. doi: 10.1016/j.vaccine.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Bhandari N, Sharma P, Taneja S, et al. A dose-escalation safety and immunogenicity study of live attenuated oral rotavirus vaccine 116E in infants: a randomized, double blind, placebo controlled trial. J Infect Dis. 2009;200:421–429. doi: 10.1086/600104. [DOI] [PubMed] [Google Scholar]
- 17.National Family Health Survey (NFHS-3), 2005–06: India: Volume I. Mumbai: IIPS.; International Institute for Population Sciences (IIPS) and Macro International. 2007. [Google Scholar]
- 18.Government of India. The Drugs and Cosmetics Act. [accessed July 4, 2013];2003 http://cdsconicin/html/copy%20of%201%20d&cact121.pdf.
- 19.Indian Council of Medical Research. Ethical Guidelines for Biomedical Research on Human Participants. [accessed July 4, 2013];2006 http://icmr.nic.in/ethical_guidelines.pdf. [Google Scholar]
- 20.Government of India: Ministry of Health and Family Welfare. Integrated Management of Neonatal and childhood Illness (IMNCI): Modules 1 to 9. [accessed July 4, 2013];2009 http://www.unicef.org/india/Training_Module_1–9.pdf.
- 21.Bines JE, Kohl KS, Forster J, et al. Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004;22:569–574. doi: 10.1016/j.vaccine.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Iturriza-Gómara M, Kang G, Gray J. Rotavirus genotyping: keeping up with an evolving population of human rotaviruses. J Clin Virol. 2004;31:259–265. doi: 10.1016/j.jcv.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee I, Ramani S, Primrose B, et al. Modification of rotavirus multiplex RT-PCR for the detection of G12 strains based on the characterization of emerging G12 rotavirus strains in south India. J Med Virol. 2007;79:1413–1421. doi: 10.1002/jmv.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukhopadhya I, Sarkar R, Menon VK, et al. Rotavirus shedding in symptomatic and asymptomatic children using reverse transcription-quantitative PCR. J Med Virol. 2013;85:1661–1668. doi: 10.1002/jmv.23641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward RL, Bernstein DI, Shukla R, et al. Effects of antibody to rotavirus on protection of adults challenged with a human rotavirus. J Infect Dis. 1989;159:79–88. doi: 10.1093/infdis/159.1.79. [DOI] [PubMed] [Google Scholar]
- 26.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22:259–267. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 27.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 28.Bender R. Calculating confidence intervals for the number needed to treat. Control Clin Trials. 2001;22:102–110. doi: 10.1016/s0197-2456(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 29.Breiman RF, Zaman K, Armah G, et al. Analyses of health outcomes from the 5 sites participating in the Africa and Asia clinical efficacy trials of the oral pentavalent rotavirus vaccine. Vaccine. 2012;30:A24–A29. doi: 10.1016/j.vaccine.2011.08.124. [DOI] [PubMed] [Google Scholar]
- 30.Soares-Weiser K, MacLehose H, Bergman H, et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database of Systematic Reviews. 2012;11 doi: 10.1002/14651858.CD008521.pub3. Art No CD008521. [DOI] [PubMed] [Google Scholar]
- 31.Vodopija I, Baklaic Z, Vlatkovic R, Bogaerts H, Delem A, Andre FE. Combined vaccination with live oral polio vaccine and the bovine rotavirus RIT 4237 strain. Vaccine. 1986;4:233–236. doi: 10.1016/0264-410x(86)90135-0. [DOI] [PubMed] [Google Scholar]
- 32.Migasena S, Simasathien S, Samakoses R, et al. Simultaneous administration of oral rhesus-human reassortant tetravalent (RRV-TV) rotavirus vaccine and oral poliovirus vaccine (OPV) in Thai infants. Vaccine. 1995;13:168–174. doi: 10.1016/0264-410x(95)93131-r. [DOI] [PubMed] [Google Scholar]
- 33.Patel M, Steele AD, Parshar UD. Influence of oral polio vaccines on performance of the monovalent and pentavalent rotavirus vaccines. Vaccine. 2012;30:A30–A35. doi: 10.1016/j.vaccine.2011.11.093. [DOI] [PubMed] [Google Scholar]
- 34.Armah GE, Breiman RF, Tapia MD, et al. Immunogenicity of the pentavalent rotavirus vaccine in African infants. Vaccine. 2012;30:A86–A93. doi: 10.1016/j.vaccine.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Steele AD, De Vos B, Tumbo J, et al. Co-administration study in South African infants of a live-attenuated oral human rotavirus vaccine (RIX4414) and poliovirus vaccines. Vaccine. 2010;28:6542–6548. doi: 10.1016/j.vaccine.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 36.Chandola TR, Taneja S, Goyal N, et al. Descriptive epidemiology of rotavirus infection in a community in North India. Epidemiol Infect. 2013;141:2094–2100. doi: 10.1017/S0950268812002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grassly NC, Fraser C, Wenger J, et al. New strategies for the elimination of polio from India. Science. 2006;314:1150–1153. doi: 10.1126/science.1130388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.