To the Editor:
Respiratory infections are responsible for tremendous mortality and morbidity worldwide (1). The advent of culture-independent techniques of bacterial identification and the discovery of the lung microbiome have prompted reconsideration of existing models of pneumonia pathogenesis (2, 3). Multiple studies have demonstrated an in vitro association between catecholamines and growth of select bacteria, including prominent members of the lung microbiome, including Pseudomonas aeruginosa and Streptococcus pneumoniae (4–6). A positive-feedback loop involving alveolar inflammation, intraalveolar catecholamines, and the selective growth promotion of pathogens has been posited as a novel mechanism of pneumonia pathogenesis (2, 4, 6). However, no study has measured intraalveolar catecholamines or determined their association with changes in the lung microbiome.
Using 40 clinically obtained bronchoalveolar lavage (BAL) specimens from lung transplant recipients, we compared intraalveolar catecholamine concentrations with the composition of the bacterial lung microbiome. Twenty-two specimens were obtained during bronchoscopies for an acute clinical indication (cough, sputum production, abnormal chest imaging, or worsened lung function), and 18 were performed on asymptomatic patients for posttransplant surveillance. No subjects were taking inhaled β-agonists at the time of bronchoscopy. All patients were receiving both systemic immunosuppression and Pneumocystis jirovecii prophylaxis at the time of bronchoscopy; additional antibiotic exposure in both groups has been previously reported (7). Patient characteristics, methods of bronchoscopy, DNA isolation and amplification, pyrosequencing, and analysis have previously been published (7, 8). Catecholamines were quantified using 2-CAT (A-N) Research ELISA (Rocky Mountain Diagnostics, Colorado Springs, CO). We measured associations between catecholamine levels and lung microbiota using linear regression. In permutation testing (permutational multivariate analysis of variance) of community composition (adonis function in vegan), we analyzed alveolar catecholamine levels as log-adjusted continuous environmental variables.
Norepinephrine was detected in 26 (65%) specimens (range, 2.6–105.0 pg/ml), and epinephrine was detected in 33 (83%) specimens (range, 0.8–40.7 pg/ml). Five (13%) specimens had no detectable norepinephrine or epinephrine; all five were obtained from asymptomatic patients undergoing surveillance bronchoscopy. Symptomatic patients had higher total alveolar catecholamines than patients undergoing surveillance bronchoscopy (P = 0.01). Intraalveolar catecholamine levels were significantly associated with culture-independent indices of acute infection, including total bacterial burden and BAL neutrophilia (Table 1).
Table 1.
Regression Analysis of Bronchoalveolar Lavage Bacterial Microbiome Features versus Intraalveolar Catecholamine Concentration
| BAL/Microbiome Feature | Norepinephrine |
Epinephrine |
Total |
Direction of Relationship | |||
|---|---|---|---|---|---|---|---|
| P Value | R2 | P Value | R2 | P Value | R2 | ||
| Bacterial (16S) DNA, log(copies/5 ml) | 0.198 | 0.043 | 0.047 | 0.100 | 0.051 | 0.097 | Positive |
| Percentage neutrophils | 0.129 | 0.063 | 0.001 | 0.280 | 0.006 | 0.193 | Positive |
| Shannon diversity index | 0.048 | 0.099 | 0.016 | 0.144 | 0.010 | 0.163 | Negative |
| Maximum OTU % | 0.003 | 0.208 | 0.014 | 0.150 | 0.001 | 0.271 | Positive |
| OTU 969 (Pseudomonas fluorescens), % total community | 0.006 | 0.183 | 0.063 | 0.088 | 0.005 | 0.190 | Negative |
Definition of abbreviations: BAL = bronchoalveolar lavage; OTU = operational taxonomic unit.
Catecholamine concentrations (pg/ml) were log-transformed before linear regression.
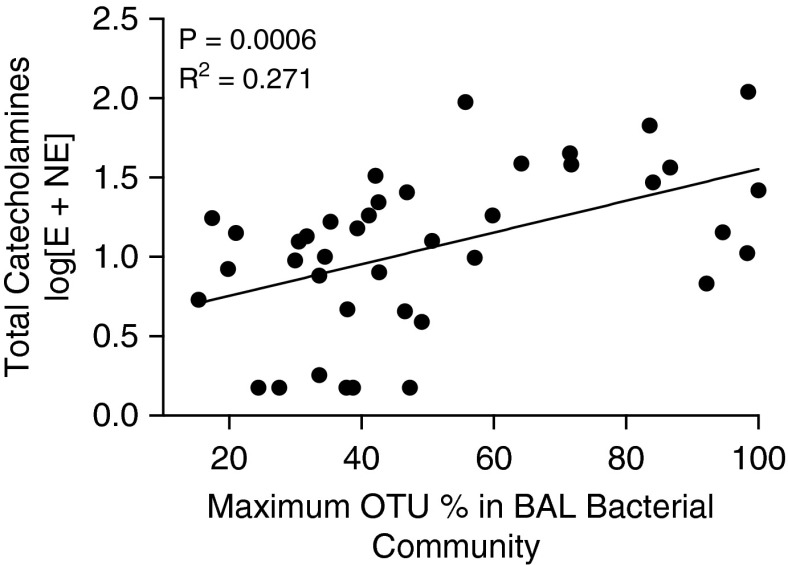
Increased intraalveolar catecholamine concentrations were strongly associated with decreased community diversity in the bacterial lung microbiome, a feature of pneumonia (8). As measured by the Shannon diversity index, diversity was negatively correlated with norepinephrine and epinephrine concentrations (P ≤ 0.05 for each) and combined catecholamine concentration (P = 0.009). The emergence of a single dominant bacterial species, as measured by maximum operational taxonomic unit (OTU) percentage (the highest relative abundance of a single community member in a bacterial community), was significantly associated with greater intraalveolar concentrations of norepinephrine (P = 0.003), epinephrine (P = 0.014), and total catecholamines (P = 0.0006) (Figure 1). Thus, intraalveolar catecholamines, both norepinephrine and epinephrine, are associated with collapse of community diversity to domination by a single bacterial species.
Figure 1.
Increased intraalveolar catecholamines in conditions of low lung microbiome diversity. High concentrations of epinephrine, norepinephrine, and total catecholamines are associated with emergence of a single dominant bacterial species. Maximum OTU percentage represents the relative abundance of most abundant single community members in a specimen’s bacterial community. Catecholamine concentrations are reported as log-transformed picograms per milliliter. BAL = bronchoalveolar lavage; E = epinephrine; NE = norepinephrine; OTU = operational taxonomic unit.
We then asked whether the community membership of the bacterial lung microbiome was associated with concentrations of intraalveolar catecholamines. As tested by adonis (permutational multivariate analysis of variance), the community membership of BAL specimens was significantly associated with differences in total catecholamine levels (P = 0.010). Specifically, high-catecholamine specimens were relatively enriched with P. aeruginosa, which was present in low abundance in low- and moderate-catecholamine specimens. Conversely, a prominent nonaeruginosa pseudomonad, Pseudomonas fluorescens, was enriched in low-catecholamine specimens and absent from high-catecholamine specimens (Table 1). Its relative abundance was negatively associated with intraalveolar norepinephrine and total catecholamine concentrations (P ≤ 0.01 for each). These associations remained significant when adjusted for indices of acute infection (Shannon diversity index and total bacterial DNA, P ≤ 0.05 for each). Thus, community membership of the bacterial lung microbiome is significantly associated with intraalveolar catecholamine concentrations.
This study is the first to report an association between increased intraalveolar catecholamines and both indices of acute infection and emergence of specific microbiome community members as a dominant species. Our results support the in vivo plausibility of a previously postulated (2, 4, 6) positive feedback loop that propels the inflammation and bacterial growth of acute pneumonia: select bacterial growth provokes alveolar inflammation, resulting in increased intraalveolar catecholamines, which in turn further provoke selective bacterial growth among catecholamine-responsive species. P. aeruginosa was the community member most enriched in high-catecholamine conditions; this finding is consistent with past in vitro demonstrations that P. aeruginosa growth is dose-dependently promoted by various catecholamines (4, 5). P. aeruginosa is the most commonly isolated respiratory pathogen in a variety of clinical settings, including ventilator-associated pneumonia; thus, our findings may have broad mechanistic implications in the pathogenesis of respiratory infections. Interestingly, we found a strong and independent negative association between intraalveolar catecholamines and P. fluorescens, which we have shown to be a prominent, nonpathogenic inhabitant of the human lung microbiome (7, 9). The mechanism and significance of this negative association is undetermined. We also observed a strong correlation between intraalveolar catecholamine levels and the abundance of alveolar neutrophils; this finding is consistent with experimental evidence that neutrophils generate catecholamines and contribute to alveolar inflammatory injury (10).
Catecholamines, both endogenous and exogenous, are prominent in the pathogenesis and treatment of respiratory disease and critical illness. This is the first report of an association between endogenous catecholamines and features of the bacterial lung microbiome. To our knowledge, nothing is known about the effects of exogenous catecholamines, adrenergic agonists, or adrenergic antagonists on the bacterial lung microbiome. Our results suggest that further study is warranted to determine whether microbe–host interactions via catecholamines contribute to the pathogenesis of respiratory infections.
Footnotes
Supported by National Institutes of Health grants T32HL00774921 (R.P.D. and H.C.P.), U01HL098961 (J.L.C. and G.B.H.), R01HL094622 (V.N.L.), R01HL118017 (V.N.L.), and R01HL114447 (G.B.H. and F.J.M.); by the Biomedical Laboratory and Clinical Science Research and Development Services, Department of Veterans Affairs (J.L.C.); and by the Host Microbiome Initiative of the University of Michigan.
Author Contributions: Conception and design: R.P.D., J.R.E.-D., G.B.H., and J.L.C.; acquisition of data: V.N.L., R.P.D., and G.B.H.; analysis and interpretation of data: R.P.D., J.R.E.-D., G.B.H., H.C.P., and J.L.C.; drafting or revising of manuscript: R.P.D., J.R.E.-D., H.C.P., F.J.M., V.N.L., J.L.C., and G.B.H.; and final approval of manuscript: R.P.D., J.R.E.-D., H.C.P., F.J.M., V.N.L., J.L.C., and G.B.H.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Mizgerd JP. Lung infection—a public health priority. PLoS Med. 2006;3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickson RP, Erb-Downward JR, Huffnagle GB. Towards an ecology of the lung: new conceptual models of pulmonary microbiology and pneumonia pathogenesis. Lancet Respir Med. 2014;2:238–246. doi: 10.1016/S2213-2600(14)70028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet. 2014;384:691–702. doi: 10.1016/S0140-6736(14)61136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freestone PP, Hirst RA, Sandrini SM, Sharaff F, Fry H, Hyman S, O’Callaghan C. Pseudomonas aeruginosa-catecholamine inotrope interactions: a contributory factor in the development of ventilator-associated pneumonia? Chest. 2012;142:1200–1210. doi: 10.1378/chest.11-2614. [DOI] [PubMed] [Google Scholar]
- 5.Lyte M, Ernst S. Catecholamine induced growth of gram negative bacteria. Life Sci. 1992;50:203–212. doi: 10.1016/0024-3205(92)90273-r. [DOI] [PubMed] [Google Scholar]
- 6.Marks LR, Davidson BA, Knight PR, Hakansson AP. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. MBio. 2013;4:4. doi: 10.1128/mBio.00438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickson RP, Erb-Downward JR, Freeman CM, Walker N, Scales BS, Beck JM, Martinez FJ, Curtis JL, Lama VN, Huffnagle GB. Changes in the lung microbiome following lung transplantation include the emergence of two distinct Pseudomonas species with distinct clinical associations. PLoS One. 2014;9:e97214. doi: 10.1371/journal.pone.0097214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickson RP, Erb-Downward JR, Prescott HC, Martinez FJ, Curtis JL, Lama VN, Huffnagle GB. Analysis of culture-dependent versus culture-independent techniques for identification of bacteria in clinically obtained bronchoalveolar lavage fluid. J Clin Microbiol. 2014;52:3605–3613. doi: 10.1128/JCM.01028-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scales BS, Dickson RP, LiPuma JJ, Huffnagle GB. Microbiology, genomics, and clinical significance of the Pseudomonas fluorescens species complex, an unappreciated colonizer of humans. Clin Microbiol Rev. 2014;27:927–948. doi: 10.1128/CMR.00044-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, McGuire SR, List RP, Day DE, Hoesel LM, et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–725. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]