Abstract
Soft tissue sarcoma (STS) is a heterogenous tumor arising from the embryonic mesoderm represented by approximately 50 histological subtypes. Effective therapeutic intervention is lacking for recurrent, late stage and metastatic disease. CD39, a cell-surface ectonucleotidase, has previously been shown to be upregulated in hematological malignancies and various epithelial tumors, but not in STS. Here, we show by mass spectrometry and immunohistochemistry that CD39 is highly expressed in primary patient sarcoma samples. Moreover, CD39 nucleotidase activity is enhanced in fibrosarcoma compared with normal control cells. We demonstrate that an inhibitory monoclonal anti-CD39 antibody, abrogates CD39 enzymatic activity significantly and prolongs survival in a lethal metastatic patient-derived sarcoma model. Taken together, the data suggest CD39 is a novel therapeutic target for the treatment of STS.
Keywords: Soft tissue sarcoma, CD39, monoclonal antibody, mass spectrometry, immunohistochemistry, in vivo efficacy
Introduction
Soft tissue sarcoma (STS) is a rare tumor group that comprises 1% of adult cancers in the US, with 11,000 new cases per year [1]. STS is primarily treated by surgical resection/radiotherapy with or without systemic chemotherapy. However, systemic chemotherapy provides an overall response rate of only 25% and the relapse rate is high [2]. Chemotherapy remains the current standard of care, as other modalities, such as therapeutic antibodies have not shown clinical efficacy in STS [3]. The poor prognosis of STS suggests that the standard of care is insufficient, and advocates for the identification of new targets to enable the development of more effective therapies.
Adenosine triphosphate (ATP) mediates a variety of biological functions including mounting an efficient immune response required for successful anticancer therapy [4,5]. The balance between ATP and its derivatives is regulated in tissues by ectonucleotidases CD39 and CD73, which are broadly expressed on the cell surface of mammalian cells [6]. CD39 is an ectonucleoside triphosphate diphosphohydrolase, type I membrane protein, which hydrolyzes extracellular ATP and/or adenosine diphosphate (ADP), which is the rate-limiting step in ATP hydrolysis [7]. Ecto-5’-nucleotidase CD73 then converts adenosine monophosphate (AMP) to adenosine, which has been identified as a universal and potent immune suppressor through its interaction with the adenosine A2A receptor on T-cells [8,9]. Upregulation of CD39 has been reported in a number of epithelial and hematological malignancies and its expression in chronic lymphocytic leukemia has been shown to correlate with poor prognosis [10-12]. Moreover, CD39 is highly expressed on regulatory T-cells (Tregs) and is required for their suppressive function as demonstrated with impaired suppressive activity of Tregs in CD39-null mice [13]. Thus, CD39 may help drive tumorigenesis by its enhanced enzymatic activity either on Tregs, tumor-associated stroma or on malignant epithelial cells, resulting in adenosine-mediated immunosuppression of anti-tumor T- and natural killer (NK) cells as well as neutralization of ATP-induced cell death by chemotherapy [11,12,14]. Modulation of the immunosuppressive CD39/CD73-adenosine pathway has been suggested as a promising immunotherapeutic strategy for cancer therapy [15]. For example, polyoxymetalates (POM), which broadly inhibit ectonucleotidases, have been proposed as chemotherapeutic agents against colon, lung and breast cancer [16,17]. Ohta et al. demonstrated that A2A-receptor-deficient mice rejected established tumors [9]. Inhibitory CD39 and CD73 antibodies have also been reported, but their characteristics were only elucidated in the context of abrogating tumor cell-mediated immunosuppression in vitro, not in the context of inhibiting CD39 expressed by sarcoma cells [12,18].
In this study we show high CD39 expression in a subset of 150 human sarcoma tumors, describe the anti-tumor effect of an inhibitory human specific anti-CD39 antibody in a metastatic patient-derived sarcoma model and propose CD39 as a novel target for the treatment of sarcoma.
Materials and methods
General materials
Recombinant His-tagged human CD39 fusion protein was purchased from Sinobiological (Beijing, China). Antibodies and dyes used in flow cytometry, immunohistochemistry or non-invasive imaging were from Sigma Aldrich (St. Louis, MO), Dianova (Hamburg, Germany), Molecular Probes (Eugene, OR) or Perkin Elmer (Waltham, MA) respectively. ATP and sodium metatungstate (POM-1) were from Tocris (Bristol, United Kingdom). The formalin-fixed paraffin-embedded sarcoma tissue microarrays were obtained from US Biomax (#SO801, #SO802, #FDA808b1-2; Rockville, MD) and Pantomics Inc. (#SFT1021, #SFT961; Richmond, CA). Murine IgG2a antibody (clone HB-121) served as an isotype control (ATCC, Manassas, VA). Human platelets were obtained from All cells (Alameda, CA). The Cooperative Human Tissue Network (CHTN) and the National Disease Research Interchange provided primary tumor tissue samples, respectively. CHTN is funded by the National Cancer Institute.
Surface antigen labeling and liquid chromatography/mass spectrometry (LC-MS) analysis
Specimens comprising tumor biopsy and matched normal adjacent tissue were received fresh in the laboratory within 6-24 hours of sample collection. Upon receipt, specimens were surface labeled and membrane-associated protein fractions isolated using methods as previously described [19].
Eluted proteins were subjected to overnight acetone precipitation at -20°C, resuspended, digested with trypsin and purified using C18 Zip-Tips (EMD Millipore, Billerica, MA). Tryptic peptides were loaded onto pulled-tip fused silica C18 nanospray columns and separated by reverse-phase gradient for 220 min using a nanoscale liquid chromatography system EASY-nLC tandem-coupled to a LTQ Orbitrap Velos Pro hybrid mass spectrometer (Thermo Fisher Scientific).
The resulting data were searched against the Uniprot human FASTA database using the SEQUEST algorithm executed on the Sorcerer platform (Sagen N Research; Milpitas, CA). The relative quantitative levels of identified proteins were determined using the spectral counting method [20].
Antibody generation
Monoclonal antibodies against CD39 were generated through immunizing mice with murine sarcoma cells expressing human CD39. Splenocytes from immunized mice were used for hybridoma generation as previously described [21].
Immunohistochemistry
Slides were deparaffinized, rehydrated and heat-induced antigen retrieval was performed (EDTA pH 9) prior to blocking and incubating with the rabbit anti-human CD39 polyclonal antibody (Sigma; #HPA014067; 1:750 dilution). CD39 expression was assessed by manually scoring intensity, location and cell types. The strength of CD39 staining was scored as negative (0), moderate (+1) or strong (+2-3).
Cryostat sections from the IGN-SRC-004 PDX model were double stained for both human CD39 (Igenica mouse anti-human CD39 monoclonal antibody at 5 μg/ml (clone#165C)) and mouse CD31 (rat anti-mouse CD31 monoclonal antibody (Dianova; #DIA-310; 1:50 dilution) as outlined by Vector (Vector Laboratories Inc., Burlingame, CA).
Flow cytometry (FC)
Flow cytometric data were acquired using a MACSQuant Analyzer 10 cytometer (Miltenyi Biotec, Cologne, Germany) operated by MACSQuantify software and at least 10,000 viable events per fluorochrome were collected per sample. Data was analyzed using FlowJo software (Version 10.0.7, Tree Star, Ashland, OR).
Platelet aggregation assay and quantitative copy-number analysis by FC
Platelet aggregation was evaluated by FC in primary peripheral blood samples as described previously [22]. Briefly, purified platelets stained with either 0.3 μM CFSE (Molecular Probes) or 2 μM PKH26 (Sigma) were combined at a 1:1 ratio. Aliquots were pre-incubated with 2 μM recombinant human CD39 and 1 mM ATP, followed by 4 μM 9-8B, 10 μM POM-1 or isotype control antibody before analysis by FC.
Quantitative flow analysis of CD39 on cells was performed as previously described and according to the manufacturer’s protocol (Bangs Laboratories, Fishers, IN) [23].
Radioactive ATPase activity assay
Ectonucleotidase activity of CD39 was assessed as previously described [24]. Briefly, cells were washed with PBS, resuspended in serum-free RPMI 1640 supplemented with 25 mM Tris-HCl, pH 8.0 (RPMI-Tris medium), combined with BSA, test or control antibody solution in Tris-CaCl2 reaction buffer and incubated at RT for 20 min. ATP reaction mixture (0.2 μCi [-33P]-ATP, 0.2 mM ATP) was added and incubated for 30 min at 37°C. Reactions were stopped by the addition of charcoal stop mix, placed on ice and centrifuged. Supernatant was placed into scintillation tubes and 33Pi was determined by liquid scintillation counting. Non-enzymatic [-33P]-ATP hydrolysis was determined in parallel reactions, where cell suspension was replaced with RPMI-Tris medium only.
Xenograft transplantation and non-invasive imaging experiments
Patient-derived tumor tissue was passaged in vivo as described previously [25]. IGN-SRC-004 is a proprietary patient-derived sarcoma tumor xenograft line that was established at Igenica Biotherapeutics. Immunocompromised female NOD-scidIL-2R γnull (NOG) mice were used for the establishment of IGN-SRC-004 tumor xenografts (Taconic, Hudson, NY). Mice were subcutaneously injected on the right flank with 5 × 106 IGN-SRC-004 cells. Once the tumor reached a size between 65-200 mm3, mice were randomized to treatment. Antibodies were administered weekly. A representative experiment was performed on 38 animals per treatment arm post-randomization.
For non-invasive imaging IGN-SRC-004 was implanted subcutaneously into the lower right flank of a NOG mouse. The tumor-bearing animal was then injected intravenously with 10 nM 2-deoxyglucose-750 probe (Perkin Elmer) and 1 nM of Alexa Fluor 647-conjugated human CD39-specific mouse monoclonal antibody (Igenica; #5-13A). Images were acquired 24 hr post-injection using IVIS Spectrum 3D (Perkin Elmer) and spectral unmixing tools were applied to acquired images. Animal experiments were performed in accordance with protocols approved by the Igenica Biotherapeutics Institutional Review Board-Animal Care and Use Committee.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). Group means were compared using Student’s 2-tailed, unpaired t-test or Mantel-Cox-test. Probability (P) values of < 0.05 were interpreted as significantly different, and not adjusted for multiple comparisons. All statistical analyses were performed using Microsoft EXCEL (Microsoft, Redmond, WA) and GraphPad Prism v.5.0f (GraphPad Software, Inc., La Jolla, CA).
Results and discussion
CD39 expression in sarcoma
Overexpression of CD39 has recently been described in a wide variety of human cancers using immunohistochemistry (IHC) [12]. In that report, CD39 was overexpressed in kidney, lung, pancreatic, thyroid and testicular tumors, as well as melanoma and different types of lymphoma. Although tumor stroma stained positive for CD39, the IHC data suggested that STS were CD39-negative, most likely because the TMA did not cover the heterogeneity of more than 50 histopathological subtypes of STS [26].
We were interested in evaluating CD39 expression in primary sarcomas using a mass spectrometry-based approach. The method, adapted for evaluation of primary tissue specimens, employs freshly isolated primary tumors and normal adjacent tissues as source material for cell surface protein profiling. Raw spectral counts were summed across technical replicates. We found 42 spectral counts assigned to CD39 in the 9 sarcoma samples and 0 spectral counts in the corresponding normal adjacent tissues, indicating selective expression in sarcoma (Figure 1A).
Figure 1.
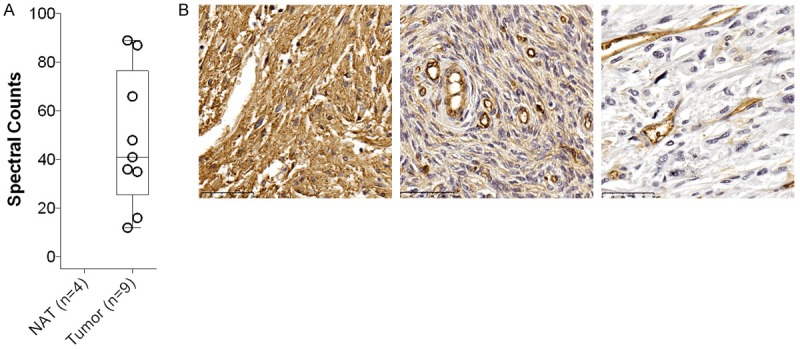
Overexpression of CD39 in Sarcoma. A. Mass-spectrometric analysis identifies CD39 expression in primary sarcoma samples, but not in non-involved adjacent tissue. B. Representative staining for CD39 on STS. Fibrosarcoma in the peritoneum with IHC score +3 (left panel), fibrosarcoma in the abdominal wall with IHC score +1 (middle panel), CD39-negative normal stroma (right panel). Please note that vasculature is CD39 positive and represents an internal control for all tissue sections. NAT, non-involved adjacent tissue; Scale bar = 50 microns.
The proteomic evaluation was subsequently corroborated by immunohistochemistry using a specific polyclonal anti-CD39 antibody and soft tissue sarcoma tumor microarrays (TMA). These sarcoma TMAs comprising 141 readable tissue cores of liposarcoma, fibrosarcoma, dermatofibrosarcoma, leiomyosarcoma and non-malignant stroma were used to assess membranous staining intensity for CD39 (Table 1; Figure 1B). Only cores that showed a high percentage of tumor or normal stroma were analyzed. CD39 staining was moderate to strong (IHC score +2/3) in 25% (Figure 1B, left panel), weakly positive (IHC score +1) in 30% (Figure 1B, middle panel) and negative in 45% of all sarcomas combined. In contrast only 24% of non-malignant stroma was weakly positive for CD39 and 76% was negative (Figure 1B, right panel). Taken together, the proteomic and immunohistochemical expression analysis both show high expression of CD39 in various STS, but not in non-malignant stroma.
Table 1.
IHC score of CD39 in various soft tissue sarcomas and normal stroma
| Tissue | N | IHC Score [%] | ||
|---|---|---|---|---|
|
| ||||
| Negative | +1 | +2-3 | ||
| Normal stroma | 42 | 76 | 24 | 0 |
| Liposarcoma | 46 | 67 | 22 | 11 |
| Fibrosarcoma | 40 | 45 | 35 | 20 |
| Dermatofibrosarcoma | 40 | 35 | 37.5 | 27.5 |
| Leiomyosarcoma | 15 | 33 | 27 | 40 |
| 141* | 45# | 30# | 25# | |
Total number of analyzed STS cores.
Mean IHC score % in analyzed STS cores.
Antibody-mediated inhibition of enzymatic activity of CD39
It has been shown that inhibition of nucleotidase activity of CD39 on tumor cells diminishes their immunosuppressive characteristics [12,18]. We generated antibodies against human CD39 and identified the inhibitory mouse monoclonal anti-CD39 IgG2a antibody 9-8B. The antibody possesses a KD of 3.1 nM against human CD39 and does not crossreact with its mouse ortholog (data not shown). Its enzymatic inhibitory function was determined with a flow-based platelet aggregation method and a highly-sensitive radioactive CD39 cell-based assay (Figure 2). The broad ectonucleotidase inhibitor, POM-1, was used as a positive control [27]. ADP-mediated platelet aggregation was induced by addition of ATP to enzymatically-active recombinant human CD39. ADP-induced platelet aggregation was increased 1.6 fold compared with untreated cells, but significantly inhibited by 67% in the presence of either 9-8B or POM-1 (both p < 0.001) (Figure 2A). We then measured the nucleotidase activity of endogenously-expressed CD39 on viable primary sarcoma cells in the absence or presence of 9-8B. As a model system we used IGN-SRC-004, a stage 3 (T2b, N1, M0), grade 2 recurrent fibrosarcoma derived from a patient who had previously received chemotherapy. First, CD39 copy-number was determined by flow cytometry using 9-8B as the detection antibody on IGN-SRC-004. Human umbilical vascular endothelial cells (HUVECs) were used as a positive control [14]. IGN-SRC-004 and HUVECs expressed 201851 ± 19475 and 16552 ± 6930 CD39 receptors per cell, respectively, confirming that CD39 expression is high in sarcoma and moderate on endothelial cells. Additionally, IGN-SRC-004 exhibited 6.3-fold higher ATPase activity than HUVECs (28.3 ± 0.3 vs 4.5 ± 1.1 pmol/min/103 cells; P < 0.001) when the molar quantities of hydrolyzed free phosphate were normalized per 1000 cells. Importantly, 9-8B and POM-1 reduced ATPase activity by 37% and 83%, respectively (17.9 ± 0.9 vs 4.9 ± 1.4 pmol/min/103 cells; P < 0.001) (Figure 2B). Both assays demonstrated that anti-CD39 antibody 9-8B specifically inhibits the nucleotidase activity of CD39.
Figure 2.
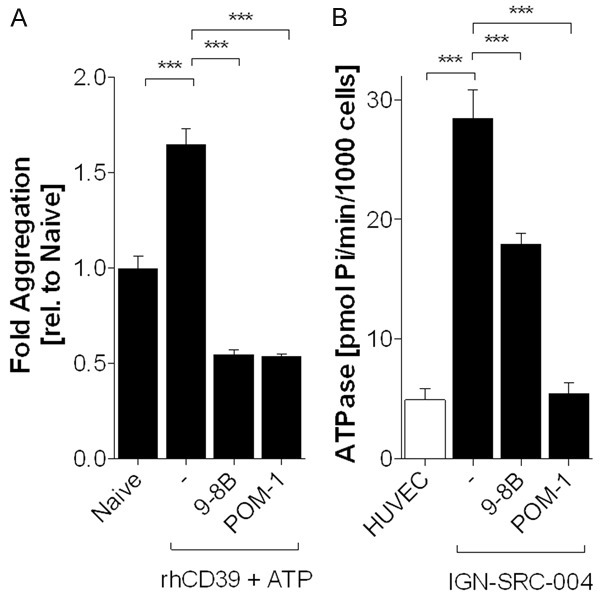
Monoclonal Antibody 9-8B Inhibits Enzymatic Activity of CD39. A. Flow-cytometric analysis shows inhibition of ADP-induced platelet aggregation by 9-8B (4 μM) and POM-1 (10 μM). Naïve; untreated platelets, -; rhCD39 (2 μM)+ ATP (1 mM) treated platelets. B. Orthogonal radioactive CD39 assay demonstrates inhibition of ATPase activity on IGN-SRC-004 cells by 9-8B (6 nM) and POM-1 (10 μM). Ectonucleotidase activity is expressed as picomolar of hydrolyzed free 33Pi per minute per 1000 cells. ***P < 0.001.
Antibody-mediated anti-tumor activity in vivo
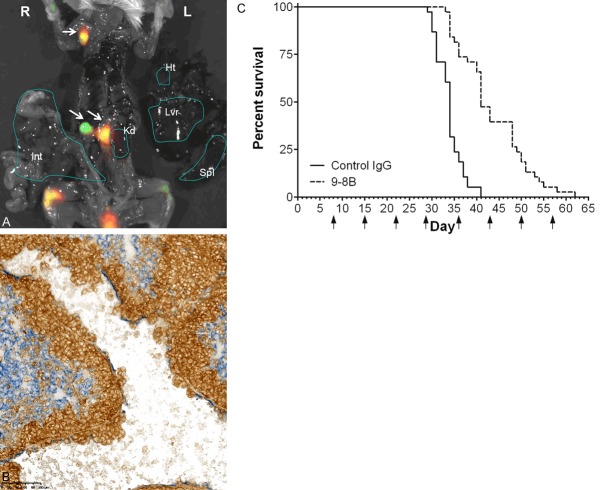
Patient-derived xenograft (PDX) models have been shown to represent the complex clinical tumor heterogeneity and molecular diversity of human cancer better than cell line-derived xenograft models [28]. To generate a STS PDX model, we first implanted IGN-SRC-004 into NOD-scidIL-2R γnull (NOG) mice. We discovered that IGN-SRC-004 is very aggressive, as disseminated cells could be detected in the lymph nodes as early as 21days post implantation (Figure 3A). After 30 days IGN-SRC-004 tumor cells had metastasized to the spleen, kidneys, liver and lungs (Figure 3B). Next, we tested if CD39 inhibition by 9-8B would increase survival of animals. Note that because NOG mice are T-, B- and NK cell deficient and macrophages are non-functional, antibody-dependent cell-mediated cytotoxicity (ADCC) can be ruled out as a possible mechanism of action for 9-8B in this model [29]. Established tumors in NOG mice were treated once weekly at 15 mg/kg with 9-8B or isotype-matched control antibody and mice were euthanized when moribund. We observed that 47% of 9-8B treated animals were alive on day 41, while 100% of the animals in the control group were moribund. The survival of the 9-8B treatment group was increased by 21 days (62 days vs 41 days; P < 0.0001) (Figure 3C). Taken together, treatment with the CD39-specific antibody 9-8B significantly improved survival in this metastatic patient-derived sarcoma model.
Figure 3.
Monoclonal Antibody 9-8B Increases Survival of Metastatic Lethal Patient-derived Sarcoma Xenograft Model IGN-SRC-004. Spontanous metastasis of IGN-SRC-004 to (A) lymph nodes after 21 days and (B) lungs after 30 days. (A) Human fibrosarcoma cells are detected in the lymph nodes using AF647-conjugated anti-human CD39 antibody 5-13 (green). Uptake of 2-deoxyglucose indicates metabolically active sites (red). Overlay (yellow) depicts metabolically active human sarcoma cells. White arrows indicate IGN-SRC-004 positive lymph nodes. L; Left, R; Right, Ht; Heart, Lvr; Liver, Kd; Kidney, Spl; Spleen, Int; Intestine. (B) Double-stained immunohistochemistry for human CD39 (brown) and mouse blood vessel marker CD31 (blue) reveals that human sarcoma cells have breached the lung vasculature. Breakdown of the mouse vascular endothelial wall is also observed. Scale bar = 100 microns. (C) Kaplan-Meier analysis of mice implanted with metastatic IGN-SRC-004. Mice with established tumors of 122 mm3 ± 21 mm3 were randomized and treated at 15 mg/kg with either 9-8B or isotype control antibody (n = 38/treatment). Mantel-Cox p < 0.0001 relative to control. Arrow; administration of antibody.
Overexpression of CD39 on hematological and epithelial malignancies, as well as its immunosuppression in the oncology setting makes it a viable and attractive target. Consequently, the CD39/CD73-adenosine pathway has evolved into an attractive strategy for cancer therapy [15]. The in vivo proof-of-concept study described here is complementary to previous publications that described in vitro enhanced tumoricidal activity of anti-CD39 antibody-treated immune cells [12,18]. Antibody-mediated CD39 intervention could not only alleviate tumor-induced immunosuppression, but also exert direct tumor cell killing. The therapeutic efficacy of an inhibitory anti-CD39 antibody could potentially be enhanced through effector functions, such as ADCC.
We demonstrated that an anti-CD39 antibody was effective in a fibrosarcoma PDX model. Future studies are warranted to evaluate efficacy in other STS models overexpressing CD39, such as leiomyosarcoma.
Here we identified expression of CD39 in STS employing a mass-spectrometric approach, and confirmed it through immunohistochemistry on a larger set of independent STS samples. The data suggest that STS could be added to CD39-overexpressing cancer types, broadening the indication potential for CD39 intervention and providing a novel target for STS treatment.
Acknowledgements
We would like to thank Joseph Zachwieja and Steven Gomez for their help generating anti-CD39 antibodies; and Jason Damiano, William Ho and Sally Bolmer for their useful comments on this manuscript.
Disclosure of conflict of interest
B.C., L.C., Z.L., J-W.T. and E.H.vdH are employees of Igenica Biotherapeutics.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Linch M, Miah AB, Thway K, Judson IR, Benson C. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat Rev Clin Oncol. 2014;11:187–202. doi: 10.1038/nrclinonc.2014.26. [DOI] [PubMed] [Google Scholar]
- 3.Juergens H, Daw NC, Geoerger B, Ferrari S, Villarroel M, Aerts I, Whelan J, Dirksen U, Hixon ML, Yin D, Wang T, Green S, Paccagnella L, Gualberto A. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J. Clin. Oncol. 2011;29:4534–4540. doi: 10.1200/JCO.2010.33.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 5.Shabbir M, Burnstock G. Purinergic receptor-mediated effects of adenosine 5’-triphosphate in urological malignant diseases. Int J Urol. 2009;16:143–150. doi: 10.1111/j.1442-2042.2008.02207.x. [DOI] [PubMed] [Google Scholar]
- 6.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Wang TF, Guidotti G. CD39 is an ecto-(Ca2+, Mg2+)-apyrase. J Biol Chem. 1996;271:9898–9901. [PubMed] [Google Scholar]
- 8.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 9.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulte D, Furman RR, Broekman MJ, Drosopoulos JH, Ballard HS, Olson KE, Kizer JR, Marcus AJ. CD39 expression on T lymphocytes correlates with severity of disease in patients with chronic lymphocytic leukemia. Clin Lymphoma Myeloma Leuk. 2011;11:367–372. doi: 10.1016/j.clml.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene. 2013;32:1743–1751. doi: 10.1038/onc.2012.269. [DOI] [PubMed] [Google Scholar]
- 12.Bastid J, Regairaz A, Bonnefoy N, Dejou C, Giustiniani J, Laheurte C, Cochaud S, Laprevotte E, Funck-Brentano E, Hemon P, Gros L, Bec N, Larroque C, Alberici G, Bensussan A, Eliaou JF. Inhibition of CD39 Enzymatic Function at the Surface of Tumor Cells Alleviates Their Immunosuppressive Activity. Cancer Immunol Res. 2015;3:254–265. doi: 10.1158/2326-6066.CIR-14-0018. [DOI] [PubMed] [Google Scholar]
- 13.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng L, Sun X, Csizmadia E, Han L, Bian S, Murakami T, Wang X, Robson SC, Wu Y. Vascular CD39/ENTPD1 directly promotes tumor cell growth by scavenging extracellular adenosine triphosphate. Neoplasia. 2011;13:206–216. doi: 10.1593/neo.101332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sitkovsky MV, Hatfield S, Abbott R, Belikoff B, Lukashev D, Ohta A. Hostile, hypoxia-A2-adenosinergic tumor biology as the next barrier to overcome for tumor immunologists. Cancer Immunol Res. 2014;2:598–605. doi: 10.1158/2326-6066.CIR-14-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhule JT, Hill CL, Judd DA, Schinazi RF. Polyoxometalates in Medicine. Chem Rev. 1998;98:327–358. doi: 10.1021/cr960396q. [DOI] [PubMed] [Google Scholar]
- 17.Hasenknopf B. Polyoxometalates: introduction to a class of inorganic compounds and their biomedical applications. Front Biosci. 2005;10:275–287. doi: 10.2741/1527. [DOI] [PubMed] [Google Scholar]
- 18.Hausler SF, Del Barrio IM, Diessner J, Stein RG, Strohschein J, Honig A, Dietl J, Wischhusen J. Anti-CD39 and anti-CD73 antibodies A1 and 7G2 improve targeted therapy in ovarian cancer by blocking adenosine-dependent immune evasion. Am J Transl Res. 2014;6:129–139. [PMC free article] [PubMed] [Google Scholar]
- 19.Weekes MP, Antrobus R, Lill JR, Duncan LM, Hor S, Lehner PJ. Comparative analysis of techniques to purify plasma membrane proteins. J Biomol Tech. 2010;21:108–115. [PMC free article] [PubMed] [Google Scholar]
- 20.Neilson KA, Ali NA, Muralidharan S, Mirzaei M, Mariani M, Assadourian G, Lee A, van Sluyter SC, Haynes PA. Less label, more free: approaches in label-free quantitative mass spectrometry. Proteomics. 2011;11:535–553. doi: 10.1002/pmic.201000553. [DOI] [PubMed] [Google Scholar]
- 21.Liao-Chan S, Zachwieja J, Gomez S, Duey D, Lippincott J, Theunissen JW. Monoclonal antibody binding-site diversity assessment with a cell-based clustering assay. J Immunol Methods. 2014;405:1–14. doi: 10.1016/j.jim.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 22.De Cuyper IM, Meinders M, van de Vijver E, de Korte D, Porcelijn L, de Haas M, Eble JA, Seeger K, Rutella S, Pagliara D, Kuijpers TW, Verhoeven AJ, van den Berg TK, Gutierrez L. A novel flow cytometry-based platelet aggregation assay. Blood. 2013;121:e70–80. doi: 10.1182/blood-2012-06-437723. [DOI] [PubMed] [Google Scholar]
- 23.D’Hautcourt JL. Quantitative flow cytometric analysis of membrane antigen expression. Curr Protoc Cytom. 2002 doi: 10.1002/0471142956.cy0612s22. Chapter 6:Unit 6.12. [DOI] [PubMed] [Google Scholar]
- 24.Pinto CS, Jinnah HA, Shirley TL, Nyhan WL, Seifert R. Altered membrane NTPase activity in Lesch-Nyhan disease fibroblasts: comparison with HPRT knockout mice and HPRT-deficient cell lines. J Neurochem. 2005;93:1579–1586. doi: 10.1111/j.1471-4159.2005.03151.x. [DOI] [PubMed] [Google Scholar]
- 25.van der Horst EH, Chinn L, Wang M, Velilla T, Tran H, Madrona Y, Lam A, Ji M, Hoey TC, Sato AK. Discovery of fully human anti-MET monoclonal antibodies with antitumor activity against colon cancer tumor models in vivo. Neoplasia. 2009;11:355–364. doi: 10.1593/neo.81536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95–104. doi: 10.1097/PAT.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 27.Muller CE, Iqbal J, Baqi Y, Zimmermann H, Rollich A, Stephan H. Polyoxometalates--a new class of potent ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) inhibitors. Bioorg Med Chem Lett. 2006;16:5943–5947. doi: 10.1016/j.bmcl.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Maelandsmo GM, Roman-Roman S, Seoane J, Trusolino L, Villanueva A. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4:998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c) (null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]