Abstract
Muscular dystrophy (MD) refers to a clinically and genetically heterogeneous group of degenerative muscle disorders characterized by progressive muscle wasting and often premature death. Although the primary defect underlying most forms of MD typically results from a loss of sarcolemmal integrity, the secondary molecular mechanisms leading to muscle degeneration and myofiber necrosis is debated. One hypothesis suggests that elevated or dysregulated cytosolic calcium is the common transducing event, resulting in myofiber necrosis in MD. Previous measurements of resting calcium levels in myofibers from dystrophic animal models or humans produced equivocal results. However, recent studies in genetically altered mouse models have largely solidified the calcium hypothesis of MD, such that models with artificially elevated calcium in skeletal muscle manifest fulminant dystrophic-like disease, whereas models with enhanced calcium clearance or inhibited calcium influx are resistant to myofiber death and MD. Here, we will review the field and the recent cadre of data from genetically altered mouse models, which we propose have collectively mostly proven the hypothesis that calcium is the primary effector of myofiber necrosis in MD. This new consensus on calcium should guide future selection of drugs to be evaluated in clinical trials as well as gene therapy-based approaches.
Facts
The primary myofiber death-inducing effect underlying muscular dystrophy (MD) is an unstable plasma membrane and an associated dysregulation in calcium handling or influx.
Genetic data in mice shows that unregulated cellular calcium entry alone is sufficient to induce myofiber death and MD.
Genetic data in mice shows that enhanced calcium clearance from the cytosol mitigates myofiber death and MD.
Genetic data in mice shows that making mitochondria insensitive to calcium overload reduces myofiber death and MD.
Open Questions
Is the calcium overload or dysregulation that occurs in MD primarily due to membrane ruptures or dysregulated ion channel and exchanger activity?
What intracellular domains of calcium dysregulation most directly couple to initiation of myofiber death in MD?
Given our recent consensus on calcium as the common mediator of myofiber death in MD, what calcium-affecting drugs might be best to attempt for use in human clinical trials?
MD is a disease of progressive muscle weakness and degeneration of myofibers caused by mutations in genes that often serve a structural role in stabilizing the plasma membrane of the myofibers (referred to as the sarcolemma). Duchenne MD (DMD) is an X-linked recessive genetic disease that is the most common form of MD in humans with an occurrence of ~1 in 3500 males.1 Dystrophin, the protein encoded by the gene mutated in DMD, functions in stabilizing the sarcolemma, as do a host of other gene products that when mutated result in limb-girdle MDs, congenital MDs, and various myopathies.2 Loss of select sarcolemmal structural gene products or even gene products involved in membrane repair, such as dysferlin, lead to membrane instability and a hypothesized influx of calcium that serves as the final common pathway leading to myofiber necrosis and muscle degeneration.3 However, this model of pathogenesis with calcium serving as the central transducer of myofiber death has remained a hypothesis, and although many biochemical lines of evidence support this hypothesis, it was not until the past few years that the use of mouse genetics allowed for a more definitive analysis of this ‘calcium hypothesis'.
The concept that membrane instability could lead to calcium overload, mitochondrial dysfunction, and ultimately the necrosis of myofibers predates the discovery of dystrophin. This calcium hypothesis was originally proposed as a final common pathway for multiple neuromuscular diseases in 1976 by Wrogemann, which remains remarkably accurate and an impressive deduction given the limited data available at the time.4 Here, we will review the body of evidence that we believe has solidified the concept that calcium serves as the common intracellular transducer of myofiber necrosis in most forms of MD, with a special emphasis placed on data derived from recent genetic studies in the mouse.
Excitation Contraction-Coupling
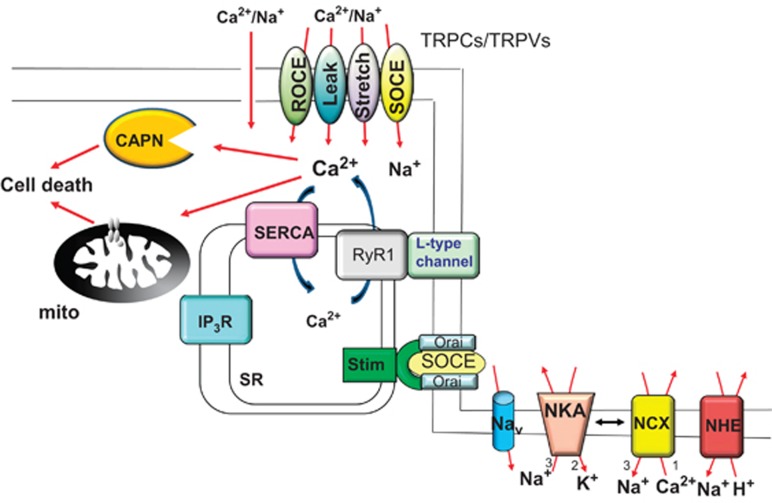
The process of muscle contraction is initiated by acetylcholine binding to the acetylcholine receptor in motor neurons at the end plates, leading to the opening of voltage-gated sodium channels across the sarcolemma and down the t-tubules into the myofibers. The wave of depolarization leads to a conformational change in the L-type calcium channel and a direct gating of the ryanodine receptor (RyR) within the sarcoplasmic reticulum (SR), allowing for a very large release of calcium causing muscle contraction. Muscle relaxation occurs as the SR calcium-ATPase (SERCA) pumps calcium from the cytoplasm back into the SR (Figure 1).
Figure 1.
Schematic of the calcium handling proteins and downstream calcium-regulated effectors that are involved in calcium dysregulation in MD, leading to myofiber necrosis. Elevations in resting calcium has been associated with increased store-operated calcium entry (SOCE), increased stretch-activated calcium entry, increased calcium leak, and increased receptor-operated calcium entry (ROCE), attributed to the activity of transient receptor potential canonical (TRPC) and vanilloid (TRPV) family members, as well as by Stim and Orai family member proteins that can directly generate a store-operated calcium entry event. The L-type calcium channel might also be responsible for some content of pathologic calcium influx, as well as leak from the RyR1 in dystrophic skeletal muscle. In addition to elevations in calcium, sodium is increased in the cytosol of dystrophic myofibers owing to increased activity of TRPC channels, sodium channels (Nav), or possibly in conjunction with less effective sodium extrusion by the sodium–potassium ATPase (NKA) pump. Elevated intracellular sodium can secondarily increase resting calcium levels by causing reverse-mode calcium influx through the sodium–calcium exchanger (NCX) as well as by altering NHE1 activity. Sarcoplasmic reticulum (SR) calcium reuptake is also reduced in MD with decreased function of the SERCA pump. Finally, pathologic calcium may also arise owing to increased IP3R activity. In response to this pathologic profile of elevated intracellular calcium, the mitochondria (mito) can swell and rupture owing to MPTP activation, and intracellular proteins can be degraded by the calpains (CAPN)
Alterations in excitation contraction-coupling have been observed in MD. Indeed, muscle weakness is a hallmark of DMD, with a slowing in relaxation that suggests a defect in SR-calcium reuptake.5, 6 Interestingly, although the mothers of boys with DMD that only contain one functional dystrophin gene do not typically show muscle weakness, their muscles do relax slower than normal controls.7 These early studies of muscle physiology in boys with DMD and their mothers provided the first evidence that there may be a deficit in calcium handling in muscular dystrophies, but it was not until the discovery of the mdx mouse that calcium handling could be more thoroughly dissected.
Like boys with DMD, the mdx mouse model of MD has a loss-of-function mutation in dystrophin. Although the mdx mouse only has a modest 10–20% deficit in specific force generation in the hindlimb musculature, it has a much more severe deficit in relaxation that is suggestive of a major problem in calcium reuptake by the SR.8, 9, 10 Thus, a deficit in relaxation appears to be an evolutionarily conserved aspect of MD that is prominent even in the mildly pathologic mdx mouse.11, 12 Such a defect in relaxation is predicted to result in prolonged elevations in cytosolic calcium under continuous contractile activity.
Initial studies with fluorescent calcium-indicator dyes reported that excitation contraction-coupling was unchanged in myofibers from mdx mice compared with wild-type controls.13 However, subsequent studies consistently observed that the decay phase of the calcium transient was prolonged in mdx muscle fibers, consistent with the profile of delayed relaxation observed in intact muscle.14, 15 The mechanism of slowed reuptake appears to be due to decreased SERCA activity, which has been observed in microsomes from boys with DMD, Sgcd−/−mice (mouse model of limb-girdle MD due to loss of δ-sarcoglycan gene, which similarly disrupts the dystrophin-glycoprotein complex similar to that observed in mdx mice with the loss of dystrophin) and dy2j/dy2j mice that have a mutation in Lama2.15, 16, 17 The slowed reuptake across a diversity of dystrophic models suggests that decreased SERCA function may be a generalizable feature of many of the muscular dystrophies.
More recent studies utilizing low-affinity calcium-indicator dyes that more faithfully measure the calcium transient, along with computer modeling to estimate calcium release, have found that calcium release is slower in mdx fibers.18 In addition to deficits in the velocity of calcium release, the localization of calcium release is also changed in mdx muscle fibers in a more diffuse pattern.19 This is interesting because dystrophin localizes to the sarcolemma junction with the SR at the triads, and thus may have a role in patterning calcium release.20 Deficits in the patterning of calcium release are likely to expose greater subcellular regions of the muscle fiber to higher concentrations of calcium than would otherwise occur. This situation could expose mitochondria to higher calcium levels, and if sustained, could lead to mitochondrial swelling, rupture, and necrosis of the muscle fiber (this issue will be discussed in greater detail later).
Resting intracellular Calcium Concentration
Although muscle utilizes calcium in a highly specialized manner to regulate contraction and relaxation, multiple other calcium-sensitive intracellular regulatory processes still proceed and must be adequately regulated. One of these processes is opening of the mitochondrial permeability transition pore (MPTP) in response to calcium overload, which causes mitochondrial depolarization and eventual swelling and rupture of this organelle.21, 22 Calcium overload also promotes activation of the calcium-activated protease calpain, which has also been shown to contribute to the pathogenesis of MD.23, 24 These calcium-regulated degenerative processes are likely governed both by the amplitude and duration of calcium present in the cytosol, likely during contraction and at rest. Initial attempts to quantify resting intracellular calcium in dystrophin-deficient myofibers utilized biopsy specimens from boys with DMD.25, 26, 27 Three techniques available at the time were X-ray fluorescence, histochemical staining, and atomic absorption spectrophotometry, all of which showed higher resting calcium in muscle from boys with DMD.25, 26, 27 However, later studies conducted with the newly available fluorescent calcium-indicator dyes such as Fura-2 and Indo-1 produced equivocal results that partially ‘unseated' the calcium hypothesis (Table 1).13, 28, 29, 30 Although it is possible that resting calcium is truly elevated as identified in later studies with arguably more definitive technical approaches (see below), it is also possible that the key biologic effect underlying myofiber degeneration is due to defects in total calcium dynamics, such as rates of calcium release and reuptake, as well as subcellular domain-specific calcium elevations.
Table 1. Initial studies examining resting calcium in dystrophic muscle based on fluorescent dyes.
| Study | mdx [Ca2+] nM | WT [Ca2+] nM | Dye | Muscle | Isolation technique | Dye loading | Calibration parameters | Temperature |
|---|---|---|---|---|---|---|---|---|
| Turner (23) | 92±9.8 | 40±2.8 | Fura-2 tetracarboxylate | FDB | Mechanical dissection | Microinjection | Identical between mdx and WT | 37 °C |
| Turner (23) | 282±13 | 201±6 | Fura-2/AM | FDB | Mechanical dissection | Passive loading | Identical between mdx and WT | 37 °C |
| Gailly (24) | 123±12 | 125±9 | Fura-2/AM | Soleus | Collagenase digestion | Passive loading | Different between mdx and WT | 20 °C |
| Gailly (24) | 45.2±3 | 44.9±4 | Fura-2/AM | FDB | Collagenase digestion | Passive loading | Different between mdx and WT | 20 °C |
| Head (12) | 45.7+4.1 | 46.2±3.9 | Fura-2 tetracarboxylate | FDB | Collagenase digestion | Microinjection | No significant difference | 22 °C |
| Collet (25) | 48±7 | 56±5 | Indo-1 | FDB and interosseous | Collagenase digestion | Microinjection | No significant difference | 20–22 °C |
Abbreviations: FDB, flexor digitorum brevis; WT, wild-type; [Ca2+], calcium concentration. The initial study in the mdx mouse by Turner found a difference in basal intracellular calcium in myofibers between the mdx and the C57 mouse. They found this difference regardless of whether they used active or passive loading. Interestingly, this study was the only study to utilize mechanical dissection and the only study to find a statistically significant difference. Overall, technical challenges associated with photometric measurement of calcium, in conjunction with challenges associated with fiber isolation and selection bias, may explain the negative data that were also observed
The recent use of calcium-sensitive microelectrodes has supported the hypothesis of increased resting calcium in dystrophic myofibers, although this method of measurement is not without some limitations.31, 32, 33 For example, Altamirano et al.34 used calcium microelectrodes to show that resting intracellular calcium was increased to 308 nM±6 nM in mdx myotubes compared with 113 nM±2 nM in wild-type myotubes, and in vivo resting calcium was measured to be 315 nM±8 nM in mdx gastrocnemius versus 112 nM±2 nM in wild-type gastrocnemius.32 We also observed a threefold elevation in intracellular resting calcium in the gastrocnemius muscle from mdx mice using microelectrode technology.33 The caveats with using microelectrode technology are twofold. First, given the known weakness of the dystrophic membrane, a leak around the microelectrode may cause a spurious increase in the intracellular calcium that is recorded. Second, puncture of the muscle cell membrane is a form of cellular injury that could also alter calcium measurements. However, measurements of resting calcium in wild-type fibers with the microelectrode approach matches those values obtained with calcium-sensitive fluorescent dyes.
Another hypothesis is that selective calcium microdomains might be altered in dystrophic myofibers leading to disease. In 2001, Robert et al. used calcium sensing aequorin protein targeted to different intracellular locations. They showed that a subsarcolemmal aequorin protein detected increased calcium levels in mdx myotubes.35 Mallouk et al.36 used a calcium-activated potassium channel to detect increased subsarcolemmal calcium concentrations in mdx mice. A membrane localized calcium-sensitive dye, FFP-18, also showed significantly elevated levels of subsarcolemmal calcium in myofibers from mdx mice.37 The concept of microdomains of calcium is well-known in cardiovascular biology but further work is still required to understand its role in the pathogenesis of MD and the potential for therapeutic applications.38
Role of the L-type Calcium Channel
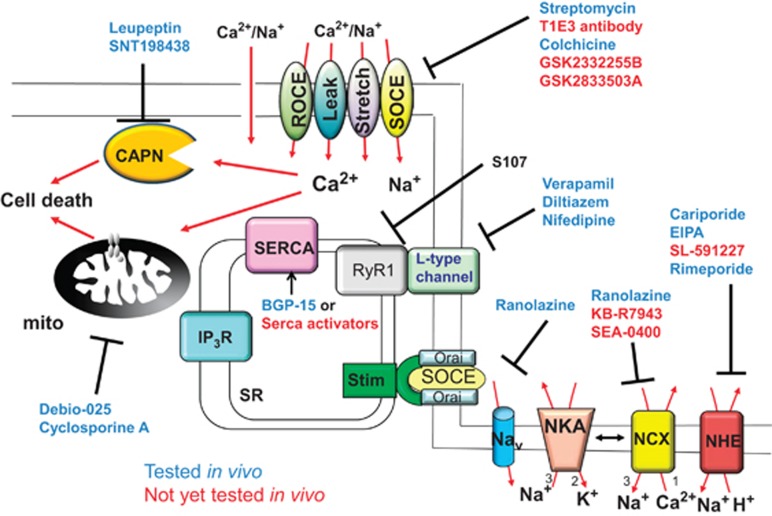
As discussed earlier, the L-type calcium channel (α1s subunit encodes the channel itself) is largely mechanically coupled to the RyR in skeletal muscle, without a requirement for external calcium to pass through the channel. Given this feature it would appear to be a relatively poor target for pharmacologic antagonism in possibly treating DMD in humans. Indeed, clinical trials undertaken with L-type calcium channel inhibitors including diltiazem, verapamil, nifedipine and flunarizine have produced mixed results (Figure 2).39, 40, 41, 42, 43 The study with verapamil reported a significant improvement in muscle strength but unfortunately this was also accompanied by cardiac side effects.43 A trial with diltiazem showed decreased deterioration of muscle from biopsies of the lower but not upper extremities, suggesting that under certain conditions there may be a small positive effect of these inhibitors.44 These mixed results are nonetheless encouraging given that even a theoretically poor target in the calcium handling pathway of skeletal muscle produced some clinical effect when inhibited.
Figure 2.
Schematic of the pharmacologic agents that have been or could be used to address a profile of elevated calcium in dystrophic muscle. Drugs previously tested in dystrophic mouse models are shown in blue text, whereas those that are more experimental are shown in red text
L-type calcium channel inhibitors have also been used in animal models of MD. In one study mdx mice were injected with saline, diltiazem, or verapamil for 18 days. The mice given either of the two calcium channel inhibitors showed decreased levels of circulating creatine kinase and decreased necrosis in the diaphragm.45 A more recent study observed that after 1 week of treatment of mdx mice with nifedipine, intracellular calcium was decreased and grip strength and swimming times were increased.32 Overall, these studies in mice and humans suggest that the small amount of calcium influx from the L-type channel may contribute to the pathogenesis of MD. L-type calcium channel inhibitors are interesting targets because of the fact that they are already clinically approved for human use (Figure 2).
SR Calcium Regulation in MD
As discussed earlier, myofibers from dystrophic mice or DMD patients show a defect in SR-calcium handling and reuptake during relaxation.11, 12, 13, 14, 15, 16, 17, 46 Indeed, we showed that myofibers from Sgcd−/− and mdx mice have significantly slower reuptake of calcium and that this defect can be corrected by overexpression of SERCA1 through transgenesis, leading to a marked lessening of myofiber necrosis and muscle wasting (Table 2).15 Furthermore, adeno-associated virus delivery of SERCA2 rescued pathology in the hindlimb of Sgcd−/− mice, and in a separate study, viral delivery of SERCA1a was shown to decrease pathology in the diaphragm of dystrophic mice.15, 47 Because the SERCA2 vector utilized is already in clinical trials for congestive heart failure,48 viral delivery of SERCA2 could be a clinical option in the future (Figure 2). Overall, the data obtained by SERCA overexpression are highly supportive of the calcium hypothesis of disease in MD, as they suggest that increasing the rate of calcium clearance during relaxation reduces myofiber necrosis. Indeed, the drug BGP-15 increased SERCA activity and reduced muscle pathology in mdx mice, resulting in greater muscle-specific force.49 Thus, in addition to gene therapy, the use of pharmacologic agents that increase SERCA expression or activity is also an interesting strategy to consider in the future.
Table 2. Summary of findings of genetic manipulations of calcium handling in transgenic used to investigate mechanisms of MD pathology.
| Genetic alteration | Year | Dystrophy model | Change in calcium handling | Change in phenotype |
|---|---|---|---|---|
| Sarcolemma | ||||
| dn TRPC6 transgenic81 | 2009 | mdx and Sgcd−/− | dnTRPC6 inhibited increased SOCE in Sgcd−/− fibers | dnTRPC6 TG reduced histopathology and serum CK |
| Trpc3 overexpression81 | 2009 | Trpc3 transgenic | Increased SOCE versus WT | TRPC3 TG caused dystrophy-like histopathology without membrane permeability |
| Adenoviral dnTRPV288 | 2008 | Bio14.6 hamster | Decreased calcium influx in high-calcium solution | dnTRVP2 decreased dystrophic histopathology |
| Transgenic dnTRPV288 | 2008 | mdx | Decreased calcium influx in high-calcium with 2-APB | dnTPV2 improved muscle function and decreased histopathology |
| Trpv2−/−89 | 2009 | mdx | Not evaluated | Trpv2−/− had increased force and decreased membrane permeability |
| Stim1 transgenic87 | 2014 | Stim1 transgenic | Stim1 overexpression increased SOCE and resting calcium | Stim1 TG led to severe dystrophy-like phenotype in muscle |
| dnOrai1 Tg87 | 2014 | mdx and Sgcd−/− | dnOrai inhibited increased SOCE in Sgcd−/− and mdx fibers | dnOrai TG decreased histopathology and CK release in muscle |
| NCX1 Tg33 | 2014 | mdx, Sgcd−/−, and Dysf−/− | NCX1 increased [Na]i and increased Na, Ca exchange | NCX1 TG worsened pathology in hindlimb but improved pathology in diaphragm |
| Slc8a1f/f with MLC-CRE33 | 2014 | Sgcd−/− | Not evaluated | Deletion of NCX1 protein improved histopathology at early time points |
| EC-coupling | ||||
| SERCA1 transgenic15 | 2011 | Sgcd−/− and mdx | SERCA1 increased rate of SR-calcium uptake | SERCA1 TG decreased histopathology and serum CK |
| SERCA1 transgenic15 | 2011 | TRPC3 | Not evaluated | SERCA1 TG rescued pathology mediated by TRPC3 overexpression. |
| AAV-SERCA215 | 2011 | mdx | Not evaluated | SERCA2a overexpression improved histopathology in gastrocnemius |
| AAV-SERCA147 | 2010 | mdx | Not evaluated | SERCA1 improved force after eccentric contraction and decreased histopathology |
| Mitochondrial | ||||
| Ppif−/−109 | 2008 | mdx, Sgcd−/− and Lama2 | Ppif deletion decreased mitochondrial swelling | Ppif−/− decreased histopathology in all MD models. Improved strength in Sgcd−/− |
| Ppif−/−110 | 2009 | Col6a1−/− | Ppif deletion decreased mitochondrial depolarization | Ppif−/− decreased histopathology and EBD uptake |
| Calpain | ||||
| Calpastatin transgenic23 | 2002 | mdx | Not evaluated | Calpastatin overexpression decreased histopathology and EBD uptake |
Modification of calcium release from the SR has also been investigated through genetic strategies. For example, calcium sparks can be readily observed in myofibers from dystrophic muscle, although they are typically never observed in wild-type myofibers.50 Sparks are attributed to unregulated opening of a group of RyRs, suggesting that in dystrophic muscle the RyR channels might be leaking calcium.50, 51 Mechanistically, calcium sparks are normally inhibited by the protein calstabin. In mdx muscle fibers, calstabin binds less avidly to the RyR leading to leak,52, 53 and restoring this interaction with the drug S107 (Figure 2)53 decreased MD disease in mdx and Sgcb−/− mice.54, 55 Thus, just as correction in calcium leak from the SR was sufficient to partially rescue dystrophic pathology, similar to how overexpression of SERCA might also be protective by better maintenance of resting calcium.
Although less studied than the RyR complex, calcium release from the SR via the inositol (1,4,5)-triphosphate receptor (IP3R) may also have an important role in the pathogenesis of MD.56, 57, 58 One study found that in dystrophin-deficient myotubes, IP3R activation events were downregulated following transfection with minidystrophin, suggesting activation of this receptor is a downstream consequence of dystrophin deficiency.59 As inhibition of calcium sparks is already known to associate with reduced dystrophic pathology, it is plausible that a strategy targeting IP3R signaling could also benefit dystrophic muscle.
Stretch and Store-Operated Calcium Entry
The first evidence for aberrant calcium entry through the sarcolemma of diseased skeletal muscle came in 1988 by Turner et al.60 working with mdx muscle fibers versus wild-type. Calcium currents were also observed to be elevated in mdx-diseased myotubes under conditions of mechanical stress.61 Previous studies have also observed that mdx muscle fibers are more sensitive to cell death due to osmotic stress than wild-type muscle fibers.62 Interestingly, calcium entry is also increased in muscle fibers from mdx mice under conditions of osmotic stress.14, 63, 64 In some of these studies, the observed current was inhibited by gadolidium and lanthanum, suggesting entry through channels of some sort.14, 63, 64 Finally, very large sodium currents also appear to be triggered by eccentric contraction, which could have implications for increased calcium influx due to sodium–calcium exchange dynamics.65
The activation of sodium and calcium entry by stretch provides a likely explanation for the damage and force decrement observed during eccentric contractions in mdx mice.65, 66 For example, muscle from wild-type mice show only a modest decrement in force after eccentric contractions, whereas muscle from mdx mice exhibits large deficits in force, as well as membrane instability and loss of intracellular enzymes.67, 68, 69 Both the elevation of sodium and calcium and the damage incurred by eccentric contraction can be inhibited by gadolidium and lanthanum.66, 70 Thus, in both intact muscles with eccentric stretch and in individual muscle fibers with osmotically mediated stress, calcium and sodium entry appear to be a primary mechanism that could directly lead to myofiber death.
The proximal mechanism linking sodium and calcium entry to membrane stress may be the recently described X-ROS (X-reactive oxygen species) pathway.71 It was also shown that calcium entry and ROS production can act in a positive feedback loop in mdx muscle under conditions of osmotic stress, showing that calcium can amplify ROS production and vice versa.72 An alternative or potentially complementary explanation of stretch-induced calcium entry was suggested by the observation that Src can phosphorylate the transient receptor potential canonical-1 channel to give greater activity.73 Finally, calcium entry in skeletal muscle has also been associated with a process known as receptor-operated calcium entry (ROCE), such as through the P2X7 ATP-activated channel in association with phospholipase A2 signaling and diacylglycerol generation.74, 75, 76
Genetic Evidence for the Calcium Hypothesis: TRP Channels and Orai1-Stim1
Members of the TRPC family form heterotetrameric calcium and sodium entry channels that open in response to stretch, decreased SR-calcium content, and diacylglycerol77, 78, 79 (Figure 1). Vanderbrouk et al.80 first hypothesized that the increased cationic currents observed in dystrophic myofibers was due to TRPC channels. A later study by Millay et al.81 showed that store-operated calcium entry was increased in myofibers from Sgcd−/− mice, and that this activity was fully inhibited with a dominant-negative (dn) TRPC channel mutant in transgenic mice (Table 2). Furthermore, overexpression of wild-type TRPC3, which is known to increase calcium influx, generated abundant store-operated calcium entry that fully induced skeletal muscle pathology in vivo that was highly reminiscent of MD (Table 2).81 These results were actually profound and proved for the first time that increased calcium entry alone was capable of mediating essentially all the disease aspects of MD at the level of the myofiber in vivo. Conversely, overexpression of dnTRPC6 ameliorated dystrophic pathology in Sgcd−/− and mdx mice (Table 2).81 Thus, TRPC protein activity is both necessary and sufficient in the development of MD, although whether this channel generates a bonafide store-operated calcium entry process is still debated.82, 83, 84 These observations suggest that pharmacologic inhibitors against TRP channels could be of clinical value in MD (Figure 2).
Although TRPC channels can result in pathologic calcium entry, the more newly identified Stim and Orai proteins are thought to be the true mediators of store-operated calcium entry85 (Figure 1). Recently, shRNA-mediated knockdown of Orai1 in vivo decreased store-operated calcium entry in myofibers from mdx mice, also reducing muscle pathology.86 Other work using skeletal muscle transgenic strategies has shown that Stim1 overexpression, which markedly increases store-operated calcium entry, is pathogenic in skeletal muscle and induces fulminant MD (Table 2).87 Moreover, expression of a dominant-negative Orai1 protein by transgenesis in mouse skeletal muscle completely blocked Stim1 transgene-induced MD disease, as well as reduced dystrophic disease in Sgcd−/− mice (Table 2).87 The results of this study provide additional genetic proof in mice that calcium entry alone is sufficient to induce the entire process of MD. Furthermore, inhibition of these key pathogenic calcium entry pathways in mdx or Sgcd−/− mice, such as through TRPC channels or Orai1-Stim1 complexes, can be strongly protective. Such results strongly suggest that calcium is the nodal mediator of myofiber necrosis and muscle degeneration in MD.
Alternatively, stretch-mediated calcium entry may also contribute to dystrophic pathology, such as through the transient receptor potential vanilloid (TRPV) family members.88 Trpv2−/− mice exhibited less-muscle pathology in the mdx background, suggesting that the TRPV2 channel itself is a critical disease determinant (Table 2).89 Ho et al.90 determined that SKF-96365 and ruthenium red both inhibited stretch-activated currents in myofibers, which were also inhibited in Trpv4−/− mice. These results suggest that broad inhibitors of the greater TRP subfamilies could be an interesting approach to attempt in treating MD. Indeed, cationic antibiotics that broadly inhibit such channels, such as streptomycin, were shown to ameliorate aspects of muscle disease in mdx mice.66, 91 Unfortunately, chronic use of streptomycin adversely affects the heart and diaphragm, likely through inhibition of mitochondrial ribosomal activity.92
Na Homeostasis and Indirect Control of Calcium and MD
The gradient of sodium ions across the plasma membrane is the basis for excitability and active transport, but this sodium gradient also serves as a co-regulator of calcium influx through the sodium–calcium exchanger (NCX), the sodium–potassium–calcium exchanger, and the sodium–hydrogen exchanger (NHE1) (Figure 1). In living organisms, the activity of the sodium–potassium ATPase (NKA) generates and maintains the plasma membrane sodium gradient. Importantly, increased intracellular sodium concentration, as measured in dystrophic myofibers, can cause sodium-dependent exchangers to function in reverse-mode and thereby lead to a net increase in intracellular calcium levels through NCX and possibly contribute to pathologic effects of MD.
The first study that measured intracellular sodium in mdx mice found a marked elevation of resting sodium levels from 13±3 mM to 24±2 mM in the gastrocnemius and from 13.0±0.3 mM to 23.5±0.7 mM in the diaphragm.93 Resting sodium levels of 11.5 mM in wild-type myofibers and 22.5 mM in mdx myofibers were subsequently measured using a dye-based method, suggesting that the above results were accurate.94 Intracellular sodium measurements have also been extended to DMD patients using sodium 23 magnetic resonance imaging, which estimated a value of 25.4 mM in control muscle versus 38.0 mM in DMD patient muscle, suggesting that sodium overload may be an even larger component of the MD disease process in humans as they appear to have even higher basal levels.95, 96 The critical concept here related to sodium is that not only could such an elevation cause cellular edema, but it would result in a secondary increase in basal calcium levels through the reversal of the NCX and NHE1 when the membrane is depolarized, augmenting calcium overload.
We observed that NCX1 protein levels were profoundly elevated in muscle tissue from dystrophic mice, which we modeled by generating transgenic mice to overexpress NCX1 in skeletal muscle.33 The overexpression of NCX1 induced a progressive dystrophic-like pathology in hindlimb skeletal muscle that was associated with greater reverse-mode calcium entry through this exchanger (Table 2).33 Not surprisingly, the overexpression of NCX1 exacerbated the pathology of the hindlimb musculature when crossed into the mdx and Sgcd−/− mouse models, again by presumably increasing calcium influx.33 Finally, the deletion of endogenous NCX1 (Slc8a gene) specifically in skeletal muscle ameliorated the early pathological profile of MD disease in Sgcd−/− mice when this type of reverse-mode calcium entry normally occurs and contributes to pathology.33 Thus, inhibitors that either selectively reduce intracellular sodium levels so that NCX remains in forward mode operation, or inhibitors against reverse-mode NCX activity, could be therapeutics to evaluate in human clinical trials. Indeed, ranolazine, a general sodium-lowering drug reduced muscle pathology in Sgcd−/− mice33 (Figure 2). It is interesting to note that because of the thermodynamics of sodium and calcium exchange mediated by NCX1, reversal will occur in dystrophic muscle at a more polarized membrane potential because intracellular sodium is elevated (calculations performed based on formula from ref. 97 not shown).
Another recent study looked at the role of the NHE1 in MD, in part because intracellular pH was observed to be elevated in dystrophic muscle.98 Iwata et al. showed that both sodium and calcium were elevated with MD, and that treatment of dystrophic myotubes with inhibitors of NHE1 decreased sodium and use of these inhibitors in vivo decreased dystrophic pathology when administered to mdx mice or BIO14.6 hamsters.98 These results are consistent with the NCX1 data discussed above and again suggest that sodium elevation is a considerable disease mechanism that can underlie secondary calcium entry, leading to myofiber necrosis and muscle degeneration in MD.
Calcium-Activated Protease Activity
The calpains are calcium-activated proteases that are critical to muscle development and homeostasis (Figure 1). Increased calpain activity can exacerbate pathology in MD by cleaving critical intracellular proteins, and not surprisingly, calpain activity is increased in muscle from mdx mice.99 To test the involvement of calpains in the MD disease process, Spencer et al.23 overexpressed the inhibitory protein calpastatin in the mdx mouse, which ameliorated dystrophic pathology (Table 2). Interestingly, calpastatin overexpressing mice had less necrotic lesions in histologic sections, but membrane instability was still present.23 A subsequent study using leupeptin, a protease inhibitor with some specificity to calpains, found less pathology in dystrophic mice.100 Recently, Briguet et al.101 repeated overexpression of calpastatin in the mdx mouse and failed to observe a difference in muscle pathology; however, when they inhibited both calpains and the 20 S proteasome with SNT198438, they were able to ameliorate the dystrophic phenotype. Despite minor inconsistencies, the overall conclusion is that calcium elevation in MD participates in calpain proteolytic activity, which contributes to myofiber dysfunction and necrosis and hence could be pharmacologically inhibited to treat MD (Figure 2).
MPTP Opening
Calcium- and ROS-induced MPTP-opening results in depolarization and swelling of the mitochondria leading to loss of energy production and ultimately the rupture of this organelle and myofiber necrosis (Figure 1). The MPTP is a multiprotein complex found within the inner membrane of mitochondria regulated by the prolyl isomerase cyclophilin D (CypD, encoded by Ppif gene). Recent data have shown that the pore itself is most likely comprised of the mitochondrial F1FO ATP synthase, which spans the inner mitochondrial membrane.102, 103 CypD sensitizes the pore to opening in response to elevated ROS or calcium. Indeed, mice lacking the gene for CypD show reduced MPTP opening to various stimuli and general protection from cardiac and brain ischemic injury in vivo.104
By using mitochondrial localized aequorin proteins it was also shown that mitochondrial calcium is increased in mdx myotubes.35 The first evidence that calcium overload of the mitochondrial may actually happen in vivo was provided through the study of a mouse model of MD owing to a deficiency in Col6a1.105, 106 Early work in the Col6a1−/− mice defined mitochondrial deficiency and apoptosis as hallmarks of this disease, clearly linking mitochondrial dysfunction to this muscle disease.106 Furthermore, they implicated CypD by finding that the mitochondrial dysfunction observed in vitro and the cell death observed in vivo was inhibited by the CypD inhibitor cyclosporine A.105, 107 The improvement in mitochondrial function and reduction in cell death was subsequently shown in patients with Ullrich's congenital MD, and this therapy was tolerated even after long-term follow-up.108
At about the same time we reported that muscle from mdx and Sgcd−/− mice had swollen mitochondria, suggesting that MPTP opening is a pathogenic occurrence in MD.109 Indeed, deletion of the Ppif gene reduced mitochondrial swelling and led to a profound reduction in the dystrophic phenotype of Sgcd−/− mice and the Lama2−/− mice, the latter of which is a model of congenital MD due to lamininα2 deficiency (Table 2).109 Ppif deletion also led to decreased muscle pathology and restoration of mitochondrial function in the Col6a1 mouse model as deletion of MD.110 The fact that four separate models of MD with potentially divergent proximal mechanisms of disease were each rescued suggested that MPTP opening due to calcium dysregulation may be the final common pathway for multiple muscle diseases. Indeed, Debio-025, a CypD inhibitor, also ameliorated dystrophic pathology in mdx mice and an Ulrich congenital MD mouse model105, 109, 111, 112, 113 (Figure 2). These results further implicate calcium as the primary second messenger in mediating myofiber necrosis and muscle degeneration in MD.
Novel Medical Treatments Based on the Calcium Hypothesis
The calcium hypothesis of MD suggests a number of potential treatment options, only a small number of which have been tested to date (Figure 2). Preclinical efficacy in the mouse has been shown for inhibitors of the MPTP (Debio-025), NHE1 (cariporide and 5-(N-ethyl-N-isopropyl)-amiloride), ryanodine leak inhibitors (S107), indirect SERCA activators (BGP-15), stretch-activated channel inhibitors (streptomycin), L-type calcium channel inhibitors (verapamil, diltiazem, and nifedipine), TRPC channel inhibitors, inhibitors of X-ROS pathway (colchicine), and reverse-mode NCX inhibitors (ranolazine) or other general inhibitors that reduce intracellular sodium (ranolazine).33, 39, 41, 42, 43, 49, 53, 54, 55, 71, 91, 92, 98, 109, 114 Many more inhibitors have yet to be tested including novel TPRC/TRPV inhibitors, SERCA activators, and other inhibitors of NCX1 including KB-R7943 and SEA0400115, 116, 117, 118, 119, 120, 121, 122, 123 (Figure 2).
Alternatively, gene therapy approaches are also rapidly maturing and could be translated into the clinic, such as SERCA2 viral vectors, which are now in phase II/III trials for human heart failure.48 SERCA gene therapy is particularly exciting to consider given the large magnitude of effect associated with increasing SERCA activity in ameliorating disease in multiple mouse models of MD, results observed across independent laboratories.15, 47 Another possibility could be adenoviral gene therapy to express dnTRPC or dnTRPV channels selectively in skeletal muscle, which appears to reduce or eliminate most of store-operated, stretch-dependent, and even ROCE pathways that are known to occur in dystrophic skeletal muscle.
Summary and Implications of the Calcium Hypothesis
The calcium hypothesis has matured greatly over the past decade; thanks to genetic models that have proven beyond a doubt the importance of calcium overload/dysregulation in mediating myofiber necrosis and MD pathogenesis. Clearly, calcium homeostasis can be corrected at multiple levels to positively impact MD, including at the level of the SR, the plasma membrane, and the mitochondria. It seems logical, given the known mechanical defects within the dystrophic plasma membrane that alterations in calcium and sodium levels likely stems from excessive activation of various channels and exchangers that then leads to alterations in SR-calcium handling and mitochondrial calcium loading. For example, it is easy to see how slowed calcium reuptake to the SR could lead to greater mitochondrial uptake and MPTP opening, which in turn could lead to reduced energy production and failure of active transport, thereby producing even greater sodium and calcium overload and eventually cellular necrosis. Although the data we presented in genetically modified mouse models makes a compelling case for the calcium hypothesis of disease pathogenesis in MD as originally proposed by Wrogemann, questions still remain. However, in the meantime we believe that the animal data are more than compelling enough to spur new clinical trials aimed at correcting defects in calcium handling and basal calcium overload, both with pharmacologic agents and with gene therapeutic approaches.
Acknowledgments
This work was supported by grants from the National Institutes of Health (to JDM). JDM is an investigator of the Howard Hughes Medical Institute.
Author Contributions
ARB and JDM wrote the manuscript.
Glossary
- CK
creatine kinase
- CypD
cyclophilin D
- DMD
Duchenne muscular dystrophy
- dn
dominant negative
- IP3R
inositol 1,4,5-triphosphate receptor
- MD
muscular dystrophy
- MPTP
mitochondrial permeability transition pore
- NADPH
nicotinamide adenine dinucleotide phosphate
- NCX
sodium–calcium exchanger
- NHE
sodium–hydrogen exchanger
- NKA
sodium–potassium ATPase
- ROCE
receptor-operated calcium entry
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
- SERCA
sarcoplasmic/endoplasmic reticulum calcium ATPase
- TRPC
transient receptor potential canonical
- TRPV
transient receptor potential vanilloid
- X-ROS
X-reactive oxygen species
The authors declare no conflict of interest.
Footnotes
Edited by L Scorrano
References
- Emery AE. Population frequencies of inherited neuromuscular diseases—a world survey. Neuromuscul Dis. 1991;1:19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev. 2002;12:349–361. doi: 10.1016/s0959-437x(02)00309-x. [DOI] [PubMed] [Google Scholar]
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- Wrogemann K, Pena SD. Mitochondrial calcium overload: a general mechanism for cell-necrosis in muscle diseases. Lancet. 1976;1:672–674. doi: 10.1016/s0140-6736(76)92781-1. [DOI] [PubMed] [Google Scholar]
- McComas AJ, Sica RE, Currie S. An electrophysiological study of Duchenne dystrophy. J Neurol Neurosurg Psychiatry. 1971;34:461–468. doi: 10.1136/jnnp.34.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComas AJ, Thomas HC. A study of the muscle twitch in the Duchenne type muscular dystrophy. J Neurol Sci. 1968;7:309–312. doi: 10.1016/0022-510x(68)90151-2. [DOI] [PubMed] [Google Scholar]
- Wood DS, Sorenson MM, Eastwood AB, Charash WE, Reuben JP. Duchenne dystrophy: abnormal generation of tension and Ca++ regulation in single skinned fibers. Neurology. 1978;28:447–457. doi: 10.1212/wnl.28.5.447. [DOI] [PubMed] [Google Scholar]
- Williams DA, Head SI, Lynch GS, Stephenson DG. Contractile properties of skinned muscle fibres from young and adult normal and dystrophic (mdx) mice. J Physiol. 1993;460:51–67. doi: 10.1113/jphysiol.1993.sp019458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco P, Jones DA, Dick JR, Vrbova G. Contractile properties and susceptibility to exercise-induced damage of normal and mdx mouse tibialis anterior muscle. Clin Sci (Lond) 1992;82:227–236. doi: 10.1042/cs0820227. [DOI] [PubMed] [Google Scholar]
- Quinlan JG, Johnson SR, McKee MK, Lyden SP. Twitch and tetanus in mdx mouse muscle. Muscle Nerve. 1992;15:837–842. doi: 10.1002/mus.880150713. [DOI] [PubMed] [Google Scholar]
- Divet A, Huchet-Cadiou C. Sarcoplasmic reticulum function in slow- and fast-twitch skeletal muscles from mdx mice. Pflugers Arch. 2002;444:634–643. doi: 10.1007/s00424-002-0854-5. [DOI] [PubMed] [Google Scholar]
- Nicolas-Metral V, Raddatz E, Kucera P, Ruegg UT. Mdx myotubes have normal excitability but show reduced contraction-relaxation dynamics. J Mus Res Cell Motil. 2001;22:69–75. doi: 10.1023/a:1010384625954. [DOI] [PubMed] [Google Scholar]
- Head SI. Membrane potential, resting calcium and calcium transients in isolated muscle fibres from normal and dystrophic mice. J Physiol. 1993;469:11–19. doi: 10.1113/jphysiol.1993.sp019801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutdibi O, Brinkmeier H, Rudel R, Fohr KJ. Increased calcium entry into dystrophin-deficient muscle fibres of MDX and ADR-MDX mice is reduced by ion channel blockers. J Physiol-London. 1999;515:859–868. doi: 10.1111/j.1469-7793.1999.859ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonasekera SA, Lam CK, Millay DP, Sargent MA, Hajjar RJ, Kranias EG, et al. Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J Clin Invest. 2011;121:1044–1052. doi: 10.1172/JCI43844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E, Hartner KT, Pette D. Postnatal development of Ca2+-sequestration by the sarcoplasmic reticulum of fast and slow muscles in normal and dystrophic mice. Eur J Biochem. 1988;174:247–253. doi: 10.1111/j.1432-1033.1988.tb14090.x. [DOI] [PubMed] [Google Scholar]
- Landi N, Nassi P, Liguri G, Bobbi S, Sbrilli C, Marconi G. Sarcoplasmic reticulum Ca2+-ATPase and acylphosphatase activities in muscle biopsies from patients with Duchenne muscular dystrophy. Clin Chim Acta. 1986;158:245–251. doi: 10.1016/0009-8981(86)90288-3. [DOI] [PubMed] [Google Scholar]
- Woods CE, Novo D, DiFranco M, Vergara JL. The action potential-evoked sarcoplasmic reticulum calcium release is impaired in mdx mouse muscle fibres. J Physiol-London. 2004;557:59–75. doi: 10.1113/jphysiol.2004.061291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez J, Neco P, DiFranco M, Vergara JL. Calcium release domains in mammalian skeletal muscle studied with two-photon imaging and spot detection techniques. J Gen Physiol. 2006;127:623–637. doi: 10.1085/jgp.200509475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EP, Knudson CM, Campbell KP, Kunkel LM. Subcellular fractionation of dystrophin to the triads of skeletal-muscle. Nature. 1987;330:754–758. doi: 10.1038/330754a0. [DOI] [PubMed] [Google Scholar]
- Karch J, Molkentin JD. Identifying the components of the elusive mitochondrial permeability transition pore. Proc Natl Acad Sci USA. 2014;111:10396–10397. doi: 10.1073/pnas.1410104111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P, Di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J Mol Cell Cardiol. 2015;78:100–106. doi: 10.1016/j.yjmcc.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer MJ, Mellgren RL. Overexpression of a calpastatin transgene in mdx muscle reduces dystrophic pathology. Hum Mol Gen. 2002;11:2645–2655. doi: 10.1093/hmg/11.21.2645. [DOI] [PubMed] [Google Scholar]
- Turner PR, Schultz R, Ganguly B, Steinhardt RA. Proteolysis results in altered leak channel kinetics and elevated free calcium in mdx muscle. J Membr Biol. 1993;133:243–251. doi: 10.1007/BF00232023. [DOI] [PubMed] [Google Scholar]
- Bodensteiner JB, Engel AG. Intracellular calcium accumulation in Duchenne dystrophy and other myopathies: a study of 567,000 muscle fibers in 114 biopsies. Neurology. 1978;28:439–446. doi: 10.1212/wnl.28.5.439. [DOI] [PubMed] [Google Scholar]
- Maunder-Sewry CA, Gorodetsky R, Yarom R, Dubowitz V. Element analysis of skeletal muscle in Duchenne muscular dystrophy using x-ray fluorescence spectrometry. Muscle Nerve. 1980;3:502–508. doi: 10.1002/mus.880030607. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Jones DA, Edwards RH. Measurements of calcium and other elements in muscle biopsy samples from patients with Duchenne muscular dystrophy. Clin Chim Acta. 1985;147:215–221. doi: 10.1016/0009-8981(85)90202-5. [DOI] [PubMed] [Google Scholar]
- Turner PR, Westwood T, Regen CM, Steinhardt RA. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature. 1988;335:735–738. doi: 10.1038/335735a0. [DOI] [PubMed] [Google Scholar]
- Gailly P, Boland B, Himpens B, Casteels R, Gillis JM. Critical evaluation of cytosolic calcium determination in resting muscle fibres from normal and dystrophic (mdx) mice. Cell Calcium. 1993;14:473–483. doi: 10.1016/0143-4160(93)90006-r. [DOI] [PubMed] [Google Scholar]
- Collet C, Allard B, Tourneur Y, Jacquemond V. Intracellular calcium signals measured with indo-1 in isolated skeletal muscle fibres from control and mdx mice. J Physiol. 1999;520 (Pt 2:417–429. doi: 10.1111/j.1469-7793.1999.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamirano F, López JR, Henríquez C, Molinski T, Allen PD, Jaimovich E. Increased resting intracellular calcium modulates NF-? B-dependent inducible nitric-oxide synthase gene expression in dystrophic mdx skeletal myotubes. J Biol Chem. 2012;287:20876–20887. doi: 10.1074/jbc.M112.344929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamirano F, Valladares D, Henriquez-Olguin C, Casas M, Lopez JR, Allen PD, et al. Nifedipine treatment reduces resting calcium concentration, oxidative and apoptotic gene expression, and improves muscle function in dystrophic mdx mice. PLoS One. 2013;8:e81222. doi: 10.1371/journal.pone.0081222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr AR, Millay DP, Goonasekera SA, Park KH, Sargent MA, Collins J, et al. Na+ dysregulation coupled with Ca2+ entry through NCX1 promotes muscular dystrophy in mice. Mol Cell Biol. 2014;34:1991–2002. doi: 10.1128/MCB.00339-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamirano F, Lopez JR, Henriquez C, Molinski T, Allen PD, Jaimovich E. Increased resting intracellular calcium modulates NF-kappaB-dependent inducible nitric-oxide synthase gene expression in dystrophic mdx skeletal myotubes. J Biol Chem. 2012;287:20876–20887. doi: 10.1074/jbc.M112.344929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V, Massimino ML, Tosello V, Marsault R, Cantini M, Sorrentino V, et al. Alteration in calcium handling at the subcellular level in mdx myotubes. J Biol Chem. 2001;276:4647–4651. doi: 10.1074/jbc.M006337200. [DOI] [PubMed] [Google Scholar]
- Mallouk N, Allard B. Ca(2+) influx and opening of Ca(2+)-activated K(+) channels in muscle fibers from control and mdx mice. Biophys J. 2002;82:3012–3021. doi: 10.1016/S0006-3495(02)75642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Grounds MD, Bakker AJ. Measurement of sub-membrane [Ca2+] in adult myofibers and cytosolic [Ca2+] in myotubes from normal and mdx mice using the Ca2+ indicator FFP-18. Cell Calcium. 2006;40:299–307. doi: 10.1016/j.ceca.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Shannon TR, Wang F, Puglisi J, Weber C, Bers DM. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys J. 2004;87:3351–3371. doi: 10.1529/biophysj.104.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernice W, Beckmann R, Ketelsen UP, Frey M, Schmidt-Redemann B, Haap KP, et al. A double-blind placebo controlled trial of diltiazem in Duchenne dystrophy. Klinische Wochenschrift. 1988;66:565–570. doi: 10.1007/BF01720830. [DOI] [PubMed] [Google Scholar]
- Phillips MF, Quinlivan R. Calcium antagonists for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008. p. CD004571. [DOI] [PMC free article] [PubMed]
- Dick DJ, Gardner-Medwin D, Gates PG, Gibson M, Simpson JM, Walls TJ. A trial of flunarizine in the treatment of Duchenne muscular dystrophy. Muscle Nerve. 1986;9:349–354. doi: 10.1002/mus.880090412. [DOI] [PubMed] [Google Scholar]
- Moxley RT, 3rd, Brooke MH, Fenichel GM, Mendell JR, Griggs RC, Miller JP, et al. Clinical investigation in Duchenne dystrophy. VI. Double-blind controlled trial of nifedipine. Muscle Nerve. 1987;10:22–33. doi: 10.1002/mus.880100106. [DOI] [PubMed] [Google Scholar]
- Emery AEH, Skinner R, Howden LC, Matthews MB. Verapamil in Duchenne muscular-dystrophy. Lancet. 1982;1:559–559. doi: 10.1016/s0140-6736(82)92063-3. [DOI] [PubMed] [Google Scholar]
- Bertorini TE, Palmieri GM, Griffin JW, Igarashi M, McGee J, Brown R, et al. Effect of chronic treatment with the calcium antagonist diltiazem in Duchenne muscular dystrophy. Neurology. 1988;38:609–613. doi: 10.1212/wnl.38.4.609. [DOI] [PubMed] [Google Scholar]
- Neto HS, Matsumura CY, Marques MJ. Diltiazem and verapamil protect dystrophin-deficient muscle fibers of mdx mice from degeneration: Potential role in calcium buffering and sarcolemmal stability. Neuromuscul Disord. 2008;18:812–812. doi: 10.1002/mus.21188. [DOI] [PubMed] [Google Scholar]
- Kargacin ME, Kargacin GJ. The sarcoplasmic reticulum calcium pump is functionally altered in dystrophic muscle. Biochim Biophys Acta. 1996;1290:4–8. doi: 10.1016/0304-4165(95)00180-8. [DOI] [PubMed] [Google Scholar]
- Morine KJ, Sleeper MM, Barton ER, Sweeney HL. Overexpression of SERCA1a in the mdx diaphragm reduces susceptibility to contraction-induced damage. Hum Gene Ther. 2010;21:1735–1739. doi: 10.1089/hum.2010.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrig SM, van der Poel C, Sayer TA, Schertzer JD, Henstridge DC, Church JE, et al. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature. 2012;484:394–398. doi: 10.1038/nature10980. [DOI] [PubMed] [Google Scholar]
- Wang X, Weisleder N, Collet C, Zhou JS, Chu Y, Hirata Y, et al. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat Cell Biol. 2005;7:525–530. doi: 10.1038/ncb1254. [DOI] [PubMed] [Google Scholar]
- Weisleder N, Wang X, Collet C, Zhou JS, Chu Y, Hirata Y, et al. Stress-induced uncontrolled calcium sparks as dystrophic signals in mammalian skeletal muscle. Biophys J. 2005;88:534a–535a. doi: 10.1038/ncb1254. [DOI] [PubMed] [Google Scholar]
- Aracena P, Tang W, Hamilton SL, Hidalgo C. Effects of S-glutathionylation and S-nitrosylation on calmodulin binding to triads and FKBP12 binding to type 1 calcium release channels. Antioxid Redox Signal. 2005;7:870–881. doi: 10.1089/ars.2005.7.870. [DOI] [PubMed] [Google Scholar]
- Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, et al. Remodeling of ryanodine receptor complex causes ‘leaky' channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci USA. 2008;105:2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu XP, Rothman L, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15:325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DC, Meli AC, Reiken S, Betzenhauser MJ, Umanskaya A, Shiomi T, et al. Leaky ryanodine receptors in beta-sarcoglycan deficient mice: a potential common defect in muscular dystrophy. Skelet Muscle. 2012;2:9. doi: 10.1186/2044-5040-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondin L, Balghi H, Constantin B, Cognard C, Sebille S. Negative modulation of inositol 1,4,5-trisphosphate type 1 receptor expression prevents dystrophin-deficient muscle cells death. Am J Physiol Cell Physiol. 2009;297:C1133–C1145. doi: 10.1152/ajpcell.00048.2009. [DOI] [PubMed] [Google Scholar]
- Balghi H, Sebille S, Constantin B, Patri S, Thoreau V, Mondin L, et al. Mini-dystrophin expression down-regulates overactivation of G protein-mediated IP3 signaling pathway in dystrophin-deficient muscle cells. J Gen Physiol. 2006;127:171–182. doi: 10.1085/jgp.200509456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjondrokoesoemo A, Li N, Lin PH, Pan Z, Ferrante CJ, Shirokova N, et al. Type 1 Inositol (1,4,5)-trisphosphate receptor activates ryanodine receptor 1 to mediate calcium spark signaling in adult mammalian skeletal muscle. J Biol Chem. 2013;288:2103–2109. doi: 10.1074/jbc.M112.425975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balghi H, Sebille S, Mondin L, Cantereau A, Constantin B, Raymond G, et al. Mini-dystrophin expression down-regulates IP3-mediated calcium release events in resting dystrophin-deficient muscle cells. J Gen Physiol. 2006;128:219–230. doi: 10.1085/jgp.200609559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PR, Fong PY, Denetclaw WF, Steinhardt RA. Increased calcium influx in dystrophic muscle. J Cell Biol. 1991;115:1701–1712. doi: 10.1083/jcb.115.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco Jr, A, Lansman JB. Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature. 1990;344:670–673. doi: 10.1038/344670a0. [DOI] [PubMed] [Google Scholar]
- Menke A, Jockusch H. Decreased osmotic stability of dystrophin-less muscle-cells from the Mdx mouse. Nature. 1991;349:69–71. doi: 10.1038/349069a0. [DOI] [PubMed] [Google Scholar]
- Franco Jr, A, Winegar BD, Lansman JB. Open channel block by gadolinium ion of the stretch-inactivated ion channel in mdx myotubes. Biophys J. 1991;59:1164–1170. doi: 10.1016/S0006-3495(91)82332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijendekker WJ, Passaquin AC, Metzinger L, Ruegg UT. Regulation of cytosolic calcium in skeletal muscle cells of the mdx mouse under conditions of stress. Br J Pharmacol. 1996;118:611–616. doi: 10.1111/j.1476-5381.1996.tb15445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung EW, Ballard HJ, Bourreau JP, Allen DG. Intracellular sodium in mammalian muscle fibers after eccentric contractions. J Appl Physiol. 2003;94:2475–2482. doi: 10.1152/japplphysiol.01128.2002. [DOI] [PubMed] [Google Scholar]
- Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol. 2005;562:367–380. doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P, Baatsen PHWW, Marechal G. Increased susceptibility of Edl muscles from Mdx mice to damage-induced by contractions with stretch. J Mus Res Cell Motil. 1993;14:446–451. doi: 10.1007/BF00121296. [DOI] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers MK, Okamura CS, Bogan DJ, Bogan JR, Petroski GF, McDonald K, et al. Eccentric contraction injury in dystrophic canine muscle. Arch Phys Med Rehab. 2002;83:1572–1578. doi: 10.1053/apmr.2002.35109. [DOI] [PubMed] [Google Scholar]
- Yeung EW, Head SI, Allen DG. Gadolinium reduces short-term stretch-induced muscle damage in isolated mdx mouse muscle fibres. J Physiol-London. 2003;552:449–458. doi: 10.1113/jphysiol.2003.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairallah RJ, Shi GL, Sbrana F, Prosser BL, Borroto C, Mazaitis MJ, et al. Microtubules underlie dysfunction in Duchenne muscular dystrophy. Sci Signal. 2012;5:ra56. doi: 10.1126/scisignal.2002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkryl VM, Martins AS, Ullrich ND, Nowycky MC, Niggli E, Shirokova N. Reciprocal amplification of ROS and Ca2+ signals in stressed mdx dystrophic skeletal muscle fibers. Pflugers Arch. 2009;458:915–928. doi: 10.1007/s00424-009-0670-2. [DOI] [PubMed] [Google Scholar]
- Gervasio OL, Whitehead NP, Yeung EW, Phillips WD, Allen DG. TRPC1 binds to caveolin-3 and is regulated by Src kinase - role in Duchenne muscular dystrophy. J Cell Sci. 2008;121:2246–2255. doi: 10.1242/jcs.032003. [DOI] [PubMed] [Google Scholar]
- Young CN, Brutkowski W, Lien CF, Arkle S, Lochmuller H, Zablocki K, et al. P2X7 purinoceptor alterations in dystrophic mdx mouse muscles: relationship to pathology and potential target for treatment. J Cell Mol Med. 2012;16:1026–1037. doi: 10.1111/j.1582-4934.2011.01397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boittin FX, Petermann O, Hirn C, Mittaud P, Dorchies OM, Roulet E, et al. Ca2+-independent phospholipase A2 enhances store-operated Ca2+ entry in dystrophic skeletal muscle fibers. J Cell Sci. 2006;119:3733–3742. doi: 10.1242/jcs.03184. [DOI] [PubMed] [Google Scholar]
- Harvey AL, Hider RC, Khader F. Effect of phospholipase A on actions of cobra venom cardiotoxins on erythrocytes and skeletal muscle. Biochim Biophys Acta. 1983;728:215–221. doi: 10.1016/0005-2736(83)90474-1. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, et al. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci USA. 2008;105:2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol. 2002;158:1089–1096. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millay DP, Goonasekera SA, Sargent MA, Maillet M, Aronow BJ, Molkentin JD. Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism. Proc Natl Acad Sci USA. 2009;106:19023–19028. doi: 10.1073/pnas.0906591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JN, Friedrich O, Cully TR, von Wegner F, Murphy RM, Launikonis BS. Upregulation of store-operated Ca2+ entry in dystrophic mdx mouse muscle. Am J Physiol Cell Physiol. 2010;299:C42–C50. doi: 10.1152/ajpcell.00524.2009. [DOI] [PubMed] [Google Scholar]
- Brechard S, Melchior C, Plancon S, Schenten V, Tschirhart EJ. Store-operated Ca2+ channels formed by TRPC1, TRPC6 and Orai1 and non-store-operated channels formed by TRPC3 are involved in the regulation of NADPH oxidase in HL-60 granulocytes. Cell Calcium. 2008;44:492–506. doi: 10.1016/j.ceca.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Antigny F, Jousset H, Konig S, Frieden M. Thapsigargin activates Ca2+ entry both by store-dependent, STIM1/Orai1-mediated, and store-independent, TRPC3/PLC/PKC-mediated pathways in human endothelial cells. Cell Calcium. 2011;49:115–127. doi: 10.1016/j.ceca.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Dirksen RT. Checking your SOCCs and feet: the molecular mechanisms of Ca2+ entry in skeletal muscle. J Physiol. 2009;587:3139–3147. doi: 10.1113/jphysiol.2009.172148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Moloughney JG, Zhang S, Komazaki S, Weisleder N. Orai1 mediates exacerbated Ca(2+) entry in dystrophic skeletal muscle. PLoS One. 2012;7:e49862. doi: 10.1371/journal.pone.0049862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonasekera SA, Davis J, Kwong JQ, Accornero F, Wei-Lapierre L, Sargent MA, et al. Enhanced Ca2+ influx from STIM1-Orai1 induces muscle pathology in mouse models of muscular dystrophy. Hum Mol Gen. 2014;23:3706–3716. doi: 10.1093/hmg/ddu079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y, Katanosaka Y, Arai Y, Shigekawa M, Wakabayashi S. Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum Mol Gen. 2009;18:824–834. doi: 10.1093/hmg/ddn408. [DOI] [PubMed] [Google Scholar]
- Zanou N, Iwata Y, Schakman O, Lebacq J, Wakabayashi S, Gailly P. Essential role of TRPV2 ion channel in the sensitivity of dystrophic muscle to eccentric contractions. FEBS Lett. 2009;583:3600–3604. doi: 10.1016/j.febslet.2009.10.033. [DOI] [PubMed] [Google Scholar]
- Ho TC, Horn NA, Huynh T, Kelava L, Lansman JB. Evidence TRPV4 contributes to mechanosensitive ion channels in mouse skeletal muscle fibers. Channels. 2012;6:246–254. doi: 10.4161/chan.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead NP, Streamer M, Lusambili LI, Sachs F, Allen DG. Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromuscul Disord. 2006;16:845–854. doi: 10.1016/j.nmd.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Jorgensen LH, Blain A, Greally E, Laval SH, Blamire AM, Davison BJ, et al. Long-term blocking of calcium channels in mdx mice results in differential effects on heart and skeletal muscle. Am J Pathol. 2011;178:273–283. doi: 10.1016/j.ajpath.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JF, Bannister N, Kemp GJ, Publicover SJ. Sodium is elevated in mdx muscles: ionic interactions in dystrophic cells. J Neurol Sci. 1993;114:76–80. doi: 10.1016/0022-510x(93)90052-z. [DOI] [PubMed] [Google Scholar]
- Miles MT, Cottey E, Cottey A, Stefanski C, Carlson CG. Reduced resting potentials in dystrophic (mdx) muscle fibers are secondary to NF-kappaB-dependent negative modulation of ouabain sensitive Na+-K+ pump activity. J Neurol Sci. 2011;303:53–60. doi: 10.1016/j.jns.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MA, Nagel AM, Jurkat-Rott K, Lehmann-Horn F. Sodium (23Na) MRI detects elevated muscular sodium concentration in Duchenne muscular dystrophy. Neurology. 2011;77:2017–2024. doi: 10.1212/WNL.0b013e31823b9c78. [DOI] [PubMed] [Google Scholar]
- Weber MA, Nagel AM, Wolf MB, Jurkat-Rott K, Kauczor HU, Semmler W, et al. Permanent muscular sodium overload and persistent muscle edema in Duchenne muscular dystrophy: a possible contributor of progressive muscle degeneration. J Neurol. 2012;259:2385–2392. doi: 10.1007/s00415-012-6512-8. [DOI] [PubMed] [Google Scholar]
- Weber CR, Ginsburg KS, Philipson KD, Shannon TR, Bers DM. Allosteric regulation of Na/Ca exchange current by cytosolic Ca in intact cardiac myocytes. J Gen Physiol. 2001;117:119–131. doi: 10.1085/jgp.117.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y, Katanosaka Y, Hisamitsu T, Wakabayashi S. Enhanced Na+/H+ exchange activity contributes to the pathogenesis of muscular dystrophy via involvement of P2 receptors. Am J Pathol. 2007;171:1576–1587. doi: 10.2353/ajpath.2007.070452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer MJ, Croall DE, Tidball JG. Calpains are activated in necrotic fibers from mdx dystrophic mice. J Biol Chem. 1995;270:10909–10914. doi: 10.1074/jbc.270.18.10909. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Ziats NP, Tierney BP, Nakabayashi N, Anderson JM. Protein adsorption from human plasma is reduced on phospholipid polymers. J Biomed Mater Res. 1991;25:1397–1407. doi: 10.1002/jbm.820251107. [DOI] [PubMed] [Google Scholar]
- Briguet A, Erb M, Courdier-Fruh I, Barzaghi P, Santos G, Herzner H, et al. Effect of calpain and proteasome inhibition on Ca2+-dependent proteolysis and muscle histopathology in the mdx mouse. FASEB J. 2008;22:4190–4200. doi: 10.1096/fj.07-099036. [DOI] [PubMed] [Google Scholar]
- Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci USA. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, et al. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci USA. 2014;111:10580–10585. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadov S, Kuznetsov A. Mitochondrial permeability transition and cell death: the role of cyclophilin d. Front Physiol. 2013;4:76. doi: 10.3389/fphys.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelin A, Tiepolo T, Sabatelli P, Grumati P, Bergamin N, Golfieri C, et al. Mitochondrial dysfunction in the pathogenesis of Ullrich congenital muscular dystrophy and prospective therapy with cyclosporins. Proc Natl Acad Sci USA. 2007;104:991–996. doi: 10.1073/pnas.0610270104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin WA, Bergamin N, Sabatelli P, Reggiani C, Megighian A, Merlini L, et al. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet. 2003;35:367–371. doi: 10.1038/ng1270. [DOI] [PubMed] [Google Scholar]
- Merlini L, Angelin A, Tiepolo T, Braghetta P, Sabatelli P, Zamparelli A, et al. Cyclosporin A corrects mitochondrial dysfunction and muscle apoptosis in patients with collagen VI myopathies. Proc Natl Acad Sci USA. 2008;105:5225–5229. doi: 10.1073/pnas.0800962105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlini L, Sabatelli P, Armaroli A, Gnudi S, Angelin A, Grumati P, et al. Cyclosporine A in Ullrich congenital muscular dystrophy: long-term results. Oxid Med Cell Longev. 2011;2011:139194. doi: 10.1155/2011/139194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millay DP, Sargent MA, Osinska H, Baines CP, Barton ER, Vuagniaux G, et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat Med. 2008;14:442–447. doi: 10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Tiepolo T, Angelin A, Sabatelli P, Maraldi NM, Basso E, et al. Genetic ablation of cyclophilin D rescues mitochondrial defects and prevents muscle apoptosis in collagen VI myopathic mice. Hum Mol Gen. 2009;18:2024–2031. doi: 10.1093/hmg/ddp126. [DOI] [PubMed] [Google Scholar]
- Wissing ER, Millay DP, Vuagniaux G, Molkentin JD. Debio-025 is more effective than prednisone in reducing muscular pathology in mdx mice. Neuromuscul Disord. 2010;20:753–760. doi: 10.1016/j.nmd.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutenauer J, Dorchies OM, Patthey-Vuadens O, Vuagniaux G, Ruegg UT. Investigation of Debio 025, a cyclophilin inhibitor, in the dystrophic mdx mouse, a model for Duchenne muscular dystrophy. Br J Pharmacol. 2008;155:574–584. doi: 10.1038/bjp.2008.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiepolo T, Angelin A, Palma E, Sabatelli P, Merlini L, Nicolosi L, et al. The cyclophilin inhibitor Debio 025 normalizes mitochondrial function, muscle apoptosis and ultrastructural defects in Col6a1−/− myopathic mice. Br J Pharmacol. 2009;157:1045–1052. doi: 10.1111/j.1476-5381.2009.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toifl K, Presterl E, Graninger W. Lack of effect of diltiazem in the treatment of Duchennes muscular-dystrophy - a double-blind placebo-controlled study. Wien Klin Wochenschr. 1991;103:232–235. [PubMed] [Google Scholar]
- Satoh H, Ginsburg KS, Qing K, Terada H, Hayashi H, Bers DM. KB-R7943 block of Ca(2+) influx via Na(+)/Ca(2+) exchange does not alter twitches or glycoside inotropy but prevents Ca(2+) overload in rat ventricular myocytes. Circulation. 2000;101:1441–1446. doi: 10.1161/01.cir.101.12.1441. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Arakawa N, Takuma K, Kishida Y, Kawasaki Y, Sakaue M, et al. SEA0400, a novel and selective inhibitor of the Na+-Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J Pharmacol Exp Ther. 2001;298:249–256. [PubMed] [Google Scholar]
- Cornea RL, Gruber SJ, Lockamy EL, Muretta JM, Jin DZ, Chen JQ, et al. High-throughput FRET assay yields allosteric SERCA activators. J Biomol Screen. 2013;18:97–107. doi: 10.1177/1087057112456878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- Beauge L, DiPolo R. SEA-0400, a potent inhibitor of the Na+/Ca2+ exchanger, as a tool to study exchanger ionic and metabolic regulation. Am J Physiol Cell Physiol. 2005;288:C1374–C1380. doi: 10.1152/ajpcell.00492.2004. [DOI] [PubMed] [Google Scholar]
- Urban N, Hill K, Wang LM, Kuebler WM, Schaefer M. Novel pharmacological TRPC inhibitors block hypoxia-induced vasoconstriction. Cell Calcium. 2012;51:194–206. doi: 10.1016/j.ceca.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Kraft R. The Na+/Ca2+ exchange inhibitor KB-R7943 potently blocks TRPC channels. Biochem Biophys Res Commun. 2007;361:230–236. doi: 10.1016/j.bbrc.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Kiyonaka S, Kato K, Nishida M, Mio K, Numaga T, Sawaguchi Y, et al. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc Natl Acad Sci USA. 2009;106:5400–5405. doi: 10.1073/pnas.0808793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harguindey S, Arranz JL, Polo Orozco JD, Rauch C, Fais S, Cardone RA, et al. Cariporide and other new and powerful NHE1 inhibitors as potentially selective anticancer drugs—an integral molecular/biochemical/metabolic/clinical approach after one hundred years of cancer research. J Trans Med. 2013;11:282. doi: 10.1186/1479-5876-11-282. [DOI] [PMC free article] [PubMed] [Google Scholar]