Abstract
Rationale: Chronic bronchitis (CB) is characterized by persistent cough and sputum production. Studies were performed to test whether mucus hyperconcentration and increased partial osmotic pressure, in part caused by abnormal purine nucleotide regulation of ion transport, contribute to the pathogenesis of CB.
Objectives: We tested the hypothesis that CB is characterized by mucus hyperconcentration, increased mucus partial osmotic pressures, and reduced mucus clearance.
Methods: We measured in subjects with CB as compared with normal and asymptomatic smoking control subjects indices of mucus concentration (hydration; i.e., percentage solids) and sputum adenine nucleotide/nucleoside concentrations. In addition, sputum partial osmotic pressures and mucus transport rates were measured in subjects with CB.
Measurements and Results: CB secretions were hyperconcentrated as indexed by an increase in percentage solids and total mucins, in part reflecting decreased extracellular nucleotide/nucleoside concentrations. CB mucus generated concentration-dependent increases in partial osmotic pressures into ranges predicted to reduce mucus transport. Mucociliary clearance (MCC) in subjects with CB was negatively correlated with mucus concentration (percentage solids). As a test of relationships between mucus concentration and disease, mucus concentrations and MCC were compared with FEV1, and both were significantly correlated.
Conclusions: Abnormal regulation of airway surface hydration may slow MCC in CB and contribute to disease pathogenesis.
Keywords: COPD, mucociliary clearance, mucus hyperconcentration
At a Glance Commentary
Scientific Knowledge on the Subject
Chronic bronchitis (CB) is characterized by symptoms of cough and sputum production. Increases in mucus concentration (i.e., dehydration) seem to contribute to failure of mucus clearance and are associated with symptoms and disease progression in CB.
What This Study Adds to the Field
Mucus clearance is a major mechanism protecting the lung from inhaled infectious and noninfectious challenges. Hydration of the mucus layer, in part regulated by extracellular nucleotide/nucleoside levels, is a major determinant of the efficacy of mucus clearance. This study characterized the hydration status of secretions obtained from subjects with CB and related CB mucus concentrations (percentage solids) to mucus partial osmotic pressures and clearance. Increased mucus concentrations, and decreased extracellular nucleotide/nucleoside levels, were a feature of CB secretions. Increased mucus concentrations in CB were positively correlated with mucus partial osmotic pressure and negatively correlated with mucus clearance rates. Abnormal regulation of airway surface hydration may slow clearance from the lung in CB and contribute to an increased risk of infection, exacerbations, and disease pathogenesis. Testing of therapies in subjects with CB to reduce mucus concentration seems rational.
Chronic bronchitis (CB) is a common respiratory disease that is often caused by exposure to environmental toxicants, especially tobacco smoke (1–3). CB is defined epidemiologically by a productive chronic cough for 3 or more consecutive months for 2 consecutive years. CB is clinically characterized by mucus hypersecretion, sputum production, and it may or may not be associated with airflow obstruction (4, 5). If airflow obstruction occurs, CB is categorized as part of the chronic obstructive pulmonary disease (COPD) spectrum. Investigations into the pathogenesis of the mucus abnormalities in CB have included studies of mucus viscoelastic properties, sputum microbiology, and mucus clearance rates (6–10).
Despite these investigations, it has been difficult to generate a formulation that quantitatively describes the pathogenesis of the mucus abnormalities in CB or identifies objective biomarkers to complement the reliance on symptoms for diagnosis (11–15). Recently, a formulation has been proposed that integrates abnormalities of airway surface hydration and mucin hypersecretion into parameters related to mucus concentration to predict mucus flow in health versus absence of flow in disease (16, 17). In this “two-gel” model, the airway surface is comprised of two osmotically active polymer hydrogels, a mucus layer gel containing secreted mucins and a “brush-like” gel comprised of tethered mucins in the periciliary (PCL) region. The distribution of water between these two apposing hydrogels is governed by the partial osmotic pressures (“water drawing power”) generated by the concentrations of the high-molecular-weight polymers (typically mucins) and proteins in each layer.
Healthy airways sense and maintain normal mucus concentrations (hydration) via interactions of cilia with the mucus layer during cilial propulsion of mucus (18). Mucus concentration-dependent cilial strain governs the rate of ATP release onto airway surfaces, which regulates, via P2Y2R, the balance between liquid absorption from versus liquid secretion onto airway surfaces. Disease can override this regulatory system to produce relative dehydration of the mucus layer, (i.e., increased mucus concentration) (19, 20). When the concentration of the mucus layer rises to a level that generates a partial osmotic pressure exceeding basal PCL values, there is osmotic compression of the PCL; slowed mucus clearance; mucus adhesion and accumulation; and, we postulate, the sputum production characteristic of CB (11, 16).
In this study, we tested the hypothesis that dehydration of the mucus lining the conducting airways is a feature of CB and produces decreased mucus transport. This hypothesis was tested using cross-sectional measurements in normal nonsmoking subjects; smokers without symptoms or lung disease; and subjects with CB, which included (1) measures of mucus concentration (e.g., percentage solids), (2) mucus adenine nucleotide/nucleoside concentrations, and (3) mucus transport rates. In parallel, we characterized the relationship between sputum percentage solids and a key biophysical property that governs mucus flow (i.e., the mucus partial osmotic pressure) (16). Some of the results of these studies have been reported in the form of an abstract (21).
Methods
Patient Recruitment
The inclusion criteria for subjects with CB included chronic mucus production and cough for at least 3 months per year for 2 successive years, age 40–70, cigarette use (≥20 pack-years), and an FEV1 greater than 35% of predicted. Subjects with a history of atopy, a greater than 200-ml post-bronchodilator increase in FEV1, significant finding on electrocardiogram, oxygen use, or a medical disorder that placed the subject at risk, interfered with study evaluations, or participation, were excluded. Asymptomatic normal nonsmoking subjects (NS) and current cigarette smokers without symptoms and normal lung function (CS-N) served as control groups. The study was approved by the University of North Carolina institutional review board, and subjects provided written informed consent.
Airway Mucus Sampling and Sputum Induction
Bronchoscopic sampling of airway surface mucus was conducted by a modification of previously described techniques (22). Fiberoptic bronchoscopy was performed according to American Thoracic Society guidelines with moderate conscious sedation (23). No local anesthesia was used below the cords. To measure mucus percentage solids, preweighed, catheter-protected filter papers were placed for 20 seconds on the surface of the bronchus intermedius. The filter paper was withdrawn, immediately reweighed, dried overnight at 100°C, and reweighed after dry weight had plateaued. Wet-to-dry ratios were calculated as percentage solids (24). Airway lavages (a single 50-ml aliquot of sterile 0.9% saline) were next obtained from the right middle lobe. Bronchoscopies were performed 7–14 days after mucociliary clearance (MCC) measurements. Sputum was induced with hypertonic saline as previously reported, following the 24-hour MCC measurement (25).
Measurement of Sputum Percentage Solids, Total Mucins, and Partial Osmotic Pressure
Sputum percentage solids was measured as previously discussed (24). The contribution of inflammatory cells to sputum percentage solids was determined by an experimentally derived correction factor (see the Methods section in the online supplement). Total mucin concentrations were measured by differential refractometry combined with size exclusion chromatography (20). CB sputum partial osmotic pressures were measured in triplicate using a custom-designed direct-membrane colloid osmometer, incorporating a 10-kD membrane (16).
Sputum Extracellular Nucleotide, Nucleoside, and Nucleotidase Analyses
Sputum adenosine (ADO) and adenine nucleotides were ethenoderivatized and measured by HPLC analysis (26). Extracellular ATPase activity was measured in randomly selected bronchial wash samples (27).
Measurement of In Vitro Mucociliary Transport Rates
Airway epithelial cells from lungs of human organ donors were isolated as previously described (28). Human airway epithelial cultures (HBE) were left unwashed for 2–3 weeks to produce mucus with greater than 10% solids (16, 29). To measure MCC, fluorescent microspheres were added to the apical surface of HBE cultures approximately 16 hours before study (18, 30). MCC rates were calculated from time-lapse fluorescent images (30). Following baseline imaging, varying volumes of phosphate-buffered saline were nebulized onto the HBE surfaces to reduce mucus concentrations. Parallel HBE cultures were used to determine the concentration of the mucus layer after phosphate-buffered saline aerosol exposure using the mesh technique (16).
Measurement of Mucus Clearance In Vivo in Subjects with CB
MCC measurements by gamma scintigraphy were performed in subjects with CB in the whole, central, and peripheral lung zones using regions of interest (ROI) analyses (31, 32). Tracheobronchial (TB) clearance (1-TB retention) was calculated for the first 60 minutes of radiotracer clearance by subtraction of the 24-hour radiotracer retention (33). The average clearance over the 0–60 time points was computed as an overall index of clearance rates.
Statistical Analysis
All statistical analyses were conducted using SAS JMP Pro 9 statistical software (Cary, NC). Correlations were determined by Spearman correlation; F ratios less than 0.05 were considered significant. Group comparisons used the nonparametric Wilcoxon/Kruskal-Wallis test for nonnormally distributed values. Tukey-Kramer was used for all-pairs comparisons. Covariates were evaluated using multiple linear regression or analysis of covariance as appropriate.
Results
Baseline demographics and pulmonary function of the three subject groups are presented in Table 1. The two asymptomatic groups (NS and CS-N) were similar in baseline lung function and age. The subjects with CB were older, had a greater pack-years smoking history, and exhibited a spectrum of lung function.
Table 1.
Demographics and Baseline Pulmonary Function
| Nonsmoking, Nonsymptomatic | Smoking, Nonsymptomatic | Chronic Bronchitis | |
|---|---|---|---|
| N | 29 | 34 | 68 |
| Age | 26.4 ± 6.0 | 30.8 ± 8.2 | 58.7 ± 10.6 |
| Sex, % male | 66 | 53 | 57 |
| Current smokers, % | 0 | 100 | 44 |
| Pack-years | 0 | 9.0 ± 8.0 | 41.7 ± 25.2 |
| Race | |||
| White, % | 66 | 68 | 87 |
| Black, % | 21 | 32 | 13 |
| Hispanic, % | 7 | 0 | 0 |
| Asian, % | 7 | 0 | 0 |
| FVC, % predicted | 4.85 ± 1.05 (96.2) | 4.55 ± 1.03 (102.4) | 3.38 ± 1.01 (82.6) |
| FEV1, % predicted | 3.91 ± 0.8 (96.5) | 3.62 ± 0.74 (99.9) | 1.96 ± 0.87 (61.6) |
| FEV1/FVC | 0.81 ± 0.06 | 0.82 ± 0.05 | 57.3 ± 15.1 |
Mean ± SD.
FEV1, FVC, and FEV1/FVC are prebronchodilator for nonsymptomatic subjects and post-bronchodilator for subjects with chronic obstructive pulmonary disease.
Mucus and Sputum Percentage Solids and Total Mucin Concentrations
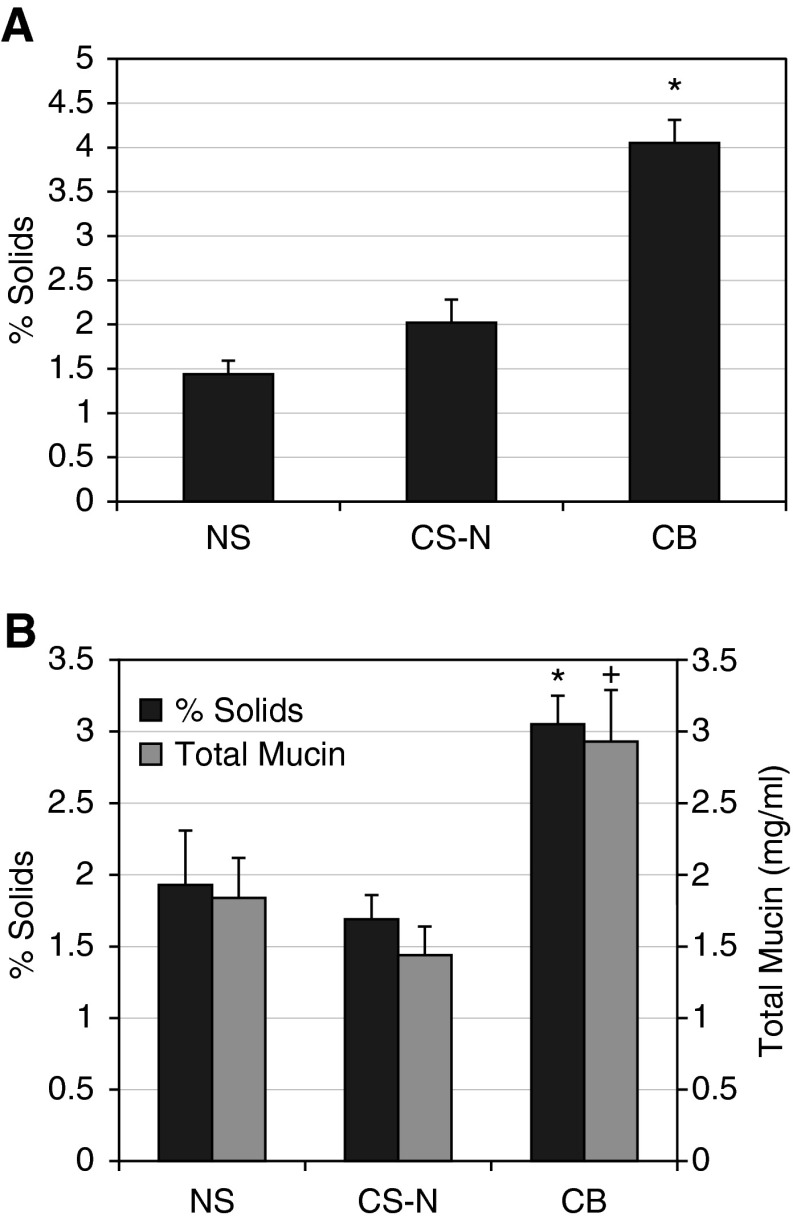
The mean percentage solids of mucus samples obtained bronchoscopically for each subject group is presented in Figure 1A. There was an approximately threefold increase in percentage solids in the subjects with CB as compared with both control groups (P < 0.0001). The smoking control subjects were not different than normal subjects. A similar pattern of percentage solids was observed for induced sputum (Figure 1B). The CB group exhibited significantly increased sputum percentage solids content versus the NS group (P = 0.006) and the CS-N group (P = 0.007). Again, the smoking control group did not differ from normal subjects.
Figure 1.
Mucus hydration and mucin concentrations in normal subjects (NS), normal smokers (CS-N), and subjects with chronic bronchitis (CB). (A) Percentage solids measured in mucus samples obtained by bronchoscopy. The data are presented as means ± SE for each of the three subject groups: NS (n = 24), CS-N (n = 22), and CB (n = 43). *CB versus NS and versus CS-N, P < 0.0001. (B) Sputum percentage solids as means ± SE NS (n = 29), CS-N (n = 29), and CB (n = 65); and total sputum mucin concentration as means ± SE NS (n = 15), CS-N (n = 15), and CB (n = 42). *The sputum percentage solids of the CB group were significantly higher than the NS (P = 0.006) and the CS-N (P = 0.0007) groups. +Similarly, the total mucin concentration was greater in the CB group versus the CS-N group (P = 0.032) and trended higher than the NS group (P = 0.15).
There was also a significant increase in sputum total mucins in the CB group versus CS-N (P = 0.032) (Figure 1B). A significant correlation between sputum percentage solids and sputum total mucins was observed in the CB group (ρ = 0.344; P = 0.03).
Sputum ATP and Metabolite Concentrations
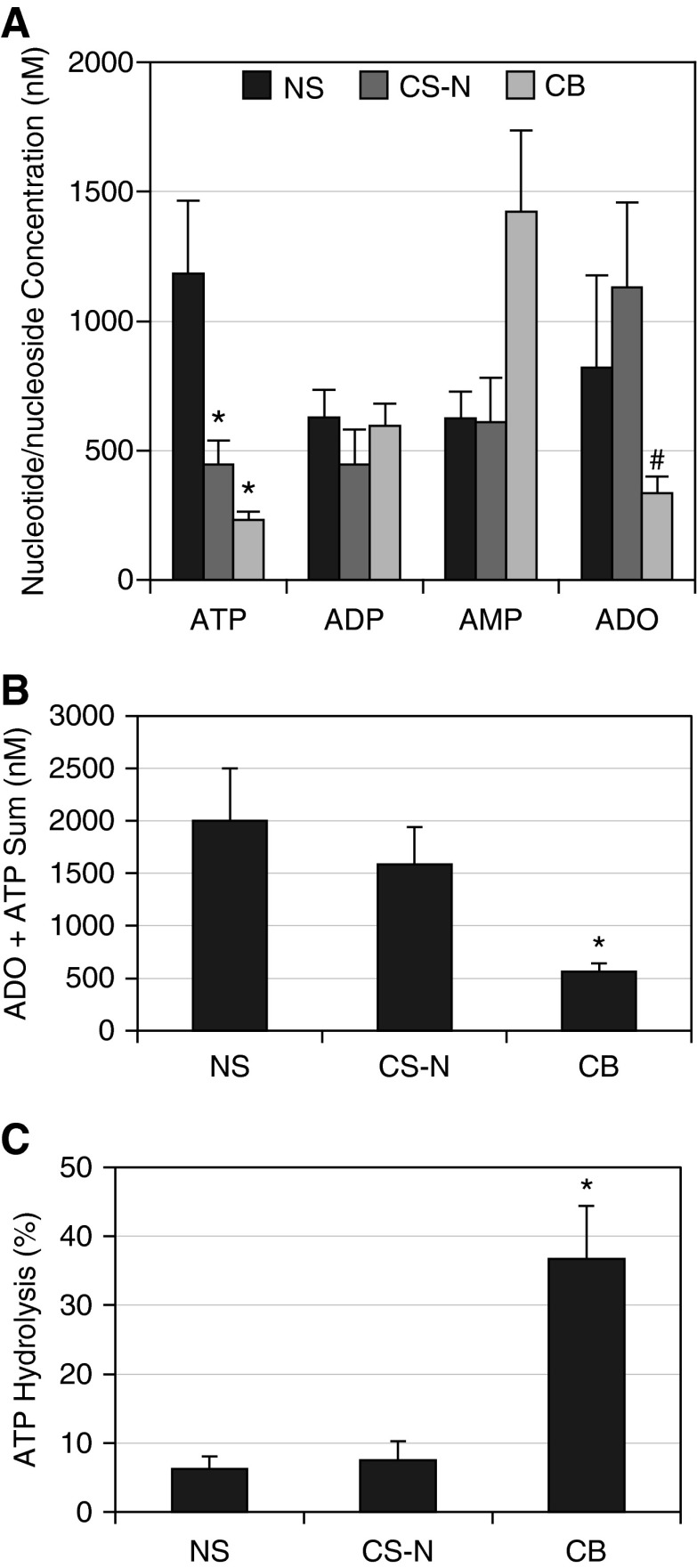
Total nucleotide concentrations in sputum were not statistically different among the three groups (P = 0.80). However, the pattern of sputum concentrations of ATP and ADO for the subject groups differed (Figure 2A). ATP was significantly reduced in the CS-N (P = 0.002) and CB (P < 0.0001) groups compared with the NS group. There were no significant differences between groups in ADP (adenosine diphosphate) or AMP (adenosine monophosphate). ADO was significant reduced in the subjects with CB as compared with the CS-N (P = 0.03). Because ATP and ADO are the two active nucleotides/nucleosides regulating airway surface liquid (ASL) volume and mucus concentration, the sum of ATP and ADO concentrations across groups was compared (Figure 2B) (18, 26). The combined ATP/ADO concentrations were significantly lower in the CB group (566 ± 73 mM) than the NS control group (2,003 ± 495 mM; P = 0.0006) or the CS-N group (1,588 ± 355 mM; P = 0.02).
Figure 2.
Sputum nucleotide/nucleoside concentrations. (A) Sputum adenyl nucleotide/nucleoside concentrations (nM) are plotted as means ± SE for the normal subjects (NS) (n = 21), normal smokers (CS-N) (n = 21), and chronic bronchitis (CB) (n = 45) groups. Comparisons for all pairs (Tukey-Kramer honestly significant difference) for ATP: *NS versus CS-N, P = 0.002; NS versus CB, P < 0.0001; ADP, no significant differences; AMP, no significant differences; ADO, #CS-N versus CB, P = 0.054. (B) Sum of ATP plus ADO concentrations presented as means ± SE for NS (n = 21), CS-N (n = 21), and CB (n = 45) groups. *The ATP/ADO sum (nM) in the CB group was significantly lower than the NS (P = 0.0006) and CS-N (P = 0.02) groups. There were no significant differences between the control groups. (C) Sputum Ecto-ATPase Activity (percentage hydrolysis): data are shown as means ± SE for the NS (n = 12), CS-N (n = 10), and CB (n = 17) groups. *Percentage hydrolysis was significantly greater in the CB group versus the NS (P = 0.005) and the CS-N (P = 0.006) groups. ADO = adenosine; ADP = adenosine diphosphate; AMP = adenosine monophosphate.
Total Ecto-ATPase activity (percentage ATP hydrolysis) was measured in a randomly selected subset of bronchial wash samples (Figure 2C). Ecto-ATPase activity was significantly raised in the CB group compared with the NS (P = 0.005) and CS-N (P = 0.006) groups.
Sputum Partial Osmotic Pressure
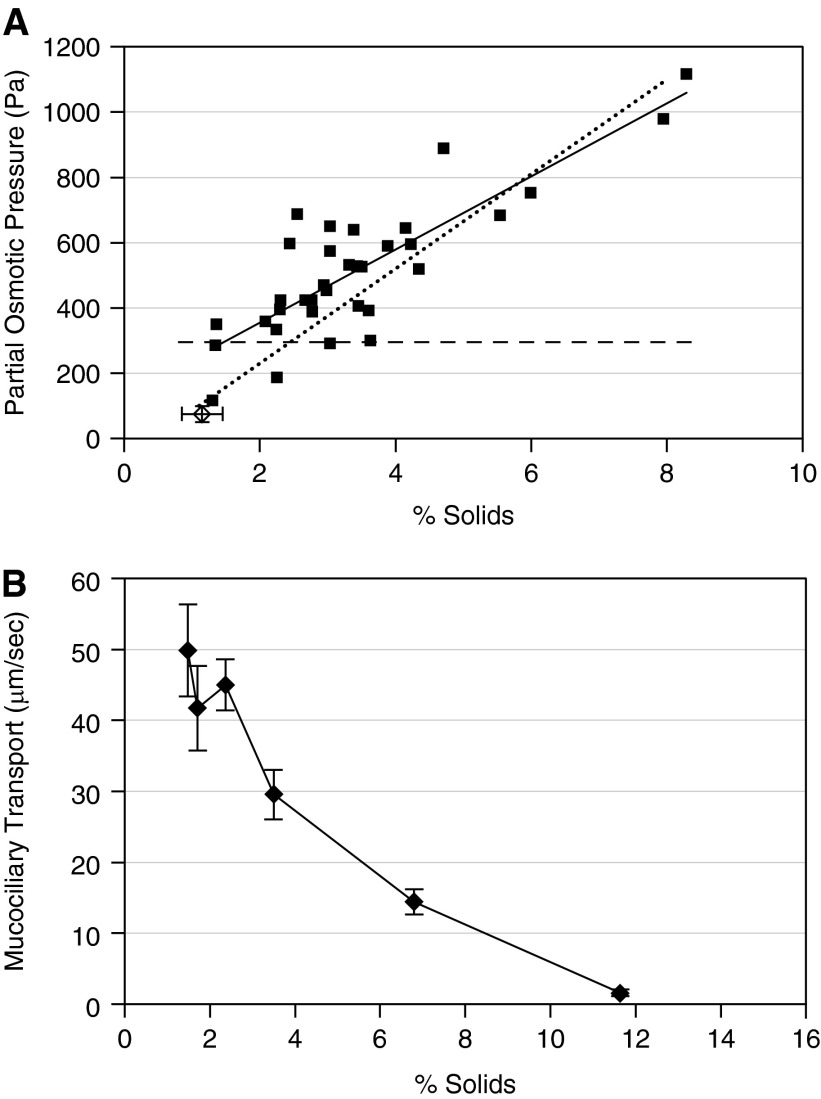
Spontaneous sputum samples were collected from six of the subjects with CB over multiple periods of time and percentage solids and partial osmotic pressures measured (Figure 3A). The individual data points are referenced to a dotted line describing the relationship between percentage solids and partial osmotic pressure of HBE mucus harvested in vitro and a dashed line depicting the basal partial osmotic pressure of the PCL (16). CB mucus samples exhibited a mucus concentration–partial osmotic pressure relationship similar to HBE mucus (solid line). The partial osmotic pressure of most spontaneously produced samples obtained from the subjects with CB (solid squares) exceeded that of previously measured basal PCL values (Figure 3A) (16). At approximately 3% solids, and above, the partial osmotic pressure of CB mucus is predicted to compress the PCL and slow MCC.
Figure 3.
Sputum percentage solids and partial osmotic pressure in subjects with chronic bronchitis and the relationship of percentage solids to mucus transport rates in vitro. (A) Correlation between percentage solids and partial osmotic pressure. Osmotic pressure was measured from a subset of the subjects on sputum samples collected at multiple visits, squares (n = 6; 34 samples). Solid line is Spearman correlation, ρ = 0.690, P < 0.0001. Dashed line is the osmotic pressure of the periciliary layer, and the dotted line is the relationship between percentage solids and mucus produced by airway epithelial cells in culture (16). The single open diamond represents the mean percentage solids ± SD (1.15 ± 0.3) and mean ± SD partial osmotic pressure (74.6 ± 24.3) for nine normal subjects reported previously (20). (B) Relationship between mucus concentration and mucociliary transport in vitro. Data are presented as means ± SEM, n = 4–6 per group.
Mucus Percentage Solids versus Mucus Clearance In Vitro and In Vivo
To test the hypothesis that increased mucus concentrations slow MCC in vitro, MCC was measured as a function of mucus concentration on HBE culture surfaces in vitro. MCC rates in vitro were consistent with in vivo rates in normal subjects in the range of 0.5–2% solids (Figure 3B) (34, 35). At higher percentage solids, MCC decreased with increased concentration and was virtually absent at percentage solids greater than 10%.
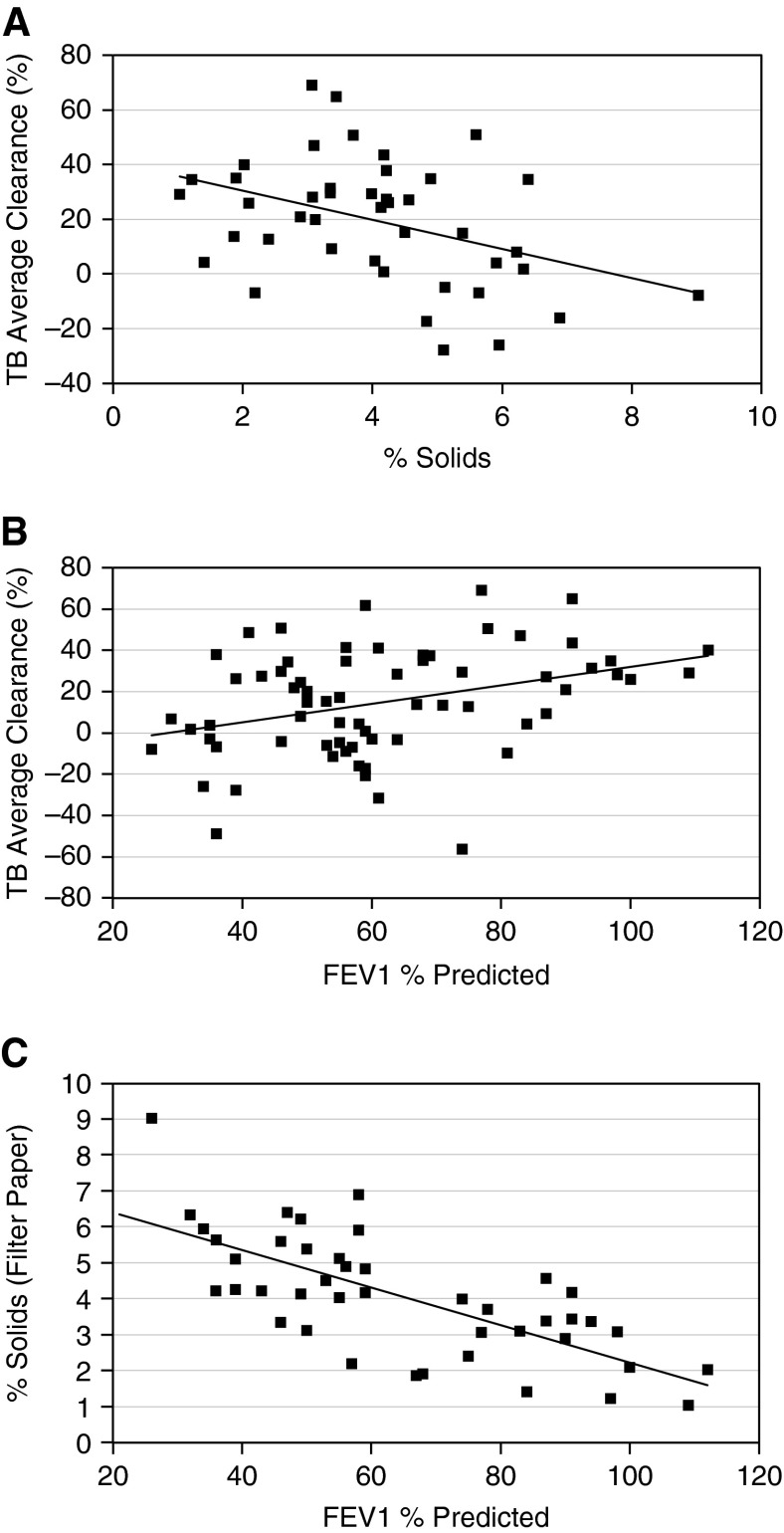
The relationship between bronchoscopic mucus percentage solids and MCC was also tested in vivo in subjects with CB (Figure 4A). The central ROI MCC data were chosen to match the source of the mucus samples. A negative correlation was observed between mucus concentration, indexed as percentage solids, and MCC for the CB group (ρ = −0.387; P = 0.01) (Figure 4A). A weaker but significant correlation was observed for induced sputum percentage solids and MCC (ρ = −0.267; P = 0.033).
Figure 4.
Relationship between mucus percentage solids, mucus clearance, and FEV1 in subjects with chronic bronchitis (CB). (A) Relationship between percentage solids (bronchoscopy) and average mucociliary clearance in the central lung region of subjects with CB (n = 43). The Spearman correlation is ρ = −0.387, P = 0.01. A simple linear regression line is added for visualization. (B) Relationship between FEV1% predicted and central lung TB average clearance for the subjects with CB. Spearman correlation coefficient, ρ = −0.338, P = 0.005. A simple linear regression line is included for visualization (n = 67). (C) Correlation of FEV1% predicted and percentage solids (bronchoscopic sample). FEV1% predicted is plotted against the percentage solids determined from the bronchoscopic samples for the subjects with CB (n = 43). ρ = −0.708; P < 0.0001. A simple linear regression line is included for visualization. TB = tracheobronchial.
Adjusting for age had minimal effect on the relationship between MCC and the bronchoscopically obtained percentage solids data. Adjusting for pack-years decreased the estimated slope as expected because of the causal role of cigarette smoke in producing the CB phenotype. Both slopes and intercepts for current and ex-smokers were not different, suggesting a similar relationship for these two groups.
Disease Status, MCC, and Mucus Percentage Solids
Slowing of MCC, as indexed by the central lung ROI, was associated with a reduced FEV1 in the subjects with CB (Figure 4B) (ρ = 0.338; P = 0.005). Significant correlations between MCC and FEV1 were also observed for the whole and peripheral lung ROIs with FEV1% predicted (ρ = 0.425, P = 0.0004; and ρ = 0.398, P = 0.001, respectively) (see Figures E1A and E1B in the online supplement). Spontaneous coughs were infrequent during the 0- to 60-minute MCC period, averaging 0.07, 0.31, and 1.58 coughs per 60 minutes for the NS, CS-N, and CB groups, respectively, indicating that cough did not influence the measured MCC rates.
Finally, we tested the relationship between mucus hydration and CB disease status. A highly significant negative correlation was observed between FEV1% predicted (an index of disease severity) and percentage solids obtained bronchoscopically (ρ = −0.708; P < 0.0001) in the CB group (Figure 4C). Similar significant correlations were observed between FEV1/FVC ratio and mucus percentage solids (ρ = 0.363; P = 0.0025).
Discussion
Efficient mucus clearance is key for the health of the lung. Genetic failures of mucus clearance (e.g., as in cystic fibrosis and primary ciliary dyskinesia) are associated with cough, sputum production, and bronchial pathology (36–39). Our study was designed to assess the relationship between an important property of mucus, its concentration (“hydration”), and mucus clearance in subjects with cigarette smoke–induced CB over a spectrum of disease severity.
The most accurate assessment of airway mucus concentration (hydration) is to directly sample the airway surface via the transbronchoscopic filter paper sampling technique (24). The concentration of normal subject’s mucus, as indexed as percentage solids, was similar to values reported previously for normal subjects (20) and was not different in asymptomatic smokers (Figure 1A). However, the concentration of CB mucus was twofold to threefold higher than normal. Because it is not practical to use bronchoscopically obtained samples for diagnosis, we also measured induced sputum percentage solids. Again, sputum percentage solids were increased in samples from subjects with CB. The modestly lower percentage solids in the CB-induced sputum versus filter paper samples may reflect hypertonic saline–induced sample dilution (20, 40). These data suggest airway mucus concentration could serve as a biomarker to complement the symptom-based diagnosis of CB.
It has been difficult to understand or quantitatively predict how “thickened” (concentrated) mucus contributes to disease pathogenesis in airways diseases, including CB. The “two-gel” hypothesis posits that the airway surface is comprised of two hydrogels whose non-Newtonian biophysical properties are largely determined by the relative concentrations of mucin polymers, and other large molecules, in the apposing mucin and PCL gel layers (Figure 5). In health the mucus layer, and its Muc5B and Muc5AC mucin polymers, are “well hydrated” (i.e., the mucus layer mucin concentrations and partial osmotic pressures are below those of the basal PCL values). In this state, the PCL is well hydrated and exhibits excellent lubricant activities, allowing the mucus layer to flow over the PCL with low friction. In disease, when epithelial ion/water transport is imbalanced to favor net absorption (e.g., because of reduced nucleotide/nucleoside signaling), water is first absorbed from the lower osmotic pressure environment of the mucus layer. When the osmotic pressure of the mucus layer rises to values equal to basal PCL values, transepithelial water absorption removes water from both layers, producing compression of and/or mucus penetration into the PCL, and slowing of MCC. Indeed, the predicted relationships between increased mucus percentage solids and decreased MCC were observed in our in vitro model system (Figure 3B).
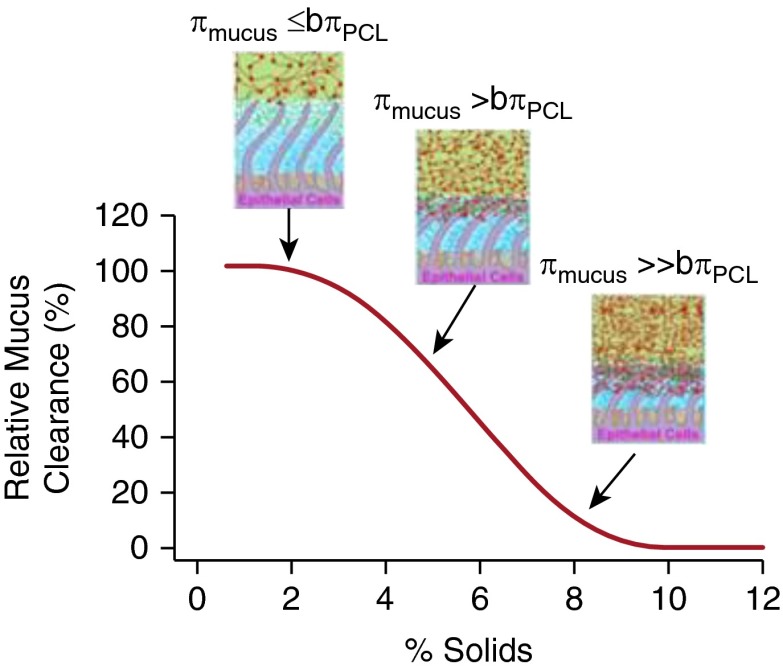
Figure 5.
Schema depicting relationships between mucus percentage solids, partial osmotic pressures of the mucus layer (πmucus), the periciliary layer (PCL) in the basal state (bπPCL), and mucus clearance rates. At normal mucus hydration (∼2% solids), the partial osmotic pressure of the mucus layer is below basal PCL values (bπPCL), the PCL is fully hydrated, and mucus transport is efficient. At modest levels of mucus dehydration (i.e., percentage solids ∼3–4%) the osmotic pressure of the mucus layer slightly exceeds the basal PCL level, modest compression of the PCL results, and mucus transport slows. When mucus dehydration is severe (i.e., percentage solids ∼7–8%) the mucus layer osmotically compresses and/or traps the cilia, producing mucus stasis and adhesion.
To link increased percentage solids to MCC rates in subjects with CB using the “two-gel” formulation, we directly tested the relationship between percentage solids and the partial osmotic pressure of CB sputum. The partial mucus osmotic pressure measures the osmotic pressure contributed by macromolecules (e.g., mucins) and not small proteins or small solutes (e.g., ions). As shown in Figure 3A, there was a strong correlation between CB sputum percentage solids and partial mucus osmotic pressures. Importantly, the relationship between CB sputum percentage solids and partial osmotic pressure did not differ significantly from the relationship between the percentage solids and partial osmotic pressures obtained from mucus of defined concentrations from HBE cultures (16). This finding suggests that there were no factors in CB sputum (e.g., cells, proteases, or DNA) that significantly affected the relationship between percentage solids and partial osmotic pressure in CB sputum. Thus, we conclude that increased mucus layer partial osmotic pressure, via PCL osmotic compression, provides a key link between sputum percentage solids and reduced MCC observed in CB in vivo (Figure 4A). The weaker correlation between mucus percentage solids and MCC observed in vivo (Figure 4A) versus in vitro (Figure 3B) may reflect technical issues inherent in in vivo measurements. For example, the mucus percentage solids measurement in vivo was obtained from a single airway, whereas the MCC measurements averaged multiple airways captured in the central ROI.
We also directly investigated one mechanism pertinent to control of airway mucus dehydration in CB. In the mammalian lung, ion transport rates and airway surface hydration are regulated in part by the extracellular purinergic system (18, 26, 41). Airway epithelial ATP release rates, balanced by extracellular metabolism, govern the levels of ATP and ADO in ASL that regulate epithelial ion and water transport via P2Y2-R and A2b-R, respectively (42). Notably, ATP is coreleased with mucins from mucus granules to regulate ciliated cell ion transport in a paracrine fashion to hydrate newly secreted mucins (43, 44). The reduced levels of ATP and ADO in CB secretions (Figure 2B) are consistent with increased Ecto-ATPase (Figure 2C) and ADO deaminase activities (40) in these secretions. The net effect of the reduced ATP and ADO concentrations in ASL is to inbalance Cl− secretion (reduced) versus Na+ absorption (increased), which reduces the volume of ASL and increases mucus percentage solids.
Our finding of lower ATP concentrations in CB sputum contrasts with previous reports of raised ATP levels in bronchoalveolar lavage from subjects with COPD (45). Nucleotide levels in extracellular fluids vary substantially based on methods of sample collection. ATP release can be increased by many stimuli, including shear stress (41, 46, 47), a variable associated with large-volume bronchoalveolar lavage. Our values for sputum ATP in normal subjects are similar to small-volume nasal lavage values (48), consistent with the notion that sputum collection had no major effects on ATP release. The lack of a difference in total adenyl nucleotides between normal and CB samples also suggests that abnormal metabolism, not release, produced the abnormal CB sputum ATP levels (Figure 2C).
The low ADO concentrations observed in CB sputum (Figure 2A) are consistent with increased ADO deaminase activity in CB sputum. However, these results differed from our exhaled breath condensate (EBC) data from subjects with COPD that showed raised ADO levels (49). Induced sputum is believed to originate from large airways, whereas EBC is thought to originate from the small airways of the peripheral lung (25, 50, 51). Thus, the disparate values for ADO in COPD sputum versus EBC samples may reflect sampling of different airway regions, with different ADO metabolic paths, affected by CB.
A key question that arose from our study was whether airway mucus hyperconcentration and decreased MCC were associated with CB disease severity. Similar to Smaldone and others (52, 53), decreased mucus clearance rates were correlated with decreased FEV1, suggesting that a decline in MCC is associated with disease severity (Figure 4B). Importantly, the relationship between reduced FEV1 and increased mucus concentration (percentage solids) in subjects with CB was if anything stronger, consistent with the notion that mucus dehydration, via reduced MCC, is also associated with disease severity (Figure 4C).
An unresolved question from our study is whether increased mucus concentration (percentage solids), reflecting in part cigarette smoke–induced effects on hydration and/or mucin hypersecretion (14, 54, 55), drives the progression of disease or results from disease progression. This question has been most rigorously studied in animal models. The βENaC transgenic mouse exhibits selective Na+ transport mediated airway dehydration (i.e., increased percentage solids) and manifests the entire spectrum of the COPD phenotype, including mucus plugging, airways inflammation, remodeling, infection, and emphysema (56, 57). These data suggest that it is plausible mucus hyperconcentration, coupled to mucus adhesion to airway surfaces, can drive disease pathogenesis. However, in a disease as complex as environmental CB, it is likely there are multiple interactions between mucus hyperconcentration and disease progression. For example, cigarette smoke produces inflammatory responses that may be distinct from, and/or interact with, the inflammatory response induced by mucus stasis per se (54, 55, 58). Similarly, adherent mucus is the site of bacterial infection in CB so concentrated mucus may promote infection in the CB lung, adding another component to disease progression (9).
Mucoactive agents including expectorants, mucolytics, mucokinetics, and mucoregulator agents have been used with only limited success in mucus hypersecretory diseases, primarily cystic fibrosis (59). Mucolytic agents were reviewed in a Cochrane Review assessing 26 clinical trials in COPD (60). A small but significant effect of mucolytic agents on exacerbation frequency was observed in the metaanalysis. Ultimately, therapies designed specifically to reduce mucus concentration (e.g., using hydrating therapies and/or suppressors of mucin secretion) may be required to rigorously evaluate the role of mucus hyperconcentration in the pathogenesis of COPD.
In summary, increases in mucus percentage solids into ranges that may produce osmotic compression of the PCL were observed in bronchoscopic and sputum samples from CB but not control subjects. These data suggest that alterations in the variables that control mucus concentration (e.g., extracellular nucleotide/nucleoside-dependent airway hydration and mucin secretion rates) may slow MCC and contribute to disease pathogenesis and loss of lung function in CB. These data also suggest that mucus concentration (percentage solids) could serve as a diagnostic biomarker for CB and may warrant testing in populations with COPD without and with the CB phenotype.
Acknowledgments
Acknowledgment
The authors thank Jihong Wu and Heather Duckworth for in vivo mucociliary clearance acquisition and analysis, and Catharina van Heusden for technical help with the HPLC-based nucleotide measurements.
Footnotes
Supported by National Institutes of Health Specialized Centers of Clinically Orientated Research (5P50HL084934), Translational Program Project Grant (P01HL108808), and Molecular Therapy Core Center (P30DK065988) grants and Cystic Fibrosis Foundation Therapeutics grant BUTTON07XX0.
Author Contributions: Concept, design, acquisition of data, analysis, and interpretation, W.H.A., A.G.H., B.B., W.D.B., K.L.Z., N.E.A., E.R.L., C.W.D., S.B., F.F., M.A., E.B., D.B.P., B.Q., R.D.C., M.K., and R.C.B. Drafting and revising, W.H.A., B.B., K.L.Z., R.D.C., W.D.B., N.E.A., E.R.L., M.R., M.K., and R.C.B. Final approval, all authors.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201412-2230OC on April 24, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Drummond MB, Buist AS, Crapo JD, Wise RA, Rennard SI. Chronic obstructive pulmonary disease: NHLBI workshop on the primary prevention of chronic lung diseases. Ann Am Thorac Soc. 2014;11:S154–S160. doi: 10.1513/AnnalsATS.201312-432LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agusti A, Sobradillo P, Celli B. Addressing the complexity of chronic obstructive pulmonary disease: from phenotypes and biomarkers to scale-free networks, systems biology, and P4 medicine. Am J Respir Crit Care Med. 2011;183:1129–1137. doi: 10.1164/rccm.201009-1414PP. [DOI] [PubMed] [Google Scholar]
- 3.Global initiative for chronic obstructive lung disease. 2014.
- 4.Innes AL, Woodruff PG, Ferrando RE, Donnelly S, Dolganov GM, Lazarus SC, Fahy JV. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest. 2006;130:1102–1108. doi: 10.1378/chest.130.4.1102. [DOI] [PubMed] [Google Scholar]
- 5.Martinez CH, Kim V, Chen Y, Kazerooni EA, Murray S, Criner GJ, Curtis JL, Regan EA, Wan E, Hersh CP, et al. COPDGene Investigators. The clinical impact of non-obstructive chronic bronchitis in current and former smokers. Respir Med. 2014;108:491–499. doi: 10.1016/j.rmed.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 7.Scheuch G, Kohlhäufl M, Möller W, Brand P, Meyer T, Häussinger K, Sommerer K, Heyder J. Particle clearance from the airways of subjects with bronchial hyperresponsiveness and with chronic obstructive pulmonary disease. Exp Lung Res. 2008;34:531–549. doi: 10.1080/01902140802341710. [DOI] [PubMed] [Google Scholar]
- 8.Foster WM. Mucociliary transport and cough in humans. Pulm Pharmacol Ther. 2002;15:277–282. doi: 10.1006/pupt.2002.0351. [DOI] [PubMed] [Google Scholar]
- 9.Sethi S. Chronic obstructive pulmonary disease and infection: disruption of the microbiome? Ann Am Thorac Soc. 2014;11:S43–S47. doi: 10.1513/AnnalsATS.201307-212MG. [DOI] [PubMed] [Google Scholar]
- 10.Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J. 2002;361:537–546. doi: 10.1042/0264-6021:3610537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012;26:533–545. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caramori G, Di Gregorio C, Carlstedt I, Casolari P, Guzzinati I, Adcock IM, Barnes PJ, Ciaccia A, Cavallesco G, Chung KF, et al. Mucin expression in peripheral airways of patients with chronic obstructive pulmonary disease. Histopathology. 2004;45:477–484. doi: 10.1111/j.1365-2559.2004.01952.x. [DOI] [PubMed] [Google Scholar]
- 13.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007;261:5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 14.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birket SE, Chu KK, Liu L, Houser GH, Diephuis BJ, Wilsterman EJ, Dierksen G, Mazur M, Shastry S, Li Y, et al. A functional anatomic defect of the cystic fibrosis airway. Am J Respir Crit Care Med. 2014;190:421–432. doi: 10.1164/rccm.201404-0670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Button B, Cai L-H, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickey BF. Walking on solid ground. Science. 2012;337:924–925. doi: 10.1126/science.1227091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Button B, Okada SF, Frederick CB, Thelin WR, Boucher RC. Mechanosensitive ATP release maintains proper mucus hydration of airways. Sci Signal. 2013;6:ra46. doi: 10.1126/scisignal.2003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill DB, Vasquez PA, Mellnik J, McKinley SA, Vose A, Mu F, Henderson AG, Donaldson SH, Alexis NE, Boucher RC, et al. A biophysical basis for mucus solids concentration as a candidate biomarker for airways disease. PLoS One. 2014;9:e87681. doi: 10.1371/journal.pone.0087681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson AG, Ehre C, Button B, Abdullah LH, Cai L-H, Leigh MW, DeMaria GC, Matsui H, Donaldson SH, Davis CW, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124:3047–3060. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson WH, Coakley RD, Button B, Henderson AG, Zeman KL, Alexis NE, Peden D, Lazarowski ER, Davis CW, Fuller F, et al. The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis [abstract] Am J Respir Crit Care Med. 2015;191:A2874. doi: 10.1164/rccm.201412-2230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noone PG, Regnis JA, Liu X, Brouwer KLR, Robinson M, Edwards L, Knowles MR. Airway deposition and clearance and systemic pharmacokinetics of amiloride following aerosolization with an ultrasonic nebulizer to normal airways. Chest. 1997;112:1283–1290. doi: 10.1378/chest.112.5.1283. [DOI] [PubMed] [Google Scholar]
- 23.Hattotuwa K, Gamble EA, O’Shaughnessy T, Jeffery PK, Barnes NC. Safety of bronchoscopy, biopsy, and BAL in research patients with COPD. Chest. 2002;122:1909–1912. doi: 10.1378/chest.122.6.1909. [DOI] [PubMed] [Google Scholar]
- 24.Knowles MR, Robinson JM, Wood RE, Pue CA, Mentz WM, Wager GC, Gatzy JT, Boucher RC. Ion composition of airway surface liquid of patients with cystic fibrosis as compared with normal and disease-control subjects. J Clin Invest. 1997;100:2588–2595. doi: 10.1172/JCI119802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexis NE, Hu S-C, Zeman K, Alter T, Bennett WD. Induced sputum derives from the central airways: confirmation using a radiolabeled aerosol bolus delivery technique. Am J Respir Crit Care Med. 2001;164:1964–1970. doi: 10.1164/ajrccm.164.10.2104051. [DOI] [PubMed] [Google Scholar]
- 26.Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem. 2004;279:36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- 28.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 29.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 30.Button B, Picher M, Boucher RC. Differential effects of cyclic and constant stress on ATP release and mucociliary transport by human airway epithelia. J Physiol. 2007;580:577–592. doi: 10.1113/jphysiol.2006.126086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett WD, Laube BL, Corcoran T, Zeman K, Sharpless G, Thomas K, Wu J, Mogayzel PJ, Jr, Pilewski J, Donaldson S. Multisite comparison of mucociliary and cough clearance measures using standardized methods. J Aerosol Med Pulm Drug Deliv. 2013;26:157–164. doi: 10.1089/jamp.2011.0909. [DOI] [PubMed] [Google Scholar]
- 32.Zeman KL, Wu J, Donaldson SH, Bennett WD. Comparison of 133 xenon ventilation equilibrium scan (XV) and 99m technetium transmission (TT) scan for use in regional lung analysis by 2D gamma scintigraphy in healthy and cystic fibrosis lungs. J Aerosol Med Pulm Drug Deliv. 2013;26:94–100. doi: 10.1089/jamp.2012.0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett WD, Almond MA, Zeman KL, Johnson JG, Donohue JF. Effect of salmeterol on mucociliary and cough clearance in chronic bronchitis. Pulm Pharmacol Ther. 2006;19:96–100. doi: 10.1016/j.pupt.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Asgharian B, Hofmann W, Miller FJ. Mucociliary clearance of insoluble particles from the tracheobronchial airways of the human lung. J Aerosol Sci. 2001;32:817–832. [Google Scholar]
- 35.Foster WM, Langenback E, Bergofsky EH. Measurement of tracheal and bronchial mucus velocities in man: relation to lung clearance. J Appl Physiol. 1980;48:965–971. doi: 10.1152/jappl.1980.48.6.965. [DOI] [PubMed] [Google Scholar]
- 36.Munkholm M, Mortensen J. Mucociliary clearance: pathophysiological aspects. Clin Physiol Funct Imaging. 2014;34:171–177. doi: 10.1111/cpf.12085. [DOI] [PubMed] [Google Scholar]
- 37.Zariwala MA, Knowles MR, Leigh MW. Primary ciliary dyskinesia. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. Seattle, WA: University of Washington; 2007. pp. 1993–2015. [PubMed] [Google Scholar]
- 38.Regnis JA, Robinson M, Bailey DL, Cook P, Hooper P, Chan H-K, Gonda I, Bautovich G, Bye PTP. Mucociliary clearance in patients with cystic fibrosis and in normal subjects. Am J Respir Crit Care Med. 1994;150:66–71. doi: 10.1164/ajrccm.150.1.8025774. [DOI] [PubMed] [Google Scholar]
- 39.Mall MA, Hartl D. CFTR: cystic fibrosis and beyond. Eur Respir J. 2014;44:1042–1054. doi: 10.1183/09031936.00228013. [DOI] [PubMed] [Google Scholar]
- 40.Henderson AG, Fuller F, Anderson WH, Alexis NE, Lazarowski ER, Kesimer M, Bordonali E, Qaqish B, Boucher RC. Differences between induced sputum in chronic obstructive pulmonary disease (COPD) [abstract] Am J Respir Crit Care Med. 2015;191:A2914. [Google Scholar]
- 41.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, et al. Normal and cystic fibrosis airway surface liquid homeostasis: the effects of phasic shear stress and viral infections. J Biol Chem. 2005;280:35751–35759. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012;8:359–373. doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sesma JI, Kreda SM, Okada SF, van Heusden C, Moussa L, Jones LC, O’Neal WK, Togawa N, Hiasa M, Moriyama Y, et al. Vesicular nucleotide transporter regulates the nucleotide content in airway epithelial mucin granules. Am J Physiol Cell Physiol. 2013;304:C976–C984. doi: 10.1152/ajpcell.00371.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kreda SM, Okada SF, van Heusden CA, O’Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol. 2007;584:245–259. doi: 10.1113/jphysiol.2007.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lommatzsch M, Cicko S, Müller T, Lucattelli M, Bratke K, Stoll P, Grimm M, Dürk T, Zissel G, Ferrari D, et al. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:928–934. doi: 10.1164/rccm.200910-1506OC. [DOI] [PubMed] [Google Scholar]
- 46.Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol. 2000;150:1349–1360. doi: 10.1083/jcb.150.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem. 2006;281:22992–23002. doi: 10.1074/jbc.M603019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donaldson SH, Lazarowski ER, Picher M, Knowles MR, Stutts MJ, Boucher RC. Basal nucleotide levels, release, and metabolism in normal and cystic fibrosis airways. Mol Med. 2000;6:969–982. [PMC free article] [PubMed] [Google Scholar]
- 49.Esther CR, Jr, Lazaar AL, Bordonali E, Qaqish B, Boucher RC. Elevated airway purines in COPD. Chest. 2011;140:954–960. doi: 10.1378/chest.10-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Almstrand A-C, Bake B, Ljungström E, Larsson P, Bredberg A, Mirgorodskaya E, Olin A-C. Effect of airway opening on production of exhaled particles. J Appl Physiol (1985) 2010;108:584–588. doi: 10.1152/japplphysiol.00873.2009. [DOI] [PubMed] [Google Scholar]
- 51.Johnson GR, Morawska L. The mechanism of breath aerosol formation. J Aerosol Med Pulm Drug Deliv. 2009;22:229–237. doi: 10.1089/jamp.2008.0720. [DOI] [PubMed] [Google Scholar]
- 52.Smaldone GC, Foster WM, O’Riordan TG, Messina MS, Perry RJ, Langenback EG. Regional impairment of mucociliary clearance in chronic obstructive pulmonary disease. Chest. 1993;103:1390–1396. doi: 10.1378/chest.103.5.1390. [DOI] [PubMed] [Google Scholar]
- 53.Goodman RM, Yergin BM, Landa JF, Golivanux MH, Sackner MA. Relationship of smoking history and pulmonary function tests to tracheal mucous velocity in nonsmokers, young smokers, ex-smokers, and patients with chronic bronchitis. Am Rev Respir Dis. 1978;117:205–214. doi: 10.1164/arrd.1978.117.2.205. [DOI] [PubMed] [Google Scholar]
- 54.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 55.Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, Tidwell S, Tang LP, Liu B, Fortenberry JA, et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med. 2013;188:1321–1330. doi: 10.1164/rccm.201304-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 57.Mall MA, Harkema JR, Trojanek JB, Treis D, Livraghi A, Schubert S, Zhou Z, Kreda SM, Tilley SL, Hudson EJ, et al. Development of chronic bronchitis and emphysema in β-epithelial Na+ channel-overexpressing mice. Am J Respir Crit Care Med. 2008;177:730–742. doi: 10.1164/rccm.200708-1233OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Livraghi-Butrico A, Grubb BR, Kelly EJ, Wilkinson KJ, Yang H, Geiser M, Randell SH, Boucher RC, O’Neal WK. Genetically determined heterogeneity of lung disease in a mouse model of airway mucus obstruction. Physiol Genomics. 2012;44:470–484. doi: 10.1152/physiolgenomics.00185.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rogers DF. Mucoactive agents for airway mucus hypersecretory diseases. Respir Care. 2007;52:1176–1193, discussion 1193–1197. [PubMed] [Google Scholar]
- 60.Poole P, Black PN. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010;(2):CD001287. doi: 10.1002/14651858.CD001287.pub3. [DOI] [PubMed] [Google Scholar]