Abstract
The high-grade glioma (HGG) remains as the greatest challenge for cancer management worldwide. Identification of novel therapeutics and diagnostic method is in urgent need. The V-set and immunoglobulin domain-containing protein 4 (VSIG4) is a complement receptor for C3b/iC3b and inhibits cytotoxic T lymphocytes activation, which may play important roles in glioma oncogenesis. In this study, we performed immunohistochemistry in tissue microarray to determine the expression of VSIG4 in malignant glioma and normal brain. We then applied univariate and multivariate analyses to evaluate the expression of VSIG4 and correlated with prognosis of glioma patients. We have shown that VSIG4 was significantly elevated in high-grade glioma compared with those of normal brain tissues (P<0.001). We have also found that high VSIG4 expression was an independent prognostic factor for a shorter progression-free survival (PFS) and overall survival (OS) in high-grade glioma patients [hazard ratio (HR) =1.786, P=0.011 and HR=2.199, P=0.001, respectively]. Patients with low VSIG4 expression had a significantly longer median OS and PFS than those with high VSIG4 expression. Subgroup analysis stratifying HGG patients by both VSIG4 expression and tumor grade further confirmed the independent prognostic role of VSIG4 in HGG patients, while no adjuvant radiotherapy, small extent of resection and higher tumor grade were other three independent risk factors for HGG poor prognosis. Similar findings were also obtained using data from Cancer Genome Atlas (TCGA). Together, our results support that VISG4 can be used as a prognostic factor and potentially an immunotherapeutic target for glioma.
Keywords: VSIG4, proliferation, human glioma, prognosis
Introduction
The glioblastoma multiforme (GBM) is the grade IV glioma and features for its highly infiltrative and destructive characteristics. Although the treatment for GBM has been a very exciting topic, it remains as the greatest challenge for cancer management worldwide. In spite of multimodality therapy including aggressive resection, chemotherapy and radiotherapy, the prognosis of GBM remains dismal. According to Stupp et al [1], patients with newly diagnosed GBM had a median life expectancy of 14.6 months and 5 years survival rate of less than 10%. Thus, identification of novel therapeutics is in urgent need.
Cancer and the immune system interrelate tightly to each other [2,3]. It has been long accepted that immune surveillance can eliminate neoplastic cells and thus inhibit tumor formation and metastases. Examples include increased tumor incidence in immuno-compromised patients [4]. Among all immune cells, the T cell and macrophage are most prevalent in tumor related inflammation [5]. In fact, the GBM is usually heavily infiltrated by macrophages [6] and T cells [7]. However, this inflammatory response in GBM is to set up a pro-tumorigenic microenvironment, which is actively triggered by the GBM instead of the immune system [5]. These infiltrated macrophages and T cells in glioma get inhibited of their normal immunity while remain active in various trophic cytokines secretion (e.g. NF-κB, STAT3 and SMAD), which overall promote glioma progression [8]. However, how the GBM inhibits only the immunity of immune cells remains largely unknown.
The B7 super family is one of the most pivotal inflammatory protein families, which provide the primary co-signals (both co-stimulatory and co-inhibitory) for immune cells modulating [9].The VSIG4 (also known as CRIg [10] or Z39Ig [11]) is a newly identified member of the B7 super family [10]. Unlike other B7 homologs leading to either activation or inhibition immune cells, the VSIG4 obtain a bidirectional function at the same time. It negatively modulates inflammation process by causing T-cell anergy [12] while activates macrophage immunity when binding to the C3b/iC3b [10]. However, role of VSIG4 in glioma remains elusive.
In this study, we reported, for the first time that, VSIG4 was highly expressed in glioma (esp. GBM) cells. We also observed correlation of high VSIG4 expression with poor prognosis of glioma, supporting a role of VSIG4 in glioma tumor progression and prognosis.
Material and methods
Patients population and tissue samples
All aspects of the current study were reviewed and approved by the Specialty Committee on Ethics of Biomedicine Research, Second Military Medical University. Human tissue acquisition and usage in the current study followed the National Regulations on the Use of Clinical Samples in China. The data was obtained from 162 patients who underwent glioma resection surgery in our department from 2007 to 2013. Before enrollment, all patients or their legal surrogates reviewed, signed and provided a consent form, informing the surgical procedures and usage permission of resected tissue specimens. 14 days prior to surgery, a complete medical record was required to evaluate each patient’s overall condition, including complete medical history, physical examination, brain magnetic resonance images (MRI) and hematology and biochemistry blood analysis. Resected tumor tissues obtained at the time of resection were snap-frozen in liquid nitrogen and transferred into -80°C freezer when arrived at lab.
The inclusion criteria for the current study included: 1) ≥18 years of age, 2) histopathological confirmation of glioma according to the 2007 WHO Classification of Tumors of the Central Nervous System [13], 3) the tissue sample was sufficient for experimental usage, and 4) post-operative follow-up at least once every 6 months. Normal brain tissues were obtained for negative control from trauma patients, who required partial resection of normal brain for relief of intracranial pressure.
Immunohistochemical staining and tissue microarray
The microarray was constructed as described previously [14]. After hematoxylin and eosin (H&E) staining to confirm histopathology, each specimen was punched (round shape with 1.5 mm in diameter) at the center of tumor foci to construct the tissue microarray slides (Shanghai Biochip Co., Shanghai, China).
The avidin-biotin complex (ABC) method (Vector Laboratories, Burlingame, CA, USA) was performed according to manufacturer’s instructions for immunohistochemical staining. The polyclonal anti-VSIG4 antibody (Abcam Inc., Cambridge, USA) was incubated with tissue specimens at a dilution of 1:100.
The results of staining were reviewed by two independent pathologists, who were blinded to the demographic and clinical data. Staining of tumor cells and glial cell were regarded as positive for glioma and normal brain, respectively. The staining intensity of tumor/glial cell cytoplasm was scored as: 0 for no staining, 1 for light-brown, 2 for medium-brown, and 3 for dark-brown. The percentage of tumor/glial cells staining was graded as 0 (<5%), 1 (5-25%), 2 (25-50%), 3 (50-75%), and 4 (75-100%). The product of the intensity and percentage stand for the final VSIG4 expression scores, which was classified as strong (+++, final score 7-9), moderate (++, final score =4-6) and weak (+, final score =0-3). For analysis, VSIG4 expression was classified into either “high” (+++) or “low” (++ and below) group. The representative figures were presented in Figure 1.
Figure 1.
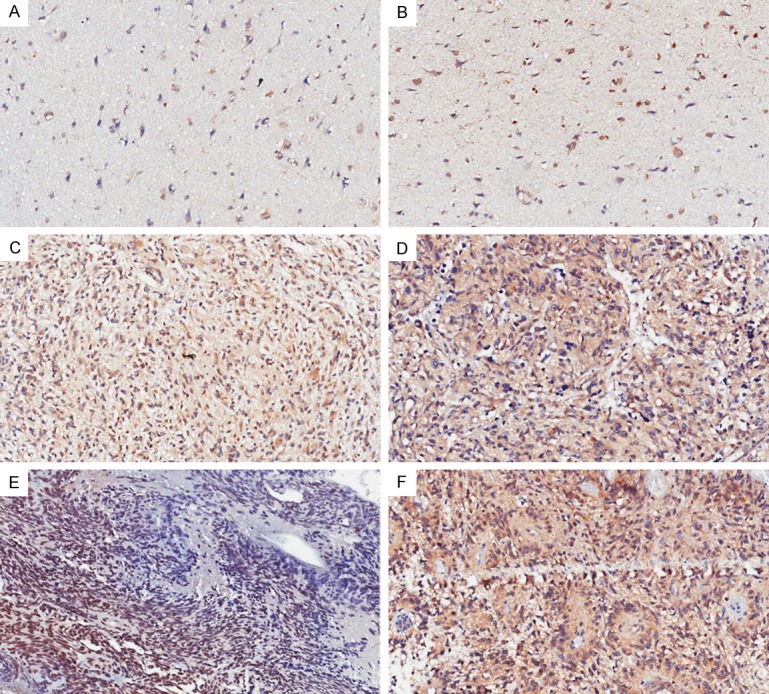
Immunohistochemical staining slides showed VSIG4 staining intensity and density in normal brain (A, B), WHO grade III (C, D), IV (E, F) glioma tissues. The scores for all six slides are A (score =0), B (score =1), C (score =4), D (score =6), E (score =6), F (score =9). Compared to the low staining intensity and density in normal brain tissues, VSIG4 showed significantly elevated staining intensity and density in high-grade gliomas.
TCGA database
In order to verify the potential role of VSIG4 in malignant glioma, we searched TCGA (www. cancaergenome.nih.gov), which provides multimodal data of more than 500 GBM cases. Available raw microarray gene expression data based on the Affymetrix microarrays (Human gene U133A), and clinical treatment and follow-up information were included. The expression of gene VSIG4 was collected for each case, and VSIG4 expression was classified as either High (expression value ≥264) or Low (expression value <264). OS and PFS were calculated in days from the data of diagnosis to the time of death and to the time of tumor progression or recurrence, or death of the patient from GBM, respectively.
Statistical analysis
All statistical analyses were performed in SPSS statistical software, version 16.0 (SPSS Inc., Chicago, IL, USA). The P-values <0.05 was considered as statistically significant. Continuous variables were reported as means ± standard error while categorical variables were reported as frequencies (%). The overall survival (OS) and the progression-free survival (PFS) were monitored for each patient. The OS denoted the time period from the day of surgery to patient’s death. The PFS measured the time length from the day of surgery to tumor progression in MRI or causing death. The correlations of VSIG4 and clinicopathologic parameters of glioma were examined by either chi-squared test or the Mann-Whitney U test. Survival rates were calculated by the Kaplan-Meier method and analyzed by log-rank test. Univariate and multivariate analyses were performed by stepwise backward Cox regression model (P<0.2 was considered as the inclusion criterion for factors that could be added into multivariate analysis).
Result
Demographic and clinicopathological characteristics of study participants
A total of 160 high glioma samples (n=45 and 115 for grade III and IV, respectively) were finally eligible for evaluating (2 spots out of 162 spots were lost in the TMA). The characteristics of these glioma tissues were elaborately recorded (Table 1). The follow-up period for all glioma patients varied from 0.5 to 119 months. At the final follow-up, 71.1% (32 cases) grade III and 93% (107 cases) grade IV patients were dead.
Table 1.
Basic characteristics of glioma tissues collected and used in current study
| The demographic and clinicopathological characteristics of enrolled high-grade glioma patients | ||
|---|---|---|
|
| ||
| Number of patients | ||
| Age (years) | ||
| <65 | 143 | |
| >65 | 17 | |
| Gender | ||
| Male | 105 | |
| Female | 55 | |
| Primary/Secondary | ||
| Primary glioma | 136 | |
| Secondary glioma | 24 | |
| Seizure | ||
| Yes | 25 | |
| No | 135 | |
| Increased intracranial pressure | ||
| Yes | 60 | |
| No | 90 | |
| Cystic degeneration on MRI | ||
| Yes | 41 | |
| No | 119 | |
| Necrosis on MRI | ||
| Yes | 29 | |
| No | 131 | |
| MTD (cm) | ||
| <5 | 101 | |
| ≥5 | 59 | |
| Resection degree | ||
| Total | 118 | |
| Sub-total | 35 | |
| Partial | 4 | |
| Biopsy | 3 | |
| WHO Grade | ||
| WHO III | 45 | |
| WHO IV | 115 | |
| Chemotherapy | ||
| Yes | 113 | |
| No | 47 | |
| Radiotherapy | ||
| Yes | 115 | |
| No | 45 | |
| Lineage | ||
| Astrocytic | 149 | |
| Oligodendroglial | 11 | |
The median PFS for all enrolled HGG patients was 12.9 months. The PFS rates for HGG patients were 52% at 1 year, 28% at 2 years, and 8% at 5 years, respectively. The OS rates for HGG patients were 59% at 1 year, 31% at 2 years, and 10% at 5 years, respectively, with a median OS of 15.9 months.
VSIG4 is highly-expressed in HGG samples
We then examined the expression of VSIG4 in high grade glioma and normal brain tissues by immunohistochemistry using tissue microarray. The detailed staining results were listed in Supplementary Table 1, representative figures were shown in Figure 1. Immunohistochemical staining showed a 3-folder elevated expression of VSIG4 in HGG tissues compared with that of normal brain tissues (P<0.001). To assess whether VSIG4 expression correlated with clinicopathological characteristics other than tumor grade, we did a correlation analysis, and found that high VSIG4 expression was not correlated with common clinicopathological items including age, gender, absence of seizure, necrosis, large MTD or astrocytic tumor lineage, indicating that VSIG4 might be an independent risk factor for HGG patients.
High VSIG4 expression correlates with poorer survival of HGG patients
We then evaluated the prognostic effects of high VSIG4 expression on HGG patients. OS and PFS were stratified by VSIG4 expression using the log-rank and Cox regression test. We found that the median PFS of HGG patients with low VSIG4 expression was 17 months, which was significantly longer that of HGG patients with high VSIG4 expression (11 months) (P=0.046) (Figure 2A). The median OS of HGG patients with low VSIG4 expression was 17 months, which was significantly longer that of HGG patients with high VSIG4 expression (14 months) (P=0.021) (Figure 2B). The univariate analysis showed high VSIG4 expression was a potential risk factor of survival and progression of HGG patients (Table 2).
Figure 2.
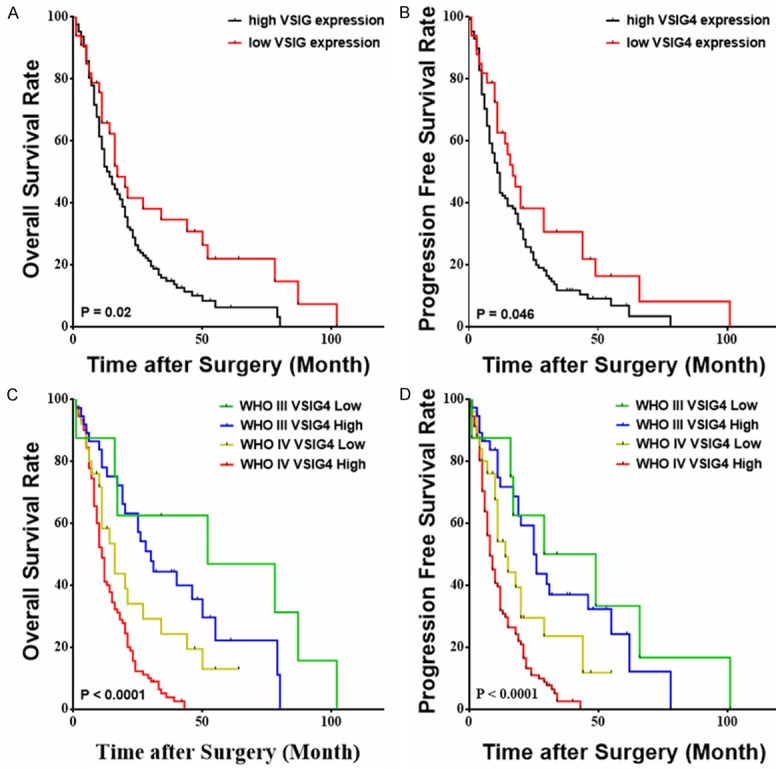
High VSIG4 expression is associated shorter overall survival and progression-free survival of glioma patients. The progression-free survival (A) and Kaplan-Meier survival curve (B) for high-grade glioma patients stratified by VSIG4 expression. The progression-free survival (C) and Kaplan-Meier survival curve (D) for WHO grade III and IV glioma patients stratified by VSIG4 expression.
Table 2.
Univariate analysis of factors associated with survival and progression in HGG patients
| Variable | HGG | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| OS | PFS | |||||
|
|
|
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (≥60 vs. <60 y) | 1.528 | 1.032-2.264 | 0.034 | 1.607 | 1.084-2.382 | 0.018 |
| VSIG4 (High vs. Low) | 1.670 | 1.066-2.619 | 0.025 | 1.530 | 0.994-2.354 | 0.053 |
| Gender (Female vs. male) | 0.814 | 0.570-1.163 | 0.258 | 0.759 | 0.533-1.081 | 0.126 |
| Primary (vs. secondary) | 0.686 | 0.426-1.104 | 0.120 | 0.734 | 0.462-1.168 | 0.192 |
| Seizure (Yes vs. no) | 0.847 | 0.521-1.377 | 0.503 | 0.974 | 0.611-1.550 | 0.910 |
| IICP (Yes vs. no) | 0.953 | 0.682-1.333 | 0.780 | 0.892 | 0.640-1.243 | 0.499 |
| Cystic Degeneration (Yes vs. no) | 0.750 | 0.510-1.102 | 0.142 | 0.818 | 0.561-1.190 | 0.293 |
| Necrosis on MRI (Yes vs. no) | 0.950 | 0.614-1.469 | 0.817 | 1.178 | 0.774-1.794 | 0.445 |
| MTD (≥5 vs <5 cm) | 1.047 | 0.745-1.472 | 0.792 | 1.209 | 0.864-1.692 | 0.268 |
| Resection Degree | 0.009 | 0.037 | ||||
| Subtotal vs. total | 1.224 | 0.816-1.836 | 0.329 | 1.223 | 0.824-1.815 | 0.318 |
| Partial vs. total | 4.312 | 1.557-11.943 | 0.005 | 3.453 | 1.253-9.516 | 0.017 |
| Biopsy vs. total | 3.222 | 1.012-10.262 | 0.048 | 2.637 | 0.803-8.385 | 0.100 |
| Chemotherapy (Yes vs. no) | 0.783 | 0.542-1.132 | 0.194 | 0.887 | 0.615-1.281 | 0.524 |
| Radiotherapy (Yes vs. no) | 0.644 | 0.450-0.921 | 0.016 | 0.708 | 0.496-1.009 | 0.056 |
| WHO Grade | ||||||
| IV vs. III | 3.051 | 1.973-4.717 | <0.001 | 2.794 | 1.837-4.250 | <0.001 |
| Lineage (Oligodendroglial vs. astrocytic) | 0.211 | 0.078-0.574 | 0.002 | 0.259 | 0.106-0.636 | 0.003 |
Note: Univariate analysis, Cox proportional hazards regression model. Abbreviations: IICP, increased intracranial pressure; MTD, mean tumor diameter; NA, not applicable; HR, Hazard ratio; OS, overall survival; PFS, progression-free survival.
In the multivariate Cox regression, we found high-expression of VSIG4, no adjuvant radiotherapy, small extent of resection and higher tumor grade as independent risk factor of both shorter PFS and OS in human HGG patients (Table 3). The hazard ratio for progression among patients with high VSIG4 expression, as compared to those with low VSIG4 expression, was 1.786 (95% CI, 1.144 to 2.788; P=0.011). The hazard ratio for death among patients with high VSIG4 expression, as compared to those with low VSIG4 expression, was 2.199 (95% CI, 1.372 to 3.523; P=0.001).
Table 3.
Multivariate analysis of factors associated with survival and progression of HGG patients
| Survival* | HR | 95% CI | P |
|---|---|---|---|
| OS | |||
| Cystic degeneration (Yes vs. No) | 0.698 | 0.47-1.037 | 0.075 |
| VSIG4 (High vs. low) | 2.199 | 1.372-3.523 | 0.001 |
| Resection | <0.001 | ||
| Subtotal vs. Total | 1.19 | 0.781-1.815 | 0.418 |
| Partial vs. Total | 4.91 | 1.755-13.737 | 0.002 |
| Biopsy vs. Total | 7.436 | 2.035-27.166 | 0.002 |
| Radiotherapy (Yes vs. No) | 0.61 | 0.419-0.887 | 0.001 |
| Grade (IV vs. III) | 3.088 | 1.86-5.126 | <0.001 |
| Lineage (astrocyte vs. oligodendrocyte) | 0.307 | 0.102-0.922 | 0.035 |
| PFS | |||
| Gender (male vs. female) | 0.643 | 0.442-0.034 | 0.021 |
| VSIG4 (High vs. low) | 1.786 | 1.144-2.788 | 0.011 |
| Resection | 0.001 | ||
| Subtotal vs. Total | 1.021 | 0.682-1.53 | 0.918 |
| Partial vs. Total | 4.019 | 1.435-11.252 | 0.008 |
| Biopsy vs. Total | 7.849 | 2.216-27.804 | 0.001 |
| Grade (IV vs. III) | 3.401 | 2.156-5.365 | <0.001 |
| Radiotherapy (Yes vs. No) | 0.654 | 0.443-0.966 | 0.033 |
| Age (≥60 vs. <60 y) | 1.579 | 1.037-2.405 | 0.033 |
Note: Multivariate analysis, Cox proportional hazards regression model. Abbreviations: HGG, high-grade glioma; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Variables were adopted for their prognostic significance by univariate analysis (P≤0.2).
We further did a subgroup analysis, stratifying HGG patients by both VSIG4 expression and tumor grade. Patients with WHO grade IV and high VSIG4 level had the significantly shortest median PFS (8 months) and OS (11 months), while those with WHO grade III and low VSIG4 level had the significantly longest median PFS (29 months) and OS (52 months). There was no difference in PFS and OS between patients with WHO grade IV, low VSIG4 level and patients with WHO grade III, high VSIG4 level (Figure 2C and 2D). The detailed results were listed in Supplementary Table 2. When the Cox regression was done separately in GBM patients only, the independent prognostic role of VSIG4 is still confirmed (Data not shown). The results established an independent prognostic role for VSIG4 in HGG patients.
Data from current study matched that from the TCGA data base
To further ensure the accuracy of this study, we analyzed the data that obtained from the TCGA database, and compared it with our results. We found that overall, the expression of VSIG4 in 528 TCGA patients exhibited significantly elevated VSIG4, compared to that of the normal brain (P<0.001). The VSIG4 expression level was highly expressed in glioma and strongly associated with poor prognosis of glioma patients. Patients with low VSIG4 have a significantly longer overall survival time (383 days versus 350 days, P=0.045) and progression-free survival time (228 days versus 183 days, P=0.017) (Figure 3A and 3B).
Figure 3.
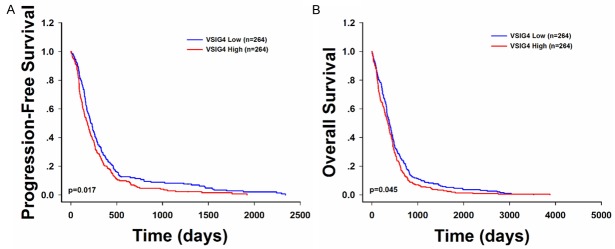
Survival and VSIG4 expression pattern of GBM patients from TCGA database showed similar pattern as that from the current study. The progression-free survival (A) and Kaplan-Meier survival curve (B) for GBM patients stratified by VSIG4 expression.
Discussion
In current study, we analyzed clinical data collected from 251 glioma patients. Our data demonstrated that higher VSIG4 expression was detected in glioma tissues than in normal brain tissues. We also showed that high VSIG4 expression correlated with poor prognosis in HGG patients, which agreed with data that obtained from the TCGA database.
Although the host immunity provides protection against cancer formation, cancer immunoediting is capable of switching immune surveillance to immune escape [15]. In normal immune surveillance condition, tumor antigen-specific T- lymphocytes actively migrate into glioma [16], recognize MHC class I antigen, receive co-stimulation signals, get activated, eradicate cancer cells, expand more tumor antigen-specific T lymphocytes and develop immune memory [17,18]. Previous studies had correlated increasing tumor antigen-specific T lymphocytes infiltration with stronger anti-glioma inflammation and better prognosis [19,20]. However, loss or down-regulation of MHC class I expression in cancer has been observed in multiple types of cancers [21], including the glioma [22,23]. Because MHC class I is the primary activation signal for T lymphocytes, eliminating MCH class I enables glioma cells to hamper antigen-specific T lymphocytes activation and subsequent anti-glioma process. Additionally, glioma cells also inhibit the expression of multiple co-stimulatory molecules, such as the CD86, CD80, and CD40 [24], which provide double assurance for antigen-specific T lymphocytes inhibition. In our study, we proposed another possible mechanism for immune escape of glioma cells: VSIG4 may cause immune escape via T lymphocytes anergy. Previous studies had already reported that VSIG4 inhibited host immune response via preventing the expansion of tumor antigen-specific helper and cytotoxic T lymphocytes [10,25] and their interleukin-2 production [25]. The underlying mechanism of this phenomenon is that VSIG4 could bind to unknown co-inhibitory receptors on T lymphocytes membrane [12], which recruit phosphatases and prohibited downstream phosphorylation cascades inside T lymphocytes [9]. Our study has shown that glioma cells over-express VSIG4, which subsequently inhibit recruited T lymphocytes’ activation. As tumor antigen-specific helper and cytotoxic T lymphocytes correlate with better survival in glioma [26], their inhibition leads to glioma progression and poorer prognosis. In addition, we have also shown that VSIG4 is significantly highly expressed in WHO grade III and IV gliomas. We therefore, hypothesize that high grade gliomas are capable of establishing an anti-immune micro-environment. Meanwhile, there must be other inhibitory factors that are synergetic with VSIG4 to cause immune escape of glioma, as high-VSIG4-expression HGG had a significant lower survival rate compared to that of LGG.
Notably, VSIG4 has been shown as a complement receptor for C3b/iC3b. Previous studies revealed increased deposition of complement, including C3b (but not the membrane attack complex) on cancer cell membranes [27]. The increased deposition of complement had been tightly related to accelerated neovascularization process via induction of vascular endothelial growth factor (VEGF) expression [28]. As VSIG4 is increased in glioma, this may cause increased C3b deposition in glioma, which subsequently promotes glioma neovascularization, although further evidence are needed to support this speculation.
In conclusion, the data from current study suggested that VSIG4 constitutes one possible mechanism of modulating immune system, which allows glioma cells to inhibit host immune system and thus escape from immune attack. VSIG4 may also promote glioma neovascularization and thus, glioma progression. Therefore, VISG4 may serve as a promising prognostic factor for glioma as well as a potential target for glioma immunotherapy.
Acknowledgements
This work was supported by the National “863” High Technique Project (2007AA02Z483), National Natural Science Foundation of China (81272781, 81101907, 81472354), the Program for academic leaders in health science (XBR2011030) and “Shu Guang” project (11SG37) in Shanghai.
Disclosure of conflict of interest
None.
References
- 1.Stupp R, Mason W, van den Bent M, Weller M, Fisher B, Taphoorn M, Belanger K, Brandes A, Marosi C, Bogdahn U, Curschmann J, Janzer R, Ludwin S, Gorlia T, Allgeier A, Lacombe D, Cairncross J, Eisenhauer E, Mirimanoff R European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber R, Old L, Smyth M. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg R. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Vajdic C, van Leeuwen M. Cancer incidence and risk factors after solid organ transplantation. Int J Cancer. 2009;125:1747–1754. doi: 10.1002/ijc.24439. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 6.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn G, Dunn I, Curry W. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun. 2007;7:12. [PMC free article] [PubMed] [Google Scholar]
- 8.Grivennikov S, Greten F, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov. 2013;12:130–146. doi: 10.1038/nrd3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helmy K, Katschke KJ, Gorgani N, Kljavin N, Elliott J, Diehl L, Scales S, Ghilardi N, van Lookeren Campagne M. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 11.Langnaese K, Colleaux L, Kloos D, Fontes M, Wieacker P. Cloning of Z39Ig, a novel gene with immunoglobulin-like domains located on human chromosome X. Biochim Biophys Acta. 2000;1492:522–525. doi: 10.1016/s0167-4781(00)00131-7. [DOI] [PubMed] [Google Scholar]
- 12.He J, Wiesmann C, van Lookeren Campagne M. A role of macrophage complement receptor CRIg in immune clearance and inflammation. Mol Immunol. 2008;45:4041–4047. doi: 10.1016/j.molimm.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Louis D, Ohgaki H, Wiestler O, Cavenee W, Burger P, Jouvet A, Scheithauer B, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu T, Qin R, Zhou J, Yan Y, Lu Y, Zhang X, Fu D, Lv Z, Li W, Xia C, Hu G, Ding X, Chen J. High bone sialoprotein (BSP) expression correlates with increased tumor grade and predicts a poorer prognosis of high-grade glioma patients. PLoS One. 2012;7:e48415. doi: 10.1371/journal.pone.0048415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ransohoff R, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 17.Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, Grau S, Hiraoka N, Eckstein V, Ecker R, Korff T, von Deimling A, Unterberg A, Beckhove P, Herold-Mende C. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin Cancer Res. 2011;17:4296–4308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- 18.Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23(Suppl 8):viii6–9. doi: 10.1093/annonc/mds256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prins R, Soto H, Konkankit V, Odesa S, Eskin A, Yong W, Nelson S, Liau L. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17:1603–1615. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prins R, Craft N, Bruhn K, Khan-Farooqi H, Koya R, Stripecke R, Miller J, Liau L. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol. 2006;176:157–164. doi: 10.4049/jimmunol.176.1.157. [DOI] [PubMed] [Google Scholar]
- 21.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 22.Wu A, Wiesner S, Xiao J, Ericson K, Chen W, Hall W, Low W, Ohlfest J. Expression of MHC I and NK ligands on human CD133+ glioma cells: possible targets of immunotherapy. J Neurooncol. 2007;83:121–131. doi: 10.1007/s11060-006-9265-3. [DOI] [PubMed] [Google Scholar]
- 23.Rees R, Mian S. Selective MHC expression in tumours modulates adaptive and innate antitumour responses. Cancer Immunol Immunother. 1999;48:374–381. doi: 10.1007/s002620050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain S, Yang D, Suki D, Aldape K, Grimm E, Heimberger A. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8:261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogt L, Schmitz N, Kurrer M, Bauer M, Hinton H, Behnke S, Gatto D, Sebbel P, Beerli R, Sonderegger I, Kopf M, Saudan P, Bachmann M. VSIG4, a B7 family-related protein, is a negative regulator of T cell activation. J Clin Invest. 2006;116:2817–2826. doi: 10.1172/JCI25673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang I, Tihan T, Han S, Wrensch M, Wiencke J, Sughrue M, Parsa A. CD8+ T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. J Clin Neurosci. 2010;17:1381–1385. doi: 10.1016/j.jocn.2010.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pio R, Corrales L, Lambris J. The role of complement in tumor growth. In: Koumenis C, Hammond E, Giaccia A, editors. Tumor Microenvironment and Cellular Stress. New York: Springer New York; 2014. pp. 229–262. [Google Scholar]
- 28.Nozaki M, Raisler B, Sakurai E, Sarma J, Barnum S, Lambris J, Chen Y, Zhang K, Ambati B, Baffi J, Ambati J. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]


