Abstract
Background and Aim: Recurrent laryngeal nerve injury is a common severe complication in neck surgery, which can cause varying degrees of vocal fold paralysis and respiratory tract problems. In present study, the effects of laminin-binding brain derived neurotrophic factor (LBD-BDNF) on recurrent laryngeal nerve regeneration were explored and its possible mechanism was investigated. Methods: LBD-BDNF or NAT-BDNF (BDNF without LBD binding) treatment was performed in laryngeal nerve injured rabbits for sixteen weeks. The laryngeal nerve was removed, and histological examination as well as laryngeal electromyography was employed to evaluate its morphology and function of conduction. PC12 cells were cultured to investigate the mechanisms underlying the effects of LBD-BDNF. Neurite outgrowth, proliferation and migration were determined in nerve cells. The expression of miRNAs and protein of mTOR was quantified by real-time PCR and western blotting respectively. Results: In vivo experiments, LBD-BDNF significantly improved the histological structure and function of recurrent laryngeal nerve compared with NAT-BDNF. LBD-BDNF also markedly promoted neurite outgrowth, proliferation and migration in PC12 cells in vitro experiments. The levels of miR-222 and p-mTOR were up-regulated by LBD-BDNF treatment in both in vivo and in vitro experiments. miR-222 inhibitor attenuated the expression of phosphorylated mTOR and miR-222 mimic enhanced its expression in PC12 cells. In addition, the improved nerve conduction by LBD-BDNF was canceled by miR-222 inhibitor, and the mTOR inhibitor reversed the effects of miR-222 inhibitor on LBD-BDNF treated cells. Conclusions: The present study revealed that LBD-BDNF promoted the recurrent laryngeal nerve regeneration in laryngeal nerve injured rabbits. The underlying mechanism was closely related to activation of p-mTOR by miR-222.
Keywords: Laryngeal nerve, nerve regeneration, laminin, brain-derived neurotrophic factor
Introduction
There are two recurrent laryngeal nerves (RLN), right and left, in the human body. The recurrent laryngeal nerves control all intrinsic muscles of the larynx except for the cricothyroid muscle [1]. Recurrent laryngeal nerve may be injured because of neck trauma, neck surgery or other factors. Injured recurrent laryngeal nerve can cause varying degrees of vocal fold paralysis and respiratory tract problems [2], which seriously affecting patients’ quality of life. In addition, laryngeal nerve injury is also one of the important factors for medical dispute from neck surgery. Except for improving surgeon operation skills, rapidly and efficiently promoting recurrent laryngeal nerve regeneration is greatly needed.
Recovery of laryngeal function is dependent on the type, extent, and site of RLN lesion [3]. It is critical for cell body to supply sufficient metabolic milieu in nerve regeneration processes. Application of neurotrophic factors is appealing strategy to protect injured neurons via improving neuronal survival and regeneration of nerve fibers. Brain-derived neurotrophic factor (BDNF) is widely used for neurogenesis both in central nervous system and the peripheral nervous system [4-6]. However, it is difficult to retain BDNF at the injury neurons, which is due to its rapid diffusion to extracellular fluids [7]. In vivo experiments, it is proved that mixture injection of nerve growth factor is more effective than a single injection in improving axonal population [8]. Therefore, to explore the way to be sustained delivery of BDNF at injury neurons is useful in clinical application.
The extracellular matrix (ECM) plays a pivotal role in offering anchorage points to maturing neurons and neurites, as well as a permissive environment for tissue formation [9]. Laminin, which is extracellular matrix glycoprotein, plays an important role in the central nervous system. There is emerging evidence that construction of laminin-binding domain (LBD) to BDNF attenuates neural-degeneration after middle cerebral artery occlusion in rat model [10]. In addition, an enhance functional restoration following nerve damages of LBD fused nerve growth factor β (NGF-β) is observed in rat sciatic nerve crush injury model [11]. However, no studies on recurrent laryngeal nerves injury by LBD-BDNF treatment have been conducted so far.
MicroRNAs (miRNAs) are involved in regulation of gene expression in various physiological processes via binding to the 3’untranslated regions of target genes. The important role of miRNAs in nerve regeneration and functional recovery is increasingly recognized [12,13], and the altered miRNAs expression are involved in the inflammation, oxidation, and apoptosis which are critical process in the pathogenesis of nerves injury [14]. Mammalian target of rapamycin (mTOR) is a key signal in regulating the process of cellular growth, nutrient metabolism and redox states. Research indicates that it is necessary to activate mTOR in axon regeneration [15], and it was also reported that mTOR was regulated by miRNAs in nerve injury [16]. Therefore, we hypothesized that miRNAs may play a role in the recurrent laryngeal nerve regeneration function of LBD-BDNF via regulation of mTOR pathway. In vivo and In vitro experiments were employed to verify this hypothesis in present study.
Materials and methods
Preparation of NAT-BDNF and LBD-BDNF
As described previous [11,17], N-terminal domain of agrin (NAT) was inserted into pET-28a (Novagen, USA) and BDNF DNA was amplified by PCR. The vectors for BDNF with or without LBD were constructed as indicated in elsewhere [10]. In brief, the escherichia coli BL21 were culured at 37°C for 3 h with vectors LBD-BDNF or NAT-BDNF. And then treated with 1 mM isopropyl β-D-thiogalactopyranoside at 37°C for 5 h. Nickel chelate chromatography was used to purify LBD-BDNF and NAT-BDNF under denaturing conditions.
Animal experiments: laryngeal nerve injured model
All animal experiments were operated according to the Guide for the Care and Use of Laboratory Animals from Shanghai Jiao Tong University Affiliated First People’s Hospital. Adult New Zealand white rabbits were anesthetized with an intramuscular injection of 1:1 10 mg/kg ketamine and 2 mg/kg xylazine. Ten rabbits with nerve section in left laryngeal nerve were considered as control group. The ones with operation in right laryngeal nerve were included in experiment group. 0.25 nmol NAT-BDNF was injected into the injured site of laryngeal nerve in ten rabbits, and the remaining ten rabbits in experiments received the injection of 0.25 nmol LBD-BDNF. The same volume of PBS was injected as control. Sixteen weeks after operation, rabbits were sacrificed and laryngeal nerves were removed to have a histological analysis and electromyography.
Histological analysis
The histological analysis was performed at weeks 16 after laryngeal nerve injury. The laryngeal nerve at the injury sites were rapidly excised and fixed in 4% (vol/vol) formaldehyde for 48 h and then embedded in paraffin. Serial section was applied and sections (3 µm) were stained by H.E to observe the histological character.
Cell culture
PC12 cells were cultured in RPMI1640 medium, which supplemented with 5% fetal bovine serum, 10% heat-inactivated horse serum, 25 U/ml penicillin and 25 U/ml streptomycin. PC12 cells were incubated in in 24-well culture plates coated with poly-D-lysine/laminin. Gradient concentration (0 μmol, 0.5 μmol, 1 μmol, 2 μmol and 4 μmol) of NAT-BDNF or LBD-BDNF was co-incubated with medium in PC12 for 48 h.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to evaluate the cell viability as described elsewhere [18]. The absorbance was determined at a wavelength of 490 nm by using SpectraMax M5 (Molecular Devices, USA). The quantification of neurite outgrowth was conducted by the instruction as mentioned in report [19].
Transwell
The effects of LBD-BNDF on the relative migration in PC12 cells were evaluated by Transwell chambers (BD Bioscience, USA). The bottom surface of each membrane was coated with 10 mg/ml fibronectin. PC12 cells (the concentration is 105 cells/ml) were resuspended in RPMI1640 and then transferred to the top chambers. The lower chambers were supplied with 600 ml complete medium. After 8 h, the upper chambers were cleaned with a cotton swab, and then counted the cells adhering to the bottom surface after staining with 0.1% Crystal Violet. Migrating cells in five fields on each chamber were counted to calculate the average.
Down-regulation and overexpression of miR-222
The expression of miR-222 in PC12 was down-regulated or overexpressed by transiently transfected with miR-222 inhibitor or miR-222 mimic using the Lipofectamine 2000 reagent (Invitrogen, USA) following the manufacturer’s instructions. The miR-222 inhibitor and miR-222 mimic were produced by RiboBio Co., Ltd. (Guangzhou, China). 100 nmol/L Rapamycin (sigma, Germany), an inhibitor of mTOR, was dissolved in DMSO and supplemented in cultured cells system to suppresses the expression of mTOR.
Real-time PCR
TRIzol reagent (Invitrogen, USA) was used to extract the miRNAs from nerve tissue and PC12 cells. 2 µg of RNA was quantified to do reverse transcription-PCR using ImProm-II™ (Promega, USA) following the manufacturer’s instructions. The expression of targets miRNAs were detected by 7500HT Real-Time PCR System (Applied Biosystems, USA), and U6 small nuclear RNA served as an internal normalized reference.
Western blotting
Protein extracts were extracted from PC12 cells or nerve tissues. Equal amounts of protein were loaded onto sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and transferred to nitrocellulose membrane (Bio-Rad, USA), which was blocked with 5% non-fat dry milk in Tris-HCl buffered saline incubated with the primary antibodies (Cell signaling, USA) according to the manufacturer’s recommendations. Immunoreactivity was detected by enhanced chemiluminescence (Millipore, USA). β-actin was served as a control protein to quantify the expression of target protein.
Statistical analysis
The results are presented as means ± standard deviation. Statistical analyses were performed by SPSS 17.0. A one-way analysis of variance (ANOVA) and Student’s t-test were utilized for comparison between groups. P < 0.05 was considered statistically significant.
Results
General observation of laryngeal recurrent nerve regeneration
The model of laryngeal recurrent nerve injured rabbits was established. As showed in Figure 1A, the laryngeal recurrent nerve with NAT-BDNF treatment had local swollen markedly and tight adherence with surround-tissue. Treated animals with LBD-BDNF, no significant scar and swell were observed in laryngeal recurrent nerve. The histological examination results (Figure 1B) showed that nerve fiber was well arranged and distributed in control rabbits. A large amount of fibrous connective tissue and sparse regenerating nerve axonal were observed in nerves treated with NAT-BDNF. LBD-BDNF prominently increased the number of regenerating fibers and axonal, while the amount of fibrous connective tissue was markedly decreased. The laryngeal electromyography (Figure 1C, 1D) presented that the amplitude and nerve conduction velocity (NCV) of RLN were significantly decreased in NAT-BDNF group compared with control, and LBD-BDNF markedly restored the amplitude and NCV.
Figure 1.
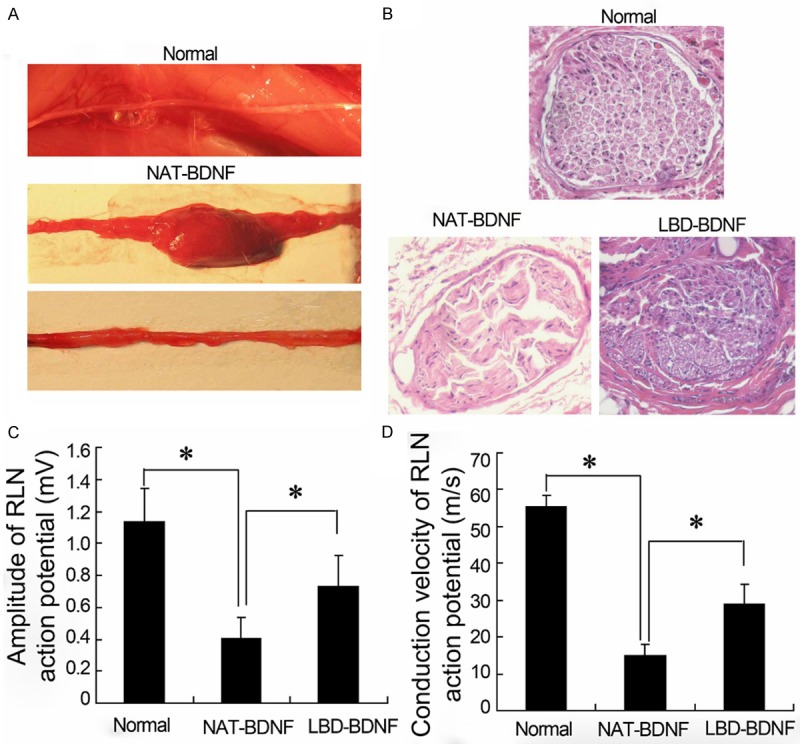
The general observation, histological examination and electromyography in laryngeal nerve of rabbits. A. The general observation of laryngeal recurrent nerve regeneration; B. Histology of laryngeal nerve; C, D. Laryngeal electromyography. NAT: N-terminal domain of agrin; BDNF: Brain Derived Neurotrophic Factor; LBD: Laminin-Binding Domain.
Expression of microRNAs in laryngeal recurrent nerve
To investigate the role of miRNAs in regeneration of laryngeal recurrent nerve by LBD-BDNF, the expression of miRNAs including miR-133b, miR-221, miR-222, miR-16 and miR-15b were detected in laryngeal recurrent nerve. The real-time PCR results showed that miR-133b, miR-221, miR-222 and miR-15b were down-regulated and miR-16 was up-regulated in NAT-BDNF group compared with the Normal. However, only the expression of miR-222 by LBD-BDNF treatment was observed significantly higher than that in NAT-BDNF group (Figure 2).
Figure 2.
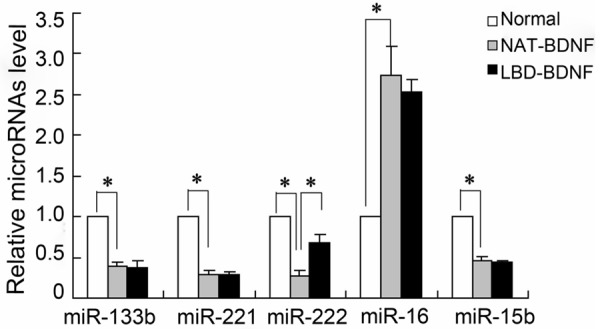
The expression of microRNAs in recurrent laryngeal nerve of rabbits. NAT: N-terminal domain of agrin; BDNF: Brain Derived Neurotrophic Factor; LBD: Laminin-Binding Domain.
Expression of mTOR in laryngeal recurrent nerve
Western blotting was used to evaluate the protein expression of p-mTOR and T-mTOR. As shown in Figure 3, all three groups had roughly an equal amount of T-mTOR expression. The level of p-mTOR was decreased in NAT-BDNF compared with Normal, and LBD-BDNF elevated the expression of p-mTOR in laryngeal recurrent nerve.
Figure 3.
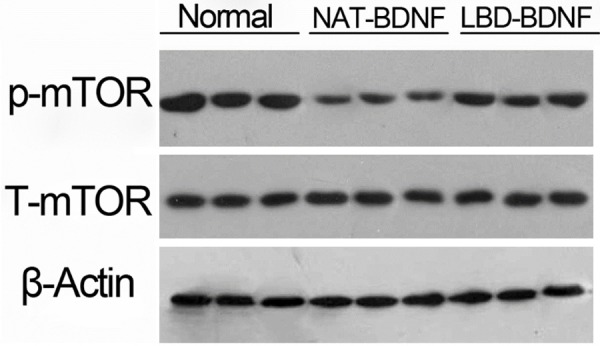
The protein expression of mTOR in laryngeal recurrent nerve. NAT: N-terminal domain of agrin; BDNF: Brain Derived Neurotrophic Factor; LBD: Laminin-Binding Domain.
Effects of laminin-binding domain on neurite outgrowth, hyperplasia and migration of PC12 cells
To better understand the mechanism of LBD-BDNF involved in the laryngeal recurrent nerve, PC12 cell line was employed. As shown in Figure 4, cells treated with LBD-BDNF had a significant increase in neurite outgrowth (Figure 4A), value of OD490 (Figure 4B) and migration rate (Figure 4C). The effects of LBD-BDNF were in a protein concentration–dependent manner.
Figure 4.
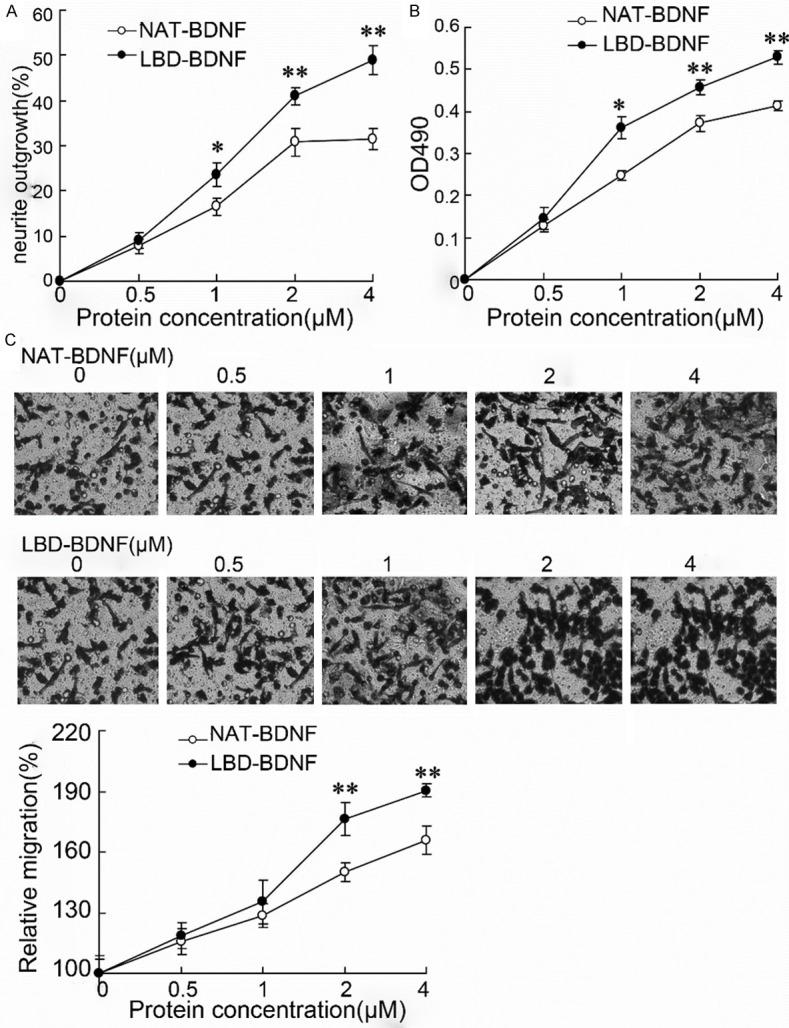
The effects of laminin-binding domain on neurite outgrowth, hyperplasia and migration of PC12 cells. A. The neurite outgrowth in PC12 cells; B. The value of OD490 was measured by MTT assay; C. The migration of neurite was evaluated by Transwell, and the group without NAT-BDNF or LBD-BDNF treatment was acted as control to calculate the relative migration. NAT: N-terminal domain of agrin; BDNF: Brain Derived Neurotrophic Factor; LBD: Laminin-Binding Domain.
Effects of miR-222 inhibitor on P12 cells treated with LBD-BDNF
Treated PC12 cells with 4 μM LBD-BDNF, The miR-222 expression was up-regulated (Figure 5A) and the level of p-mTOR was also increased (Figure 5B) compared with cells exposed to NAT-BDNF. The miR-222 inhibitor effectively silenced the miR-222 expression in PC12 cells, which resulted in significant down-regulation of the miR-222 level (Figure 5C). The silence of miR-222 significantly abolished LBD-induced increase of neurite outgrowth level (Figure 5D), cell survival rate (Figure 5E) and relative migration (Figure 5F). However, these effects of miR-222 inhibitor on cells were reversed by rapamycin, an inhibitor of mTOR.
Figure 5.
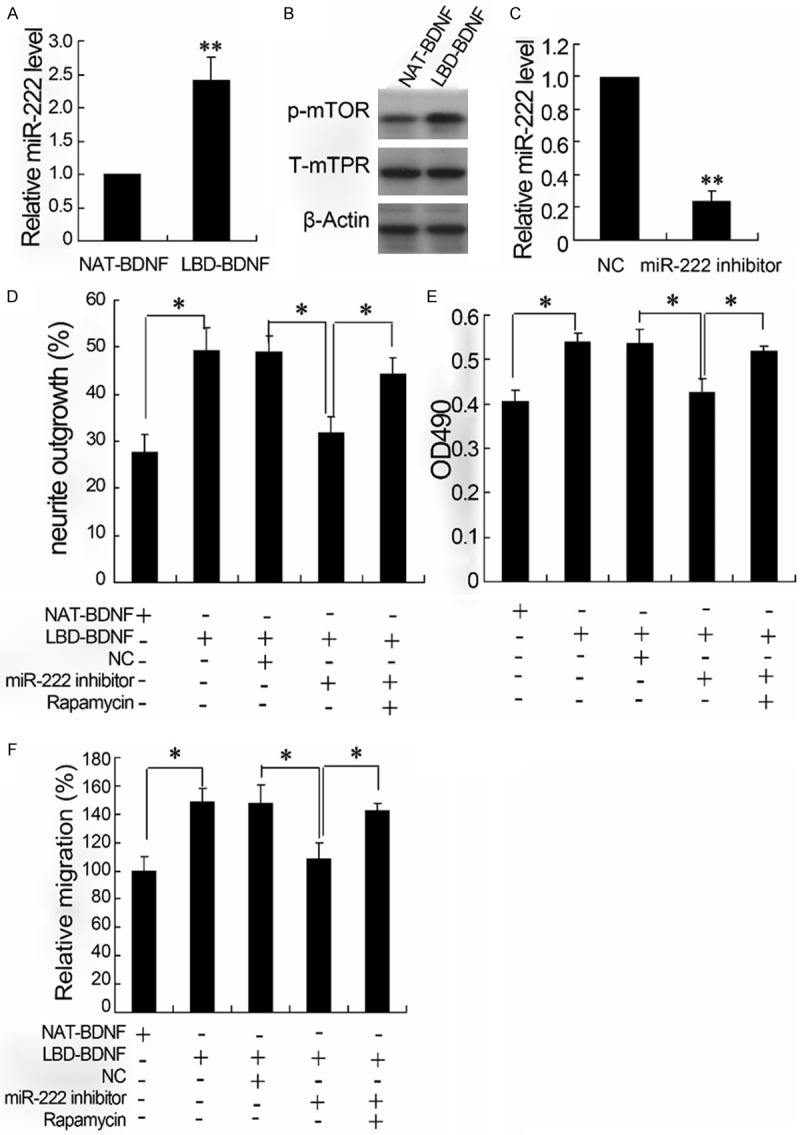
The effects of miR-222 inhibitor on P12 cells treated with LBD-BDNF. The relative miR-222 level was detected by real-time PCR (A and C); the expression of p-mTOR and T-mTOR was quantified by western blotting (B). The neurite outgrowth detected by neurite outgrowth assay (D); the hyperplasia of neurite detected by MTT (E); The migration of neurite evaluated by Transwell (F).
Effects of miR-222 inhibitor and mimic on p-mTOR expression of P12 cells
To investigate the effects of miR-222 on mTOR expression, cells were treated with miR-222 inhibitor or miR-222 mimic. As shown in Figure 6, miR-222 inhibitor decreased the expression of p-mTOR (Figure 6A) and overexpression of miR-222 increased the level of p-mTOR (Figure 6B, 6C).
Figure 6.
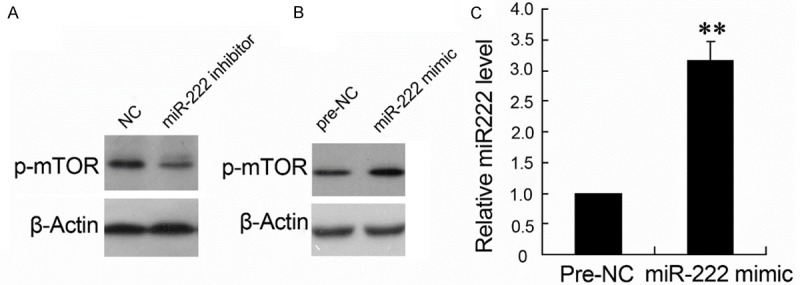
The effects of miR-222 inhibitor and mimic on p-mTOR expression of P12 cells. The expression of p-mTOR was quantified by western blotting (A and B); the relative miR-222 level was quantified by real-time PCR (C).
Discussion
Recurrent laryngeal nerve (RLN) injury, which can cause varying degrees of vocal fold paralysis and respiratory tract problems, is a common severe complication in neck surgery and seriously affects the quality of life in patients [20,21]. In present study, The LBD fused BDNF was used to promote recurrent laryngeal nerve regeneration. In vivo experiments, LBD-BDNF significantly improved the histological structure and function of recurrent laryngeal nerve in rabbits after 16 weeks following surgery. LBD-BDNF also markedly promoted neurite outgrowth, proliferation and migration in PC12 cells in vitro experiments. The effects of LBD-BDNF on nerve regeneration were probably related to the activation of mTOR signal pathway by miR-222.
Neurotrophic factors are a family of proteins that are responsible for the growth and survival of developing neurons and the maintenance of mature neurons [22]. BDNF, a member of neurotrophic factors, which is widely used in the recovery of injured neurons [10,23,24]. However, the injury site treated with BDNF alone is difficult to be retained, and previous studies proved that mixture injections are needed to achieve the therapeutic concentration [24]. Laminin is extra cellular matrix glycoproteins with multiple functions in the central nervous system and laminin is rapidly produced in neurons after the CNS injury and neuronal degeneration. LBD-BDNF is actually a tripartite fusion protein containing laminin, N-terminal domain of agrin and BDNF, which is reported to attenuate neural-degeneration and improve neurogenesis in dentate gyrus of hippocampi after middle cerebral artery occlusion in rats recently [10]. In present study, the numbers of regenerating fibers and axonal are significantly elevated and nerve conduction was restored by LBD-BDNF after recurrent laryngeal nerve injury in rabbits. In vitro experiments confirmed that LBD-BDNF could promote neurite outgrowth, proliferation and migration in PC12 cells, which implies that LBD-BDNF has a potential strategy to regenerate the injured recurrent laryngeal nerve.
Even so, the molecule mechanisms of LBD-BDNF involved in laryngeal nerve regeneration remains unclear. Studies have found that miRNAs are essential for functional recovery of peripheral nerve injury [25,26]. Schachner et al reported that miR-133b play an important role in regulating regenerative capacity of zebrafish after spinal cord injury via directly increasing the expression of RhoA [27], which is a small GTPase protein known to regulate the actin cytoskeleton in the formation of stress fibers. A study also showed that longevity assurance homologue 2 was a direct target of miR-221/222 to promote Schwann cells proliferation and migration [25]. In current research, the levels of miR-133b, miR-221, miR-222, miR-16 and miR-15b were quantified in laryngeal nerve of rabbits. The present data showed that only the expression level of miR-222 was increased by LBD-BDNF treatment after laryngeal nerve injury in vivo. Therefore, the further experiments of miR-222 were performed to explore the possible molecular mechanisms underlying the effects of LBD-BDNF on laryngeal nerve regeneration.
mTOR is a key factor in an intracellular signaling pathway that regulates protein synthesis, cell growth and proliferation. It has been known that mTOR signaling pathway is involved in the process of neurite outgrowth, nerve regeneration and synaptic plasticity, which acts as a critical role in the injured neuron and its axon [15]. It was also reported that mTOR was regulated by miRNAs in spinal cord injury [16]. In present study, the level of phosphorylated mTOR as well as the expression of miR-222 was elevated by LBD-BDNF treatment in laryngeal nerve injured rabbits compared with NAT-BDNF group, and the up-regulated levels of phosphorylated mTOR and miR-222 were also observed in PC12 cells treated with LBD-BDNF. The further study in vitro experiments showed that miR-222 inhibitor attenuated the expression of phosphorylated mTOR while miR-222 mimic enhanced its expression in PC12 cells. These results suggest that miR-222 is required in neuron for the regulation of mTOR. In addition, LBD-BDNF increased the levels of neurite outgrowth, proliferation and migration in PC12 cells as previously mentioned, while the effects of which was canceled by miR-222 inhibitor. In addition, rapamycin, an inhibitor of mTOR, reversed the effects of miR-222 inhibitor on LBD-BDNF induced cells. The above findings imply that the regulation of miR-222 and mTOR takes part in the protective effects of LBD-BDNF on injured neuron.
In conclusion, the present study revealed that LBD-BDNF promote the recurrent laryngeal nerve regeneration in laryngeal nerve injured rabbits. The underlying mechanism was closely related to activation of p-mTOR by miR-222. The present findings offer a theoretical basis for better application of LBD-BDNF to recurrent laryngeal nerve regeneration and recovery of damaged peripheral nerve.
Disclosure of conflict of interest
None.
References
- 1.Shinners MJ, Goding GS, McLoon LK. Effect of recurrent laryngeal nerve section on the laryngeal muscles of adult rabbits. Otolaryngol Head Neck Surg. 2006;134:413–418. doi: 10.1016/j.otohns.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 2.Ardito G, Revelli L, D’Alatri L, Lerro V, Guidi ML, Ardito F. Revisited anatomy of the recurrent laryngeal nerves. Am J Surg. 2004;187:249–253. doi: 10.1016/j.amjsurg.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Woodson GE. Spontaneous laryngeal reinnervation after recurrent laryngeal or vagus nerve injury. Ann Otol Rhinol Laryngol. 2007;116:57–65. doi: 10.1177/000348940711600110. [DOI] [PubMed] [Google Scholar]
- 4.Kirschenbaum B, Goldman SA. Brainderived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc Natl Acad Sci U S A. 1995;92:210–214. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schabitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, Kuhn HG. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38:2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- 6.Vogelin E, Baker JM, Gates J, Dixit V, Constantinescu MA, Jones NF. Effects of local continuous release of brain derived neurotrophic factor (BDNF) on peripheral nerve regeneration in a rat model. Exp Neurol. 2006;199:348–353. doi: 10.1016/j.expneurol.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 7.Han QQ, Jin W, Xiao ZF, Huang JC, Ni HB, Kong J, Wu J, Chen B, Liang WB, Dai JW. The promotion of neurological recovery in an intracerebral hemorrhage model using fibrin-binding brain derived neurotrophic factor. Biomaterials. 2011;32:3244–3252. doi: 10.1016/j.biomaterials.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 8.Sayer FT, Oudega M, Hagg T. Neurotrophins reduce degeneration of injured ascending sensory and corticospinal motor axons in adult rat spinal cord. Exp Neurol. 2002;175:282–296. doi: 10.1006/exnr.2002.7901. [DOI] [PubMed] [Google Scholar]
- 9.Yu LMY, Leipzig ND, Shoicheta MS. Promoting neuron adhesion and growth. Materialstoday. 2008;11:36–43. [Google Scholar]
- 10.Han Q, Li B, Feng H, Xiao Z, Chen B, Zhao Y, Huang J, Dai J. The promotion of cerebral ischemia recovery in rats by laminin-binding BDNF. Biomaterials. 2011;32:5077–5085. doi: 10.1016/j.biomaterials.2011.03.072. [DOI] [PubMed] [Google Scholar]
- 11.Sun W, Sun C, Zhao H, Lin H, Han Q, Wang J, Ma H, Chen B, Xiao Z, Dai J. Improvement of Sciatic Nerve Regeneration Using Laminin-Binding Human NGF-β. PLoS One. 2009;4:e1680. doi: 10.1371/journal.pone.0006180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eacker SM, Dawson TM, Dawson VL. Understanding microRNAs in neurodegeneration. Nat Rev Neurosci. 2009;10:837–841. doi: 10.1038/nrn2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fineberg SK, Kosik KS, Davidson BL. MicroRNAs potentiate neural development. Neuron. 2009;64:303–309. doi: 10.1016/j.neuron.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Liu NK, Wang XF, Lu QB, Xu XM. Altered microRNA expression following traumatic spinal cord injury. Exp Neurol. 2009;219:424–429. doi: 10.1016/j.expneurol.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park KK, Liu K, Hu Y, Kanter JL, He Z. PTEN/mTOR and axon regeneration. Exp Neurol. 2010;223:45–50. doi: 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 16.Liu G, Detloff MR, Miller KN, Santi L, Houle JD. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Exp Neurol. 2012;233:447–456. doi: 10.1016/j.expneurol.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han Q, Sun W, Lin H, Zhao W, Gao Y, Zhao Y, Chen B, Xiao Z, Hu W, Li Y, Yang B, Dai J. Linear ordered collagen scaffolds loaded with collagen-binding brain-derived neurotrophic factor improve the recovery of spinal cord injury in rats. Tissue Eng Part A. 2009;15:2927–2935. doi: 10.1089/ten.TEA.2008.0506. [DOI] [PubMed] [Google Scholar]
- 18.Lin LF, Chiu SP, Wu MJ, Chen PY, Yen JH. Luteolin induces microRNA-132 expression and modulates neurite outgrowth in PC12 cells. PLoS One. 2012;7:e43304. doi: 10.1371/journal.pone.0043304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watkins RJ, Patil R, Goult BT, Thomas MG, Gottlob I, Shackleton S. A novel interaction between FRMD7 and CASK: evidence for a causal role in idiopathic infantile nystagmus. Hum Mol Genet. 2013;22:2105–2118. doi: 10.1093/hmg/ddt060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Y, Gao B, Zhang X, Zhao J, Chen J, Zhang S, Luo D. Prevention and treatment of recurrent laryngeal nerve injury in thyroid surgery. Int J Clin Exp Med. 2014;7:101–107. [PMC free article] [PubMed] [Google Scholar]
- 21.Dispenza F, Dispenza C, Marchese D, Kulamarva G, Saraniti C. Treatment of bilateral vocal cord paralysis following permanent recurrent laryngeal nerve injury. Am J Otolaryngol. 2012;33:285–288. doi: 10.1016/j.amjoto.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Deister C, Schmidt CE. Optimizing neurotrophic factor combinations for neurite outgrowth. J Neural Eng. 2006;3:172–179. doi: 10.1088/1741-2560/3/2/011. [DOI] [PubMed] [Google Scholar]
- 23.Liang W, Han Q, Jin W, Xiao Z, Huang J, Ni H, Chen B, Kong J, Wu J, Dai J. The promotion of neurological recovery in the rat spinal cord crushed injury model by collagen-binding BDNF. Biomaterials. 2010;31:8634–8641. doi: 10.1016/j.biomaterials.2010.07.084. [DOI] [PubMed] [Google Scholar]
- 24.Park J, Lim E, Back S, Na H, Park Y, Sun K. Nerve regeneration following spinal cord injury using matrix metalloproteinase-sensitive, hyaluronic acid-based biomimetic hydrogel scaffold containing brain-derived neurotrophic factor. J Biomed Mater Res A. 2010;93:1091–1099. doi: 10.1002/jbm.a.32519. [DOI] [PubMed] [Google Scholar]
- 25.Yu B, Zhou S, Wang Y, Qian T, Ding G, Ding F, Gu X. miR-221 and miR-222 promote Schwann cell proliferation and migration by targeting LASS2 after sciatic nerve injury. J Cell Sci. 2012;125:2675–2683. doi: 10.1242/jcs.098996. [DOI] [PubMed] [Google Scholar]
- 26.Liu G, Keeler BE, Zhukareva V, Houle JD. Cycling exercise affects the expression of apoptosis-associated microRNAs after spinal cord injury in rats. Exp Neurol. 2010;226:200–206. doi: 10.1016/j.expneurol.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu YM, Gibbs KM, Davila J, Campbell N, Sung S, Todorova TI, Otsuka S, Sabaawy HE, Hart RP, Schachner M. MicroRNA miR-133b is essential for functional recovery after spinal cord injury in adult zebrafish. Eur J Neurosci. 2011;33:1587–1597. doi: 10.1111/j.1460-9568.2011.07643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


