Abstract
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) such as gefitinib, erlotinib, and afatinib are standard-of-care for first-line treatment of EGFR-mutant advanced non-small cell lung cancer (NSCLC). These drugs have a proven benefit in terms of higher response rate, delaying progression and improvement of quality of life over palliative platinum-based chemotherapy. The most common adverse events (AEs) are gastrointestinal (GI) (diarrhoea and stomatitis/mucositis) and cutaneous (rash, dry skin and paronychia). These are usually mild, but if they become moderate or severe, they can have a negative impact on the patient’s quality of life (QOL) and lead to dose modifications or drug discontinuation. Appropriate management of AEs, including prophylactic measures, supportive medications, treatment delays and dose reductions, is essential. A consensus meeting of a UK-based multidisciplinary panel composed of medical and clinical oncologists with a special interest in lung cancer, dermatologists, gastroenterologists, lung cancer nurse specialists and oncology pharmacists was held to develop guidelines on prevention and management of cutaneous (rash, dry skin and paronychia) and GI (diarrhoea, stomatitis and mucositis) AEs associated with the administration of EGFR-TKIs. These guidelines detail supportive measures, treatment delays and dose reductions for EGFR-TKIs. Although the focus of the guidelines is to support healthcare professionals in UK clinical practice, it is anticipated that the management strategies proposed will also be applicable in non-UK settings.
Key Points
| Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), i.e. gefitinib, erlotinib and afatinib, are the standard-of-care for first-line treatment of EGFR-mutant, advanced non-small cell lung cancer (NSCLC). |
| A consensus meeting of a UK-based multidisciplinary panel was held to develop guidelines on prevention and management of cutaneous (rash, dry skin and paronychia) and GI (diarrhoea, stomatitis and mucositis) adverse events associated with the administration of EGFR-TKIs. |
| Cutaneous adverse events can be prevented with regular use of emollients. Dose reduction or interruption of the EGFR-TKI might be appropriate if grade 2 toxicity is prolonged or intolerable. Use of topical corticosteroids/antibiotics and oral antibiotics are indicated to manage these adverse events. |
| The majority of patients will experience any grade diarrhoea. A low-fat, low-fibre diet and minimising intake of fruit, red meat, alcohol, spicy food and caffeine may be a sensible approach for patients experiencing diarrhoea. Loperamide, together with oral isotonic solution, is indicated for diarrhoea persisting >48 h. If no improvement, the drug should be discontinued and re-started, with appropriate dose reduction, when toxicity returns to G1 or baseline bowel habits. |
Introduction
Lung cancer remains the leading cause of cancer-related death worldwide [1]. Non-small cell lung cancer (NSCLC) represents 85 % of all lung cancer diagnoses and is a heterogeneous disease with several biological events driving tumour growth and progression [2].
Activating epidermal growth factor receptor (EGFR) gene mutations have emerged as the most relevant predictor of response to the EGFR tyrosine kinase inhibitors (EGFR-TKIs) gefitinib, erlotinib and afatinib [3–5]. These mutations are present in 10–35 % of NSCLC patients and more frequent in never/light smokers, women, patients with adenocarcinoma histology and patients with East Asian ethnicity. Several randomised phase III trials have consistently shown that gefitinib, erlotinib and, more recently, afatinib are more effective in terms of response rate (RR) and progression-free survival (PFS), less toxic and better tolerated than standard platinum-based doublet chemotherapy when given to untreated advanced NSCLC patients harbouring an activating EGFR mutation [6–15]. Furthermore, after a median follow-up of 36.5 months, a prespecified analysis of LUX-Lung 3 and LUX-Lung 6 studies demonstrated longer overall survival (OS) favouring the afatinib arm over chemotherapy for patients with a tumour harbouring an exon 19 deletion (LUX-Lung 3: 33.3 vs. 21.1 months, p = 0.0015; LUX-Lung 6: 31.4 vs. 18.4 months, p = 0.02) [16].
On the basis of these phase III clinical data [6–15], the American Society of Clinical Oncology (ASCO) [17] and European Society for Medical Oncology (ESMO) [2] recommend EGFR mutation analysis to determine whether an EGFR-TKI or chemotherapy is the appropriate first-line treatment for advanced NSCLC.
Gefitinib, erlotinib and afatinib are all approved by the European Medicine Agency (EMA) for use in the first-line setting for EGFR mutation positive advanced NSCLC patients [18–20]. The most common adverse events (AEs) associated with the use of these drugs are GI (diarrhoea and stomatitis/mucositis) and cutaneous (rash, dry skin and paronychia). These AEs are usually mild, but if they become moderate or severe, they can have a negative impact on the patient’s quality of life (QOL) and lead to dose modifications or drug discontinuation. Given that therapy is likely to continue for at least 10 months, appropriate management of AEs, including prophylactic measures, supportive medications, treatment delays and dose reductions, is essential.
Table 1 summarises the incidence of drug reductions/modifications and discontinuations in patients with EGFR mutation positive advanced NSCLC taking EGFR-TKIs first line in phase III randomised clinical trials [6–15].
Table 1.
Incidence of drug reductions/modifications and discontinuations in patients with EGFR mutation positivea advanced NSCLC taking EGFR-TKIs first line in phase III randomised clinical trials
| Study | Drug | Any grade 3 or 4 or 5 AE (%) | Drug-related AEs (all grades) (%) | Dose reduction/ modification due to AE (%) | Dose reduction/ modification due to drug-related AE (%) | Drug discontinuation due to AE (%) | Drug discontinuation due to drug-related AE (%) | AE leading to death (%) |
|---|---|---|---|---|---|---|---|---|
| IPASS [6] | Gefitinib | 16.3 | NS | 16.1 | NS | 6.9 | NS | 3.8 |
| First-SIGNAL [7] | Gefitinib | 28.9 | NS | NS | NS | NS | NS | 0.6 (1/159 treatment related death) |
| NEJ002 [8, 9] | Gefitinib | 41.2 (1 pt. had grade 5 AE) | NS | NS | NS | NS | NS | 0.88 (1/114 interstitial lung disease) |
| WJTOG3405 [10, 11] | Gefitinib | 41.4 | NS | NS | NS | NS | NS | NS |
| OPTIMAL [12] | Erlotinib | 17 | 87 | 6 | 6 | 1 | 0 | 0 |
| EURTAC [13] | Erlotinib | 32 | 93 | 21 | 21 | 13 | 6 | 1 (treatment-related death) |
| LUX-Lung 3 [14] | Afatinib | 49 | NS | NS | NS | NS | 8 | 1.7 (4/229 deaths: 2 respiratory decompensation, 1 sepsis, 1 unknown) |
| LUX-Lung 6 [15] | Afatinib | 36 | 98.8 | NS | NS | NS | 2.1 (rash) 0 (diarrhoea) | 0.4 (1/239 (sudden death) |
EGFR epidermal growth factor receptor, TKIs tyrosine kinase inhibitors, NSCLC non-small cell lung cancer, NS not stated, AE adverse event
aIPASS and FIRST-SIGNAL STUDY also enrolled patients with EGFR wild type tumours
Supportive care, dose reductions and treatment interruptions are appropriate strategies to manage EGFR-TKI-associated AEs [21]. The management goals for these patients are to support them throughout their treatment so that they can derive the maximum benefit from the therapy while maintaining a good QOL, and to avoid premature discontinuation of these drugs because of the potential loss of clinical benefit [21–23]. For the appropriate management of AEs, it is important that patients are followed up closely (i.e. bi-weekly) during the first 6 weeks of treatment. After that, clinical reviews can take place on a monthly basis.
In 2009, an expert consensus group published guidelines on the management of erlotinib-associated cutaneous toxicity in the UK [24]. By 2014, three EGFR-TKIs were available in the UK and it was considered that a review of management strategies for all of the AEs associated with these drugs would be beneficial.
A consensus meeting of a UK-based multidisciplinary panel composed of medical and clinical oncologists with a special interest in lung cancer, dermatologists, gastroenterologists, lung cancer nurse specialists and oncology pharmacists, was held to develop guidelines on prevention and management of cutaneous and GI AEs associated with the administration of EGFR-TKIs. These guidelines detail supportive treatment measures, treatment delays and dose reductions for EGFR-TKIs. Although the focus of the guidelines is to support healthcare professionals in UK clinical practice, it is anticipated that the management strategies proposed will also be applicable in non-UK settings.
Cutaneous Adverse Events
The EGFR has multiple effects on skin physiology, including stimulation of epidermal growth, inhibition of differentiation, and acceleration of wound healing [23, 25]. Inhibition of EGFR activity leads to a cascade of cellular events that results in multiple cutaneous AEs including rash, dry skin, pruritus and inflammation of nail/periungual tissues [23, 25]. In phase III clinical studies of EGFR-TKIs, 54–89 % of patients experienced any grade of skin AEs and 0–16.2 % experienced grade ≥3 skin AEs [6–15, 18–20]. The incidence of skin-related AEs in phase III studies of EGFR-TKIs is presented in Table 2. The prevention and management of EGFR-TKI-related cutaneous AEs is summarised in Fig. 1.
Table 2.
Incidence of skin adverse events in patients taking EGFR-TKIs in first-line phase III randomised clinical trials in EGFR mutation positivea advanced NSCLC [6–15]
| Study | Drug | Skin AE | All grades (%) | Grade 1–2 (%) | Grade 3–4 (%) |
|---|---|---|---|---|---|
| IPASS [6] | Gefitinib | Rash/acneb | 66.2 | NS | 3.1 |
| First-SIGNAL [7] | Gefitinib | Rash | 72.4 | NS | 13.3 |
| NEJ002 [8, 9] | Gefitinib | Rash | 71.1 | 65.8 | 0.5 |
| WJTOG3405 [10, 11] | Gefitinib | Dry skin | 54 | NS | 0 |
| OPTIMAL [12] | Erlotinib | Rash | 73 | NS | 2 |
| EURTAC [13] | Erlotinib | Rash | 80 | 67 | 13 |
| LUX-Lung 3 [14] | Afatinib | Rash/acneb | 89.1 | 72.9 | 16.2 |
| LUX-Lung 6 [15] | Afatinib | Rash/acneb | 80.8 | 66.1 | 14.6 |
EGFR epidermal growth factor receptor, TKIs tyrosine kinase inhibitors, NSCLC non small cell lung cancer, NS not stated, AE adverse event
aIPASS and FIRST-SIGNAL STUDY also enrolled patients with EGFR wild type tumours
b acne acneiform rash, i.e. a papulo pustular, rosacea-like rash or a maculopapular rash
Fig. 1.
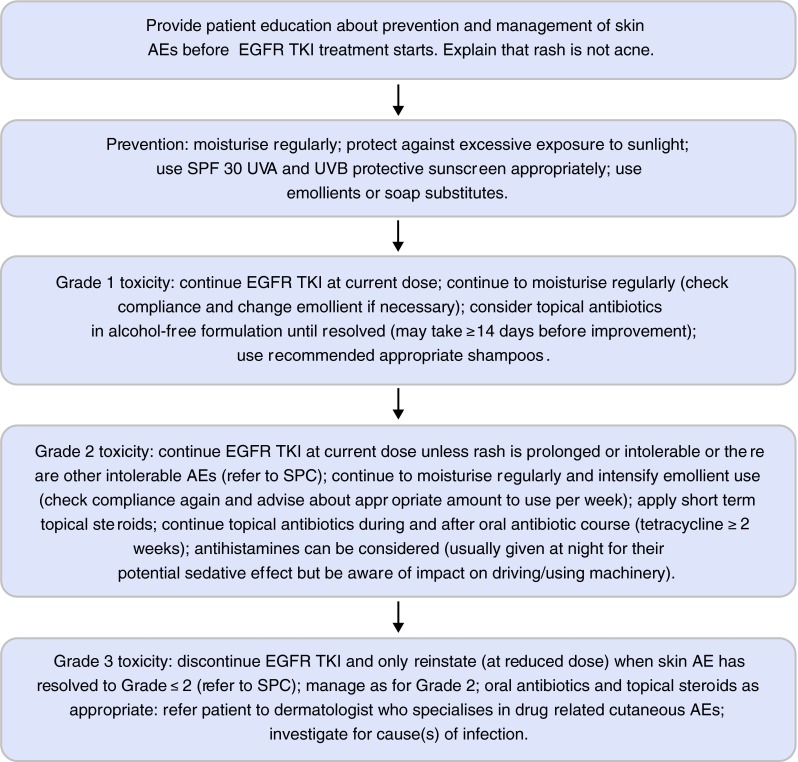
Management of skin adverse events in patients taking EGFR-TKIs (UK practice) [26, 27]. EGFR epidermal growth factor receptor, TKis tyrosine kinase inhibitors, AEs adverse events, SPF sun protection factor, UVA ultra-violet A, UVB ultra-violet B, SPC summary of product characteristics
Prevention
To reduce the risk of cutaneous AEs, patients should be advised to moisturise the skin intensively and to protect the skin from excessive exposure to sunshine. Tables 3 and 4 detail creams, gels, ointments and soap substitutes that are suitable for patients taking EGFR-TKIs [24, 26–28]. Patients should use an emollient several times a day (Table 5). Ointments are generally more effective for dry, irritable rashes because they have a hydrating effect by improving the skin’s lipid barrier. By contrast, water-based creams can further dry the skin and very greasy emollients may increase the risk of folliculitis.
Table 3.
Examples of emollients and soap substitutes that are suitable for patients taking EGFR-TKIs
| Lotions | Creams and gels | Ointments | Soap substitutes |
|---|---|---|---|
| Eucerin® intensive lotion (10 % urea) | Balneum Plus®
(5 % urea, lauromacrigols 3 %)] [includes anti-pruritic] |
50 % white soft paraffin/liquid paraffin | Balnuem Plus® bath oil |
| E45 Lotion® | Ungmentum M® | White soft paraffin | Aqueous cream |
| Dermol® 500 lotion (used as a soap substitute) (contains Benzalkonium Chloride 0.1 % and chlorhexidine 0.1 %) |
Doublebase® gel Doublebase Dayleve |
Emulsifying ointment | Doublebase® emollient shower gel |
| Aveeno® lotion | Dermol® 500 cream (contains benzalkonium chloride 0.1 % and chlorhexidine 0.1 % | Yellow soft paraffin | Doublebase® bath additive |
| Vaseline Dermacare® | Epaderm® cream | Epaderm® ointment | E45 bath additive® |
| Diprobase® cream | Diprobase® ointment | Oilatum® shower gel | |
| Cetraben® | Hydrous ointment | Cetraben® emollient | |
| Hydromol® cream | Hydromol® ointment | Oilatum® bath; hydromol® oil |
Usage at TWICE daily dosing estimated at 200–400 g (mL for lotions) per week, rounded to nearest container
EGFR epidermal growth factor receptor, tkis tyrosine kinase inhibitors
Table 4.
Examples of topical corticosteroid, corticosteroid/antimicrobial and other corticosteroid combination preparations that are suitable for patients taking EGFR-TKIs
| Mild | Moderate | Potent | Very potent |
|---|---|---|---|
| Hydrocortisone 1 % (range 0.1–2.5 %) | Eumovate® (clobetasone butyrate 0.05 %) | Betnovate® (betometasone Valerate 0.1 %) | Dermovate® (clobetasol propionate 0.05 %) |
| Dioderm® (hydrocortisone 0.1 %)—clinical activity equivalent to Hydrocortisone 1 % | Betnovate-RD® (betometasone Valerate 0.025 %) | Elocon® (Mometasone 0.1 %) Beclometasone (beclometasone dipropionate 0.025 %) |
Nerisone Forte® (diflucortolone valerate 0.3 %) |
| Canesten HC® (hydrocortisone 1 % + clotrimazole 1 %) Daktacort® (Hydrocortisone 1 % + miconazole nitrate 2 %) Fucidin H® (hydrocortisone1 % + fusidic acid 2 %) |
Trimovate® (clobetasone 0.05 % + oxytetracycline 3 % + nystatin 100,000 units/g) | Betnovate-C®
(Betamethasone valerate 0.1 % + Clioquinol 3 %) Betnovate-N® (betamethasone valerate 0.1 % + neomycin sulphate 0.5 %) Fucibet® (Betamethasone valerate 0.1 % + fusidic acid 2 %) |
Dermovate-NN® (clobetasol propionate 0.05 %, neomycin sulphate 0.5 %, nystatin 100,000 units/g) |
| Calmurid HC® (hydrocortisone 1 % + urea 10 % + lactic acid 5 %) |
Most preparations are available as creams (use if skin is weeping) or ointments (use if skin is dry)
EGFR epidermal growth factor receptor, TKIs tyrosine kinase inhibitors
Table 5.
Guidance for amounts of emollients that patients taking EGFR-TKIs should use per 2 weeks
| Area of body | Creams and ointments |
|---|---|
| Face and neck | 15–30 g |
| Both hands | 15–30 g |
| Scalp | 15–30 g |
| Groins and genitalia | 15–30 g |
| Both arms | 30–60 g |
| Both legs | 100 g |
| Trunk | 100 g |
| These amounts are usually suitable for an adult for a single daily application for 2 weeks | |
EGFR epidermal growth factor receptor, TKIs tyrosine kinase inhibitors
Although there is limited evidence that EGFR-TKIs trigger a photosensitive reaction, encouraging patients to cover their skin and to wear SPF 30 UVA/UVB non-occlusive sunscreen, especially in strong sunshine, is a pragmatic approach [24, 27, 28]. For personal hygiene, aqueous emollients and soap substitutes are less dehydrating for the skin than normal soaps; shampoos that reduce the risk of scalp folliculitis, e.g. ketoconazole, betadine and ceanel, should be recommended.
Management of Rash
Patients with grade 1 rash should continue their EGFR-TKI therapy and apply an emollient regularly (Fig. 1) [18–20, 24, 26–28]. If there are signs of superadded infection, the application of topical antibiotics in alcohol-free formulations, as recommended in local guidelines, should be considered for at least 14 days.
If the rash has progressed to grade 2, the EGFR-TKI can be continued at the current dose as the rash improves within 2 weeks in the majority of cases [18–20, 24, 26]. Dose reduction or interruption of the EGFR-TKI might be appropriate if grade 2 rash is prolonged or intolerable. Physicians should refer to the current EGFR-TKI summary of product characteristics (SPC) for prescribing advice [18–20]. If a chronic grade 2 rash develops, a dermatologist should be consulted as the rash can have a deleterious effect on the patient’s QOL. Moisturising should be intensified and topical steroids (e.g. 1–2.5 % hydrocortisone or eumovate ointment to the face; betnovate, elocon or dermovate ointment to the body) can be applied on a short-term basis (i.e. for 2–3 weeks), and then the patient’s condition should be reviewed. Topical antibiotics (as alcohol-free formulations), in accordance with local guidelines, and/or a course of oral antibiotics (e.g. tetracycline ≥2 weeks) may be indicated. Oral antihistamines are sometimes prescribed for patients with grade 2 itchy rash, but only a limited proportion of the patients derive symptomatic benefit. Patients should be advised about the possible sedative effects of antihistamines on their ability to drive or operate machinery.
In case of grade 3 rash, EGFR-TKI therapy should be temporarily interrupted and the treating physician should refer to the current SPC for each EGFR-TKI for prescribing advice [18–20, 24, 26, 27]. We recommend restarting EGFR-TKI therapy only when the rash has improved to grade ≤2 [18–20]. Dose reductions are recommended in the SPCs for erlotinib and afatinib, but not for gefitinib [18–20]1. It is not uncommon in clinical practice to restart gefitinib on alternate days but this is not recommended in the SPC. The rash should be managed as recommended for grade 2 rash, with oral antibiotics and topical corticosteroids as appropriate and referral to a dermatologist who specialises in drug-related cutaneous AEs. Any potential infection associated with the rash should be identified and appropriately treated, as recommended in local guidelines.
Paronychia
Paronychia is a disorder characterised by an inflammatory process involving the soft tissues around the nail. It is a relatively common AE in EGFR-TKI-treated patients because EGFR-TKIs prevent normal EGFR signalling and this leads to alterations in, and inflammation of, the periungual tissues [23].
In phase III studies, between 4 and 56.8 % of patients experienced any grade of paronychia and 0–11.4 % experienced grade ≥3 paronychia (Table 6) [6–15, 18–20]. Paronychia can severely impact on a patient’s QOL and ability to carry out his/her activities of daily living. It usually emerges 1–6 months after the initiation of EGFR-TKI therapy [25, 28]. Prevention and management of EGFR-TKI- related paronychia is summarised in Fig. 2.
Table 6.
Incidence of paronychia in patients taking EGFR-TKIs in first-line phase III randomised clinical trials in EGFR mutation positivea advanced NSCLC [6–15]
| Study | Drug | All grades (%) | Grade 1–2 (%) | Grade 3–4 (%) |
|---|---|---|---|---|
| IPASS [6] | Gefitinib | 13.5 | NS | 0.3 |
| First-SIGNAL [7] | Gefitinib | NS | NS | NS |
| NEJ002 [8, 9] | Gefitinib | NS | NS | NS |
| WJTOG3405 [10, 11] | Gefitinib | 32 | 30.9 | 1.1 |
| OPTIMAL [12] | Erlotinib | 4 | 4 | 0 |
| EURTAC [13] | Erlotinib | NS | NS | NS |
| LUX-Lung 3 [14] | Afatinib | 56.8 | NS | 11.4 |
| LUX-Lung 6 [15] | Afatinib | 32.6 | 32.6 | 0 |
EGFR epidermal growth factor receptor, TKIs tyrosine kinase inhibitors, NSCLC non-small cell lung cancer, NS not stated
aIPASS and FIRST-SIGNAL STUDY also enrolled patients with EGFR wild type tumours
Fig. 2.
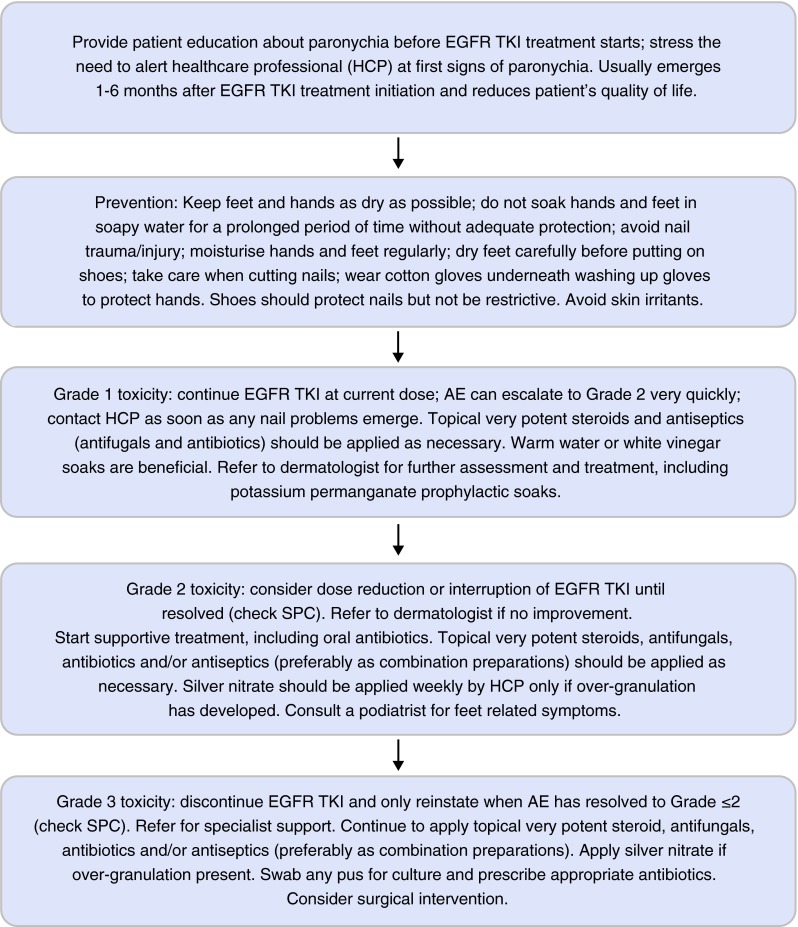
Management of paronychia in patients taking EGFR-TKIs (UK practice). EGFR epidermal growth factor receptor, TKis tyrosine kinase inhibitors, AEs adverse events, SPC summary of product characteristics
Prevention
Educating patients about the risk of paronychia during EGFR-TKI therapy and how to prevent it is essential (Fig. 2) [28]. Careful hand protection (as for hand dermatitis) is advisable. Patients should keep their hands and feet as dry as possible and not soak them in soapy water for prolonged periods of time without adequate protection, e.g. wearing cotton-lined washing up gloves. Nails should be cut carefully, and nail trauma or injury should be avoided. Emollients should be applied regularly. Shoes should not be restrictive but should protect the nails. Exposure to skin irritants should be avoided.
Management
The prescribed dose of the EGFR-TKI can usually be maintained in the presence of grade 1 paronychia, but dose reductions and/or interruptions should be considered if toxicity becomes grade ≥2 (consult the summary of product characteristics (SPC) for each EGFR-TKI) [18–20]. At the first signs of nail problems, patients should inform their medical team as grade 1 paronychia can escalate to grade 2 very quickly.
For grade 1 toxicity, some patients find warm water or white vinegar soaks beneficial [29–31]. The affected area should be soaked in warm water for approximately 15 min 3–4 times per day. White vinegar soaks (1:1 white vinegar:water) can be used for 15 min every day.
Consult a dermatologist for further assessment and management, such as potassium permanganate soaks. For grade 1–2 paronychia, topical very potent corticosteroids and antimicrobials (antifungals and antibiotics), preferably as combination preparations and as recommended in local guidelines, should be applied as necessary (Table 4). For grade 2 toxicity, supportive treatment, including oral antibiotics, should be started and silver nitrate should be applied by a healthcare professional if over-granulation has developed. Consulting a podiatrist may be useful if the patient experiences feet-related symptoms.
For grade 3 toxicity, the EGFR-TKI should be discontinued and only reinstated when toxicity has improved to grade ≤2 (consult SPC for details) [18–20]. The patient should be referred for specialist support (a dermatologist or podiatrist as appropriate). Continued application of topical very potent combinations of steroids, antifungals, antibiotics and/or antiseptics, as recommended in local guidelines, and of silver nitrate, if over-granulation is present, is advised. Any pus should be swabbed for culture and antibiotics prescribed accordingly. Surgical intervention, with or without intravenous antibiotics, should be considered if toxicity does not improve.
Gastrointestinal Adverse Events
Diarrhoea
Diarrhoea is the most common AE experienced by patients treated with an EGFR-TKI. The underlying mechanism for this AE is poorly understood, but it is believed that EGFR-TKI-associated diarrhoea is due to excessive chloride secretion, which leads to a secretory form of diarrhoea [22, 32]. In phase III studies, between 25 and 95 % of patients experienced any grade of GI AEs and 1–14 % experienced grade ≥3 GI AEs (Table 7) [6–15, 18–20, 32]. Prevention and management of EGFR-TKI-related diarrhoea is summarised in Fig. 3.
Table 7.
Incidence of gastrointestinal adverse events in patients taking EGFR-TKIs in first-line phase III randomised clinical trials in EGFR mutation positivea advanced NSCLC [6–15]
| Study | Drug | All grades (%) | Grade 1–2 (%) | Grade 3–4 (%) |
|---|---|---|---|---|
| IPASS [6] | Gefitinib | 46.6 | NS | 3.8 |
| First-SIGNAL [7] | Gefitinib | 49.7 | NS | 2.5 |
| NEJ002 [8, 9] | Gefitinib | 34.2 | 33.3 | 0.9 |
| WJTOG3405 [10, 11] | Gefitinib | 54 | NS | 1 |
| OPTIMAL [12] | Erlotinib | 25 | NS | 1 |
| EURTAC [13] | Erlotinib | 57 | 52 | 5 |
| LUX-Lung 3 [14] | Afatinib | 95 | 80.6 | 14.4 |
| LUX-Lung 6 [15] | Afatinib | 88.3 | 82.8 | 5.4 |
EGFR epidermal growth factor receptor, TKIs tyrosine kinase inhibitors, NSCLC non-small cell lung cancer, NS not stated
aIPASS and FIRST-SIGNAL STUDY also enrolled patients with EGFR wild type tumours
Fig. 3.
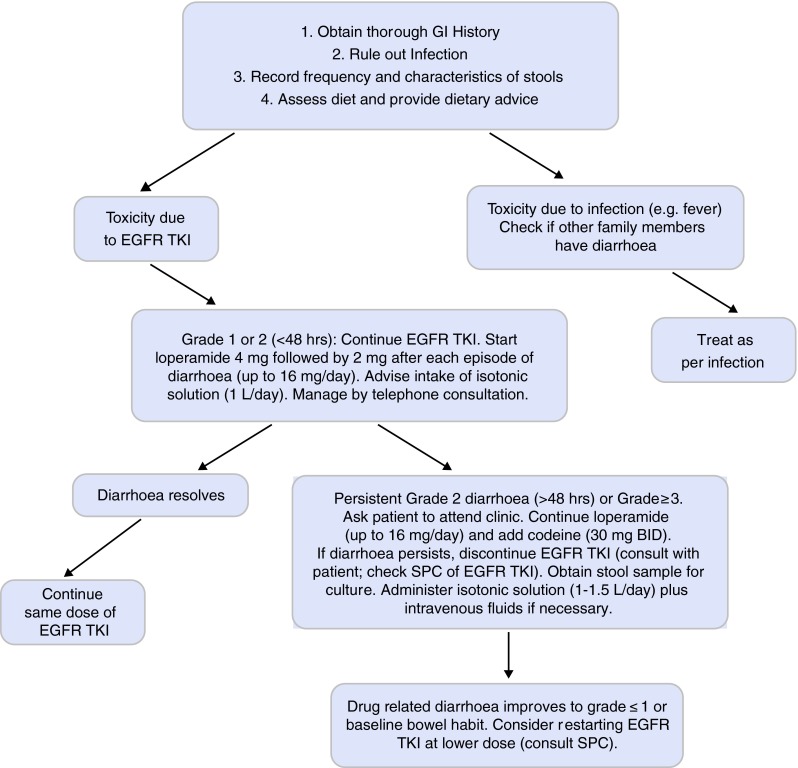
Management of diarrhoea in patients taking EGFR-TKIs (UK practice) [32]. EGFR epidermal growth factor receptor, BID twice daily, TKis tyrosine kinase inhibitors, SPC summary of product characteristics
Prevention
Prior to initiating EGFR-TKI therapy, the patient’s baseline bowel history should be obtained [32]. Although there is no validated questionnaire for this patient group, obtaining information about the patient’s bowel habits for the 6-week period prior to starting EGFR-TKI treatment will provide a baseline against which any EGFR-TKI-induced GI changes can be assessed (Fig. 3). Information on the patient’s concomitant medications and other clinical conditions should be collected at baseline and assessed for their potential impact on the GI tract by checking the prescribing information for each drug. Drug:drug interactions that might lead to GI AEs should also be evaluated.
The evidence base for interventions (e.g. special diets) to prevent EGFR-TKI-associated GI AEs is very limited [26]. Following a low-fat, low-fibre diet and minimising intake of fruit, red meat, alcohol, spicy food and caffeine may be a sensible approach for patients experiencing diarrhoea during EGFR-TKI treatment. If feasible, referral to a dietician will provide patients with the most up-to-date information about reducing the risk of diarrhoea and/or managing GI AEs. However, the physician must always be aware that dietary restrictions might have a negative impact on the patient’s QOL and promote weight loss in a patient population where maintaining body weight can already be difficult.
Management
If diarrhoea occurs during EGFR-TKI therapy, it is important to determine whether this is drug-related or infective [26]. If there is evidence of a GI infection, the patient should be treated appropriately. The aim of managing GI AEs is to return the patient to his/her baseline state or to grade ≤1.
If grade 1 or grade 2 (for less than 48 h) diarrhoea occurs, patients should be advised to take loperamide at a dose of 4 mg, followed by 2 mg after each episode of diarrhoea, up to a maximum of 16 mg/day (up to 20 mg/day in some countries outside the UK). Drinking 1–1.5 l per day of isotonic, oral rehydration salts (ORS) is recommended; patients should be advised not to drink more than 0.5 l of hypotonic fluids (e.g. water, tea, fruit juice) as this can make the diarrhoea worse [32].
Most cases of grade 1 or short duration grade 2 diarrhoea resolve quickly and can be managed via telephone consultation. Patients should be advised to inform the medical team if they develop grade 1 or 2 diarrhoea that does not resolve within 48 h, or if they develop diarrhoea with fever.
If the diarrhoea persists for >48 h, despite administration of the maximum daily dose of loperamide, or is grade 3–4, the patient’s condition should be reviewed and EGFR-TKI should be discontinued [18–20, 26]. Patients should be advised to phone their clinical nurse specialist if diarrhoea persists for >48 h or is grade 3–4. Adding codeine (starting with 30 mg/day which can be increased up to 60 mg four times a day) to the patient’s regimen, on a short-term basis, may be beneficial [33]. If codeine has been added to loperamide, it should be discontinued when the EGFR-TKI is stopped. Stool cultures should be performed and patients should be hospitalised and rehydrated with oral and intravenous fluids if necessary [32]. They should be referred to a gastroenterologist if the diarrhoea does not improve despite discontinuing EGFR-TKI therapy.
The EGFR-TKI should only be restarted when the patient has returned to baseline bowel habits or grade ≤1 toxicity [18–20, 32]. Dose reductions to improve treatment tolerability are recommended in the SPC for erlotinib and afatinib, but not for gefitinib [18–20].
Stomatitis and Mucositis
In phase III studies, between 13 and 72.1 % of patients experienced any grade of stomatitis/mucositis and up to 8.7 % experienced grade ≥3 toxicity (Table 8) [6–15, 18–20]. Prevention and management of EGFR-TKI- related stomatitis and mucositis is summarised in Fig. 4.
Table 8.
Incidence of stomatitis/mucositis in patients taking EGFR-TKIs in first-line phase III randomised clinical trials in EGFR mutation positivea advanced NSCLC [6–15]
| Study | Drug | All grades (%) | Grade 1–2 (%) | Grade 3–4 (%) |
|---|---|---|---|---|
| IPASS [6] | Gefitinib | 17 | NS | 0.2 |
| First-SIGNAL [7] | Gefitinib | 40.2 | NS | 1.9 |
| NEJ002 [8, 9] | Gefitinib | NS | NS | NS |
| WJTOG3405 [10, 11] | Gefitinib | 21.8 | 21.8 | 0 |
| OPTIMAL [12] | Erlotinib | 13 | 12 | 1 |
| EURTAC [13] | Erlotinib | NS | NS | NS |
| LUX-Lung 3 [14] | Afatinib | 72.1 | 63.4 | 8.7 |
| LUX-Lung 6 [15] | Afatinib | 51.9 | 46.4 | 5.4 |
EGFR epidermal growth factor receptor, TKIs tyrosine kinase inhibitors, NSCLC non-small cell lung cancer, NS not stated
aIPASS and FIRST-SIGNAL STUDY also enrolled patients with EGFR wild type tumours
Fig. 4.
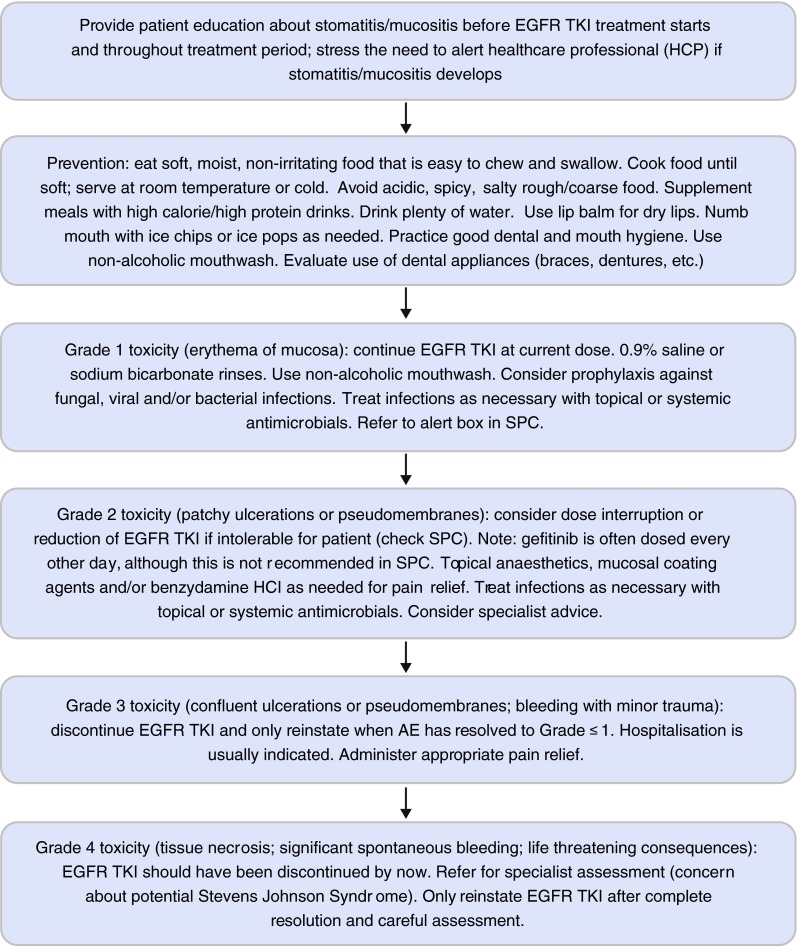
Management of stomatitis/mucositis in patients taking EGFR-TKIs (UK practice) [34, 35]. EGFR epidermal growth factor receptor, TKis tyrosine kinase inhibitors, SPC summary of product characteristics
A summary of healthcare products that are suitable for use in patients with mucositis is shown in Table 9.
Table 9.
| Prevention of mucositis | Treatment of oral mucositis | Treatment of anorectal mucositis |
|---|---|---|
| Review drug history | Suitable antacid therapy—see local formulary | Prednisolone 20 mg/100 ml retention enema, nocte |
| Dental review (pre-treatment) | Saline mouth wash 10 ml QDS (mix 1 teaspoon of table salt into 500 ml water) | Prednisolone (Predsol) 5 mg suppository mane and nocte (after a bowel movement) |
| Baseline bowel habit review | Sodium chloride mouthwash compound BP (contains bicarbonate) 10 ml QDS (mix 1 teaspoon of table salt and three-quarter teaspoon of bicarbonate of soda [baking soda] into 500 ml water) |
Mesalazine 1 g/100 ml retention enema, nocte |
| Soft bristle tooth brush | Saline-peroxide MW 10 ml QDS Hydrogen peroxide mouthwash BP—can be prescribed, 15 ml (diluted in 250 ml water) BD-TDS Peroxyl mouthwash is available OTC 10 mL QDS |
|
| Maintain adequate oral fluid intake (1.5 l/day) | Benzydamine 0.15 % mouthwash 15 ml QDS | |
| Ice cubes/ice chips/ice lollies (including for secondary prophylaxis) | Benzydamine 0.15 % mouthwash spray 4–8 sprays every 1.5–3 h |
|
| Limit consumption of tobacco, alcohol, acidic food, spicy food and hot foods/beverages | Antacid and oxcetacaine 15 ml mouthwash QDS (before food) (Unlicensed Special, Rosemont Pharmaceuticals Ltd.) |
|
| Avoid alcohol-based mouthwashes | Caphosol mixed as directed 15 ml mouthwash 4–10 times daily as required (where available) | |
| Primary prophylaxis: Caphosol (mixed as directed) 15 ml mouthwash 4–10 times daily as required (where available) | Sucralfate 1 g QDS (before meals) | |
| Secondary prophylaxis: Gelclair 15 ml mouthwash TDS | If oral candida: nystatin suspension 1 ml QDS for 7 days (oral local formulary alternative—caution: check potential interactions with EGFR-TKI) | |
| Systemic antifungal as required Systemic antibacterial as required According to local formulary. Caution: check potential interactions with EGFR-TKI |
Nocte at night, mane in the morning, QDS four times daily, TDS three times daily, BD twice daily, OTC over the counter
Prevention
Patient education about the risk and causes of stomatitis/mucositis is essential before starting therapy (Fig. 4; Table 9) [34, 35]. Patients must be aware of the need to alert a healthcare professional at the first signs of stomatitis/mucositis. Maintaining good oral hygiene is essential; non-alcoholic mouthwashes are recommended. It may be necessary to evaluate the use of dental appliances (braces, dentures, retainers, etc.) before therapy begins, as they can aggravate oral mucositis. Patients should be advised to eat food that will not cause oral lesions, i.e. soft, moist, non-irritating food that is easy to chew and swallow. Patients should drink plenty of water and lip balms can help to reduce mouth dryness.
Management
Patients with grade 1 stomatitis/mucositis (erythema of the mucosa) can usually continue the EGFR-TKI at the current dose (Fig. 4) [18–20]. Oral rinses (0.9 % saline or sodium bicarbonate) can soothe the mouth [34, 35] and only non-alcoholic mouthwashes should be used. Prophylaxis against fungal, viral and/or bacterial infections can be considered; infections must be treated as appropriate with topical or systemic antimicrobials, as recommended in local guidelines.
For grade 2 stomatitis/mucositis, it may be necessary to stop the treatment or, if the SPC recommends it, reduce the dose [18–20]. In the UK, it is common practice to reduce the dose of gefitinib with administration of a tablet every other day, although this is not advised in the SPC2 and there are no data to support this dosing schedule [19]. EGFR-TKI should be restarted when the stomatitis/mucositis has improved to grade ≤1. Topical anaesthetics, mucosal coating agents and/or benzydamine HCl may be administered as needed for pain relief [34]. Infections should be treated with topical or systemic antimicrobials. Obtaining specialist advice should be considered.
In the case of a grade 3 stomatitis/mucositis, treatment with an EGFR-TKI should be discontinued and the patient is usually hospitalised to receive supportive care [18–20]. Appropriate pain relief and antimicrobials should be administered [34]. The EGFR-TKI can be restarted, at a lower dose as per the SPC, once the toxicity has resolved to grade ≤1. If grade 4 stomatitis/mucositis develops, a specialist dermatology assessment should be sought, especially if Stevens-Johnson Syndrome is suspected [34]. EGFR-TKI therapy should have already been discontinued, and restarting treatment at a reduced dose should only be attempted after complete resolution of toxicity and a careful assessment of the patient.
Conclusions
EGFR-TKIs have changed the natural history of EGFR mutation-positive advanced NSCLC patients. It is likely patients will be treated for many months before developing progressive disease. These therapies are associated with a number of AEs that are usually mild-moderate, manageable and with low discontinuation rates. Nonetheless, these AEs may impact on patient’s QOL and increase the risk of non-compliance or drug discontinuation that, in turn, may affect patient’s clinical outcome [24, 27, 28, 32, 46].
Patient education, early recognition and proactive management of EGFR-TKI-related AEs are crucial. Treating physicians should strive to manage patients appropriately, possibly within a multi-specialty team, in order to minimise the impact of these AEs and preserve the patient’s QOL, trying to avoid unnecessary dose reductions or early discontinuation of these effective therapies.
Compliance with Ethical Standards
Funding
Boehringer Ingelheim provided financial support for medical writing, authorship meetings and submission costs. Boehringer Ingelheim also completed a factual review with respect to afatinib data within the article. Boehringer Ingelheim had no editorial input into the content.
Conflict of interest
R. Califano: Honoraria: Astra Zeneca, Roche and Boehringer Ingelheim Ltd. Travel support: Boehringer Ingelheim Ltd. Lecture fees: Roche, Astra Zeneca, Boehringer Ingelheim Ltd. No COI for employment; grants received or pending; participation in review activities; writing or reviewing the manuscript; provision of writing assistance, medicines, equipment or administrative support; stock or stock options; expert testimony; patents (planned, pending or issued); royalties; or other potential COIs.
N. Tariq: Honoraria: Boehringer Ingelheim Ltd. Travel support: Boehringer Ingelheim Ltd. No COI for employment; grants received or pending; participation in review activities; writing or reviewing the manuscript; provision of writing assistance, medicines, equipment or administrative support; payment for lectures; stock or stock options; expert testimony; patents (planned, pending or issued); royalties; or other potential COIs.
S. Compton: Honoraria: Boehringer Ingelheim Ltd. Travel support: Boehringer Ingelheim Ltd. No COI for employment; grants received or pending; participation in review activities; writing or reviewing the manuscript; provision of writing assistance, medicines, equipment or administrative support; payment for lectures; stock or stock options; expert testimony; patents (planned, pending or issued); royalties; or other potential COIs.
D.A. Fitzgerald: Honoraria: Boehringer Ingelheim Ltd. Travel support: Boehringer Ingelheim Ltd. No COI for employment; grants received or pending; participation in review activities; writing or reviewing the manuscript; provision of writing assistance, medicines, equipment or administrative support; payment for lectures; stock or stock options; expert testimony; patents (planned, pending or issued); royalties; or other potential COIs.
C.A. Harwood: Honoraria from Novartis, Roche, Leo, Meda, Almirall and Boehringer Ingelheim Limited. No COI for employment; grants received or pending; consulting fees or honoraria; support for travel to meetings; participation in review activities; writing or reviewing the manuscript; provision of writing assistance, medicines, equipment or administrative support; stock or stock options; expert testimony; patents (planned, pending or issued); royalties; or other potential COIs.
R. Lal: Payment for lectures: Boehringer Ingelheim Ltd (Advisory Board). No COI for employment; grants received or pending; consulting fees or honoraria; support for travel to meetings; participation in review activities; writing or reviewing the manuscript; provision of writing assistance, medicines, equipment or administrative support; stock or stock options; expert testimony; patents (planned, pending or issued); royalties; or other potential COIs.
J. Lester: Honoraria: Astra Zeneca, Roche and Boehringer Ingelheim Ltd. Travel support: Boehringer Ingelheim Ltd. Payment for lectures: AstraZeneca, Roche, Boehringer Ingelheim Ltd. No COI for employment; grants received or pending; participation in review activities; writing or reviewing the manuscript; provision of writing assistance, medicines, equipment or administrative support; stock or stock options; expert testimony; patents (planned, pending or issued); royalties; or other potential COIs.
J. McPhelim: Honoraria: Boehringer Ingelheim Ltd. Travel support: Boehringer Ingelheim Ltd. No COI for employment; grants received or pending; participation in review activities; writing or reviewing the manuscript; provision of writing assistance, medicines, equipment or administrative support; stock or stock options; expert testimony; patents (planned, pending or issued); royalties; or other potential COIs.
C. Mulatero: Grant: Boehringer Ingelheim Ltd (support for Phase II trial). Honoraria: Boehringer Ingelheim Ltd, Roche, Astra Zeneca. Travel support: Boehringer Ingelheim Ltd, Roche, Astra Zeneca, Bristol Myers Squibb, GE Healthcare, GlaxoSmith Kline, Lilly. Provision of writing assistance, medicines, equipment or administrative support: Boehringer Ingelheim Ltd. Payment for lectures: AstraZeneca, Roche, Boehringer Ingelheim Ltd. No COI for employment; participation in review activities; writing or reviewing the manuscript; stock or stock options; expert testimony; patents (planned, pending or issued); royalties; or other potential COIs.
S. Subramanian: Honoraria (for Advisory Boards) Dr Falk, AbbVie, Vitfor Pharmaceuticals, Boehringer Ingelheim Ltd, Janssen. Educational grants: Dr Falk, Warner Chillcott (Actavis), Merck Sharpe Dohme, AbbVie, Shire Pharmaceuticals. Lecture Fees: Merck Sharpe and Dohme, AbbVie, Warner Chillcott (Actavis), Dr Falk. No COI for employment; grants received or pending; participation in review activities; writing or reviewing the manuscript; provision of writing assistance, medicines, equipment or administrative support; stock or stock options; expert testimony; patents (planned, pending or issued); royalties; or other potential COIs.
A. Thomas: Grant: Roche Ltd (to attend symposia). Honoraria: Boehringer Ingelheim Ltd. Travel support: Boehringer Ingelheim Ltd. Provision of writing assistance, medicines, equipment or administrative support: a medical writer was provided to take notes on the meetings about the consensus guidelines manuscript. No COI for employment; participation in review activities; writing or reviewing the manuscript; payment for lectures; stock or stock options; expert testimony; patents (planned, pending or issued); royalties; or other potential COIs.
N. Thatcher: Honoraria: Lily, Roche, Teva, Novartis, Astra Zeneca and Boehringer Ingelheim Ltd. Travel support: Lilly, Roche, Teva, Novartis, Astra Zeneca and Boehringer Ingelheim Ltd. Fees for participation in review activities: Lilly, Roche, Teva, Novartis, Astra Zeneca and Boehringer Ingelheim Ltd. Payment for writing or reviewing manuscripts: Lilly, Roche, Teva, Novartis, Astra Zeneca and Boehringer Ingelheim Ltd. Lecture fees: Lily, Roche, Teva, Novartis, Astra Zeneca, Boehringer Ingelheim Ltd. No COI for employment; grants received or pending; provision of writing assistance, medicines, equipment or administrative support; stock or stock options; expert testimony; patents (planned, pending or issued); royalties; or other potential COIs.
M. Nicolson: Educational Grants: Boehringer Ingelheim Limited, Lilly, Roche, BMS, Pfozer, GSK, MSD, Bioven and Novartis. Honoraria: Boehringer Ingelheim Ltd, Lilly, Roche, Amgen, Bristol Myers Squibb, Pfizer, GlaxoSmith Kline, Merck Sharpe and Dohme. Travel support: Boehringer Ingelheim Ltd, Lilly. No COI for employment; grants received or pending; participation in review activities; writing or reviewing the manuscript; provision of medicines, equipment or administrative support; stock or stock options; expert testimony; patents (planned, pending or issued); royalties; or other potential COIs.
Footnotes
Please note that the SPC for gefitinib does not recommend dose reductions. The SPC should be consulted for full information.
Please note that the SPC for gefitinib does not recommend dose reductions. The SPC should be consulted for full information.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann oncol. 2012;23(Suppl 7):vii56–64. [DOI] [PubMed]
- 3.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 4.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci. 2004;101(36):13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 6.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 7.Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–1128. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 8.Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, Isobe H, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002) Ann Oncol. 2013;24(1):54–59. doi: 10.1093/annonc/mds214. [DOI] [PubMed] [Google Scholar]
- 9.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 10.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Seto T, et al. Updated overall survival results of WJTOG 3405, a randomized phase III trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer harboring mutations of the epidermal growth factor receptor (EGFR). J Clin Oncol. 2012;30(15 suppl):Abstr 7521.
- 11.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 12.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 13.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 14.Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 15.Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 16.Yang J-H, Sequist LV, Schuler M, Mok T, Yamamoto N, O’Byrne K, et al. Overall survival (OS) in patients (pts) with advanced non-small cell lung cancer (NSCLC) harboring common (Del19/L858R) Epidermal Growth Factor Receptor mutations (EGFR mut): pooled analysis of two large open-label phase III studies (LUX-Lung 3 [LL3] and LUX-Lung 6 [LL6]) comparingafatinib with chemotherapy (CT). In: 50th American Society of Clinical Oncology (ASCO) Annual Meeting; 2014 30 May–3 June; Chicago; 2014. p. Abstract 21366.
- 17.Keedy V, Temin S, Somerfield M, Beasley M, Johnson D, McShane L, et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) mutation testing for patients with advanced non–small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol. 2011;29(15):2121–2127. doi: 10.1200/JCO.2010.31.8923. [DOI] [PubMed] [Google Scholar]
- 18.European Medicines Agency. Summary of Product Characterisitics—Tarceva (erlotinib). 2005 (2014 version accessed). cited; Available from: http://www.ema.europa.eu.
- 19.European Medicines Agency. Summary of Product Characterisitics—Iressa (gefitinib). 2009 (2014 version accessed). cited; Available from: http://www.ema.europa.eu.
- 20.European Medicines Agency. Summary of Product Characterisitics—Giotrif (afatinib). 2014. cited; Available from: http://www.ema.europa.eu.
- 21.Arriola E, Reguart N, Artal A, Cobo M, Garcia-Campelo R, Esteban E. Management of the adverse events of afatinib: a consensus of the recommendations of the Spanish expert panel. Future Oncol. 2015;11(2):267–277. doi: 10.2217/fon.14.214. [DOI] [PubMed] [Google Scholar]
- 22.Harandi A, Zaidi AS, Stocker AM, Laber DA. Clinical efficacy and toxicity of anti-EGFR therapy in common cancers. J Oncol. 2009;2009:567486. doi: 10.1155/2009/567486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6(10):803–812. doi: 10.1038/nrc1970. [DOI] [PubMed] [Google Scholar]
- 24.Thatcher N, Nicolson M, Groves RW, Steele J, Eaby B, Dunlop J, et al. Expert consensus on the management of erlotinib-associated cutaneous toxicity in the U.K. Oncologist. 2009;14(8):840–847. doi: 10.1634/theoncologist.2009-0055. [DOI] [PubMed] [Google Scholar]
- 25.Lynch TJ, Jr, Kim ES, Eaby B, Garey J, West DP, Lacouture ME. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12(5):610–621. doi: 10.1634/theoncologist.12-5-610. [DOI] [PubMed] [Google Scholar]
- 26.Hirsh V. Managing treatment-related adverse events associated with EGFR tyrosine kinase inhibitors in advanced non-small-cell lung cancer. Curr Oncol. 2011;18(3):126–138. doi: 10.3747/co.v18i3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacouture M, Schadendorf D, Chu C-Y, Uttenreuther-Fischer M, O’Brien D, Hauschild A. Dermatologic adverse events associated with afatinib: an oral ErbB family blocker. Expert Rev Anticancer Ther. 2013;13(6):1–8. [DOI] [PubMed]
- 28.Potthoff K, Hofheinz R, Hassel JC, Volkenandt M, Lordick F, Hartmann JT, et al. Interdisciplinary management of EGFR-inhibitor-induced skin reactions: a German expert opinion. Ann Oncol. 2011;22(3):524–535. doi: 10.1093/annonc/mdq387. [DOI] [PubMed] [Google Scholar]
- 29.Anon. Paronychia. [cited 17.07.2014]; Available from: http://www.drugs.com/healthguide/paronychia.html.
- 30.NICE. Paronychia—acute. 2011 [cited 17.07.2014]; Available from: http://cks.nice.org.uk/paronychia-acute-!scenariorecommendation.
- 31.Rockwell PG. Acute and chronic paronychia. Am Fam Physician. 2001;63(6):1113–1116. [PubMed] [Google Scholar]
- 32.Yang JC, Reguart N, Barinoff J, Kohler J, Uttenreuther-Fischer M, Stammberger U, et al. Diarrhea associated with afatinib: an oral ErbB family blocker. Expert Rev Anticancer Ther. 2013;13(6):729–736. doi: 10.1586/era.13.31. [DOI] [PubMed] [Google Scholar]
- 33.Nightingale JM. The medical management of intestinal failure: methods to reduce the severity. Proc Nutr Soc. 2003;62(3):703–710. doi: 10.1079/PNS2003283. [DOI] [PubMed] [Google Scholar]
- 34.Bensinger W, Schubert M, Ang K, Brizel D, Brown E, Eilers J, et al. NCCN Task Force Report: prevention and management of mucositis in cancer care. J Natl Compr Canc Netw. 2008;6(Suppl 1):S1–S21. [PubMed] [Google Scholar]
- 35.Nutrition in Cancer Care (PDQ)., National Cancer Institute. Nutrition therapy. 2014 Updated 26.02.2014 [cited Accessed 25.04.2014]; Available from: http://www.cancer.gov/cancertopics/pdq/supportivecare/nutrition/HealthProfessional/page4.
- 36.NHS. [cited; Available from: http://www.nhs.uk/Conditions/Mucositis/Pages/Treatment.aspx.
- 37.Papas AS, Clark RE, Martuscelli G, O’Loughlin KT, Johansen E, Miller KB. A prospective, randomized trial for the prevention of mucositis in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;31(8):705–712. doi: 10.1038/sj.bmt.1703870. [DOI] [PubMed] [Google Scholar]
- 38.Tombes M, Gallucci B. The effects of hydrogen peroxide rinses on the normal oral mucosa. Nurs Res. 1993;42(6):332–337. doi: 10.1097/00006199-199311000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003;98(7):1531–1539. doi: 10.1002/cncr.11671. [DOI] [PubMed] [Google Scholar]
- 40.Turhal NS, Erdal S, Karacay S. Efficacy of treatment to relieve mucositis-induced discomfort. Support Care Cancer. 2000;8(1):55–58. doi: 10.1007/s005209900076. [DOI] [PubMed] [Google Scholar]
- 41.Choi S-E, Kim H-S. Sodium bicarbonate solution versus chlorhexidine mouthwash in oral care of acute leukemia patients undergoing induction chemotherapy: a randomized controlled trial. Asian Nurs Res. 2012;6(2):60–66. doi: 10.1016/j.anr.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Sonis S. Regimen-related gastrointestinal toxicities in cancer patients. Curr Opin Support Palliat Care. 2010;4(1):26–30. doi: 10.1097/SPC.0b013e328335fb76. [DOI] [PubMed] [Google Scholar]
- 43.Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109(5):820–831. doi: 10.1002/cncr.22484. [DOI] [PubMed] [Google Scholar]
- 44.Lalla R, Sonis S, Peterson D. Management of oral mucositis in patients who have cancer. Dent Clin N Am. 2008;52(1):61–77. doi: 10.1016/j.cden.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manojlovic N, Babic D. Radiation-induced rectal ulcer–prognostic factors and medical treatment. Hepato-gastroenterology. 2004;51(56):447–450. [PubMed] [Google Scholar]
- 46.Hirsh V. Encouraging data out of the 2010 Congress of the European Society for Medical Oncology with respect to non-small-cell lung cancer. Curr Oncol. 2010;17(6):7–8. doi: 10.3747/co.v17i6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]


