Abstract
Deficits in impulse control are increasingly recognized in association with posttraumatic stress disorder (PTSD). To our further understanding of the neurobiology of PTSD‐related disinhibition, we examined alterations in brain morphology and network connectivity associated with response inhibition failures and PTSD severity. The sample consisted of 189 trauma‐exposed Operation Enduring Freedom/Operation Iraqi Freedom veterans (89% male, ages 19–62) presenting with a range of current PTSD severity. Disinhibition was measured using commission errors on a Go/No‐Go (GNG) task with emotional stimuli, and PTSD was assessed using a measure of current symptom severity. Whole‐brain vertex‐wise analyses of cortical thickness revealed two clusters associated with PTSD‐related disinhibition (Monte Carlo cluster corrected P < 0.05). The first cluster included portions of right inferior and middle frontal gyri and frontal pole. The second cluster spanned portions of left medial orbital frontal, rostral anterior cingulate, and superior frontal gyrus. In both clusters, commission errors were associated with reduced cortical thickness at higher (but not lower) levels of PTSD symptoms. Resting‐state functional magnetic resonance imaging analyses revealed alterations in the functional connectivity of the right frontal cluster. Together, study findings suggest that reductions in cortical thickness in regions involved in flexible decision‐making, emotion regulation, and response inhibition contribute to impulse control deficits in PTSD. Furthermore, aberrant coupling between frontal regions and networks involved in selective attention, memory/learning, and response preparation suggest disruptions in functional connectivity may also play a role. Hum Brain Mapp 36:3076–3086, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: cortical thickness, resting‐state connectivity, impulsivity, veterans, go/no‐go, trauma
Neurobiological indicators of disinhibition in posttraumatic stress disorder
Posttraumatic stress disorder (PTSD) is increasingly recognized as involving deficits in impulse control and self‐regulation, as evidenced by inclusion of the new “reckless and self‐destructive behavior” symptom in DSM‐5 (American Psychiatric Association, 2013). It is associated with elevated rates of substance abuse, violent outbursts, impulsive self‐injury, and other behaviors marked by impulse control problems [Elbogen et al., 2010; Jacobsen et al., 2001; Miller et al., 2006; Nock and Prinstein, 2005; Wolf et al., 2012]. However, research on the neurobiology of impulse control failures and disinhibition in the context of PTSD is relatively sparse, which presents a significant barrier to understanding the mechanisms that initiate and maintain impulsive behavior in this disorder.
Inhibition is a multifaceted higher‐order cognitive function that is essential for self‐control, and it can be parsed into overlapping, yet distinct, inhibitory control processes [Nigg, 2000]. Proposals on the taxonomy of inhibition‐related processes typically distinguish between response inhibition, which involves control of an automatic or dominant motor response, and interference control or resistance to distractor interference, which involve the ability to resolve conflicting information [e.g., Nigg, 2000; Friedman and Miyake, 2004]. In regards to PTSD, impaired inhibitory processes have been identified as both a potential vulnerability for the development of the disorder and implicated in the maintenance of posttraumatic stress reactions over time [Aupperle et al., 2012; Jovanovic and Ressler, 2010; Verwoerd et al., 2009]. For example, diminished control of cognitive, emotional, and behavioral reactions to trauma‐related stimuli have been linked to reexperiencing symptoms (e.g., failure to suppress intrusive trauma‐related memories) [Verwoerd et al., 2009] and hyperarousal symptoms (e.g., failure to suppress fear responses in the presence of safety cues) [Jovanovic and Ressler, 2010]. Thus, inhibitory dysfunction appears to play a central role in the etiology and progression of PTSD. The purpose of this study was to examine the neurobiology of response inhibition in PTSD, because this component of inhibition has been closely tied to problems with impulsivity [Keilp et al., 2005] but has not been as thoroughly characterized in neurobiological studies of PTSD as other types of inhibition (e.g., interference control with the Stroop).
A sizeable body of research has focused on identifying areas of the brain that mediate response inhibition and impulsivity. The GNG task is one of the most widely studied measures of response inhibition, as it measures effortful control of a motor response without imposing demands on other high‐level cognitive control systems (e.g., distractor suppression, interference control) [Rubia et al., 2001; Schulz et al., 2007]. Functional magnetic resonance imaging (fMRI) studies using this task have consistently found task‐based activation differences in prefrontal cortex (PFC), particularly right inferior frontal gyrus (IFG) [Aron et al., 2004]. According to a recent meta‐analysis of 30 neuroimaging studies, activation in response to No‐Go stimuli is most consistently seen in a predominately right‐lateralized network of brain regions in healthy adults, including bilateral IFG, right dorsolateral PFC, superior temporal gyrus, supplementary motor cortex, anterior cingulate cortex (ACC), and left insula [Criaud and Boulinguez, 2013]. Inhibitory control has also been related to IFG activation during emotional processing, with inhibition and emotional processing showing additive activation effects in this region [Brown et al., 2012]. These findings converge with prior work implicating IFG in inhibitory control and emotional regulation [Fortier et al., 2014; Ochsner et al., 2004].
To our knowledge, only three prior neuroimaging studies have examined response inhibition using GNG tasks in PTSD, and none have examined these processes in relation to structural brain morphology. In a sample of adolescents, PTSD symptoms during a GNG task were associated with decreased No‐Go activation in left middle frontal cortex as well as greater activation in left cuneus, left inferior occipital/temporal gyri, and bilateral medial frontal gyrus/ACC [Carrion et al., 2008]. Consistent with these findings, research suggests that adults with PTSD demonstrate relatively reduced No‐Go task activation in right ventral and medial PFC, dorsolateral PFC, and temporoparietal junction, and relatively greater activation in postcentral gyrus and cuneus compared to those without PTSD [Falconer et al., 2008; Jovanovic et al., 2013]. Thus, studies published to date suggests that PTSD is associated with less activation in frontal brain regions that are typically recruited during response inhibition in healthy controls and greater activation in motor areas.
Structural differences associated with response inhibition in PTSD have yet to be examined but are an important next step for several reasons. First, structural variation may partially or completely explain differences in functional activation. For instance, individuals with PTSD may activate the cortex to the same degree as healthy controls but show weaker activation, because loss of cortical thickness dilutes the strength of the activation signal. Second, it is crucial to differentiate between functional and structural differences, as structural differences may be less amenable to treatment and thus may require a different strength, duration, and/or type of intervention. Finally, not all structural differences are reflected in functional activation. Thus, it is necessary to directly examine brain structure to form a more comprehensive model of the disinhibition‐related neural disturbances that occur in the context of PTSD symptoms. The primary aims of this study were to investigate the cortical substrates of response disinhibition in PTSD and the impact of PTSD symptom severity on disinhibition‐related variation in morphology. To better understand the functional significance of variations in morphology, we conducted exploratory resting‐state functional connectivity analyses to examine whether regions with altered cortical thickness, in turn, displayed disruptions in interregional communication. This allowed us to gain insight into the impact of morphological variation within the larger context of neural circuitry. Resting‐state fMRI was examined (as opposed to task fMRI), because resting‐state coupling is thought to represent stable individual differences, similar to the structural morphology that was also examined. To rule‐out potential confounds, we examined whether conditions that influence brain structure and frequently co‐occur with PTSD symptoms, specifically depression symptoms, alcohol consumption, and mild traumatic brain injury (mTBI), could account for our findings. We recruited a large sample of trauma‐exposed Operation Enduring Freedom or Operation Iraqi Freedom (OEF/OIF) service members, presenting with a range of current PTSD symptom severity. Participants completed a GNG task with emotional stimuli and then underwent magnetic resonance imaging. Unlike the task‐based fMRI studies reviewed above, this study examined whether disinhibition (measured by GNG commission errors on a task performed outside the scanner) and PTSD symptom severity interacted to predict individual variation in brain structure and connectivity in the resting state (as opposed to functional activation to Go vs. No‐Go stimuli). We selected commission errors as our measure of disinhibition, because previous studies have shown that commission errors are elevated in syndromes associated with impulse control problems (e.g., borderline personality disorder, attention deficit hyperactivity disorder) [Moeller et al., 2001; Swann et al., 2002] and correlate with trait measures of disinhibition (e.g., impulsive personality traits) [Keilp et al., 2005]. We used a GNG task with arousing stimuli (emotional words) given that impulse control failures during emotional processing may be particularly relevant to PTSD.
We hypothesized that the degree of disinhibition on the emotional GNG task would relate to cortical thickness in brain regions consistently linked to inhibitory control, most notably IFG. Specifically, we predicted that higher levels of disinhibition would be associated with reduced cortical thickness. We also expected that greater PTSD symptoms would predict reduced structural integrity and abnormal functional connectivity in regions associated with disinhibition based on prior functional neuroimaging work examining GNG task activation and PTSD.
MATERIALS AND METHODS
Sample
Participants were 205 OEF/OIF service members who were primarily veterans (93%) consecutively enrolled in the Veterans Affairs (VA) RR&D Traumatic Brain Injury Center of Excellence, Translational Research Center for traumatic brain injury (TBI) and Stress Disorders at VA Boston Healthcare System. Individuals were eligible to participate if they did not have a history of seizures or serious physical illness, a current psychiatric condition requiring crisis intervention, current DSM‐IV diagnosis of bipolar disorder, schizophrenia, or other psychotic disorder, or a cognitive disorder due to general medical condition. Six participants were excluded from structural analyses due to missing data on the GNG task and 10 were excluded for a history of moderate/severe TBI. The final sample for structural analyses consisted of the remaining 189 predominately male (89%) OEF/OIF veterans ages 19 to 62 (M = 32.2, SD = 8.7). Demographic characteristics of the final sample are presented in Table 1. The majority of participants self‐identified as White (70%), followed by Black/African American (11%), Hispanic/Latino (16%), American Indian (0.5%), and Asian (2.5%). Based on the DSM‐IV criteria, 25% met criteria for a current mood disorder (33% lifetime), and 14% met criteria for a current substance use disorder (62% lifetime). OEF/OIF lifetime service deployment ranged from 0 to 56 months, and the average length per deployment was 13 months (M = 12.9, SD = 9.1). For the resting state connectivity analyses, data were available for 166 participants who did not differ from the larger sample on the demographic or clinical characteristics assessed.
Table 1.
Descriptive characteristics (N = 189)
| Age (M/SD) | 32.2/8.7 |
| Male (n, %) | 168/88.9% |
| Ethnicity (n, %) | |
| White | 129/68.3% |
| Black/African‐American | 21/11.1% |
| Hispanic/Latino | 30/15.9% |
| American Indian | 1/0.5% |
| Asian | 5/2.6% |
| Current mental health diagnosis (n, %) | |
| Posttraumatic stress disorder | 94/49.7% |
| Major depressive disorder | 46/24.3% |
| Substance use disorder | 26/13.8% |
| Anxiety disorder | 32/16.9% |
| Mild traumatic brain injury (n, %) | 123/65.1% |
| Medication use (n, %) | |
| Antidepressant medication | 30/15.9% |
| Antiepileptic medication | 7/3.7% |
| Sedative/hypnotic medication | 12/6.3% |
| Estimated verbal IQ (M/SD) | 103.3/10.0 |
| Years of education (M/SD) | 14.0/1.97 |
| Full time employment (n, %) | 93/49.2% |
| Months deployed (M/SD) | 12.9/9.1 |
Note. Participants with a diagnosis of current bipolar disorder, schizophrenia or psychotic disorder were ineligible to participate. Three participants did not report ethnicity.
Participants completed a series of clinical interviews, a battery of self‐report measures and neuropsychological tests, and underwent magnetic resonance imaging scans. All relevant Institutional Review Boards and regulatory committees approved the study procedures, and informed consent was obtained from all participants.
Measures
PTSD symptoms
Current PTSD symptom severity was assessed by a doctoral‐level psychologist using the Clinician Administered PTSD Scale (CAPS) [Blake et al., 1993], a diagnostic interview used to assess the frequency and intensity of the 17 DSM‐IV PTSD criteria each on a 5‐point scale. Past‐month dimensional severity scores were used in analyses and calculated by summing the frequency and intensity ratings for each of the 17 symptoms. All participants experienced a DSM‐IV PTSD Criterion A event. Fifty percent of the sample met DSM‐IV criteria for current PTSD.
GNG task
Participants completed a computer‐administered GNG task that consisted of emotionally‐arousing words presented serially for 300 ms each [Robbins et al., 1998]. We used a GNG task with arousing stimuli, because impulse control failures during emotional processing may be particularly relevant for understanding impulsivity in PTSD. This task was completed outside of the MRI scanner. Participants were informed of the target valence (pleasant or unpleasant) at the beginning of each block and told to respond via button press if the word matched the target valence (Go condition) or to withhold the motor response if the word did not match (No‐Go condition). Stimuli were presented in 10 blocks (five pleasant, five unpleasant, two practice) of 18 words each, and each block consisted of nine “Go” and nine “No‐Go” trials (there were no neutral trials). Order of presentation was counterbalanced across participants. Additional details are available in Amick et al. [2013], who examined relationships between performance on this task, PTSD, and military TBI.1
Potential confounds
Participants completed the self‐report Depression Anxiety Stress Scale [Lovibond and Lovibond, 1995], the structured Lifetime Drinking History interview [Skinner and Sheu, 1982], the Boston Assessment of TBI‐Lifetime clinical interview [Fortier et al., 2013], the Wechsler Test of Adult Reading (WRAT) [Wechsler, 2001], and the Color‐word Interference Test, Verbal Fluency Test, and Trail Making Test from the Delis‐Kaplan Executive Function System (D‐KEFS) [Delis et al., in press]. Information was also obtained about psychiatric medication use and handedness. The depression subscale total score (measuring current symptoms), total lifetime alcohol consumption (weight corrected), lifetime history of mTBI (present or absent), handedness (based on the hand used to write letters), psychiatric medication use (using three present or absent variables for current antidepressant medication use, antiepileptic medication use, and sedative/hypnotic medication use), estimated verbal IQ from the WRAT, and a composite executive functioning index derived from the D‐KEFS (summed standard scores from the Inhibition, Inhibition/Switching, Letter Fluency, Category Fluency, Category Switching, and Number/Letter Switching subtests) were used to assess for potential confounds in subsidiary analyses.
MRI acquisition
Participants were instructed to remain still with their eyes open while 2 EPI runs (voxel size = 3 × 3 × 3mm, TR = 3000 ms, TE = 30 ms, scan time per run = 360 s) were acquired on a Siemens 3T TIM Trio scanner. Two MPRAGEs (voxel size = 1 × 1 × 1 mm T1 = 1000 ms, TR = 2530 s, TE = 3.32 ms) were acquired and averaged to create a single high contrast‐to‐noise image.
Data Analysis
Morphometric processing
Individualized cortical parcellations and subcortical segmentations were created via FreeSurfer [Salat et al., 2004], including spatial smoothing of 20 mm FWHM. Cortical surface models were manually checked slice‐by‐slice and edited for accuracy.
Based on the study aims, we used total commission errors to measure disinhibition.2 Age, gender, and number of months deployed to OEF/OIF service were entered as covariates in all analyses. Per our first two aims, vertex‐wise analyses were computed across the entire cortex to search for brain regions where PTSD symptom severity moderated the association of disinhibition with cortical thickness. Specifically, general linear model analyses were run using the FreeSurfer application Qdec with commission errors, continuous PTSD symptom severity scores, and the interaction of PTSD severity × commission errors entered as predictors in steps. The vertex‐wise significance threshold was set at P < 0.01. We applied a Monte Carlo simulation with 10,000 iterations to correct for multiple comparisons using a cluster‐wise threshold of P < 0.05. Regions that survived correction for multiple comparisons are depicted on the cortical thickness significance maps (Fig. 1) and in Table 2.
Figure 1.
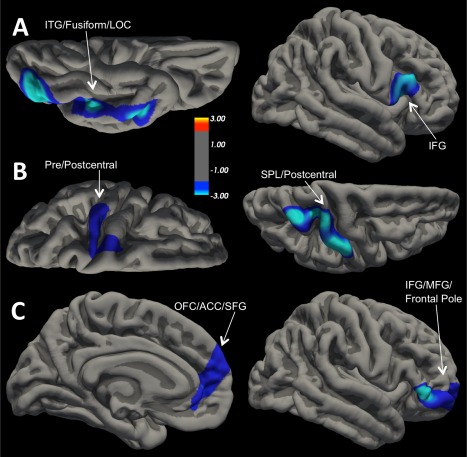
Significant Cortical Thickness Clusters Projected onto the Pial Surface. Clusters that survived cluster‐wise correction (P < 0.05). (A) Left = ventral surface of left hemisphere; (A) Right = lateral surface of right hemisphere; (B) Left = dorsal surface of left hemisphere; (B) Right = dorsal surface of right hemisphere; (C) Left = medial surface of left hemisphere; (C) Right = lateral surface of right hemisphere. For all views, anterior is on the right. (A) Cortical thickness decreased as Go/No‐Go commission errors increased in both clusters. (B) Cortical thickness decreased as PTSD symptom severity scores increased in both clusters. (C) Cortical thickness decreased as Go/No‐Go commission errors increased in both clusters, but only for individuals with high levels of PTSD symptoms. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Significant cortical thickness clusters corrected for multiple comparisons for go/no‐go disinhibition and PTSD symptom severity scores
| Peak F‐value | Peak (x,y,z) | No. of vertices | Cluster size (mm2) | |
|---|---|---|---|---|
| Disinhibition | ||||
| RH pars triangularis/pars opercularis | −3.06 | 42,18,7 | 1940 | 1102 |
| LH inferior temporal/fusiform/lateral occipital | −4.13 | −39,−74,−8 | 4456 | 2912 |
| PTSD severity | ||||
| RH superior parietal/postcentral | −3.68 | 21,−34,55 | 4452 | 2059 |
| LH precentral/postcentral | −2.61 | −38,−25,51 | 2865 | 1154 |
| Disinhibition × PTSD severity | ||||
| RH inferior frontal gyrus (pars triangularis and orbitalis)/rostral middle frontal/frontal pole | −3.92 | 51,34,−3 | 2283 | 1663 |
| LH medial orbital frontal/rostral anterior cingulate/superior frontal | −2.65 | −10,42,.7 | 2016 | 1117 |
Note: N = 189. All clusters survived Monte Carlo Simulation correction for multiple comparisons (P < 0.05). Disinhibition = total commission errors on the Go/No‐Go task. PTSD = Posttraumatic Stress Disorder. RH = right hemisphere. LH = left hemisphere.
For the sake of thoroughness, we also examined valence contrasts [pleasant vs. unpleasant words] using the same general linear model analyses described for total commission errors. Given that valence effects on disinhibition was not the primary focus of this study, the results of these analyses are provided in supplemental material.
We examined potential confounds by examining effects of depression, alcohol consumption, psychiatric medication use, handedness, verbal IQ, mTBI history, and overall executive function ability. We extracted each cluster and ran a hierarchical linear regression analysis with the covariates entered in block 1 (age, gender, deployment duration), explanatory variables in block 2 (potential confounds, commission errors, PTSD severity scores), and the interaction of commission errors and PTSD severity score in block 3. These analyses were conducted using SPSS v22 [IBM Corp, 2013].
Resting state fMRI processing
Data were preprocessed using the Graph Theoretic GLM tool [Spielberg, 2014]. Data were motion corrected, detrended (linear and quadratic), bandpass filtered (retaining 0.1‐0.10Hz), wavelet despiked, the first five principal components of the ventricular and white matter signals were partialled out, along with estimated motion parameters, and were spatially smoothed (FWHM = 5 mm; this occurred after seed timeseries were extracted).
Clusters that emerged as significant in the structural analyses for the PTSD × total commission errors were used as seed clusters for resting state connectivity analyses. These clusters were warped into each participant's 3d structural space via FreeSurfer's mri_label2vol. This procedure accounts for the thickness of each participant's cortical mantle in these regions such that analyses will not be biased by differences in the cortical thickness of seed regions. These clusters were then transformed into functional space, and the timeseries for each was extracted separately for each functional run. Each timeseries was entered as a predictor variable in FSL's FILM [Jenkinson et al., 2012], with the 3d functional data as the dependent variable. The two functional runs were entered into a fixed‐effects model in FEAT to obtain the mean effect across runs. The results of these analyses were then entered as dependent variables into a mixed‐effects model in FEAT, with the same set of predictors used in the structural analyses and the addition of a voxel specific predictor modeling the partial gray matter in that voxel. This predictor was included to account for potential differences in cortical thickness in the target regions. Gaussian Random Field correction for multiple comparisons (via FSL's cluster), with a voxel level threshold of 2.05. A gray matter mask (computed by taking the average partial gray matter maps and thresholded at 15%) was used to constrain the voxels under consideration.
To aid in the interpretation of significant interactions between PTSD symptom severity and commission errors, we examined the strength of the association between commission errors and cortical thickness/resting‐state connectivity in extreme groups comprising individuals who scored plus (CAPS total score > 74; n = 46) or minus (CAPS total score < 15; n = 47) one standard deviation from the sample mean on PTSD symptom severity. Everyone in the “high” PTSD severity group met criteria for a current diagnosis, and none of the individuals in the “low” PTSD severity group met criteria for a current PTSD diagnosis. We tested for, and did not find, multicollinearity problems in the analyses, as evidenced by tolerance levels all above 0.80 [Gaur and Gaur, 2006] and predictor intercorrelations within acceptable ranges (r < 0.20) [Leahy, 2000].
RESULTS
Behavioral Results
Descriptive statistics for the GNG task are presented in Table 3. PTSD severity scores correlated positively with total number of commission errors (r = 0.19, P = 0.01), but not RT for correct responses or number of omission errors (Ps > 0.22). PTSD severity scores did not correlate differentially with unpleasant versus pleasant words for any of the GNG variables (Ps > 0.27).
Table 3.
Descriptive statistics for performance on the go/no‐go task
| Unpleasant words | Pleasant words | Total words | |
|---|---|---|---|
| Commission errors | |||
| M/SD | 5.5/4.5 | 5.7/4.6 | 11.2/8.6 |
| Min/Max | 0/22 | 0/22 | 0/41 |
| Omission errors | |||
| M/SD | 3.1/3.9 | 3.6/4.5 | 6.6/7.9 |
| Min/Max | 0/17 | 0/23 | 0/39 |
| Reaction time | |||
| M/SD | 494.9/71.6 | 490.5/74.0 | 492.6/70.7 |
| Min/Max | 250.7/709.6 | 258.8/696.9 | 254.7/703.3 |
Cortical Thickness Results
Vertex‐wise analysis produced two clusters in which GNG commission errors correlated negatively with cortical thickness (Fig. 1‐A; Monte Carlo corrected P < 0.05). The first cluster was located in right IFG and included pars triangularis and pars opercularis (mean r = −0.26, P < 0.001). The second cluster spanned left inferior temporal gyrus, fusiform, and lateral occipital cortex (mean r = −0.33, P < 0.001).
Analysis of the relationship between PTSD severity score and cortical thickness identified two clusters in which PTSD symptoms correlated negatively with cortical thickness (Fig. 1‐B; Monte Carlo corrected P < 0.05). The first cluster was located in left precentral and postcentral gyrus (mean r = −0.31, P < 0.001). The second cluster spanned portions of right superior parietal cortex and postcentral gyrus (mean r = −0.26, P < 0.001).
In addition to these main effects, the interaction of PTSD symptom severity and disinhibition was associated with alterations in cortical thickness. The two clusters that survived correction for multiple comparisons are presented in Figure 1‐C. The first cluster included portions of right IFG (pars triangularis/orbitalis), as well as rostral middle frontal gyrus and frontal pole. To decompose the interaction, we examined the strength of the association between commission errors and cortical thickness in individuals low vs. high on PTSD symptom severity (i.e., 1 SD above or below the mean on the CAPS). Commission errors were related to reduced cortical thickness for individuals with high PTSD severity scores (β = −0.45, P = 0.001), but not trauma‐exposed individuals with low PTSD severity scores (β = 0.25, P = 0.09). A similar pattern of results emerged for the second cluster, which was located in the left medial OFC, rostral ACC, and superior frontal gyrus (Fig. 1‐C). Again, commission errors were related to reduced cortical thickness for individuals with high levels of PTSD symptoms (β = −0.39, P = 0.009) but not those with low levels of PTSD symptoms (β = 0.10, P = 0.51).3
We next assessed whether depression symptoms, lifetime alcohol consumption, verbal IQ, handedness, executive function ability, psychiatric medication use, and mTBI history could account for our findings by adding them all as predictors in the regression model for each cluster. All of the associations between commission errors, PTSD, and cortical thickness reported above remained significant when these potential confounds were included in the models, and no new results emerged.
Resting‐State fMRI Results
Next, we examined whether the two frontal clusters related to the interaction of disinhibition and PTSD severity in the cortical thickness analyses were associated with disruptions in functional connectivity. Specifically, we tested whether the interaction of disinhibition and PTSD symptoms moderated resting state connectivity with these frontal clusters (via voxel‐wise analyses with the two frontal clusters as seeds). No significant results emerged for the left seed cluster.
Three clusters emerged in which connectivity with the right frontal seed cluster varied as a function of the interaction of PTSD and disinhibition (Table 4). To interpret these effects, we examined coupling in individuals low versus high on PTSD symptom severity using (+/− 1 SD on the CAPS). The first cluster was located in right frontal pole, superior frontal gyrus, paracingulate, and rostral ACC, superior to the seed cluster. For individuals with relatively greater PTSD symptoms, disinhibition was associated with stronger positive coupling between the first cluster and the right seed cluster (β = 0.55, P = 0.001), whereas the opposite was true for trauma‐exposed individuals with few PTSD symptoms (β = −0.32, P = 0.041; Fig. 2‐A). The second cluster was located in bilateral occipital pole and intracalcarine and left lingual gyrus. Disinhibition was associated with stronger negative coupling between the second cluster and the right seed cluster in individuals with greater PTSD symptoms (β = −0.56, P < 0.001), but not associated in individuals with fewer PTSD symptoms (β = 0.21, P = 0.20; Fig. 2‐B). The third cluster to emerge was located in left hippocampus, temporal pole, insula, parahippocampal gyrus, and temporal fusiform. In individuals with greater PTSD symptoms, disinhibition was associated with stronger negative coupling between the third cluster and the right seed cluster (β = −0.55, P < 0.001), whereas the opposite was true for fewer PTSD symptoms (β = 0.55, P < 0.001; Fig. 2‐C). Thus, in addition to reduced cortical thickness, the right seed cluster showed increased positive coupling with right frontal regions and negative coupling with occipital and temporal regions in individuals with high levels of disinhibition and PTSD symptoms.
Table 4.
Resting‐state functional connectivity clusters for the right frontal seed cluster
| Peak z‐value | Peak (x,y,z) | No. of voxels | Cluster size (mm3) | |
|---|---|---|---|---|
| RH frontal pole/superior frontal/paracingulate/rostral anterior cingulate | 4.74 | 16,42,20 | 809 | 6,472 |
| RH occipital pole/medial intracalcarine/LH lingual/LH occipital pole | 3.66 | −4,−80,2 | 619 | 4,952 |
| LH hippocampus/temporal pole/insula parahippocampal/temporal fusiform | 4.52 | −36,−22,−12 | 762 | 6,096 |
Note: N = 166. Significant clusters where commission errors and PTSD severity moderated resting‐state connectivity with the right frontal seed cluster identified in the cortical thickness analyses. RH = right hemisphere. LH = left hemisphere.
Figure 2.
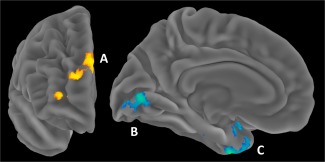
Disinhibition and PTSD Moderation of Functional Connectivity with Right Frontal Seed Cluster. Left = anterior surface of right hemisphere; Right = medial surface of left hemisphere. (A) = Cluster in RH Frontal Pole/Superior Frontal/Paracingulate/Rostral Anterior Cingulate. (B) = RH Occipital Pole/Medial Intracalcarine/LH Lingual/LH Occipital Pole. (C) = LH Hippocampus/Temporal Pole/Insula Parahippocampal/Temporal Fusiform. RH = right hemisphere. LH = left hemisphere. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Next, we examined depression symptoms, alcohol use, a history of mild TBI, handedness, verbal IQ, executive function ability, and psychiatric medication use to rule these out as potential confounds. The resting fMRI findings remained significant when these variables were included in the analyses.
DISCUSSION
Findings from this study suggest that impulsivity in PTSD is associated with atypical brain morphology and resting‐state functional coupling. Specifically, disinhibition in the presence of high PTSD severity was associated with cortical thinning in two clusters in PFC (Fig. 1‐C): a right hemisphere cluster that included right IFG, rostral MFG, and frontal pole, and a left hemisphere cluster that spanned medial OFC, rACC, and superior frontal gyrus. In contrast, disinhibited individuals on the GNG task with few PTSD symptoms showed no such reduction in cortical thickness. Notably, these prefrontal clusters were distinct from those associated with the main effects of disinhibition and overall PTSD symptom severity (Fig. 1‐A‐B), suggesting specificity in the brain morphology of PTSD‐related disinhibition. Furthermore, alterations in stable functional coupling emerged between the right frontal cluster and regions involved in cognitive control, visual attention, memory, and learning. Connectivity findings suggest that, in addition to reduced cortical thickness, disruptions in the functional coupling between the right frontal region and other key regions involved in regulating behavior may contribute to impulse control deficits in PTSD. Broadly speaking, findings indicate that response inhibition deficits in PTSD are associated with distinct neural abnormalities that are not apparent in trauma‐exposed individuals without PTSD and not associated with other common comorbidities.
Although the cross‐sectional nature of the data makes it impossible to infer causal relationships among the variables, it is plausible to assume that deficits in response inhibition in PTSD depend on cortical integrity in PFC. Indeed, our findings are consistent with a model whereby PTSD severity is associated with inhibitory dysfunction, but only in the presence of cortical thinning in the identified prefrontal regions (see Footnote 3). Because the association between reduced cortical thickness and disinihibition was stronger in these regions in individuals with severe PTSD, this may suggest that PTSD exerts neurodegenerative effects that compromise this circuitry [e.g., Miller and Sadeh, 2014], although this interpretation is purely speculative in lieu of corroborating longitudinal evidence. Nonetheless, loss of integrity in the identified brain regions would produce deficits in cognitive and emotional processes consistent with those observed in PTSD and linked to impulsivity.
The right frontal cluster observed in the present study encompasses regions important for inhibiting impulsive actions and inappropriate thoughts, monitoring goals, and flexibly switching between response sets [Aron, 2011; Banich and Depue, 2015; Tsujimoto et al., 2011], whereas the left frontal cluster includes regions important for identifying the motivational significance of stimuli, regulating attentional control, and monitoring errors [Liddle et al., 2001]. Of note, the two frontal clusters overlapped with regions that have been associated with abnormal activation in functional neuroimaging studies of inhibitory control in PTSD, including No‐Go activation in left middle frontal cortex and ACC [Carrion et al., 2008] and right ventral, dorsolateral, and medial PFC [Falconer et al., 2008; Jovanovic et al., 2013]. Our results suggest that cortical thinning in these regions may partially explain this aberrant functional activation. Future research examining how the structural integrity of these frontal regions influences activation during inhibitory control tasks as a function of PTSD status is important for clarifying how structural alterations are reflected in functional differences and vice versa.
Analysis of resting‐state connectivity provided additional insight into the functional significance of these frontal clusters. In individuals with more severe PTSD symptoms, greater disinhibition was linked to stronger positive coupling between the right frontal (seed) cluster and a more superior region of right PFC. Other studies suggest that this strengthened coupling may reflect a compensatory mechanism for cortical thinning in the seed cluster (i.e., top‐down regulatory processing in the seed cluster is degraded, and the more superior PFC region comes online to compensate) [Koechlin et al., 2003]. Individuals with more severe PTSD symptoms also exhibited stronger negative functional coupling between the right seed cluster and two clusters, one in left occipital visual regions and the second in left medial‐temporal lobe (MTL). De‐coupling with the occipital cluster may indicate that impulsivity in PTSD is related to a decreased reliance by frontal executive regions on attentional information provided in visual areas [Wager et al., 2004] and/or decreased top‐down direction of attention by the frontal executive region. Similarly, de‐coupling with the MTL cluster suggests that impulsivity in PTSD is related to a decreased reliance by frontal executive regions on contextual information provided in MTL [Konkel and Cohen, 2009] and/or decreased top‐down direction of contextual processing. De‐coupling with both regions may contribute to failures to attend to changes in the motivational significance of stimuli and to learn from maladaptive responses over time by, for instance, interfering with the ability of disinhibited individuals to interrupt a prepotent response set when cued by the environment (e.g., inhibiting responses on No‐Go trials).
Together, results suggest that the loss of structural integrity and network dysfunction in bilateral frontal regions may partially explain PTSD‐related deficits in inhibiting impulsive behavioral reactions. Furthermore, they provide context for previous research showing PTSD‐related reductions in frontal activation and increases in regions of motor activation during inhibitory control tasks [Carrion et al., 2008; Falconer et al., 2008; Jovanovic et al., 2013]. From a clinical perspective, the presence of PTSD‐associated neural mechanisms suggests that treatment and intervention for inhibitory control deficits likely need to be tailored differently in PTSD than for individuals who show impulse control deficits but are not affected by traumatic stress. For example, the brain regions identified in this study suggest that efficacious interventions for impulsivity in PTSD may need to target response inhibition deficits in the context of emotional dysregulation. Furthermore, although we identified the brain regions in this study by looking explicitly at failures in response inhibition, the cognitive functions supported by the identified clusters suggest the findings have implications that extend beyond just impulsivity in PTSD. For example, PTSD‐related disinhibition was associated with loss of integrity in rostral ACC and medial OFC, which have neuroanatomical and functional connections with amygdala and other subcortical components of emotional response systems [Bush et al., 2000; Etkin et al., 2006]. Hypoactivation in these prefrontal regions are thought to contribute to the emotion regulation and fear extinction deficits observed in PTSD [Patel et al., 2012]. Thus, our findings have broad implications for understanding inhibitory processes in PTSD across the symptom clusters.
In addition to interactive effects, we observed direct associations between disinhibition/PTSD severity and cortical integrity. Consistent with previous research showing the importance of right IFG for successful inhibitory control [Aron, 2011; Aron et al., 2004; Criaud and Boulinguez, 2013], we found that inhibition failures were associated with reduced cortical thickness in right IFG. Previous research indicates that decreased structural integrity in right IFG (in gray and adjacent white matter) [Ersche et al., 2012; Tabibnia et al., 2011] may be a useful endophenotype for the study of self‐control deficits, which converges with our finding. Disinhibition was also associated with reduced thickness in a left occipito‐temporal cluster overlapping with regions shown to be important for detecting stimuli salience and higher‐order perceptual processing, including visual word recognition [McCandliss et al., 2003], and regions activated during No‐Go trials [Simmonds et al., 2008]. In contrast, current PTSD severity was negatively associated with cortical thickness in bilateral postcentral gyri and right superior parietal cortex. This finding is consistent with a prior study conducted with a portion of this sample using an overlapping measure of PTSD [Lindemer et al., 2013] and replicates previous research demonstrating reduced cortical thickness in regions involved in attentional control in PTSD [Qi et al., 2013].
Study findings should be considered in light of several limitations. First, our measure of disinhibition was limited to performance on the GNG task and we did not systematically assess reckless and self‐destructive behaviors associated with PTSD. An important next step would be to examine whether the observed alterations in brain morphology and network connectivity associated with inhibitory control on the GNG task also relate to real‐world behaviors, such as reckless driving, impulsive self‐harm, and angry outbursts. Second, the GNG task used in this study contained an equal number of Go and No‐Go trials, which may have reduced the inhibitory control demands of this task relative to other GNG tasks that present fewer No‐Go than Go trials. This may have restricted our ability to detect inhibitory control deficits at the low end of the severity spectrum, because the task was relatively easy. On the other hand, utilization of this GNG task from the CANTAB to assess neuropsychological function is a strength of the study, because it has been widely implemented in previous research and permits comparison of the current results to prior research in a wide range of clinical populations as well as healthy individuals. Future research examining how performance on a GNG task with less frequent No‐Go trials and other tasks that measure behavioral inhibition, such as stop signal tasks, are needed to examine the reliability and generalizability of our results. Finally, we cannot determine, on the basis of these cross‐sectional data, whether the observed neural abnormalities in cortical thickness and resting‐state connectivity represent vulnerabilities for, or consequences of, PTSD and impulsivity. Despite these limitations, this study featured several notable strengths including a large, clinically‐relevant sample of trauma‐exposed veterans, use of a well‐validated indicator of behavior disinhibition, a detailed assessment of PTSD, TBI, and other psychiatric disorders by clinical interview, and integration of multiple neuroimaging modalities.
In summary, findings demonstrate that the neural substrates associated with impulsivity differ in the presence of PTSD. They provide preliminary evidence that the observed alterations in cortical thickness and related dysfunctional network connectivity represent neural markers of PTSD‐related disinhibition. Findings advance our understanding of the causes of impulsive behavior in traumatized adults and are particularly timely given the recent influx of returning veterans struggling with these difficulties.
Supporting information
Supporting Information
ACKNOWLEDGMENT
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Financial Disclosures: Drs. Sadeh, Spielberg, Miller, Milberg, Salat, Amick, Fortier, and McGlinchey reported no biomedical financial interests or potential conflicts of interest.
Footnotes
Based on research showing the mTBI and PTSD symptoms interact to influence performance on the GNG task [Amick et al., 2013], we examined whether mTBI moderated any of the neuroimaging findings. Results of these analyses indicated that a history of mTBI could not account for the reported findings nor did it interact with PTSD to produce new findings.
Given that we were interested specifically in disinhibition, we did not focus on omission errors and reaction time in our primary analyses. For descriptive purposes, we included these variables in the behavioral results. Subsidiary analyses performed with omissions errors and reaction times for correct responses did not yield significant results in the neuroimaging analyses. Thus, our findings appear to be specific to commission errors.
We conducted post hoc linear regression analyses to test an alternative theoretical model whereby cortical thickness (in the clusters identified in the vertex‐wise analyses) moderate the relationship of PTSD severity with inhibitory function. These analyses showed that PTSD severity predicted inhibitory dysfunction, but only in the presence of cortical thinning in the prefrontal clusters. Specifically, the interaction between PTSD severity and thickness in the right IFG/rostral MFG/Frontal Pole cluster predicted inhibition performance on the GNG task (β = 4.88, P < 0.001), with PTSD symptoms predicting greater commission errors only in the presence of cortical thinning (assessed using a median split on cortical thickness: below median: β = 0.43, P < 0.001; above median: β = 0.04, P = 0.71). A similar pattern of findings emerged for the left medial OFC/rostral ACC/Superior Frontal cluster (β = 2.78, P = 0.003), with PTSD symptoms predicting greater commission errors in the presence of cortical thinning (below median: β = 0.32, P = 0.004; above median: β = 0.15, P = 0.15). Thus, our findings can be interpreted as PTSD leading to inhibitory dysfunction only in the presence of reduced cortical thickness.
REFERENCES
- American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders, 5th ed Washington, DC: American Psychiatric Press. [Google Scholar]
- Amick MM, Clark A, Fortier CB, Esterman M, Rasmusson AM, Kenna A, Milberg WP, McGlinchey R (2013): PTSD modifies performance on a task of affective executive control among deployed OEF/OIF veterans with mild traumatic brain injury. J Int Neuropsychol Soc 19:792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR (2011): From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol Psychiatry 69:e55–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA (2004): Inhibition and the right inferior frontal cortex. Trends Cog Sci 8:170–177. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Melrose AJ, Stein MB, Paulus MP (2012): Executive function and PTSD: Disengaging from trauma. Neuropharmacology 62:686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Depue BE (2015): Recent advances in understanding neural systems that support inhibitory control. Curr Opin Behav Sci 1:17–22. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM (1993): A clinician rating scale for assessing current and lifetime PTSD: the CAPS‐I. Behav Ther 18:187–188. [Google Scholar]
- Brown MR, Lebel RM, Dolcos F, Wilman AH, Silverstone PH, Pazderka H, Fujiwawa E, Wild TC, Carroll AM, Hodlevskyy O, Zedkova L, Zwaigenbaum L, Thompson AH, Greenshaw AJ, Dursun SM (2012): Effects of emotional context on impulse control. Neuroimage 63:434–446. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–222. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Garrett A, Menon V, Weems CF, Reiss AL (2008): Posttraumatic stress symptoms and brain function during a response‐inhibition task: An fMRI study in youth. Depr Anx 25:514–526. [DOI] [PubMed] [Google Scholar]
- Criaud M, Boulinguez P (2013): Have we been asking the right questions when assessing response inhibition in go/no‐go tasks with fMRI? a meta‐analysis and critical review. Neurosci Biobehav Rev 37:11–23. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH (2001): Delis‐kaplan executive function system (D‐KEFS). Psychological Corporation (in press). [Google Scholar]
- Elbogen EB, Fuller S, Johnson SC, Brooks S, Kinneer P, Calhoun PS, Beckham JC (2010): Improving risk assessment of violence among military veterans: An evidence‐based approach for clinical decision‐making. Clin Psychol Rev 30:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET (2012): Abnormal brain structure implicated in stimulant drug addiction. Science 335:601–604. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J (2006): Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51:871–882. [DOI] [PubMed] [Google Scholar]
- Falconer E, Bryant R, Felmingham KL, Kemp AH, Gordon E, Peduto A, Olivieri G, Williams LM (2008): The neural networks of inhibitory control in posttraumatic stress disorder. J Psychiatry Neurosci 33:413−422. [PMC free article] [PubMed] [Google Scholar]
- Fortier CB, Amick M, Grande L, McGlynn S, Kenna A, Morra L, Clark A, Milberg WP, McGlinchey RE (2013): The boston assessment of traumatic brain Injury‐lifetime (BAT‐L) Semi‐structured interview: Evidence of research utility and validity. J Head Trauma Rehabil 66:1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier CB, Leritz EC, Salat DH, Lindemer E, Maksimovskiy A, Shepel J, Williams V, Venne JR, Milberg WP, McGlinchey RE (2014): Widespread effects of alcohol on white matter microstructure. Alcoholism Clin Exp Res 38:2925–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A (2004): The relations among inhibition and interference control functions: A latent‐variable analysis. J Exp Psychol 133:101–135. [DOI] [PubMed] [Google Scholar]
- Gaur AS, Gaur SS (2006): Statistical Methods for Practice and Research: A Guide to Data Analysis Using SPSS. New Delhi, India: Sage. [Google Scholar]
- IBM Corp. (2013): Released IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. [Google Scholar]
- Jacobsen LK, Southwick SM, Kosten TR (2001): Substance use disorders in patients with posttraumatic stress disorder: A review of the literature. Am J Psychiatry 158:1184–1190. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012): Fsl. Neuroimage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ (2010): How the neurocircuitry and genetics of fear inhibition may inform out understanding of PTSD. Am J Psychiatry 167:782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, Norrholm SD, Bradley B, Ressler KJ (2013): Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex 49:1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilp JG, Sackeim HA, Mann JJ (2005): Correlates of trait impulsiveness in performance measures and neuropsychological tests. Psychiatry Res 135:191–201. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F (2003): The architecture of cognitive control in the human prefrontal cortex. Science 302:1181–1185. [DOI] [PubMed] [Google Scholar]
- Konkel A, Cohen NJ (2009): Relational memory and the hippocampus: Representations and methods. Front Neurosci 3:166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy K (2000). Multicollinearity: When the solution is the problem In: Rud OP, editor. Data Mining Cookbook. New York, NY: Wiley; pp 106–108. [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM (2001): Event‐related fMRI study of response inhibition. Hum Brain Mapp 12:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemer ER, Salat DH, Leritz EC, McGlinchey RE, Milberg WP (2013): Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF veterans and the impact of comorbid TBI. NeuroImage: Clin 2:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF (1995): Manual for the Depression Anxiety Stress Scales, 2nd ed Sydney: Psychology Foundation. [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S (2003): The visual word form area: Expertise for reading in the fusiform gyrus. Trends Cogn Sci 7:293–299. [DOI] [PubMed] [Google Scholar]
- Miller MW, Sadeh N (2014): Traumatic stress, oxidative stress and posttraumatic stress disorder: Neurodegeneration and the accelerated‐aging hypothesis. Mol Psychiatry 19:1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Vogt DS, Mozley SL, Kaloupek DG, Keane TM (2006): PTSD and substance‐related problems: The mediating roles of disconstraint and negative emotionality. J Abnorm Psychol 115:369–379. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC (2001): Psychiatric aspects of impulsivity. Am J Psychiatry 158:1783–1793. [DOI] [PubMed] [Google Scholar]
- Nigg JT (2000): On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull 126:220–246. [DOI] [PubMed] [Google Scholar]
- Nock MK, Prinstein MJ (2005): Contextual features and behavioral functions of self‐mutilation among adolescents. J Abnorm Psychol 114:140–146. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross LL (2004): For better or for worse: Neural systems supporting the cognitive down‐and up‐regulation of negative emotion. Neuroimage 23:483–499. [DOI] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA (2012): Neurocircuitry models of posttraumatic stress disorder and beyond: A meta‐analysis of functional neuroimaging studies. Neurosci Biobehav Rev 36:2130–2142. [DOI] [PubMed] [Google Scholar]
- Qi S, Mu Y, Liu K, Zhang J, Huan Y, Tan Q, Shi M, Wang Q, Chen Y, Wang H, Wang H, Zhang N, Zhang X, Xiong L, Yin H. (2013): Cortical inhibition deficits in recent onset PTSD after a single prolonged trauma exposure. NeuroImage: Clin 3:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, McInnes L Rabbit PM (1998): A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: Implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J Int Neuropsychol Soc 4:474–490. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Taylor E (2001): Mapping motor inhibition: Conjunctive brain activations across different versions of go/no‐go and stop tasks. Neuroimage 13:250–261. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B (2004): Thinning of the cerebral cortex in aging. Cereb Cortex 14:721–730. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Fan J, Magidina O, Marks DJ, Hahn B, Halperin JM (2007): Does the emotional go/no‐go task really measure behavioral inhibition? Convergence with measures on a non‐emotional analog. Arch Clin Neuropsychol 22:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH (2008): Meta‐analysis of go/No‐go tasks demonstrating that fMRI activation associated with response inhibition is task‐dependent. Neuropsychologia 46:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ (1982): Reliability of alcohol use indices. The lifetime drinking history and the MAST. J Studies Alcohol Drugs 43:1157–1170. [DOI] [PubMed] [Google Scholar]
- Spielberg JM (2014). Graph theoretic general linear model (GTG): A MATLAB toolbox. Brain Connectivity. Poster presented at the biennial Resting State/Brain Connectivity meeting. Available at: http://www.nitrc.org/projects/metalab_gtg. Last accessed 23 November 2014
- Swann AC, Bjork JM, Moeller FG, Dougherty DM (2002): Two models of impulsivity: Relationship to personality traits and psychopathology. Biol Psychiatry 51:988–994. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, Lee B, London ED (2011): Different forms of self‐control share a neurocognitive substrate. J Neurosci 31:4805–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto S, Genovesio A, Wise SP (2011): Frontal pole cortex: Encoding ends at the end of the endbrain. Trends Cog Sci 15:169–176. [DOI] [PubMed] [Google Scholar]
- Verwoerd J, Wessel I, de Jong PJ (2009): Individual differences in experiencing intrusive memories: The role of the ability to resist proactive interference. J Behav Ther Exp Psychiatry 40:189–201. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S (2004): Neuroimaging studies of shifting attention: A meta‐analysis. Neuroimage 22:1679–1693. [DOI] [PubMed] [Google Scholar]
- Wechsler, D (2001). Wechsler Test of Adult Reading. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wolf EJ, Miller MW, Harrington KM, Reardon A (2012): Personality‐based latent classes of posttraumatic psychopathology: Personality disorders and the internalizing/externalizing model. J Abnorm Psychol 121:256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


