Abstract
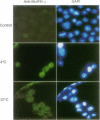
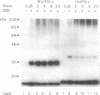
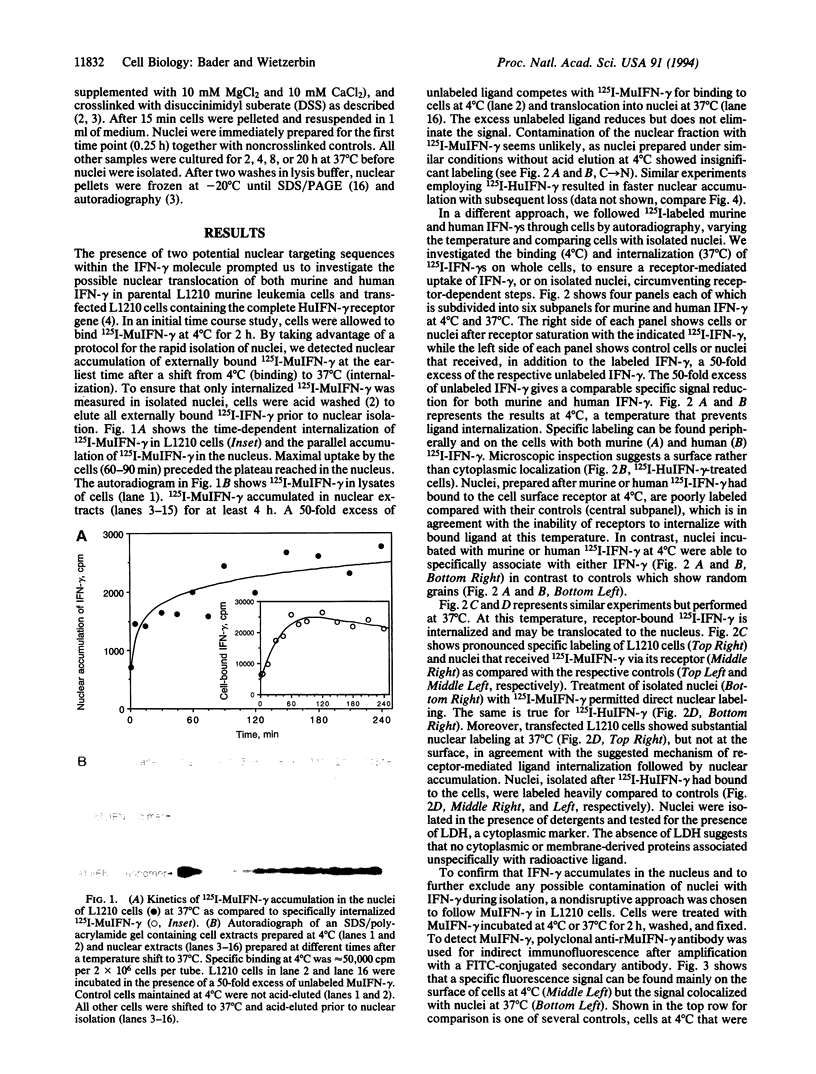
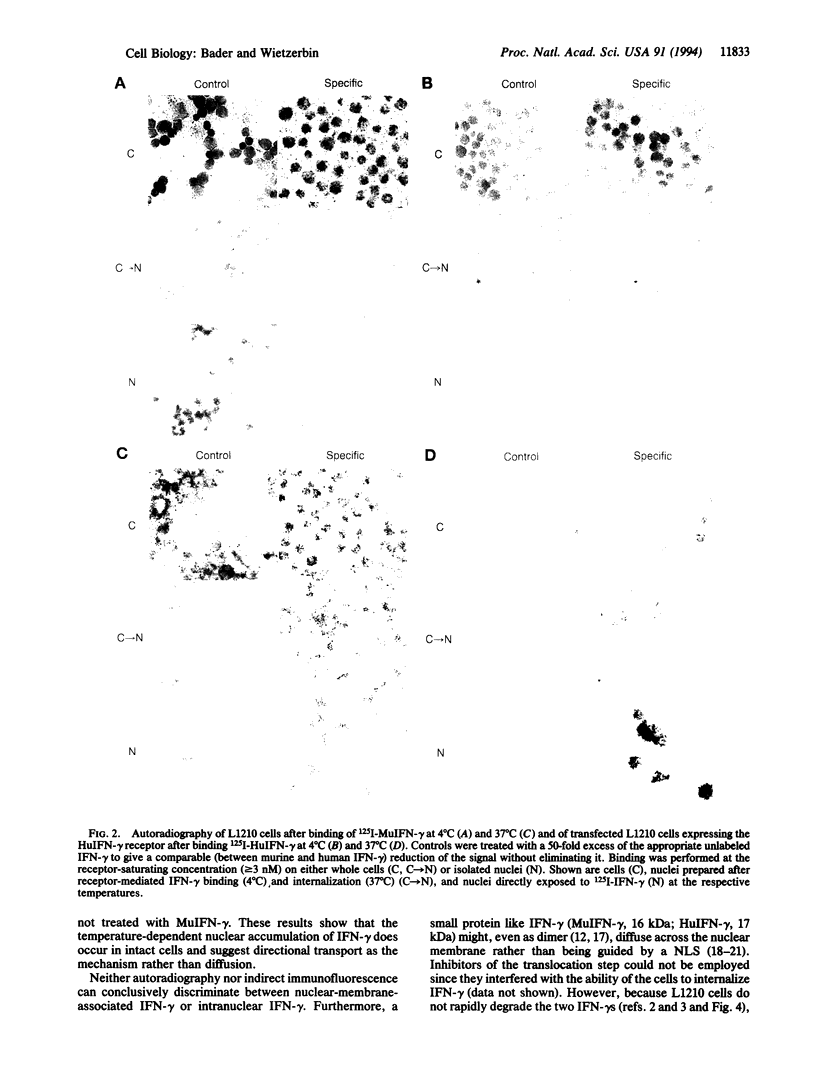
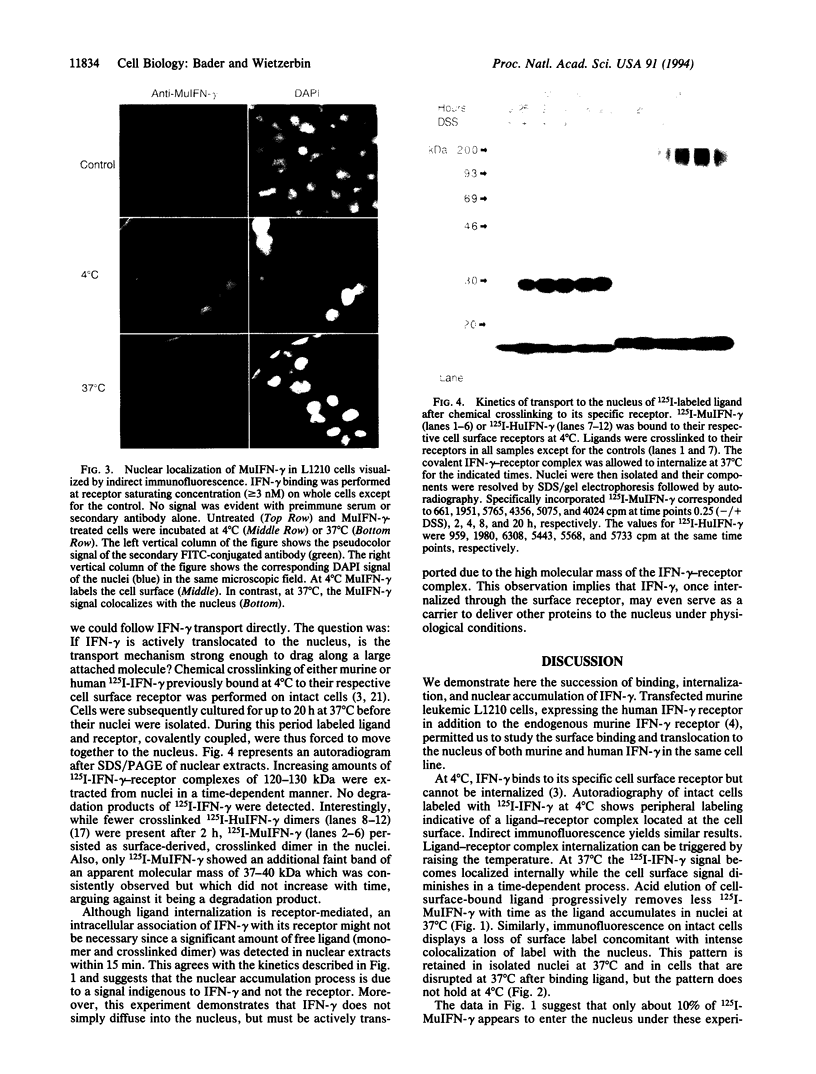
Examination of the interferon gamma (IFN-gamma) amino acid sequence revealed two conserved basic amino acid clusters similar to the prototype nuclear localization signal. We followed the fate of cell surface receptor-bound IFN-gamma in murine leukemia L1210 cells. A time- and temperature-dependent accumulation of murine IFN-gamma in the cell nucleus could be demonstrated by autoradiography and indirect immunofluorescence after the rapid isolation of nuclei. Human IFN-gamma was also internalized and translocated to the nucleus of murine L1210 cells transfected with and expressing the human IFN-gamma receptor, but it appeared to be retained by the nucleus only transiently. IFN-gamma molecules chemically crosslinked to their cell surface receptor remain capable of being translocated to the nucleus even as part of a receptor-ligand complex. Thus, the bipartite nuclear localization signal sequence appears to be functional and suggests that nuclear targeting could participate in IFN-gamma signal transduction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Lobl T. J., Mitchell M. A., Gerace L. Identification of specific binding proteins for a nuclear location sequence. Nature. 1989 Jan 19;337(6204):276–279. doi: 10.1038/337276a0. [DOI] [PubMed] [Google Scholar]
- Aguet M., Dembić Z., Merlin G. Molecular cloning and expression of the human interferon-gamma receptor. Cell. 1988 Oct 21;55(2):273–280. doi: 10.1016/0092-8674(88)90050-5. [DOI] [PubMed] [Google Scholar]
- Anderson P., Yip Y. K., Vilcek J. Specific binding of 125I-human interferon-gamma to high affinity receptors on human fibroblasts. J Biol Chem. 1982 Oct 10;257(19):11301–11304. [PubMed] [Google Scholar]
- Bader T., Wietzerbin J. Modulation of murine and human interferon-gamma receptor expression by their ligands or phorbol ester. Cytokine. 1994 Jan;6(1):70–78. doi: 10.1016/1043-4666(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Bugler B., Amalric F., Prats H. Alternative initiation of translation determines cytoplasmic or nuclear localization of basic fibroblast growth factor. Mol Cell Biol. 1991 Jan;11(1):573–577. doi: 10.1128/mcb.11.1.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M., Larner A. C. Activation of transcription factors by interferon-alpha in a cell-free system. Science. 1992 Aug 7;257(5071):813–815. doi: 10.1126/science.1496402. [DOI] [PubMed] [Google Scholar]
- Delic J., Coppey J., Magdelenat H., Coppey-Moisan M. Impossibility of acridine orange intercalation in nuclear DNA of the living cell. Exp Cell Res. 1991 May;194(1):147–153. doi: 10.1016/0014-4827(91)90144-j. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. The nuclear membrane. Science. 1992 Nov 6;258(5084):942–947. doi: 10.1126/science.1439805. [DOI] [PubMed] [Google Scholar]
- Ealick S. E., Cook W. J., Vijay-Kumar S., Carson M., Nagabhushan T. L., Trotta P. P., Bugg C. E. Three-dimensional structure of recombinant human interferon-gamma. Science. 1991 May 3;252(5006):698–702. doi: 10.1126/science.1902591. [DOI] [PubMed] [Google Scholar]
- Feldherr C. M., Akin D. Signal-mediated nuclear transport in proliferating and growth-arrested BALB/c 3T3 cells. J Cell Biol. 1991 Nov;115(4):933–939. doi: 10.1083/jcb.115.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T., Rehm A., Aguet M., Pfizenmaier K. Human chromosome 21 is necessary and sufficient to confer human IFN gamma responsiveness to somatic cell hybrids expressing the cloned human IFN gamma receptor gene. Cytokine. 1990 May;2(3):157–161. doi: 10.1016/1043-4666(90)90010-q. [DOI] [PubMed] [Google Scholar]
- Fountoulakis M., Juranville J. F., Maris A., Ozmen L., Garotta G. One interferon gamma receptor binds one interferon gamma dimer. J Biol Chem. 1990 Nov 15;265(32):19758–19767. [PubMed] [Google Scholar]
- Gibbs V. C., Williams S. R., Gray P. W., Schreiber R. D., Pennica D., Rice G., Goeddel D. V. The extracellular domain of the human interferon gamma receptor interacts with a species-specific signal transducer. Mol Cell Biol. 1991 Dec;11(12):5860–5866. doi: 10.1128/mcb.11.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi S., Merlin G., Aguet M. Functional characterization of a hybrid human-mouse interferon gamma receptor: evidence for species-specific interaction of the extracellular receptor domain with a putative signal transducer. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2737–2741. doi: 10.1073/pnas.89.7.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino Y., Kumar C. S., Mariano T. M., Lai D. H., Pestka S. Chimeric interferon-gamma receptors demonstrate that an accessory factor required for activity interacts with the extracellular domain. J Biol Chem. 1992 Feb 25;267(6):3741–3749. [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Jung V., Rashidbaigi A., Jones C., Tischfield J. A., Shows T. B., Pestka S. Human chromosomes 6 and 21 are required for sensitivity to human interferon gamma. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4151–4155. doi: 10.1073/pnas.84.12.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnaryov V. M., MacDonald H. S., Sedmak J. J., Grossberg S. E. Murine interferon-beta receptor-mediated endocytosis and nuclear membrane binding. Proc Natl Acad Sci U S A. 1985 May;82(10):3281–3285. doi: 10.1073/pnas.82.10.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnaryov V. M., MacDonald H. S., Sedmak J. J., Grossberg S. E. The cellular internalization of recombinant gamma interferon differs from that of natural interferon gamma. Biochem Biophys Res Commun. 1988 Nov 30;157(1):109–114. doi: 10.1016/s0006-291x(88)80019-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Kanda P., Kennedy R. C. Induction of nuclear transport with a synthetic peptide homologous to the SV40 T antigen transport signal. Cell. 1986 Aug 15;46(4):575–582. doi: 10.1016/0092-8674(86)90883-4. [DOI] [PubMed] [Google Scholar]
- Maher D. W., Lee B. A., Donoghue D. J. The alternatively spliced exon of the platelet-derived growth factor A chain encodes a nuclear targeting signal. Mol Cell Biol. 1989 May;9(5):2251–2253. doi: 10.1128/mcb.9.5.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. S., Blobel G. The two steps of nuclear import, targeting to the nuclear envelope and translocation through the nuclear pore, require different cytosolic factors. Cell. 1992 Jun 12;69(6):939–950. doi: 10.1016/0092-8674(92)90613-h. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Baeuerle P. A., Lührmann R. Nuclear import-export: in search of signals and mechanisms. Cell. 1991 Jul 12;66(1):15–22. doi: 10.1016/0092-8674(91)90135-l. [DOI] [PubMed] [Google Scholar]
- Sancéau J., Sondermeyer P., Béranger F., Falcoff R., Vaquero C. Intracellular human gamma-interferon triggers an antiviral state in transformed murine L cells. Proc Natl Acad Sci U S A. 1987 May;84(9):2906–2910. doi: 10.1073/pnas.84.9.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Shuai K., Schindler C., Prezioso V. R., Darnell J. E., Jr Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992 Dec 11;258(5089):1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Silver P. A. How proteins enter the nucleus. Cell. 1991 Feb 8;64(3):489–497. doi: 10.1016/0092-8674(91)90233-o. [DOI] [PubMed] [Google Scholar]
- Slodowski O., Böhm J., Schöne B., Otto B. Carboxy-terminal truncated rhuIFN-gamma with a substitution of Gln133 or Ser132 to leucine leads to higher biological activity than in the wild type. Eur J Biochem. 1991 Dec 18;202(3):1133–1140. doi: 10.1111/j.1432-1033.1991.tb16481.x. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Muegge K., Keller J. R., Kung H. F., Young H. A., Durum S. K. Direct evidence for an intracellular role for IFN-gamma. Microinjection of human IFN-gamma induces Ia expression on murine macrophages. J Immunol. 1990 Mar 1;144(5):1777–1782. [PubMed] [Google Scholar]
- Wietzerbin J., Gaudelet C., Aguet M., Falcoff E. Binding and cross-linking of recombinant mouse interferon-gamma to receptors in mouse leukemic L1210 cells; interferon-gamma internalization and receptor down-regulation. J Immunol. 1986 Apr 1;136(7):2451–2455. [PubMed] [Google Scholar]
- Wietzerbin J., Stefanos S., Lucero M., Falcoff E., Thang D. C., Thang M. N. Presence of a polynucleotide binding site on murine immune interferon (T-type). Biochem Biophys Res Commun. 1978 Nov 14;85(1):480–489. doi: 10.1016/s0006-291x(78)80067-9. [DOI] [PubMed] [Google Scholar]
- Zu X. W., Jay F. T. The E1 functional epitope of the human interferon gamma is a nuclear targeting signal-like element. Mapping of the E1 epitope. J Biol Chem. 1991 Apr 5;266(10):6023–6026. [PubMed] [Google Scholar]