Abstract
We studied patient outcomes by type of referral site following 2 years of combination antiretroviral therapy (cART) during scale-up from June 2006 to July 2011 in Mozambique's rural Zambézia Province. Loss to follow-up (LTFU) was defined as no contact within 60 days after scheduled medication pickup. Endpoints included LTFU, mortality, and combined mortality/LTFU; we used Kaplan–Meier and cumulative incidence estimates. The referral site was the source of HIV testing. We modeled 2-year outcomes using Cox regression stratified by district, adjusting for sociodemographics and health status. Of 7,615 HIV-infected patients ≥15 years starting cART, 61% were female and the median age was 30 years. Two-year LTFU was 38.1% (95% CI: 36.9–39.3%) and mortality was 14.2% (95% CI 13.2–15.2%). Patients arrived from voluntary counseling and testing (VCT) sites (51%), general outpatient clinics (21%), antenatal care (8%), inpatient care (3%), HIV/tuberculosis/laboratory facilities (<4%), or other sources of referral (14%). Compared with VCT, patients referred from inpatient, tuberculosis, or antenatal care had higher hazards of LTFU. Adjusted hazard ratios (AHR; 95% CI) for 2-year mortality by referral site (VCT as referent) were inpatient 1.87 (1.36–2.58), outpatient 1.44 (1.11–1.85), and antenatal care 0.69 (0.43–1.11) and for mortality/LTFU were inpatient 1.60 (1.34–1.91), outpatient 1.17 (1.02–1.33), tuberculosis care 1.38 (1.08–1.75), and antenatal care 1.24 (1.06–1.44). That source of referral was associated with mortality/LTFU after adjusting for patient characteristics at cART initiation suggests that (1) additional unmeasured factors are influential, and (2) retention programs may benefit from targeting patient populations based on source of referral with focused counseling and/or social support.
Introduction
Following the rapid scale-up of combination antiretroviral therapy (cART) for HIV, programs throughout Africa have received additional resources from donors and governments to improve testing and linkage to care, most notably from the U.S. President's Emergency Plan for AIDS Relief (PEPFAR) and from the Global Fund to Fight AIDS, Tuberculosis, and Malaria (Global Fund). Earlier testing and treatment and long-term retention in care improve patient outcomes and reduce HIV transmission (i.e., treatment as prevention).1–4 Mozambique has one of the world's worst HIV epidemics, with an estimated national HIV prevalence of 11.5% and 1.4 million persons living with HIV in 2009, the most recent year for estimation.5 Referral sites to HIV care in Mozambique identify patients in all disease stages, from early asymptomatic disease to near-death conditions.6 Treatment scale-up efforts should give consideration to sources of referral as the entry point to HIV services as patients may require modified provision of care and support.
As a PEPFAR implementing partner, our group sought to describe the magnitude of loss to follow-up (LTFU) and death during the first 2 years of cART, and their association with types of referral sites. By establishing the risk of LTFU and/or mortality by the source of referral, quality improvement interventions may be appropriately tailored to improve HIV care and treatment program retention. We used data from 5 years of cART scale-up in Mozambique's rural Zambézia Province to assess these clinical outcomes.
Materials and Methods
Study setting and population
This is an observational cohort study using patient-level data routinely collected for program monitoring and evaluation purposes. We included all HIV-infected patients aged 15 years or older registering at an HIV care center in clinics supported by Vanderbilt University through its in-country nongovernmental organization (NGO), Friends in Global Health (FGH). Only persons initiating cART from June 1, 2006 through July 1, 2011 were included. Data were collected at FGH-supported clinics located in 10 rural districts of Zambézia Province, including Alto Molócuè, Gilé, Ile, Inhassunge, Lugela, Maganja da Costa, Morrumbala, Mopeia, Namacurra, and Pebane.7 Analyses included data from each district seat's hospital/health center and six peripheral health centers.
Details of our clinical program have been described elsewhere.8–10 Briefly, patients enrolled into HIV care have a record opened at the time of registration following Mozambican Ministry of Health [Ministério de Saúde (MISAU)] protocols and using MISAU forms. Patients were initiated on treatment based on eligibility criteria. Prior to May 2009, patients in this Mozambican cohort were eligible for cART if they had a CD4 count <200 cells/μl and/or WHO stage IV, or 200–350 CD4 cells/μl and WHO stage III. As of May 2009, patients were determined eligible for ART if they had a CD4 count <250 cells/μl independent of clinical status, a CD4 count ≤350 cells/μl, and a WHO stage III or WHO stage IV independent of CD4 count.11 Between enrollment in HIV care and initiation of treatment, patients received clinical care according to MISAU guidelines, which includes provision of prophylaxis, clinical care, and routine CD4+ cell count monitoring. All care is documented in the patient medical record.
Data collection
Paper clinical record forms designed by MISAU were completed by clinicians, laboratory technicians, pharmacists, and counselors. At the end of each patient encounter, data entry personnel based at the health facility entered information collected on the paper forms into Microsoft Access or OpenMRS electronic databases. Data quality audits occurred every 6 months by comparing the paper clinical case report forms with the database contents for accuracy. During each audit, a random sample of 10–20% of adult records with recent services was assessed for missing forms, missing data (on forms or in database), and incorrect data entry. Errors discovered in the audit were corrected in the database, and data were deemed suitable for research. From August 2009 to September 2010, 2,113 records from adult patients recently initiating ART were examined during three rounds of routine data quality audits. Marked improvements were seen by the third round such that patient sex, age, district, marital status, CD4+ cell count, dates of enrollment, and ART initiation all had over 90% concordance. WHO stage at first visit was documented correctly in 81% of patients, 17% lacked the required information on the paper form, and 1% had a data entry error.
Source of referral
Source of referral to HIV care was recorded at the first visit to the FGH-supported clinic. The sources of referral to HIV care that were documented on MISAU forms included opt-in voluntary counseling and testing (VCT) centers, general outpatient clinics, prevention of mother to child transmission (PMTCT) in antenatal care programs, medical inpatients at health facilities, tuberculosis services, laboratories/blood banks, and transfer from other HIV care programs. The source of referral may have been marked as “other” if the code was not available on the paper clinical record form. Possible sources for “other” include community-based counseling and testing and traditional healer referral, which were not available as options on the MISAU designed forms at the time of study. Medical inpatient, general outpatient clinic, PMTCT, and tuberculosis service may be considered provider initiated, i.e., a form of opt-out provider-initiated testing and counseling (PITC).
Outcomes
Mozambican national guidelines define LTFU as no effective clinical contact within 60 days after the last scheduled medication pickup.12 This definition is retrospective because patients are allowed back into the cohort if they reengage into care before the database is closed; thus, patients who reengage in care during the period of observation are not counted as LTFU. Weekly lists of patients LTFU were generated to prioritize active case finding by nonclinician community health workers and volunteers. A death report to the health facility typically occurs during a home visit or active case finding. A patient death record could result from family member or confidant notification to the health facility. The current death registry in Mozambique was not referenced for this study as it captures in-hospital deaths at major provincial and central hospitals that do not report back to the district facilities. Patients could be followed across FGH-supported clinics and transfers were recorded during active case finding, although some patients LTFU may be in care at other facilities without our knowledge. In a 2011 program report on active case finding, 70% of patients were located while only 26% of these patients returned to care. Among those patients not located, major reasons included wrong address (27%), death (25%), and travel (17%); health facility transfer was 2%.13
We also used two additional secondary definitions of LTFU for the purpose of cross-cohort comparisons in the literature.12 A proposed “universal” definition classifies patients as LTFU if there is no effective clinical contact within 180 days of the database closure (i.e., July 1, 2011).14 The “reference” definition assigns 1 day of follow-up to any individual who does not return following treatment initiation, includes only individuals initiating ART 6 months prior to the database closure (i.e., on or before January 1, 2011), and classifies patients as LTFU if there is no effective clinical contact within 180 days of database closure.15 All three LTFU definitions are retrospective and deem the patient lost at the date of last contact.
Statistical analysis
Patient characteristics at treatment initiation were compared across 2-year outcome and source of referral using rank sum and chi-square tests. LTFU in this descriptive portion of the analyses uses only one definition based on Mozambican national guidelines. Kaplan–Meier estimates were used to compute mortality and the combined endpoint of mortality and LTFU. The cumulative incidence of LTFU was calculated by treating death as a competing risk and is calculated for all three LTFU definitions.
Multivariable Cox regression stratified by district was used to assess whether the source of referral, demographics, baseline laboratory results, and clinical assessments were independently associated with LTFU, death, or death/LTFU at 2 years following initiation of antiretroviral therapy. LTFU in this multivariable portion of the analyses uses only one definition based on Mozambican national guidelines. To relax linearity assumptions, we modeled age, square root CD4 count, and date of cART initiation using restricted cubic splines.16 Multiple imputation was used to account for missing values of covariates and to prevent case-wise deletion of missing data; 1,070 (14%) patients had complete data for all covariates. Covariates were identified a priori; those with more than 60% missing were excluded from multivariable analysis. Sociodemographic covariates included sex, age, marital status, education, and date of ART initiation. Health status covariates included body mass index (BMI), hemoglobin, cART regimen, WHO stage, and CD4 count at cART initiation. We used the functions “aregImpute” and “fit.mult.impute” from the Hmisc package in R that used predictive mean matching to take random draws from imputation models; 25 imputation data sets were used in the analysis.17 All hypothesis testing was two-sided with a level of significance set at 0.05. We employed R-software 2.15.1 (www.r-project.org) for all data analyses. Analysis scripts are available at http://biostat.mc.vanderbilt.edu/ArchivedAnalyses.
Results
Our study includes 7,615 HIV-infected patients who initiated antiretroviral therapy in 10 districts of Zambézia Province in Mozambique during the study period (Table 1). The cohort was 61% female with a median (interquartile range, IQR) age of 30 years (25–38). The median (IQR) BMI was 19.6 kg/m2 (17.8–21.6). World Health Organization (WHO) stage, CD4+ count, and hemoglobin were collected from 90 days before to 14 days after cART initiation and were incomplete for 39%, 44%, and 60% of patients. The median (IQR) CD4+ cell counts were 179 cells/μl (98–269) and 61% of patients were symptomatic at the WHO Stage III or IV classification.
Table 1.
Patient Characteristics by 2 Year Outcome, Zambézia, Mozambique 2006–2011
| Alive(n=4279) | Dead(n=797) | Losta(n=2539) | Combined(n=7615) | p valueb | |
|---|---|---|---|---|---|
| Referral type, n (%) | <0.001 | ||||
| Missing | 401 (9%) | 86 (11%) | 268 (11%) | 755 (10%) | |
| Medical inpatient | 81 (2%) | 55 (8%) | 100 (4%) | 236 (3%) | |
| General outpatient clinic | 787 (20%) | 229 (32%) | 399 (18%) | 1,415 (21%) | |
| Tuberculosis care | 73 (2%) | 18 (3%) | 60 (3%) | 151 (2%) | |
| Voluntary counseling and testing | 2,009 (52%) | 293 (41%) | 1,177 (52%) | 3,479 (51%) | |
| Prevention mother–child transmission | 336 (9%) | 20 (3%) | 195 (9%) | 551 (8%) | |
| Laboratory | 22 (1%) | 4 (1%) | 18 (1%) | 44 (1%) | |
| Transfer from other facility | 17 (<1%) | 2 (<1%) | 8 (<1%) | 27 (<1%) | |
| Other | 553 (14%) | 90 (13%) | 314 (14%) | 957 (14%) | |
| Female, n (%) | 2,795 (65%) | 406 (51%) | 1,417 (56%) | 4,618 (61%) | <0.001 |
| Age, median (IQR) | 31 (25, 38) | 29 (24, 38) | 29 (24, 36) | 30 (25, 38) | <0.001 |
| Education, n (%) | 0.25 | ||||
| Missing | 849 (20%) | 165 (21%) | 619 (24%) | 1,633 (21%) | |
| No formal education | 525 (15%) | 89 (14%) | 272 (14%) | 886 (15%) | |
| Primary school | 2,256 (66%) | 417 (66%) | 1,298 (68%) | 3,971 (66%) | |
| Secondary school, basic level | 588 (17%) | 117 (19%) | 302 (16%) | 1,007 (17%) | |
| Secondary school, level medium | 54 (2%) | 7 (1%) | 44 (2%) | 105 (2%) | |
| University | 7 (<1%) | 2 (<1%) | 4 (<1%) | 13 (<1%) | |
| Marital status, n (%) | <0.001 | ||||
| Missing | 674 (16%) | 141 (18%) | 491 (19%) | 1,306 (17%) | |
| Living with partner | 1,296 (36%) | 217 (33%) | 690 (34%) | 2,203 (35%) | |
| Married | 1,013 (28%) | 149 (23%) | 578 (28%) | 1,740 (28%) | |
| Never married | 930 (26%) | 231 (35%) | 603 (29%) | 1,764 (28%) | |
| Widowed | 366 (10%) | 59 (9%) | 177 (9%) | 602 (10%) | |
| District, n (%) | <0.001 | ||||
| Alto Molócuè | 375 (9%) | 83 (10%) | 176 (7%) | 634 (8%) | |
| Gilé | 179 (4%) | 63 (8%) | 107 (4%) | 349 (5%) | |
| Ile | 266 (6%) | 54 (7%) | 98 (4%) | 418 (5%) | |
| Inhassunge | 815 (19%) | 201 (25%) | 346 (14%) | 1,362 (18%) | |
| Lugela | 353 (8%) | 80 (10%) | 251 (10%) | 684 (9%) | |
| Maganja | 569 (13%) | 24 (3%) | 403 (16%) | 996 (13%) | |
| Mopeia | 158 (4%) | 42 (5%) | 138 (5%) | 338 (4%) | |
| Morrumbala | 728 (17%) | 102 (13%) | 458 (18%) | 1288 (17%) | |
| Namacurra | 519 (12%) | 93 (12%) | 298 (12%) | 910 (12%) | |
| Pebane | 317 (7%) | 55 (7%) | 264 (10%) | 636 (8%) | |
| Time from enrollment to ART (days), median (IQR) | 73 (23, 244) | 38 (9, 125) | 47 (12–157) | 59 (17, 198) | <0.001 |
| d4T-based regimen, n (%) | <0.001 | ||||
| Missing | 251 (6%) | 41 (5%) | 203 (8%) | 495 (7%) | |
| d4T | 3,255 (81%) | 694 (92%) | 2,034 (87%) | 5,983 (84%) | |
| Not d4T | 773 (19%) | 62 (8%) | 302 (13%) | 1,137 (16%) | |
| BMI (kg/m2),c median (IQR) | 20 (18, 22) | 19 (17, 21) | 19 (18, 21) | 20 (18, 22) | <0.001 |
| Missing, n (%) | 646 (15%) | 393 (49%) | 1,308 (52%) | 2,347 (31%) | |
| CD4 count (cells/μl),d median (IQR) | 191 (110, 276) | 130 (46, 252) | 172 (92, 260) | 179 (98, 269) | <0.001 |
| Missing, n (%) | 1,799 (42%) | 358 (45%) | 1,187 (47%) | 3,344 (44%) | |
| Hemoglobin (g/dl),d median (IQR) | 10.1 (8.9, 11.6) | 9 (7.6, 10.4) | 9.8 (8.3, 11.0) | 10 (8.6, 11.3) | <0.001 |
| Missing, n (%) | 2501 (58%) | 471 (59%) | 1,561 (61%) | 4533 (60%) | |
| WHO stage,dn (%) | <0.001 | ||||
| Missing | 1,800 (42%) | 269 (34%) | 921 (36%) | 2,990 (39%) | |
| I | 606 (24%) | 52 (10%) | 274 (17%) | 932 (20%) | |
| II | 533 (22%) | 70 (13%) | 284 (18%) | 887 (19%) | |
| III | 1,025 (41%) | 198 (38%) | 663 (41%) | 1,886 (41%) | |
| IV | 315 (13%) | 208 (39%) | 397 (25%) | 920 (20%) |
Patients are lost who have not returned for medication pickup in over 60 days from the date of last scheduled medication pickup.
To compare the distribution of study characteristics for participants by outcome, we employ chi-square tests. Similarly, we use Kruskal–Wallis tests for continuous variables by outcome.
BMI is collected at enrollment.
Collected within 90 days before and 14 days after ART initiation.
Percentages are computed using the number of patients with a nonmissing value. Continuous variables are reported as median with interquartile range (i.e., the 25th and 75th percentiles).
ART, antiretroviral therapy; d4T, stavudine; BMI, body mass index.
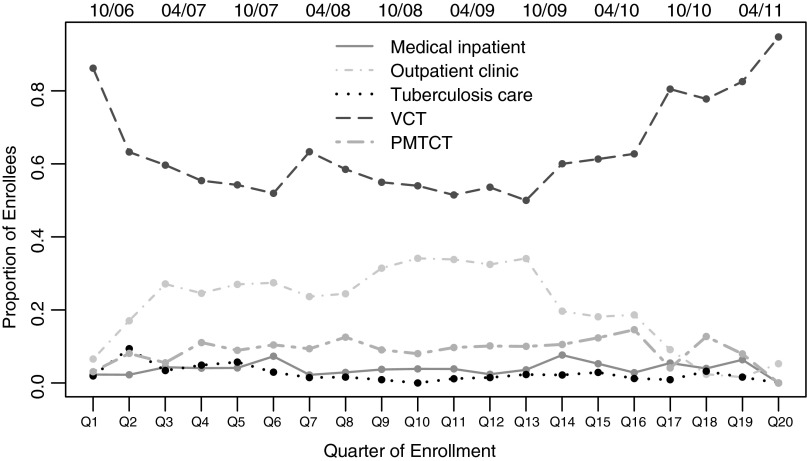
The sources of referral to HIV care included VCT centers (51%), general outpatient clinic (21%), PMTCT programs (8%), medical inpatients at health facilities (3%), tuberculosis service (2%), laboratories/blood banks (1%), and transfer from other HIV care program (<1%). The source of referral may have been marked as “other” if the code was not available on the paper clinical record form; this indication was selected for 14% of patients (equally selected for both men and women). The source of referral was missing completely for 10% of patients. The source of referral was balanced across sex with the exception of PMTCT where 537 women and only 14 men were referred. VCT was consistently the largest source of referral over time (Fig. 1). General outpatient clinic as a source of referral saw a steady decrease and VCT an increase beginning October 2010 (Fig. 1). Patient characteristics were different by source of referral to HIV care (Table 2). In addition to capturing primarily women, PMTCT captured a younger patient population. Patients referred as medical inpatients or from tuberculosis services had more advanced stage disease. Patients referred from PMTCT and VCT had higher median CD4+ cell counts. Patients referred by general outpatient clinic or PMTCT were in care for an average of 2–3 months prior to ART initiation compared to 3–4 weeks for medical inpatients and VCT referrals (Table 2).
FIG. 1.
Quarterly trends in source of referral to HIV care among patients starting treatment, Zambézia, Mozambique 2006–2011.
Table 2.
Patient Characteristics by Five Sources of Referral to HIV Care and Treatment, Zambézia, Mozambique 2006–2011
| Medical inpatient(n=236) | Outpatient clinic(n=1,415) | Tuberculosis care(n=151) | VCT(n=3,479) | PMTCT(n=551) | p-valuea | |
|---|---|---|---|---|---|---|
| Female, n (%) | 121 (51%) | 850 (60%) | 66 (44%) | 1,972 (57%) | 537 (97%) | <0.001 |
| Age, median (IQR) | 31 (25, 40) | 30 (25, 38) | 31 (25, 39) | 30 (25, 38) | 26 (22, 30) | <0.001 |
| Education, n (%) | <0.001 | |||||
| Missing | 33 (14%) | 282 (20%) | 41 (27%) | 724 (21%) | 87 (16%) | |
| No formal education | 36 (18%) | 229 (20%) | 20 (18%) | 362 (13%) | 95 (20%) | |
| Primary school | 129 (64%) | 738 (65%) | 69 (63%) | 1,860 (68%) | 277 (60%) | |
| Secondary school, basic level | 33 (16%) | 154 (14%) | 17 (15%) | 475 (17%) | 78 (17%) | |
| Secondary school, level medium | 5 (2%) | 9 (1%) | 4 (4%) | 52 (2%) | 14 (3%) | |
| University | 0 (0%) | 3 (<1%) | 0 (0%) | 6 (<1%) | 0 (0%) | |
| Marital status, n (%) | <0.001 | |||||
| Missing | 35 (15%) | 234 (17%) | 34 (23%) | 563 (16%) | 61 (11%) | |
| Living with partner | 71 (35%) | 479 (41%) | 47 (40%) | 971 (33%) | 248 (51%) | |
| Married | 53 (26%) | 215 (18%) | 31 (26%) | 846 (29%) | 141 (29%) | |
| Never married | 60 (30%) | 355 (30%) | 30 (26%) | 802 (28%) | 89 (18%) | |
| Widowed | 17 (8%) | 132 (11%) | 9 (8%) | 297 (10%) | 12 (2%) | |
| District, n (%) | <0.001 | |||||
| Alto Molócuè | 18 (8%) | 24 (2%) | 5 (3%) | 425 (12%) | 85 (15%) | |
| Gilé | 28 (12%) | 105 (7%) | 4 (3%) | 68 (2%) | 27 (5%) | |
| Ile | 3 (1%) | 4 (<1%) | 0 (0%) | 62 (2%) | 13 (2%) | |
| Inhassunge | 62 (26%) | 878 (62%) | 78 (52%) | 86 (2%) | 116 (21%) | |
| Lugela | 22 (9%) | 113 (8%) | 4 (3%) | 399 (11%) | 63 (11%) | |
| Maganja | 17 (7%) | 7 (<1%) | 9 (6%) | 710 (20%) | 45 (8%) | |
| Mopeia | 22 (9%) | 93 (7%) | 0 (0%) | 50 (1%) | 43 (8%) | |
| Morrumbala | 3 (1%) | 53 (4%) | 31 (21%) | 830 (24%) | 63 (11%) | |
| Namacurra | 27 (11%) | 94 (7%) | 8 (5%) | 486 (14%) | 65 (12%) | |
| Pebane | 34 (14%) | 44 (3%) | 12 (8%) | 363 (10%) | 31 (6%) | |
| Time from enrollment to ART (days), median (IQR) | 22 (0, 102) | 71 (25, 211) | 56 (18, 181) | 28 (0, 87) | 90 (30, 352) | <0.001 |
| d4T-based regimen, n(%) | <0.001 | |||||
| Missing | 12 (5%) | 22 (2%) | 7 (5%) | 330 (9%) | 29 (5%) | |
| d4T | 191 (85%) | 1,251 (90%) | 137 (95%) | 2,618 (83%) | 344 (66%) | |
| Not d4T | 33 (15%) | 142 (10%) | 7 (5%) | 531 (17%) | 178 (34%) | |
| BMI (kg/m2),b median (IQR) | 18 (16, 20) | 19 (18, 21) | 19 (17, 21) | 20 (18, 22) | 22 (20, 24) | <0.001 |
| Missing, n (%) | 114 (48%) | 303 (21%) | 59 (39%) | 1,129 (32%) | 149 (27%) | |
| CD4 count (cells/μl),c median (IQR) | 153 (67, 253) | 165 (89, 248) | 169 (79, 285) | 174 (95, 266) | 219 (153, 293) | <0.001 |
| Missing, n (%) | 130 (55%) | 509 (36%) | 77 (51%) | 1,522 (44%) | 246 (45%) | |
| Hemoglobin (g/dl),c median (IQR) | 9.3 (7.9, 11.0) | 9.7 (8.2, 11.2) | 10.0 (8.2, 11.4) | 10.0 (8.8, 11.5) | 10.0 (8.6, 11.0) | 0.002 |
| Missing, n (%) | 154 (65%) | 764 (54%) | 100 (66%) | 2,043 (59%) | 321 (58%) | |
| WHO Stage,cn (%) | <0.001 | |||||
| Missing | 93 (39%) | 622 (44%) | 66 (44%) | 1,282 (37%) | 213 (39%) | |
| I | 24 (17%) | 155 (20%) | 5 (6%) | 421 (19%) | 173 (51%) | |
| II | 16 (11%) | 200 (25%) | 5 (6%) | 419 (19%) | 59 (17%) | |
| III | 48 (34%) | 281 (35%) | 62 (73%) | 940 (43%) | 75 (22%) | |
| IV | 55 (38%) | 157 (20%) | 13 (15%) | 417 (19%) | 31 (9%) | |
| Outcome (24 months), n (%) | <0.001 | |||||
| Alive | 81 (34%) | 787 (56%) | 73 (48%) | 2,009 (58%) | 336 (61%) | |
| Dead | 55 (23%) | 229 (16%) | 18 (12%) | 293 (8%) | 20 (4%) | |
| Lost | 100 (42%) | 399 (28%) | 60 (40%) | 1,177 (34%) | 195 (35%) |
To compare the distribution of study characteristics for participants by source of referral, we employ chi-square tests. Similarly, we use Kruskal–Wallis tests for continuous variables by source of referral.
Height, weight, and BMI are collected at enrollment.
Collected within 90 days before and 14 days after ART initiation.
Percentages are computed using the number of patients with a nonmissing value. Continuous variables are reported as median with interquartile range (i.e., the 25th and 75th percentiles).
VCT, voluntary counseling and testing; PMTCT, prevention of mother to child transmission.
The cumulative incidence of 2-year LTFU (accounting for death as a competing risk), per Mozambique's definition as no effective clinical contact within 60 days after the last scheduled medication pickup, was 38.1% (95% CI: 36.9–39.3%). The cumulative incidence of LTFU was lower when applying two definitions from the literature. The cumulative incidence of LTFU using the “universal” definition14 was 30.7% (95% CI: 29.6–31.9%) at 2 years. The cumulative incidence of LTFU using the “reference” definition15 was 31.0% (95% CI: 29.8–32.1%) at 2 years. The 2-year probability of death was 14.2% (95% CI: 13.2–15.1%) and the probability of death/LTFU was 49.7% (95% CI: 48.4–50.9%). There was evidence that 2-year probabilities of death, cumulative LTFU, and death or LTFU were different by source of referral (Fig. 2, p<0.001 for all). Patients referred while they were medical inpatients or during antenatal care (PMTCT) had the highest and lowest probabilities of death, respectively. Patients referred by a general outpatient clinic had the lowest cumulative incidence of LTFU. Unadjusted hazard ratios (95% CI) for mortality in 2 years following treatment initiation by referral site with VCT as referent category were as follows: medical inpatient 2.68 (1.97–3.64), general outpatient clinic 1.42 (1.12–1.80), tuberculosis service 1.30 (0.79–2.13), and PMTCT 0.35 (0.22–0.55).
FIG. 2.
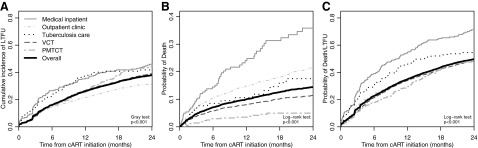
Two year outcomes by referral to HIV care and treatment, Zambézia, Mozambique 2006–2011. Subfigures demonstrate the following: cumulative incidence of loss to follow-up (LTFU) (A), probability of death (B), and probability of death or LTFU at 24 months for each source of referral to HIV care (C). Mozambican national guidelines define LTFU as no effective clinical contact within 60 days after the last scheduled medication pickup.
Table 3 reports adjusted hazard ratios for LTFU using Cox regression and censoring at date of death for those who died in the first 2 years following treatment initiation. After controlling for other variables, the referral site remained strongly associated with LTFU during the first 2 years (p<0.001). Compared with VCT, patients referred from medical inpatient, tuberculosis care, and PMTCT had 43%, 50%, and 34% higher rates of LTFU, respectively. Other factors associated with a higher hazard of LTFU were male sex, younger age, more advanced stage of diseases, and more recent year of cART initiation.
Table 3.
Adjusted Hazard Ratios from Cox Regression Models of 2 Year Loss to Follow-Up, Mortality, and Mortality/Loss to Follow-Up (n=7,615), Zambézia, Mozambique 2006–2011
| LTFUa | Mortality | Mortality/LTFUa | ||||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Referral site | <0.001 | <0.001 | <0.001 | |||
| VCT (ref) | 1 | 1 | 1 | |||
| Laboratory | 1.31 (0.79, 2.18) | 0.92 (0.34, 2.48) | 1.33 (0.86, 2.06) | |||
| Medical inpatient | 1.43 (1.15, 1.78) | 1.87 (1.36, 2.58) | 1.60 (1.34, 1.91) | |||
| General outpatient clinic | 1.04 (0.90, 1.21) | 1.44 (1.11, 1.85) | 1.17 (1.02, 1.33) | |||
| Tuberculosis care | 1.50 (1.15, 1.95) | 1.04 (0.61, 1.77) | 1.38 (1.08, 1.75) | |||
| PMTCT | 1.34 (1.14, 1.57) | 0.69 (0.43, 1.11) | 1.24 (1.06, 1.44) | |||
| Transfer from other facility | 1.28 (0.62, 2.65) | 0.76 (0.18, 3.12) | 1.13 (0.59, 2.17) | |||
| Other | 1.05 (0.92, 1.20) | 0.90 (0.70, 1.17) | 1.02 (0.91, 1.14) | |||
| Male | 1.59 (1.45, 1.75) | <0.001 | 1.64 (1.39, 1.94) | <0.001 | 1.61 (1.48, 1.75) | <0.001 |
| Age | <0.001 | 0.02 | <0.001 | |||
| 20 years (ref) | 1 | 1 | 1 | |||
| 40 years | 0.60 (0.53, 0.67) | 0.72 (0.58, 0.89) | 0.62 (0.56, 0.69) | |||
| 60 years | 0.63 (0.51, 0.78) | 0.82 (0.58, 1.16) | 0.67 (0.56, 0.80) | |||
| Marital status | 0.62 | 0.28 | 0.37 | |||
| Married (ref) | 1 | 1 | 1 | |||
| Living with partner | 1.05 (0.93, 1.18) | 1.14 (0.91, 1.43) | 1.06 (0.95, 1.19) | |||
| Never married | 1.08 (0.96, 1.21) | 1.25 (1.00, 1.55) | 1.11 (0.98, 1.26) | |||
| Widowed | 1.08 (0.91, 1.30) | 1.21 (0.87, 1.69) | 1.10 (0.94, 1.29) | |||
| Education | 0.18 | 0.22 | 0.31 | |||
| Primary school (ref) | 1 | 1 | 1 | |||
| No formal education | 1.10 (0.95, 1.27) | 0.93 (0.72, 1.19) | 1.08 (0.97, 1.21) | |||
| Secondary school or higher | 0.92 (0.81, 1.04) | 1.17 (0.95, 1.42) | 0.98 (0.87, 1.09) | |||
| BMI at enrollment (per 1 kg/m2) | 0.96 (0.94, 0.98) | <0.001 | 0.94 (0.90, 0.98) | 0.003 | 0.96 (0.94, 0.97) | <0.001 |
| Hemoglobin (per 1 g/dl) | 0.94 (0.91, 0.97) | <0.001 | 0.85 (0.79, 0.90) | <0.001 | 0.92 (0.89, 0.95) | <0.001 |
| cART regimen | 0.58 | 0.93 | 0.54 | |||
| Not d4T-based (ref) | 1 | 1 | 1 | |||
| d4T-based | 1.04 (0.90, 1.21) | 1.01 (0.75, 1.37) | 1.04 (0.91, 1.19) | |||
| WHO stage | <0.001 | <0.001 | <0.001 | |||
| I (ref) | 1 | 1 | 1 | |||
| II | 1.01 (0.85, 1.20) | 1.19 (0.81, 1.74) | 1.07 (0.93, 1.24) | |||
| III | 1.15 (1.00, 1.33) | 1.70 (1.26, 2.28) | 1.27 (1.12, 1.44) | |||
| IV | 1.62 (1.35, 1.94) | 3.32 (2.40, 4.58) | 1.97 (1.63, 2.37) | |||
| CD4 count (cells/μl) | 0.006 | <0.001 | <0.001 | |||
| 25 | 1.23 (1.08, 1.40) | 1.82 (1.40, 2.35) | 1.38 (1.21, 1.56) | |||
| 50 | 1.22 (1.07, 1.40) | 1.58 (1.21, 2.05) | 1.31 (1.14, 1.52) | |||
| 100 | 1.20 (1.02, 1.40) | 1.31 (1.00, 1.70) | 1.23 (1.05, 1.43) | |||
| 200 | 1.10 (1.02, 1.19) | 1.07 (0.94, 1.20) | 1.11 (1.03, 1.19) | |||
| 350 (ref) | 1 | 1 | 1 | |||
| Year of initiationb | <0.001 | 0.77 | <0.001 | |||
| 2007 (ref) | 1 | 1 | 1 | |||
| 2008 | 1.73 (1.49, 2.02) | 1.10 (0.88, 1.38) | 1.52 (1.33, 1.72) | |||
| 2009 | 1.65 (1.42, 1.92) | 1.18 (0.92, 1.50) | 1.48 (1.30, 1.69) | |||
| 2010 | 2.17 (1.85, 2.54) | 1.07 (0.83, 1.38) | 1.78 (1.55, 2.04) | |||
| 2011 | 3.46 (2.76, 4.35) | 1.12 (0.73, 1.72) | 2.65 (2.17, 3.23) | |||
Mozambican national guidelines define loss to follow-up (LTFU) as no effective clinical contact within 60 days after the last scheduled medication pickup.
January 1 serves as the reference date for the corresponding year.
Adjusted hazard ratios for mortality in 2 years following treatment initiation are given in Table 3. Adjusting for demographics and health status, the source of referral to HIV care remained associated with the hazard of death (p<0.001), such that hazard ratios (95% CI) for 2-year mortality with VCT as referent were medical inpatient 1.87 (1.36–2.48), general outpatient clinic 1.44 (1.11–1.85), and antenatal care 0.69 (0.43–1.11). Male sex and younger age were also associated with a higher hazard of death, as were standard measures of poor patient health including low BMI, low hemoglobin, low CD4 count, and advanced WHO stage.
With respect to the combined endpoint of mortality and LTFU, the source of referral to HIV care remained associated with 2-year retention after adjusting for demographics and health status indicators (p<0.001). Compared with VCT, hazards of 2-year mortality/LTFU were medical inpatient 1.60 (1.34–1.91), general outpatient clinic 1.17 (1.02–1.33), tuberculosis care 1.38 (1.08–1.75), and antenatal care 1.24 (1.06–1.44). Reduced and increased risk factors from all three models (LTFU, mortality, and mortality/LTFU) were generally similar with the exception of PMTCT as protective for mortality and a risk factor for LTFU and the combined endpoint.
Discussion
The source of referral in rural Mozambique was highly predictive of LTFU, mortality, and mortality/LTFU. Patients who were referred from an inpatient medical facility or general outpatient clinic had higher risks of mortality and LTFU even after controlling for standard indicators of patient health. Patients referred to care from these sites generally have more advanced HIV disease, as evidenced by higher WHO stage and lower BMI, hemoglobin, and CD4 count at cART initiation. There are likely other unmeasured attributes in these referral populations that are also associated with poorer health and subsequently higher risks of mortality and LTFU, and understanding these attributes may lead to interventions that target patient populations at the source of referral.
Obviously, HIV testing should occur before people become symptomatic. Clinical implications of late presentation include more opportunistic infections, concurrent illnesses, high short-term mortality, higher cost of care, and cART resistance.18 The clinical implications for patients who return to care after being LTFU are similar: higher mortality, more opportunistic infections, and increased risk of HIV transmission.4 With evidence that patients referred in medical inpatient facilities, tuberculosis care, and PMTCT have a higher incidence of LTFU than VCT, programs aimed at increasing retention may benefit from targeting these patient populations with more focused counseling and/or social support. These findings have important implications for planning and targeting strategies to retain newly enrolled HIV-infected patients in HIV care and treatment.
Patients in PMTCT are generally diagnosed earlier in disease progression (i.e., asymptomatic) and may not stay in care due to fear of stigma, discontinuation of cART postdelivery, and not feeling ill.19,20 Owing to new WHO recommendations for Option B+, the lifelong administration of cART for all HIV-infected women diagnosed during pregnancy, our findings of a lower risk of death and rates of LTFU similar to the overall program among women referred from antenatal care (PMTCT) is of interest to policymakers.21 The observed LTFU in antenatal care is less affected by unrecorded mortality and is more likely the result of disengagement from care. Our results suggest that this PMTCT referral population at high risk for LTFU may require assistance, such as active case finding, health systems navigation, and increased HIV counseling or social support.22
Sex differences in mortality and LTFU may be due to delayed HIV testing and social support, and interventions could be targeted at the source of referral. Men were found to have both a higher hazard of LTFU (AHR: 1.58) and death (AHR: 1.65) compared to women. Partners of women in PMTCT were typically referred to VCT for testing rather than testing at the PMTCT site. Beginning in 2012, FGH is currently piloting a program that employs “male champions” and traditional birth attendants to provide community-based HIV education services to pregnant women and their partners, while providing education to the wider community about the importance of male partner support during pregnancy. As a result, couples counseling has increased in early 2013, resulting in higher rates of HIV testing and ART acceptance among pregnant women and their partners with antenatal care as the source of referral. The “male champion” pilot program is an example of an intervention that may be targeted at the source of referral to improve outcomes and reduce gender disparities.
In early 2011, FGH began quality improvement activities to improve counseling and testing at the clinics that resulted from health worker referral. Prior to this initiative the uptake of PITC was inconsistent within clinics and across clinics. Poor uptake was mainly due to health worker overload and practices in which clinicians at the various sectors would send their patients to the VCT for testing rather than perform the tests themselves due to time constraints. This likely resulted in an artificial elevation in the number of referrals to HIV care from VCT (as displayed in Fig. 1) when in reality the process of identification of patients in need of HIV testing was initiated in the inpatient services, tuberculosis services, general outpatient clinics, and PMTCT services. This indirect referral likely results in further delays to HIV testing among those motivated enough to follow up on provider recommendations, which affects the composition of the VCT group. With VCT as the referent in all models, there is the potential to bias results toward the null because the group contains individuals who would have been referred elsewhere had the provider consistently initiated testing.
The major strength of our study is the large cohort of adults initiating therapy during 5 years of ART scale-up in a rural, sub-Saharan African setting. The quality of the data recorded is good with routine audits and daily, onsite electronic data entry, though missing data are still common.
The limitations of this study include over 30% undocumented information on major health status indicators at ART initiation. Since a complete case analysis would drop 86% of the patients, multiple imputation (using patient characteristics to predict missing values and properly incorporating missing-data uncertainty) was necessary to avoid substantial loss of power. Multiple imputation assumes that after accounting for patient characteristics, whether or not a variable is missing is independent of patient outcomes.23 This assumption may be unrealistic in our setting, and therefore imputation models may not fully adjust estimates of association between the source of referral and patient outcomes.
The referral site is recorded at the first HIV care visit and there is no way of validating whether the documented source is accurate (i.e., no referral system). Because patients enter the database through enrollment into care, we are unable to account for potential selection bias that might result from differential rates of linkage to care by referral site. In Fig. 1, the low proportions of non-VCT source of referral in the last quarters among cART initiators suggest that there may be a lag between non-VCT HIV entry into HIV care and cART initiation (i.e., more outpatient consults and PMTCT referrals enrolled during quarters 17–20 have not yet initiated therapy). The temporal trend in which LTFU rates increased during rapid scale-up may indicate an increasingly constrained health system and has been observed in other cohorts from resource-limited settings.24–26 Additionally, patients who initiated ART earlier in the program scale-up had a longer window of opportunity to reengage into care.
Mortality and LTFU are all too common among HIV-infected adults in rural Mozambique. Of study patients fortunate to receive testing and counseling and who enter care, only half were alive and remained in the program 2 years hence. We do not think that we are overestimating this much27,28 since there are very few alternative sources of ART care other than these PEPFAR-supported venues. Furthermore, the mobility of rural subsistence farmers and fishermen and families is low in this region, in contrast to circumstances during the civil war.29,30 Estimates and figures of mortality make an assumption of noninformative censoring—that patients LTFU had an incidence of death similar to patients not LTFU. Such an assumption is dubious for our rural Mozambican cohort. Instead of correcting mortality estimates for this bias using some common techniques (e.g., inverse probability censor weighting or nomogram-correction for 1 year mortality31,32), we present estimates for the combined endpoint of mortality/LTFU that serves as a crude sensitivity analysis. Because of the high rates of LTFU, hazard ratios for mortality/LTFU more closely resemble the results for LTFU in magnitude, although results were generally similar across mortality and LTFU Cox regression models.
We do not presume a causal effect of referral site on 2-year mortality, and so we expect that unmeasured confounding may explain the residual association after covariate adjustment. Unmeasured confounding would be of great interest in explaining why the referral source remains associated with 2-year outcomes. Important confounders mentioned in this discussion that are not measured in our cohort include but are not limited to duration of symptoms, fear of stigma, comorbidities, cART adherence, psychosocial support, traditional medicine use, and satisfaction with the health facility. Future research needs to explore unmeasured attributes among these patient populations that lead to poor treatment outcomes.
We believe that PEPFAR-supported initiatives in the least well-capacitated regions of rural Africa will need many more years of capacity building, health care worker training, and shifting of health care adherence norms before we can expect self-sustaining programs that do not have high mortality and LTFU rates.33 That source of referral was associated with mortality and LTFU after adjusting for sociodemographic characteristics, clinical status at treatment initiation, location, and date of cART initiation suggests that (1) additional unmeasured factors are influential, and (2) interventions targeted at increasing retention may benefit from targeting patient populations, based on the source of referral, with more focused counseling and/or social support.
Acknowledgments
We thank Ferreira Gonçalves Ferreira, Linda Moiane, Tito Jecquicene, Carlos Castel Branco, Wilson Silva, and Jairzinho Tereso for their assistance. We are sincerely grateful to the excellent peer reviewers for improving the quality of this article.
This research has been supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Department of Health and Human Services, Centers for Disease Control and Prevention under the terms of Cooperative Agreement #U2GPS000631. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Presented, in part, as a poster at the IAS meeting in Washington, DC on July 23, 2012.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Donnell D, Baeten JM, Kiarie J, et al. : Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: A prospective cohort analysis. Lancet 2010;375(9731):2092–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Romero J, Castilla J, Hernando V, Rodriguez C, and Garcia S: Combined antiretroviral treatment and heterosexual transmission of HIV-1: Cross sectional and prospective cohort study. BMJ 2010;340:c2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds SJ, Makumbi F, Nakigozi G, et al. : HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS 2011;25(4):473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ndiaye B, Ould-Kaci K, Salleron J, et al. : Characteristics of and outcomes in HIV-infected patients who return to care after loss to follow-up. AIDS 2009;23(13):1786–1789 [DOI] [PubMed] [Google Scholar]
- 5.Ministério da Saúde Instituto Nacional de Saúde (INS), Instituto Nacional de Estatística (INE), ICF Macro : Inquérito Nacional de Prevalência, Riscos Comportamentais e Informação sobre o HIV e SIDA (INSIDA) em Moçambique 2009. Calverton, Maryland, EUA: INS, INE, e ICF Macro, 2010 [Google Scholar]
- 6.Micek MA, Gimbel-Sherr K, Baptista AJ, et al. : Loss to follow-up of adults in public HIV care systems in central Mozambique: Identifying obstacles to treatment. J Acquir Immune Defic Syndr 2009;52(3):397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manders EJ, Jose E, Solis M, et al. : Implementing open MRS for patient monitoring in an HIV/AIDS care and treatment program in rural Mozambique. Stud Health Technol Inform 2010;160(Pt 1):411–415 [PubMed] [Google Scholar]
- 8.Moon TD, Burlison JR, Blevins M, et al. : Enrollment and programmatic trends and predictors of antiretroviral therapy initiation from President's Emergency Plan for AIDS Relief (PEPFAR)-supported public HIV care and treatment sites in rural Mozambique. Int J STD AIDS 2011;22(11):621–627 [DOI] [PubMed] [Google Scholar]
- 9.Moon TD, Burlison JR, Sidat M, et al. : Lessons learned while implementing an HIV/AIDS care and treatment program in rural Mozambique. Retrovirology 2010;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Silva M, Blevins M, Wester CW, et al. : Patient loss to follow-up before antiretroviral therapy initiation in rural Mozambique. AIDS Behav 2014;1–13: [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.de Bolso Guião: Tratamento Antiretroviral e Infecções Oportunistas, Adulto e Adolescente. Maputo, Mozambique: Ministério da Saúde, 2010 [Google Scholar]
- 12.Shepherd BE, Blevins M, Vaz LM, et al. : Impact of definitions of loss to follow-up on estimates of retention, disease progression, and mortality: Application to an HIV program in Mozambique. Am J Epidemiol 2013;178(5):819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Psychosocial Support Report. Friends in Global Health, 2011
- 14.Chi BH, Yiannoutsos CT, Westfall AO, et al. : Universal definition of loss to follow-up in HIV treatment programs: A statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS Med 2011;8(10):e1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimsrud AT, Cornell M, Egger M, et al. : Impact of definitions of loss to follow-up (LTFU) in antiretroviral therapy program evaluation: Variation in the definition can have an appreciable impact on estimated proportions of LTFU. J Clin Epidemiol 2013;66:1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrell FE: Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer, New York, 2001 [Google Scholar]
- 17.Harrell FE., Jr and Dupont MC: Package ‘Hmisc’. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2012 [Google Scholar]
- 18.Girardi E, Sabin CA, and Antonella d'Arminio Monforte M: Late diagnosis of HIV infection: Epidemiological features, consequences and strategies to encourage earlier testing. JAIDS J Acquir Immune Defic Syndr 2007;46:S3–S8 [DOI] [PubMed] [Google Scholar]
- 19.Ciampa PJ, Burlison JR, Blevins M, et al. : Improving retention in the early infant diagnosis of HIV program in rural Mozambique by better service integration. J AIDS J Acquir Immune Defic Syndr 2011;58(1):115–119 [DOI] [PubMed] [Google Scholar]
- 20.Watson-Jones D, Balira R, Ross DA, et al. : Missed opportunities: Poor linkage into ongoing care for HIV-positive pregnant women in Mwanza, Tanzania. PLoS One 2012;7(7):e40091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang CH, Chen CH, and Chiang PH: Primary urothelial carcinoma of the upper urinary tract in dialysis patients with 5-year follow-up. Jpn J Clin Oncol 2010;40(3):241–246 [DOI] [PubMed] [Google Scholar]
- 22.Rajabiun S, Coleman S, and Drainoni M-L: Keeping at-risk persons living with HIV/AIDS in care: A qualitative study of staff perspectives. J HIV/AIDS Social Serv 2011;10(2):120–138 [Google Scholar]
- 23.Little RJA. and Rubin DB: Statistical Analysis with Missing Data, 2nd ed. Wiley, Hoboken, NJ, 2002 [Google Scholar]
- 24.Boulle A, Van Cutsem G, Hilderbrand K, et al. : Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS 2010;24(4):563–572 [DOI] [PubMed] [Google Scholar]
- 25.Forster M, Bailey C, Brinkhof MW, et al. : Electronic medical record systems, data quality and loss to follow-up: Survey of antiretroviral therapy programmes in resource-limited settings. Bull World Health Organ 2008;86(12):939–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auld AF, Mbofana F, Shiraishi RW, et al. : Four-year treatment outcomes of adult patients enrolled in Mozambique's rapidly expanding antiretroviral therapy program. PLoS One 2011;6(4):e18453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geng EH, Glidden DV, Bangsberg DR, et al. : A causal framework for understanding the effect of losses to follow-up on epidemiologic analyses in clinic-based cohorts: The case of HIV-infected patients on antiretroviral therapy in Africa. Am J Epidemiol 2012;175(10):1080–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geng EH, Glidden DV, Bwana MB, et al. : Retention in care and connection to care among HIV-infected patients on antiretroviral therapy in Africa: Estimation via a sampling-based approach. PLoS One 2011;6(7):e21797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girdler‐Brown B: Eastern and southern Africa. Int Migration 1998;36(4):513–551 [DOI] [PubMed] [Google Scholar]
- 30.Audet CM, Burlison J, Moon TD, et al. : Sociocultural and epidemiological aspects of HIV/AIDS in Mozambique. BMC Int Health Human Rights 2010;10(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robins JM. and Finkelstein DM: Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics 2000;56(3):779–788 [DOI] [PubMed] [Google Scholar]
- 32.Egger M, Spycher BD, Sidle J, et al. : Correcting mortality for loss to follow-up: A nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med 2011;8(1):e1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vermund SH, Sidat M, Weil LF, et al. : Transitioning HIV care and treatment programs in southern Africa to full local management. AIDS (London, England) 2012;26(10):1303. [DOI] [PMC free article] [PubMed] [Google Scholar]