Abstract
Tools to measure care coordination are needed to evaluate federal, state, and private sector efforts encouraging coordination to improve health outcomes and contain costs. Administrative data are a rich source of data for studying the use of medical services, thus allowing for measurement of patient level, provider level, and system measures of care coordination. Based on a review the literature and input from an expert panel, this article describes 4 key components—building blocks—of care coordination and corresponding measures. These building blocks should have utility across clinical conditions. They may be used to test hypotheses about the impact of coordinated care on medication utilization, adherence to medications, and clinical outcomes. (Population Health Management 2014;17:247–252)
Introduction
Tools to assess provider and system measures of care coordination are increasingly needed as health reform legislation and efforts in the private sector encourage coordination of services to improve health outcomes and contain costs. New federal investments in the patient-centered medical home (PCMH) model and Accountable Care Organizations are examples of this new focus on coordinated care. This article describes the key components of care coordination measurement, herein referred to as building blocks, and identifies measures that can be constructed using administrative data to assess aspects of care coordination.
A technical report funded by the Agency for Healthcare Research and Quality (AHRQ) in 2007 collected definitions of care coordination.1 The investigators identified more than 40 distinct and heterogeneous definitions of care coordination. From these, they identified elements that are common to care coordination: (1) numerous participants are typically involved; (2) coordination is necessary when participants are dependent upon each other to carry out disparate activities in a patient's care; (3) each participant needs adequate knowledge about their own and others' roles and available resources; (4) participants rely on exchange of information; and (5) integration of care activities has the goal of facilitating appropriate delivery of health care services. This AHRQ report defined care coordination as the deliberate organization of patient care activities between two or more participants (including the patient) involved in a patient's care to facilitate the appropriate delivery of health care services.
Throughout this article, the study team considers provider and system-level measures in the context of diabetes care. Patients with diabetes have a serious chronic condition, often have important comorbid illnesses, and require care from multiple providers. Thus, coordination of care between primary care providers (PCPs), specialist physicians, and the patient and caregivers should optimize patient outcomes.2 The term providers is used to be inclusive of physicians and nurse practitioners, although the study team recognizes that a nurse practitioner's ability to bill independently varies by state. Previous studies have described tools for assessing care coordination at the patient level.3–5
For a health plan or health system, an example of a question that might be asked is whether there are differences in care delivered by a PCP who has a wide network of specialist consultants compared to care delivered by a PCP who has a limited network of specialist consultants, and whether this affects patient outcomes. The answer to this question could encourage structural changes within referral networks. This article provides preliminary guidance about how to describe these relationships using administrative claims data. Previous studies of care coordination have focused on patient and provider measures. System level measures of the provider network or medical neighborhood are relatively new tools in measuring care coordination; this article describes the building blocks for operationalizing these system-level measures.
Methods
The study team conducted a targeted search for publications describing and evaluating measurement and patient attribution approaches in care coordination programs in the peer-reviewed and non-peer-reviewed literature. The team used Google to identify current practices in patient attribution for PCMH programs using search terms including pay for performance, patient centered medical home, PCMH, and the names of individual payers or programs (eg, Medicare, Blue Cross Blue Shield, Cigna, New Hampshire). The team conducted a broad search of the care coordination literature using PubMed to identify systematic reviews using pay for performance, patient attribution, care continuity, continuity of care, and care coordination. The team supplemented this literature sample using snowball sampling and expert opinion.
The study team reviewed and synthesized practices described in the literature, and then assembled a team of experts in health services research and diabetes management from across the United States, including experts from the National Institutes of Health, AHRQ, the Centers for Disease Control and Prevention, the Veteran's Administration, 2 academic institutions, 2 large managed health care organizations, the American Diabetes Association, and the Centers for Medicare and Medicaid Services. The team asked the experts to reflect on the key concepts described in the literature, and then to suggest which building blocks are most vital to describing the care coordination of individuals with diabetes. The 4 concepts that will be described reflect the refinements proposed by the experts.
Results
From the literature review and expert input, the study team proposes the key elements measurable in administrative data that may allow one to characterize the coordination of a patient's care. These are: (1) the accurate attribution of an episode of care to a unique provider; (2) the accurate identification of the PCP; (3) the identification of a patient's medical home and medical neighborhood, if they exist; and (4) the identification of a network of providers who share patients (Table 1). These are considered by the study team to be the building blocks for describing the coordination of the care provided to an individual.
Table 1.
Care Coordination Building Blocks and Example Measures and Study Questions
| Building Blocks | ||||
|---|---|---|---|---|
| (1) Assignment of Care Episode to a Provider | (2) Assignment of a Primary Provider | (3) Medical Neighborhood | (4) Provider Networks | |
| Measure | Primary Provider for a Care Episode | Primary Care Provider | Medical Neighborhood | Centrality |
| Measure Definition | Plurality Rule—The episode of care is assigned to the provider who accounts for the plurality of the patient's costs | Plurality Algorithm—A patient is assigned to the provider with whom he or she has the largest number of visits during the study period | Density of providers in a 30-mile radius | The number of ties a physician has with a greater number of ties indicating a more central position in the network |
| Key Data Elements | 1. Unique identifier for all health care providers 2. Incurred costs for the episode 3. Dates of service |
1. Unique identifier for all health care providers 2. Procedure codes 3. Physician specialty codes 4. Dates of service |
1. Unique identifier for all health care providers 2. Provider geo code or zip code 3. Physician specialty codes |
1. Unique identifier for all health care providers 2. Physician specialty codes 3. Shared patients |
| Example research question | What provider characteristics, as identified by the plurality rule, affect the patient health outcomes? | In a health system, does the plurality provider correspond to the assigned primary provider? | Is the structure of the medical neighborhood for diabetics associated with preventable hospitalizations? | Do care coordination interventions affect the structure of provider networks? |
Building Block 1: Attribution of care
If the goal is to describe the relationships between key providers caring for individuals with diabetes, one needs to identify the providers who are involved in the patient's care including those who may be acting as consultants and those who may be delivering and coordinating substantial amounts of a patient's care.
In order to overcome the natural fluctuations in a patient's care needs, an assortment of methods have been used with claims data to attribute claims for services to the provider who had responsibility for the care administered. For pay for performance, this is a requirement in order to compensate providers for high-quality care and/or good patient outcomes. The literature suggests that most groups involved in these activities begin by using episode grouping software to group services into discrete episodes of care. The Leapfrog Group describes methods that they recommend for attributing care to certain physicians.6 They suggest that a minimum of a year's worth of claims is needed. An episode of care, as identified by grouper software, may include claims from multiple physicians. (Grouper software classifies hospital case types into groups expected to have similar hospital resource use.) The physician with the highest percentage of professional claims within an episode is assigned the attribution for that patient for that episode, as long as that percentage exceeds 25% of all available professional claims within that episode.
This Leapfrog Group acknowledges that there are other ways to assign attribution. Possibilities include (1) assigning attribution to the highest cost provider, (2) using a threshold percentage of total eligible provider fees, (3) assigning the PCP and specialists if it is a plan that requires members to select a PCP, (4) attributing episodes of costs to a virtual PCP based on the patient's history of contact with that provider, (5) attributing the costs of that episode to everyone involved in that episode, (6) attributing costs to the provider who was responsible for a “significant procedure,” or (7) attributing costs to the provider having the most face-to-face encounters with the patient.
Mehrotra et al empirically tested different attribution rules and their impact on individual physician cost profiles.7 These authors used 2 years of data from 4 health plans in Massachusetts. They found that 54% of episodes involved multiple physicians including 9% in which 5 or more physicians were involved with care. Among the attribution strategies they tested were ones that attribute the physician with the episode based on a plurality (largest share) of costs in that episode, or based on having the majority of costs in the episode. Other strategies used individual patient encounters and assigned attribution based on the professional costs of that visit or based on the physician having an “evaluation and management” (E&M) claim for that visit.
The aforementioned methods are for assigning costs to a physician; however, the implication is that that physician is responsible for the services billed to that patient and the outcomes he or she experiences. Related methods are used to assign individual patients to group practices. These methods parallel those described but instead of assigning provisions of services to an individual physician, they are assigned to the practice at which the patient received care. Kautter et al concluded that the practice that provided the plurality of outpatient evaluation and management services to the beneficiary be considered to be the practice “responsible” for the patient.8 This included evaluation and management services by specialists in addition to PCPs.
The study team is not presently recommending any of these strategies for assigning attribution but suggests that careful consideration of these strategies is a useful starting point for describing care coordination; the choice may depend on the patient population of interest.
Building Block 2: Primary care provider attribution
Closely related to the previous discussion is attribution of care of a patient to his or her PCP. This requires being able to identify the patient's PCP. This is relevant to individuals with diabetes as much of diabetes care is provided by general internists and family physicians. In addition, nurse practitioners and physician assistants are increasingly providing primary care services to individuals with chronic conditions. A complete review of different approaches is outside the scope of this article, which presents 3 approaches used for assigning patients. The first method is a landmark study by Pham and colleagues that illustrates the challenges in assigning patients to a PCP using administrative data. The second and third are methods that were validated with patient self-report data.
Pham and colleagues aimed to assign beneficiaries with Medicare to the physician who had the most responsibility for their care.9 They assigned beneficiaries to physicians by Unique Physician Identification Number (UPIN) and to practices by tax identification numbers. They compared several algorithms to assign beneficiaries to physicians (Table 2) and compared results to the Community Tracking Study Physician Survey, which is linked to Medicare claims.
Table 2.
Algorithms for Assigning Beneficiaries
| Algorithm Title | Instructions | Example |
|---|---|---|
| Plurality provider | Provider who billed the greatest number of E&M visits in a year for the beneficiary (includes specialists) | Gynecologist: 3 visits Internist: 4 visits Ophthalmologist: 3 visits (Beneficiary would be attributed to internist) |
| Plurality primary care provider | Provider who billed the greatest number of E&M visits in a year for the beneficiary, including only primary care providers* | Gynecologist: 3 visits Internist: 4 visits (Beneficiary would be attributed to internist) |
| Majority provider | Provider who billed for the plurality of E&M visits (must be>50% of all visits) | Gynecologist: 3 visits Internist: 4 visits Ophthalmologist: 3 visits (No majority provider—no assignment can be made) |
| Multiple provider | Provider who billed for at least 25% of E&M visits (may be more than 1 provider per beneficiary | Gynecologist: 3 visits Internist: 4 visits Ophthalmologist: 3 visits (Beneficiary would be assigned to all 3 providers) |
Primary care physicians have Health Care Financing Administration specialty codes of: 01, 08, 11, 12, 37, 38, 60, 84.
E&M, evaluation and management.
Pham et al found that as compared with the plurality algorithm, the majority provider and the multiple provider algorithms assigned a higher proportion of beneficiaries' visits to a primary provider, but assigned fewer beneficiaries to any physician at all. They observed a great deal of care dispersion across physicians and found that this is even more the case among beneficiaries with chronic illness. Therefore, these algorithms were least useful for assigning chronically ill individuals to a PCP. The UPIN has largely been replaced with the National Provider Identification number.
A similar approach was used by Shah and colleagues,10 who tested several algorithms to assign a beneficiary to a regular physician. They found that assigning a participant to the physician with whom he or she had the largest number of visits (similar to Pham's plurality provider) was concordant with the self-report of the “regular primary care physician” in 82.6% of cases. They applied the algorithm as well to assign participants to diabetes specialists by identifying the specialist with the greatest number of visits (2 or more required). There was a 78.5% concordance rate with this algorithm and patient self-report, including 58% who reported that they had no regular diabetes specialist.
Sharma et al defined PCP as being a general practitioner, family physician, general internist, or a geriatrician who had billed an outpatient E&M code for the patient on 3 or more occasions in the year before the hospitalization.11 They found that this definition had 76% concordance with the patient's self-identified PCP but was relevant only for patients who were hospitalized.
As already discussed, selection of the strategy for assigning attribution to a PCP may be dictated by the patient population. Supplementary Appendix 1 provides an example of identifying individuals with diabetes and the process by which an individual's care could be attributed to the PCP most responsible for his or her care (Supplementary Appendix 1 is available in the online article at www.liebertpub.com/pop).
Building Block 3: Medical homes and neighborhoods
Recent efforts to coordinate patients' care have included centralizing their care in PCMHs. This concept began many years ago for the care of chronically ill or complicated children but has more recently been adopted for the care of adults, particularly adults with Medicare. PCMHs are not yet commonplace for the care of older adults. The Patient Protection and Affordable Care Act has provided funding for medical home pilot projects that are testing the effectiveness of doctors and other health professionals working in 500 teams to improve care for 195,000 Medicare patients.12
Investigators also have described the care that is delivered outside of the medical home but within a so-called “medical neighborhood.”13 The medical neighborhood is the established or informal network of doctors who care for a group of patients centered in a PCMH. The existence of a neighborhood supporting the PCMH and providing needed services is a system-level factor that may be changeable. Closed network models are the most restrictive neighborhood structure in that the network of consultants is carefully controlled, sometimes with the PCP or nurse practitioner acting as a gatekeeper to access specialists. At the other extreme are fee-for-service insurance plans that do not regulate the consultants seen by their enrolled patients and allow for informal networks to develop around patients and their PCPs.
It may be important to consider how neighborhoods form as this may impact care coordination for patients cared for within the neighborhood. For discussion, the study team considers physicians or other providers who are not PCPs to be neighbors. This is not necessarily a geographic description but, presumably, proximity contributes to some extent. Neighbors can self-designate and present themselves to the PCMH as a potential neighbor who would accept referrals of patients. Alternatively, a PCMH may invite specialists and other providers to be neighbors and request that they accept referrals from their home. More commonly, neighbors are neighbors by default because they treat some percentage of patients from a PCMH. There may be contractual relationships, as well, that describe accountability and responsibilities of the neighbors to the PCMH.
Pham suggests that the size of neighborhood may be measurable as the number of neighbors per 100 patients served by a PCMH.13 For example, if there is a PCMH that has 3000 patients and there are 12 endocrinologists within a 30-mile radius, the size of this neighborhood of endocrinologists is 12/3000*100, or 0.28 relative to the PCMH. In a rural region, there may be only 1 endocrinologist in a 30-mile radius of a PCMH with 400 patients. The size of this neighborhood is 1/400*100 or 0.25. Supplementary Appendix 2 demonstrates the identification of medical specialists (Supplementary Appendix 2 is available in the online article at www.liebertpub.com/pop).
Building Block 4: Networks of providers
To understand the networks of care that surround individuals with diabetes, one must be able to describe the relationships of the providers involved in the care of these patients. For a patient with diabetes, it is reasonable to consider that he or she will have long-standing relationships with a PCP, endocrinologist, ophthalmologist, and podiatrist. This activity is less developed than those already described but may be increasingly important as Accountable Care Organizations form.
Bynum et al created physician-hospital networks using Medicare data and the UPINs of the doctors.14 The authors used a 2-step process. First, they assigned patients to an ambulatory care provider using an algorithm similar to that described under Building Block 2. Then they applied similar logic to assign physicians to hospitals. Based on the hospitalizations of the assigned patients, physicians were assigned to the hospital with the majority of admissions incurred by his patient panel. Ties were broken by duration of time between the first and last admission by hospital and then by most recent admission.
Increasingly used in health services research, social network analysis also may be used to explore these relationships. Social network analysis has long been used in the social sciences and is increasingly used in other disciplines. It is a set of methods for mapping, measuring, and analyzing the social relationships between people, groups, and organizations.15,16 These methods can be used descriptively to describe the degree of connectedness between individuals or organizations (called “actors”) or can be used to test hypotheses about the relationships. The data that are most often used in social network analysis are data from surveys that ask individuals to describe their sources of information or to describe their relationships within an organization.
In a recent study, Barnett et al evaluated how well administrative measures of physician connectedness correspond with physician self-report.17 They found that the proportion of physicians who identified themselves as having a relationship to another physician ranged from 19% when there was 1 shared patient to 82% when there were 9 or more shared patients. In general, PCPs acknowledged significantly more relationships than medical specialists or surgeons. They also found an inverse relationship between the number of connections identified using administrative data and the proportion of connections identified by the physician.
Barnett et al used broad criteria to identify physicians to be included as eligible to be in a patient's network. All physicians who had face-to-face visits in an office or hospital or submitted a claim with a procedure code that had a relative value unit of at least 2 were included. Anesthesiologists, emergency medicine physicians, radiologists, and pathologists were excluded.
Social network analysis tools, in readily available software such as UCINET, provide statistics to quantify the degree of connectedness in a network.18 Some key descriptors are: (1) betweenness: a measure that indicates how much a node (an actor) is in the path between other actors or how much a node connects other nodes with each other; (2) centrality: the number of ties a node has with a greater number of ties, indicating a central position in the network; (3) density: the number of ties divided by the number of possible ties; (4) distance: the number of ties that separate 2 actors (if 2 actors are directly connected, the distance equals 1); and (5) reachability: the degree by which a node can be reached by other nodes; if some nodes are unreachable, then this network is fragmented as would be the case if a doctor-patient pair has no interaction with any other doctors or patients (described in Blanchet and James16).
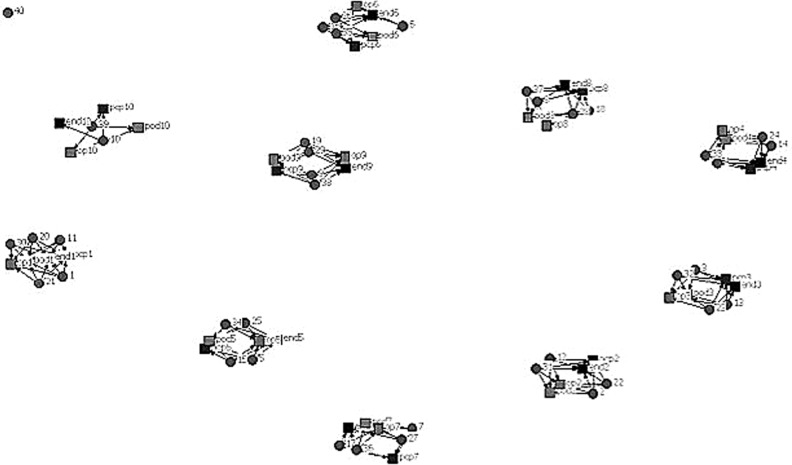
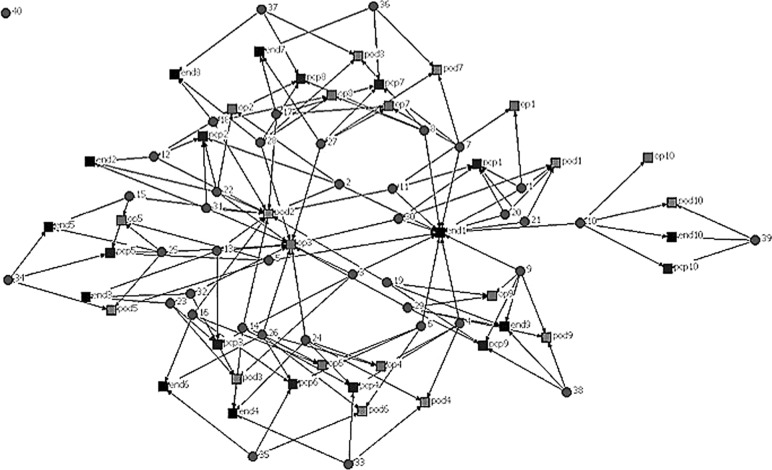
In Figures 1 and 2, patients are represented by circles and doctors by squares. The figures include physician specialties relevant to diabetes care: primary care providers, endocrinologists, ophthalmologists, and podiatrists. In the first simulated diagram, the degree of connectedness between doctors is very low; there is a set of doctors caring for a collection of patients and there is little connection between these teams. Once a network is described in these terms, then one can test hypotheses about what influences the shape of the network (geography, penetration of managed care, health reform), as well as hypotheses about the influence of the network structure on clinical outcomes. For example, a study of the degree of connectedness of providers on outcomes such as utilization of emergency care or hospitalization rates or mortality would be informative for the structuring of care of patients with diabetes.
FIG. 1.
One network of providers and patients. Circles=patients, squares=providers, connecting lines indicate the existence of a provider-patient relationship from a clinical encounter.
FIG. 2.
A second network of providers and patients. Circles=patients, squares=providers, connecting lines indicate the existence of a provider-patient relationship from a clinical encounter.
Discussion
This article provides a template for describing the care structure of patients based on individuals with diabetes. Through a review of the literature and consultation with clinical experts, the study team identified 4 key building blocks for describing care coordination: (1) attribution of care to a particular provider; (2) the identification of the primary provider; (3) the identification of a patient's medical home and medical neighborhood; and (4) a description of provider networks.
One may use 1 or more of these building blocks to assess care coordination. In studying care coordination, the measure of care coordination may be either a predictor of a patient or provider outcome or may serve as an outcome in itself. In analyzing care coordination, it is important for the researcher to consider issues of endogeneity (ie, it is not clear if poorly coordinated care causes bad outcomes or if bad outcomes result in what appears to be poorly coordinated care). In addition, researchers should carefully consider the sensitivity of their model to omitted variables such as illness severity and disability.
It is acknowledged that a systematic review of the literature was not conducted, and therefore the study team has not identified all possible care coordination measures. The team relied on expert opinion in the development of the 4 key building blocks. Different experts may have grouped or separated the key components of care coordination in more or fewer components, but the team believes that the same elements would remain. Lastly, although the study team approached measuring care coordination from the context of diabetes care, the team believes these measures are generally applicable to patients with other chronic conditions and may be easily modified based on the characteristics of the population.
Future research is needed to compare this care coordination measurement construct in different contexts and populations. Previous studies suggest that care-seeking patterns are different in pediatric populations than in adult populations, affecting the measurement of patient-level care continuity.4 Future research should investigate whether these building blocks are relevant to describing care in unique chronically ill populations such as patients with serious mental illness. In addition, this framework also should be evaluated in the context of a population with a broad range of chronic conditions and comorbidities, such as those intended for enrollment into Accountable Care Organizations.
The building blocks described here can be generated with medical claims, which are generally readily available, although access to data from multiple different payors may be needed. Comprehensive and readily accessible measures of care coordination are important for providers interested in evaluating their own care, health plans and Medicare assessing their own care coordination interventions, and researchers looking to describe what aspects of care coordination are most important to health outcomes and cost.
Conclusions
As the Affordable Care Act is put into practice, there will be changes in how care is coordinated and delivered. The building blocks described here may help with the assessment of the impact of such changes. In addition, these tools may be used to generate new research on the influences of network structure and how network structure impacts on clinical outcomes. The optimal strategy, built with the approaches described in this article, is yet to be determined and may be unique to a given patient population.
Supplementary Material
Author Disclosure Statement
Dr. Segal and Ms. DuGoff declared no conflicts of interest with respect to the research, authorship, and/or publication of this article.
This study was supported under contract R01 HS019859 to the Kaiser Foundation Research Network from the Agency for Healthcare Research and Quality for the Multi-Institutional Consortium for Comparative Effectiveness Research in Diabetes Treatment and Prevention. Johns Hopkins University was a subcontractor on this award. Ms. DuGoff received the Alvin R. Tarlov & John E. Ware Jr. Doctoral Dissertation Award in Patient Reported Outcomes from the Health Assessment Laboratory, a nonprofit organization. This award supports her dissertation research on care coordination in older adults with multiple chronic conditions.
References
- 1.McDonald KM, Sundaram V, Bravata DM, et al. Volume 7—care coordination: Stanford University–UCSF Evidence-based Practice Center, Stanford, CA, 2007. Rockville, MD: Agency for Healthcare Research and Quality, June 2007 [Google Scholar]
- 2.Beaser RS, Okeke E, Neighbours J, Brown J, Ronk K, Wolyniec WW. Coordinated primary and specialty care for type 2 diabetes mellitus, guidelines, and systems: an educational needs assessment. Endocr Pract 2011;17:880–890 [DOI] [PubMed] [Google Scholar]
- 3.McDonald K, Schultz E, Albin L, et al. Care coordination atlas version 3: Stanford University under subcontract to Battelle, 2010. Rockville, MD: Agency for Healthcare Research and Quality, January 2011 [Google Scholar]
- 4.Jee SH, Cabana MD. Indices for continuity of care: a systematic review of the literature. Med Care Res Rev 2006;63(2):158–188 [DOI] [PubMed] [Google Scholar]
- 5.Pollack CE, Weissman G, Bekelman J, Liao K, Armstrong K. Physician social networks and variation in prostate cancer treatment in three cities. Health Serv Res 2012;47(1 pt2):380–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Leapfrog Group, and Bridges to Excellence. Measuring provider efficiency version 1.0: a collaborative multi-stakeholder effort. Available at: <http://www.commonwealthfund.org/∼/media/Files/Publications/Other/2004/Dec/Measuring%20Provider%20Efficiency%20%20Version%201%200%20%20A%20Collaborative%20Multi%20Stakeholder%20Effort/measurproviderefficiency1%2012312004%20pdf.pdf>. Accessed September24, 2011
- 7.Mehrotra A, Adams JL, Thomas JW, McGlynn EA. The effect of different attribution rules on individual physician cost profiles. Ann Intern Med 2010;152(10): 649–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kautter J, Pope GC, Trisolini M, Grund S. Medicare physician group practice demonstration design: quality and efficiency pay-for-performance. Health Care Financ Rev 2007;29(1):15–29 [PMC free article] [PubMed] [Google Scholar]
- 9.Pham HH, Schrag D, O'Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. N Engl J Med 2007;356(11): 1130–1139 [DOI] [PubMed] [Google Scholar]
- 10.Shah BR, Hux JE, Laupacis A, Zinman B, Cauch-Dudek K, Booth GL. Administrative data algorithms can describe ambulatory physician utilization. Health Serv Res 2007;42:1783–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma G, Fletcher KE, Zhang D, Kuo YF, Freeman JL, Goodwin JS. Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults. JAMA 2009;301:1671–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Postal AD. HHS launches Medicare medical home pilot. Available at: <http://www.lifehealthpro.com/2011/06/06/hhs-launches-medicare-medical-home-pilot>. Accessed September24, 2011
- 13.Pham HH. Good neighbors: how will the patient-centered medical home relate to the rest of the health-care delivery system? J Gen Intern Med 2010;25:630–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bynum JP, Bernal-Delgado E, Gottlieb D, Fisher E. Assigning ambulatory patients and their physicians to hospitals: a method for obtaining population-based provider performance measurements. Health Serv Res 2007;42(1 Pt 1):45–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Malley AJ. The analysis of social network data: an exciting frontier for statisticians. Stat Med 2013;32:539–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanchet K, James P. How to do (or not to do)…a social network analysis in health systems research. Health Policy Plan 2012;27:438–446 [DOI] [PubMed] [Google Scholar]
- 17.Barnett ML, Landon BE, O'Malley AJ, Keating NL, Christakis NA. Mapping physician networks with self-reported and administrative data. Health Serv Res 2011;46:1592–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borgatti SP, Everett MG, Freeman LC. Ucinet for Windows: Software for Social Network Analysis. Harvard, MA: Analytic Technologies, 2002 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.