Abstract
Therapeutic angiogenic effects of low-intensity ultrasound have been reported in endothelial cells and animal models of hind limb ischemia. It has been shown that the proliferation, migration, and tube formation of endothelial cells play critical roles in angiogenesis. The purpose of this study was to determine the underlying mechanism of low-intensity continuous therapeutic ultrasound on angiogenesis in endothelial cells. In the present study, human umbilical vein endothelial cells (HUVECs) were simulated of low-intensity therapeutic ultrasound (TUS, 1 MHz, 0.3 W/cm2, 9 minute per day) for 3 days, and we observed migration, tube formation, and expression of endothelial nitric oxide synthase (eNOS) and serine/threonine kinase (Akt) in HUVECs. Specific inhibitors of eNOS and phosphoinositide 3-kinase (PI3K) were added to the culture medium and TUS-induced changes in the pathways that mediate angiogenesis were investigated. After exposure to TUS, HUVECs tube formation and migration were significantly promoted, which was blocked by the eNOS inhibitor Immunofluorescence assay and Western blotting analysis demonstrated that eNOS expression in the HUVECs was significantly increased after TUS exhibition. Proteins of phosphorylated eNOS and Akt were both up-regulated after TUS stimulation. However, the specific inhibitor of PI3K not only significantly decreased the expression of p-Akt, but also down-regulated the p-eNOS. This suggested that the PI3K/Akt signal pathway might participate in modulating the activity of eNOS. In short, TUS therapy promotes angiogenesis through activation of the PI3K-Akt-eNOS signal cascade in HUVECs.
Keywords: Therapeutic ultrasound, angiogenesis, migration, human umbilical vein endothelial cells
Introduction
Ischemic vascular diseases remain a leading cause of mortality and morbidity worldwide. Restoration of blood flow to ischemic organs is vital to prevent tissue death after arterial occlusion. Despite significant advances in medical and surgical intervention, there is lack of specialized treatment strategies. As a new strategy for the treatment of acute and chronic ischemia, therapeutic angiogenic effects of low-intensity ultrasound (US) have been reported in endothelial cells (ECs), chick chorioallantoic membrane, and animal model of hind limb ischemia [1,2]. Previous studies have shown that endothelial cell (EC) proliferation, migration and tube formation play critical roles in US-induced angiogenesis [3-5]. Endothelium-derived nitric oxide (NO), originally identified as endothelium-derived relaxing factor, promotes angiogenesis and plays an important role in vascular remodeling and the maintenance of vascular integrity. In ECs, NO is a product of the conversion of L-arginine to L-citrulline by endothelial NO synthase (eNOS). eNOS produces low levels of NO constitutively but can be transiently stimulated to produce high levels by various hormones and environmental stimuli such as vascular endothelial growth factor (VEGF), angiopoietin-1, hypoxia, mechanical forces, and chemical agonists [6,7]. NOS inhibitors block ultrasound-induced EC migration, proliferation, and tube formation in vitro [8]. The phosphoinositide-3-kinase-Serine/threonine protein kinase (PI3K-Akt) is involved in multiple signal pathways to regulate cell proliferation, differentiation and migration. It is closely related to the occurrence and development of angiogenesis. Akt activates downstream of eNOS; promotes the release of NO and eNOS phosphorylation at serine 1177; accelerates endothelial cell proliferation and migration; and promotes angiogenesis [9,10]. Dimmeler et al demonstrated that shear stress activates eNOS by phosphorylation of the enzyme through the PI3K-Akt pathway [11]. Although a few literary reports indicate ultrasound has a beneficial effect on angiogenesis, it is still not completely clear how TUS influences endothelial cell angiogenesis and migration process.
In the present study, we hypothesized that the up-regulation of eNOS induced by low-intensity therapeutic ultrasound (TUS) might be mediated through activation of PI3K-Akt-eNOS signal pathway, thereby promoting the angiogenesis and migration of endothelial cells. In order to confirm our hypothesis, we designed the present studies seek to address whether TUS interferes with endothelial cell functions in terms of migration and angiogenesis through in vitro tube formation and transwell experiments. In addition, we used many cell biology and molecular techniques to determine the underlying mechanisms.
Material and methods
Materials
MatrigelTM matrix (basement membrane) and transwell migration chambers were obtained from BD Biosciences (Franklin Lakes, New Jersey, U.S.). eNOS inhibitor, N-nitro-Larginine methylester hydrochloride (L-NAME) was obtained from Sigma (St. Louis, MO, U.S.), which was dissolved in water and stored at -20°C. LY294002 (PI3K inhibitor) was obtained from Cell Signaling, dissolved in DMSO, and stored at -20°C. Antibodies specific for eNOS, phospho-eNOS (Ser1177) (p-eNOS), Akt, phospho-Akt (Ser473) (p-Akt), and GAPDH were all from Cell Signaling Technology (Beverly, CA, U.S.). Cy3-conjugated goat anti-rabbit IgG and 4’,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics (San Diego, CA, USA) and were cultivated in DMEM low-glucose medium supplemented with 10% fetal bovine serum and 100 U/ml penicillin and 100 μg/ml streptomycin under standard culture conditions (37°C, 5% CO2). To investigate the role of eNOS in TUS-induced angiogenesis, HUVECs were treated with 1 mM L-NAME in TUS+L-NAME group. The control cells were cultured in the medium absence of the eNOS or L-NAME inhibitor. The involvement of PI3K/Akt in the increases in eNOS was analyzed by using 50 μM LY294002 in TUS+LY294002 group.
Therapeutic ultrasound stimulation of cells in culture
TUS were generated by a device with applicator designed and made by Institute of Acoustics, Tongji University (Shanghai, China). Ultrasound was delivered to the 6 well cell culture plates with an energy flux density of 0.3 W/cm2 at a frequency of 1.0 MHz [5]. Common ultrasound gel pad was put on a circular ultrasound transducer (2.0 cm in diameter), and they were laid together under the base of the culture plate (Figure 1A). HUVECs were reseeded into plates overnight and stimulated for 9 minutes of TUS exposure per day for 3 days. The first TUS stimulation was performed at 24 h after the beginning of HUVECs culture, and then second and third TUS sessions were carried out 24 h apart (Figure 1B). Control cells were routinely plated in wells furthest from those exposed to TUS, and there was no detectable TUS exposure at this site. HUVECs were collected at 4 days after reseeding.
Figure 1.
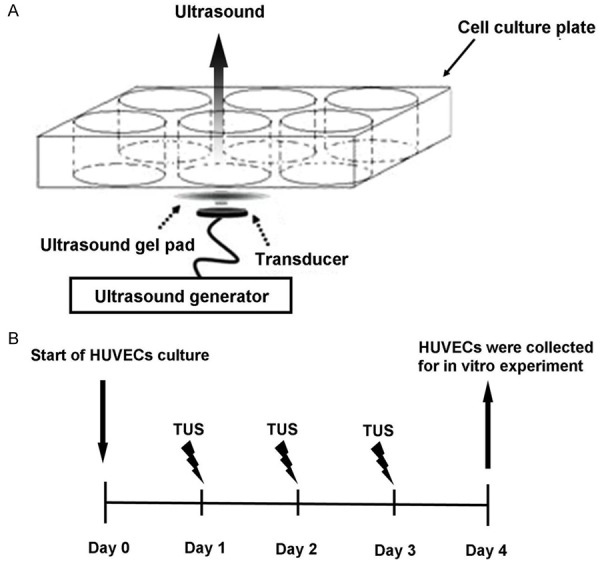
An illustration of the system of therapeutic ultrasound (TUS) stimulation of the cells in culture (A) and the experimental protocol (B). (A) Ultrasound was applied from a circular ultrasound transducer for the cells in culture through the base of the culture plate. An ultrasound gel pad was laid between the plate and the transducer. (B) Three sessions of TUS were carried out on the cells in culture and then HUVECs were collected on day 4. The collected HUVECs were used for in vitro experiments.
Endothelial cell tube formation assay
To examine the effect of TUS on in vitro angiogenesis, capillary-like tube formation assay was performed as described previously [5]. Matrigel-Matrix was pipetted into pre-chilled 96-well plates (50 µL matrigel per well) and polymerized for 45 min at 37°C. HUVEC (2 × 104 per well) in complete media were simultaneously seeded in matrigel coated plates. Then culture plates were exposed to TUS (1 MHz, 0.3 W/cm2) for 9 minutes. Common ultrasound gel pad was used for coupling. After 6 h of incubation, tubular structures were photographed. Images were acquired under a fluorescent microscope (IX-71; Olympus, Tokyo, Japan) with 12.8 M pixel recording digital color cooled camera (DP72; Olympus). The control sample was defined as 100% tube formation, and the increase or decrease in tube formation relative to the control was calculated for each sample. Each experiment was repeated at least three times under similar conditions [2].
Endothelial cell transwell migration assay
The chemotactic motility of HUVEC was determined using Transwell migration chambers with 6.5-mm-diameter polycarbonate filters (8-µm pore size) as described previously [5]. In brief, the bottom chambers were filled with 600 µL of DMEM media containing all supplements. HUVEC (3 × 104 per well) were seeded in top chambers in 100 µL DMEM media without serum. Thereafter, cells were treated with external TUS as indicated (9 minutes, 1 MHz, 0.3 W/cm2). Cells were allowed to migrate for 8 h. Non-migrated cells were removed with cotton swabs, and migrated cells were fixed with ice cold ethanol and stained with 0.01% crystal violet. Images were captured using Olympus DP72 digital camera on Olympus microscope with magnification x 200 and migrate cells were quantified by manual counting.
Immunofluorescence staining
Immunofluorescence staining was performed as described previously [12]. Briefly, cover slips were removed from the culture medium and washed with PBS. Cells were fixed with cold 4% paraformaldehyde, blocked by 8% normal goat serum, and incubated in rabbit polyclonal anti-eNOS antibody (dilution 1:200). After being washed three times with PBS, the cells were incubated with Cy3-conjugated goat anti-rabbit IgG (dilution 1:200). HUVECs nuclei were stained for 10 min with DAPI. Slides were washed another three times with PBS and then postfixed with Vectashield mounting medium, covered with glass cover slips, and subjected to fluorescence microscopy. Images were collected on Olympus DP72 digital camera on Olympus microscope. Arbitary units (AU)/μm2 were used to analysis fluorescence intensity quantitatively.
Western blotting analysis
After TUS treatment, HUVECs were scraped off and lysed with the cell lysis buffer (Cell Signaling Technology, Beverly, CA, U.S.) with PMSF [13]. The lysates were centrifuged at 12,000 x g at 4°C for 10 min to remove the insoluble material. The protein concentration was then measured with the BCA Protein Assay Reagent (Pierce, Rockford, IL, U.S.). Then equal amounts of protein (30 mg) from each sample were separated by 10% SDS-PAGE and transferred to nitrocellulose filter membrane (Millipore Co., Billerica, MA, U.S.) at 100 mV for 75 min at 4°C. The membranes were incubated with one of the following antibodies: anti-eNOS antibody (1:500), anti-p-eNOS antibody (1:500), anti-Akt antibody (1:500), anti-p-Akt antibody (1:500), anti-GAPDH antibody (1:1000) prior to incubation with IRDye800CW-conjugated secondary antibody (Rockland). The image was captured by the Odyssey infrared imaging system (Li-Cor Bioscience, Lincoln, NE, U.S.). The data were analyzed using ImageJ software. All western blotting experiments were repeated three times.
Statistic analysis
Multiple groups were analyzed by one-way ANOVA test followed by appropriate post hoc tests to determine statistical significance. Probability values < 0.05 were considered statistically significant. Data are expressed as means ± SEM. All experiments were repeated at least in triplicate. SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA) was used.
Results
Increase of capillary-like tube formation of HUVECs
To demonstrate the angiogenic potential of TUS, we performed Matrigel angiogenesis assay in vitro with HUVECs. The numbers of tube-like structures after building up by TUS for 9 minutes were comparable with those for control conditions. By 3 h after plating, the TUS treated HUVECs had differentiated into an expansive and sharply defined tube network, but the majority of the control cells remained in individual clusters. After 6 h of incubation, the TUS group showed a more extensive cellular network compared with the control group (Figure 2A and 2B). TUS treatment increased HUVEC tube length significantly (Figure 2D). These findings suggest that simulated TUS enhances the ability of HUVECs to form tube-like structures.
Figure 2.
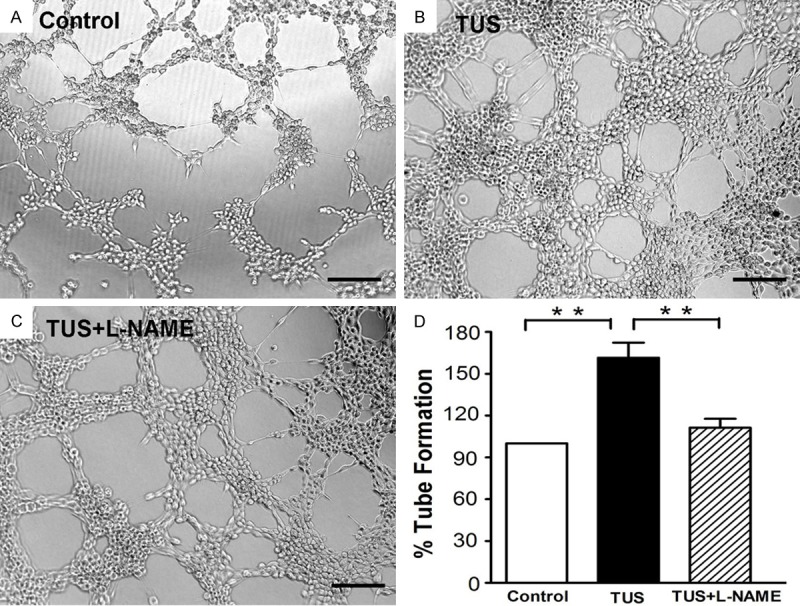
Effect of TUS on HUVECs tube formation. After 6 h, tube-like structures of the control group (A), TUS group (B) and TUS+L-NAME group (C) were photographed at 100 x magnification and tube length was measured as described (D). TUS significantly promoted the tube formation of HUVECs. Co-incubation with L-NAME, the specific inhibitor of eNOS, dramatically suppressed the tube formation. Values are mean ± SEM; n = 5, ** means p < 0.01. Scale bars = 200 μm.
In order to determine whether eNOS is involved in angiogenesis promoted by TUS in HUVECs, L-NAME was added into the culture medium and its effects were examined through tube formation assay. TUS-induced HUVECs tube formation was dramatically suppressed in TUS+L-NAME group compared with the TUS group (Figure 2C). There were no significantly tube length difference between control HUVECs and TUS+L-NAME group (Figure 2D), which suggests L-NAME significantly blocked TUS-enhanced HUVECs tube formation.
Improvement of HUVECs migration capacity
Cell motility was assessed with transwell migration assay by plating HUVECs in the upper chamber and DMEM media containing all supplements in the lower chamber, and HUVECs were treated with 9 minutes TUS. We observed that the presence of TUS in the lower chambers results in higher invasion of HUVECs through polyester layer compared to control (Figure 3A, 3B and 3D). L-NAME inhibited the TUS induced migration enhancement (Figure 3C). Overall, these results clearly indicated that TUS treatment promotes the migration of HUVECs, and L-NAME can block TUS induced endothelial cell migration.
Figure 3.
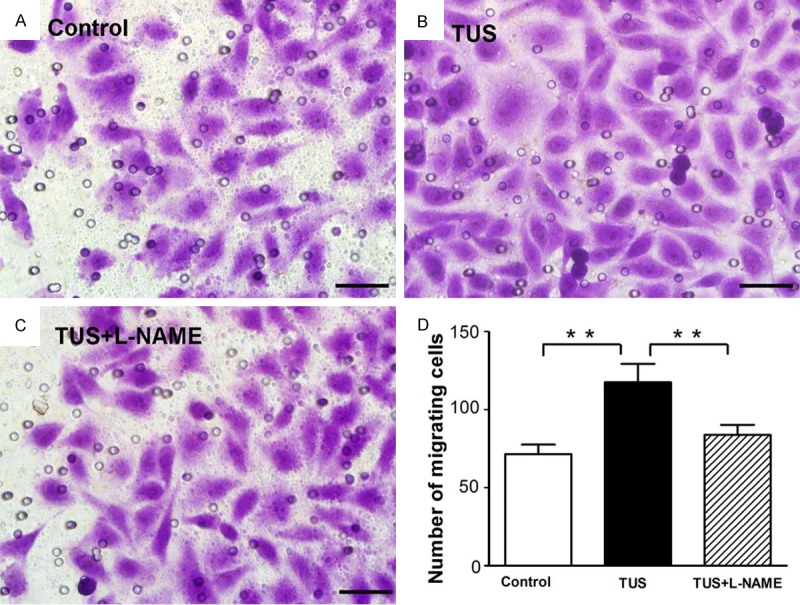
TUS effect on migration of HUVECs. Effect of TUS treatment on the migratory potential of HUVEC was examined using Transwell migration chambers as detailed in ‘Materials and Methods’. Control group (A), TUS group (B), and TUS+L-NAME group (C) were photographed at 200 x magnification. (D) Result depicted that TUS caused an obviously increase in cell migration which was blocked by L-NAME treatment. Values are mean ± SEM; n = 5, ** means p < 0.01. Scale bars = 100 μm.
Upregulation of eNOS expression of HUVECs
We performed immunofluorescence and western blot to study the effect of TUS on eNOS expression and localization in HUVECs. As shown in Figure 4, the fluorescence intensity of eNOS in the cytoplasm near the nucleus and the protein abundance of eNOS were obviously elevated in TUS-treated HUVECs relative to those in control group. However, in TUS and L-NAME co-treated group, the fluorescence intensity and protein abundance of eNOS were similar to control group, obviously weaker compared to TUS group. Therefore, these results implied that L-NAME inhibited the expression of eNOS protein induced by TUS.
Figure 4.
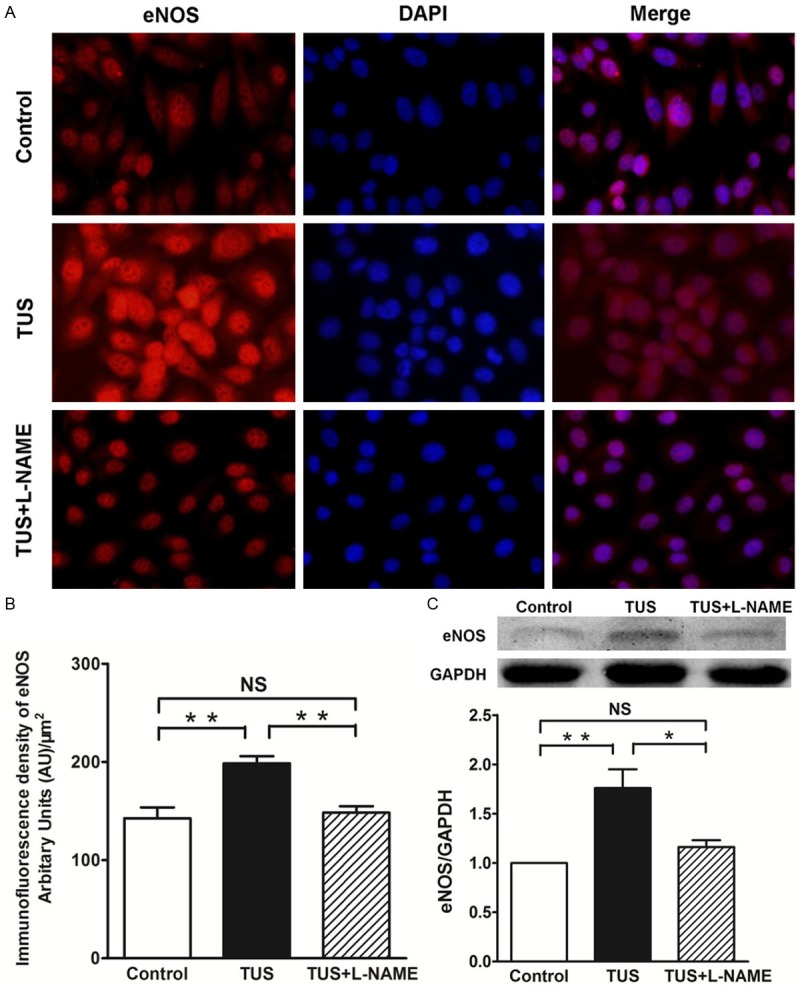
TUS increases eNOS expression of HUVECs in situ. Immunofluorescence staining of HUVECs pretreated with TUS demonstrated increase of eNOS expression (Cy3, represented in red), and L-NAME significantly inhibited TUS-induced eNOS expression. Immunofluorescence staining for eNOS-positive cells revealed significantly difference in the TUS group compared to untreated controls.
Activation of eNOS via the PI3K/Akt-dependent signaling pathway
To determine if TUS could mediate eNOS activation through Akt dependent pathways, the expression of key molecules in the PI3K/Akt signaling pathways were examined by western blot analysis. As shown in Figure 5, the treatment of TUS increased the eNOS protein levels relative to control, which indicated that TUS can stimulate eNOS expression in HUVECs and that it may be a promoter of eNOS expression. It has been shown that activation of the PI3K/Akt signal pathway and activation of Akt has been reported to stimulate phosphorylation of eNOS [14]. Moreover, the endothelial specific eNOS/NO pathway is closely associated with postnatal angiogenesis. Thus, to elucidate the underlying mechanism of eNOS activation, we determined whether phosphorylation of Akt and eNOS was regulated by TUS stimulation.
Figure 5.
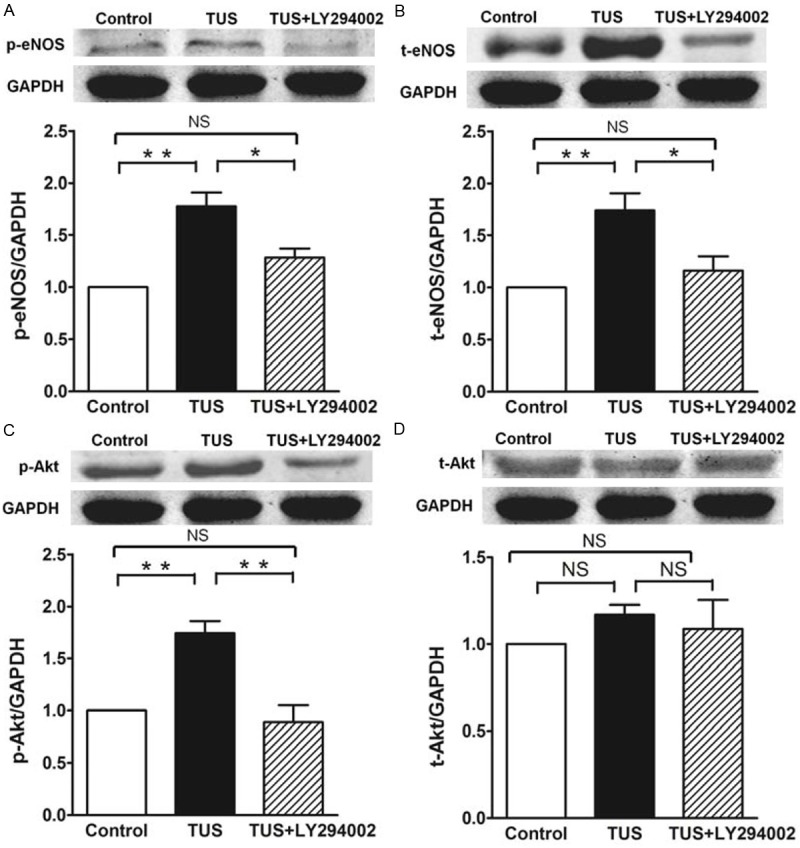
Effects of TUS on PI3K-Akt-eNOS signal pathway in HUVECs. Quantitative results of protein expression of eNOS, p-eNOS, p-Akt and total Akt. TUS promoted the expression of eNOS, p-eNOS, and p-Akt protein significantly. TUS-induced protein changes were significantly suppressed by LY294002 treatment. Data of Western blotting were represented as as percentages of expression in control group. Values are mean ± SEM; n = 5, * means p < 0.05, **means p < 0.01.
In order to evaluate this possibility, HUVECs were subjected to TUS in the presence or absence of LY294002 (a specific PI3K inhibitor). After exposure to TUS, except for total Akt, the expression of total eNOS, p-eNOS and p-Akt in HUVECs were all significantly increased (Figure 5). Interestingly, treatment of HUVECs with LY294002 suppresses the p-Akt expression. Similarly, eNOS and p-eNOS expression were stopped by blocking the PI3K/Akt kinase signaling transduction cascade, indicating the TUS’s proangiogenic effect were abolished by blocking PI3K/Akt/eNOS pathway with LY294002. It is suggested that TUS induces the activation of eNOS via the PI3K/Akt signal pathway.
Discussion
The most significant and novel of the findings discussed in this manuscript are that 1) TUS induced angiogenesis and migrate in HUVECs through promoting eNOS expression and that 2) the increase of eNOS induced by TUS is mediated by PI3K-Akt signal pathway. It is suggested underlying mechanisms and novel complementary explanations to TUS-induced postnatal neovascularization.
Angiogenesis is a complex process involving endothelial cell proliferation, migration, tube formation, and matrix reconstruction. It has been reported that ECs are highly sensitive to ultrasound wave, including the alterations in morphology and gene expression [15]. Serizawa et al have demonstrated that ultrasound wave exposure might increase the possibility of cell-to-cell interaction so that triggered cell proliferation and regeneration [16]. Those findings led to the idea that ECs under condition of ultrasound wave could become a new strategy for therapeutic angiogenesis. To investigate the effects of TUS on ECs function, we carried out in vitro tube formation and transwell migration experiment to evaluate the effects of TUS on HUVECs proangiogenic function. Our results showed that 9 minutes TUS per day for 3 days enhanced angiogenesis and migration in vitro. Our data obtained here are in agreement with previous reports [5,17].
In endotheliocytes, eNOS is the rate limiting enzyme in NO synthesis and plays an important role in regulating NO synthesis. The increase of eNOS activity will lead to more endothelial NO synthesis. NO produced by the eNOS is a fundamental determinant of cardiovascular homesotasis: it regulates systemic blood pressure, vascular remodeling, vascular permeability, and angiogenesis [7]. It has shown that the eNOS/NO pathway was important for angiogenesis in wound healing, chronic inflammation and ischemic diseases [18,19]. Several studies have found that inhibition of endogenous NO or suppression of eNOS activation can inhibit angiogenesis [20]. In the present study, we evaluated the protein expression of eNOS using immunofluorescence staining and western blotting to analysis whether eNOS activation is involved in the TUS induced angiogenic responses. After TUS treatment, eNOS protein expression was significantly increased in HUVECs. We then examined the angiogenesis after co-culturing with L-NAME, a specific inhibitor of eNOS. It is interesting to note that the activation of eNOS is required for angiogenesis, because eNOS inhibitor reduced TUS-induced tube formation and migration. These results confirmed that up-regulation of eNOS was correlated with angiogenesis after TUS exposure. The mechanisms underlying the up-regulation of eNOS were then investigated. It has been reported that in ECs, eNOS is phosphorylated by the Akt (protein kinase B), resulting in an increase in eNOS activity, which plays a crucial role in the regulation of vascular tone, vascular remodeling, angiogenesis, and NO production [11,21]. Previous studies have demonstrated that Akt mediates the activation of eNOS, leading to increased NO production. Inhibition of the PI3K/Akt pathway or mutation of the Akt site on eNOS protein (serine 1177) attenuates the serine phosphorylation and prevents the activation of eNOS [11,22]. It is reported that the phosphorylation of Ser 1177 enhanced eNOS activity and changed the sensitivity of the enzyme to Ca2+. They confered phosphorylation of eNOS by Akt represented a Ca2+ independent regulatory mechanism for activation of eNOS [11]. It also has been shown that ultrasound may activate mediators or membrane cation channels by recruiting a greater number of mechanoreceptors, resulting an increase in the intracellular concentration of free Ca2+ [23]. Taken together, our results suggest that TUS activates PI3K/Akt, which in turn is responsible for regulating the activation of eNOS.
To examine the functional involvement of Akt and eNOS in TUS-induced angiogenesis, we incubated endothelial cells with the PI3-kinase inhibitor LY294002 abrogated Akt phosphorylation induced by TUS. After exposure to TUS, the protein expressions of both p-Akt and p-eNOS were increased significantly compared with control group. Co-incubate with LY294002 not only decreased the expression of p-Akt, but also inhibited p-eNOS. These results suggest that the TUS-induced Akt-dependent phosphorylation of Ser 1177 enhances eNOS enzyme activity.
In conclusion, these experiments provide the first evidence that activation of PI3K-Akt-eNOS is a crucial signal pathway in the TUS-mediated signal transduction leads to angiogenesis. Collectively, our observations highlighted the importance of PI3K/Akt pathway in TUS-induced NO production and appeared to be necessary for vascular endothelial angiogenesis.
Acknowledgements
This work was supported by the China National Natural Science Foundation (11374213) and Foundation of National Lab for Infrared Physics (200901).
Disclosure of conflict of interest
None.
References
- 1.Atar S, Siegel RJ, Akel R, Ye Y, Lin Y, Modi SA, Sewani A, Tuero E, Birnbaum Y. Ultrasound at 27 kHz increases tissue expression and activity of nitric oxide synthases in acute limb ischemia in rabbits. Ultrasound Med Biol. 2007;33:1483–1488. doi: 10.1016/j.ultrasmedbio.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Xu P, Gul-Uludag H, Ang WT, Yang X, Huang M, Marquez-Curtis L, McGann L, Janowska-Wieczorek A, Xing J, Swanson E, Chen J. Low-intensity pulsed ultrasound-mediated stimulation of hematopoietic stem/progenitor cell viability, proliferation and differentiation in vitro. Biotechnol Lett. 2012;34:1965–1973. doi: 10.1007/s10529-012-0984-6. [DOI] [PubMed] [Google Scholar]
- 3.Hanawa K, Ito K, Aizawa K, Shindo T, Nishimiya K, Hasebe Y, Tuburaya R, Hasegawa H, Yasuda S, Kanai H, Shimokawa H. Low-intensity pulsed ultrasound induces angiogenesis and ameliorates left ventricular dysfunction in a porcine model of chronic myocardial ischemia. PLoS One. 2014;9:e104863. doi: 10.1371/journal.pone.0104863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holfeld J, Tepekoylu C, Blunder S, Lobenwein D, Kirchmair E, Dietl M, Kozaryn R, Lener D, Theurl M, Paulus P, Kirchmair R, Grimm M. Low Energy Shock Wave Therapy Induces Angiogenesis in Acute Hind-Limb Ischemia via VEGF Receptor 2 Phosphorylation. PLoS One. 2014;9:e103982. doi: 10.1371/journal.pone.0103982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang JJ, Shi YQ, Li RL, Hu A, Zhou HS, Cheng Q, Xu Z, Yang ZM, Hao CN, Duan JL. Angiogenesis effect of therapeutic ultrasound on ischemic hind limb in mice. Am J Transl Res. 2014;6:703–713. [PMC free article] [PubMed] [Google Scholar]
- 6.Sasore T, Reynolds AL, Kennedy BN. Targeting the PI3K/Akt/mTOR pathway in ocular neovascularization. Adv Exp Med Biol. 2014;801:805–811. doi: 10.1007/978-1-4614-3209-8_101. [DOI] [PubMed] [Google Scholar]
- 7.Fraisl P. Crosstalk between oxygen- and nitric oxide-dependent signaling pathways in angiogenesis. Exp Cell Res. 2013;319:1331–1339. doi: 10.1016/j.yexcr.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Hsu SH, Huang TB, Chuang SC, Tsai IJ, Chen DC. Ultrasound preexposure improves endothelial cell binding and retention on biomaterial surfaces. J Biomed Mater Res B Appl Biomater. 2006;76:85–92. doi: 10.1002/jbm.b.30345. [DOI] [PubMed] [Google Scholar]
- 9.Trouillon R, Kang DK, Park H, Chang SI, O’Hare D. Angiogenin induces nitric oxide synthesis in endothelial cells through PI-3 and Akt kinases. Biochemistry. 2010;49:3282–3288. doi: 10.1021/bi902122w. [DOI] [PubMed] [Google Scholar]
- 10.Kang Z, Zhu H, Jiang W, Zhang S. Protocatechuic acid induces angiogenesis through PI3K-Akt-eNOS-VEGF signalling pathway. Basic Clin Pharmacol Toxicol. 2013;113:221–227. doi: 10.1111/bcpt.12094. [DOI] [PubMed] [Google Scholar]
- 11.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 12.Guo JM, Liu AJ, Zang P, Dong WZ, Ying L, Wang W, Xu P, Song XR, Cai J, Zhang SQ, Duan JL, Mehta JL, Su DF. ALDH2 protects against stroke by clearing 4-HNE. Cell Res. 2013;23:915–930. doi: 10.1038/cr.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo JM, Dong WZ, Liu AJ, Cheng MH, Su DF. Nicotinamide postpones stroke in stroke-prone spontaneously hypertensive rats. CNS Neurosci Ther. 2012;18:267–268. doi: 10.1111/j.1755-5949.2011.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu FY, Song XX, Zheng H, Zhao YB, Fu GS. Thymosin beta4 induces endothelial progenitor cell migration via PI3K/Akt/eNOS signal transduction pathway. J Cardiovasc Pharmacol. 2009;53:209–214. doi: 10.1097/FJC.0b013e318199f326. [DOI] [PubMed] [Google Scholar]
- 15.Lawrie A, Brisken AF, Francis SE, Tayler DI, Chamberlain J, Crossman DC, Cumberland DC, Newman CM. Ultrasound enhances reporter gene expression after transfection of vascular cells in vitro. Circulation. 1999;99:2617–2620. doi: 10.1161/01.cir.99.20.2617. [DOI] [PubMed] [Google Scholar]
- 16.Serizawa F, Ito K, Kawamura K, Tsuchida K, Hamada Y, Zukeran T, Shimizu T, Akamatsu D, Hashimoto M, Goto H, Watanabe T, Sato A, Shimokawa H, Satomi S. Extracorporeal shock wave therapy improves the walking ability of patients with peripheral artery disease and intermittent claudication. Circ J. 2012;76:1486–1493. doi: 10.1253/circj.cj-11-1216. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Chen X, Yu A, Zhang Y, Ding J, Lu W. Highly sensitive and wide-band tunable terahertz response of plasma waves based on graphene field effect transistors. Sci Rep. 2014;4:5470. doi: 10.1038/srep05470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isenberg JS. Nitric oxide modulation of early angiogenesis. Microsurgery. 2004;24:385–391. doi: 10.1002/micr.20051. [DOI] [PubMed] [Google Scholar]
- 19.Li ST, Pan J, Hua XM, Liu H, Shen S, Liu JF, Li B, Tao BB, Ge XL, Wang XH, Shi JH, Wang XQ. Endothelial nitric oxide synthase protects neurons against ischemic injury through regulation of brain-derived neurotrophic factor expression. CNS Neurosci Ther. 2014;20:154–164. doi: 10.1111/cns.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luque Contreras D, Vargas Robles H, Romo E, Rios A, Escalante B. The role of nitric oxide in the post-ischemic revascularization process. Pharmacol Ther. 2006;112:553–563. doi: 10.1016/j.pharmthera.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Sun CK, Shao PL, Wang CJ, Yip HK. Study of vascular injuries using endothelial denudation model and the therapeutic application of shock wave: a review. Am J Transl Res. 2011;3:259–268. [PMC free article] [PubMed] [Google Scholar]
- 22.Petrilli AM, Fuse MA, Donnan MS, Bott M, Sparrow NA, Tondera D, Huffziger J, Frenzel C, Malany CS, Echeverri CJ, Smith L, Fernandez-Valle C. A chemical biology approach identified PI3K as a potential therapeutic target for neurofibromatosis type 2. Am J Transl Res. 2014;6:471–493. [PMC free article] [PubMed] [Google Scholar]
- 23.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]


