Abstract
Background: Microglia are involved in immune surveillance in intact brains and become activated in response to inflammation and neurodegeneration. Microglia have different functions, neuroprotective or neurotoxic, according to aging in patients with PD. The clinical effect of microglia in patients with Alzheimer's disease (AD) is poorly defined. This prospective study was conducted to investigate the clinical effects of microglia according to the aging process in newly diagnosed AD.
Methods: We examined 532 patients with newly diagnosed AD and 119 healthy controls, and the differences in hs-CRP between these groups were investigated. The patients with AD were classified into 3 subgroups according to age of newly diagnosed AD to investigate the relationship between hs-CRP and the aging process in newly diagnosed AD.
Results: There was significantly higher serum high-sensitivity C-reactive protein (hs-CRP), levels in patients with AD compared with healthy controls. A post-hoc analysis of the 3 AD subgroups showed no significant differences in serum hs-CRP level between each group.
Conclusion: We assumed that neuroinflammation play a role in the pathogenesis of AD, but found no clinical evidence that microglia senescence underlies the microglia switch from neuroprotective in young brains to neurotoxic in aged brains. To clarify the role of microglia and aging in the pathogenesis of AD, future longitudinal studies involving a large cohort are required.
Keywords: Alzheimer's disease, high-sensitivity C-reactive protein, Microglia, Aging process
Introduction
Previous studies have shown that hepatic synthesis of acute-phase proteins, such as high-sensitivity C-reactive protein (hs-CRP), occurs during the inflammatory response 1, 2. Therefore, hs-CRP is an exquisitely sensitive systemic marker of inflammation, infection, and tissue damage1. Increased levels of hs-CRP are strongly associated with inflammatory reactions 1, 2. Many previous studies have suggested that high concentrations of hs-CRP are associated with increased risk of cerebrovascular and neurodegenerative diseases, because hs-CRP increases the paracellular permeability of the blood-brain barrier (BBB) when its concentration exceeds the threshold required to impair BBB function 1, 3, 4.
Microglia are involved in immune surveillance in intact brains and become activated in response to inflammation, trauma, ischemia, tumor, and neurodegeneration 5. Similar to macrophages in the periphery, microglia are part of the macrophage lineage. Microglia is the first and main form of active immune defense in the CNS1. The mechanism of neuronal death, an inflammatory process in the brain, involves changes in cytokine and neurotropin levels as well as the presence of activated microglia. This process has gained a great deal of attention in neurodegenerative diseases such as Alzheimer's disease (AD) or Parkinson's disease (PD). Aging involves profound age-associated changes in the immune system that contribute to increased susceptibility to infection in the elderly6,7. Activated microglia may play neurotoxic roles by producing proinflammatory cytokines such as tumor necrosis factor-α and interleukin (IL)-68,9. Conversely, activated microglia may also play neuroprotective roles by producing neurotrophic compounds such as brain-derived neurotrophic factor 8,9. Previous reports suggest that that the inflammatory state of microglia in aged brains primes them to over-respond to minor stimuli that are otherwise well-controlled in young brains10-12. However, the clinical effect of migroglia in patients with AD in keeping with aging process is poorly understood to date, since there has been little clinical research regarding relationship between microglia-mediated neuroinflammation and senescence in AD. Therefore, we conducted this prospective study to clinically investigate the neuroinflammatory effects of microglia on aging process in patients with AD through evaluation and analysis of the association between serum concentration of hs-CRP as representative systemic inflammatory marker and age at first diagnosis of AD.
Methods
The local ethics committee approved this study, and each patient provided written informed consent for participation. All consecutive newly diagnosed AD patients who presented to the department of neurology in the Catholic Medical Center with complaints of cognitive decline including memory impairment between May 2012 and December 2013 were prospectively included. Data for 532 newly diagnosed AD patients recruited for this study were compared with those of 319 healthy subjects. There were no significant differences in age or sex between the healthy controls and AD patients. All of AD patients were also assessed by experienced neurologists at the dementia and memory clinic. The evaluation procedures other than brain MRI consisted of a detailed medical history, physical and neurological examinations, general neuropsychological assessments, which is included the Mini-Mental State Examination (MMSE), the Clinical Dementia Scale (CDR) with the sum of box of the CDR (SOB) and Global Deterioration Scale (GDS), and laboratory tests. The history of medical and neurological problems was obtained from the patient and their family members or from their other caregivers. The 532 AD patients met the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria for AD and also the NINCDS-ADRDA criteria for probable AD13. The probable AD patients never had focal neurological signs and symptoms or radiologically observed lesions of cerebrovascular disease. In this study, none of the patients fulfilled the criteria of mixed dementia or vascular dementia according to the NINDS-AIREN criteria and the Hachinski ischemic scale score (less than score 4 for AD)14. Experienced radiologist, who blinded to the clinical features of all subjects, assessed all brain images with regard to the presence of cerebrovascular diseases. None of the subjects included in this study had a history of recent infection as outpatients or inpatients, surgery or trauma in the previous month, cardiovascular disease or use of NSAIDs, such as ibuprofen or aspirin. And we also excluded patients with history of use of acetylcholinesterase inhibitors, such as donepezil, galantamine or rivstigmine, because of anti-inflammatory effects of acetylcholinesterase inhibitor. The normal controls were free of any medical abnormality, such as an infection or neurological deficit. The normal controls were determined to be free of risk factors of stroke based on their self-reported or family-reported medical history and detailed neurological examination performed by a neurologist. Previous infections were monitored by medical history obtained from the subjects and their family members, chest X-ray, 12-lead electrocardiogram, transthoracic echocardiography, routine blood biochemistry, complete blood count, routine urine analysis with microscopic examination, and a complete physical examination. In addition, all patients in the AD group were classified into 3 subgroups to evaluate changes in hs-CRP levels according to age. The subgroups were defined as follows: group I, less than 70-years-old; group II, 70-years-old to 79-years-old; group III, more than 80-years-old.
Serum hs-CRP levels were routinely measured in all patients and healthy controls. Venous blood samples were collected from all subjects in tubes containing ethylenediaminetetraacetic acid. The samples were separated immediately after collection by centrifugation at 3,000 rpm for 10 minutes. Separated sera were stored at -70℃ until laboratory evaluation. An examiner who was blinded to the clinical details collected laboratory data and patient information. And all subjects with hypertension, diabetes mellitus, hypercholesterolemia and cigarette smoking were excluded in this study because these risk factors can affect hs-CRP levels.
The statistical analysis were performed with the SPSS software version 18.0 package using analysis of variance (ANOVA) with post hoc analysis, co-variance analysis for age and MMSE and independent T-test for comparing the continuous variables, and Pearson chi-square analysis was used for comparing the categorical variables in each group, including all AD group, 3 AD subgroups and healthy controls. Statistical significance was assumed at the 5% error level.
Result
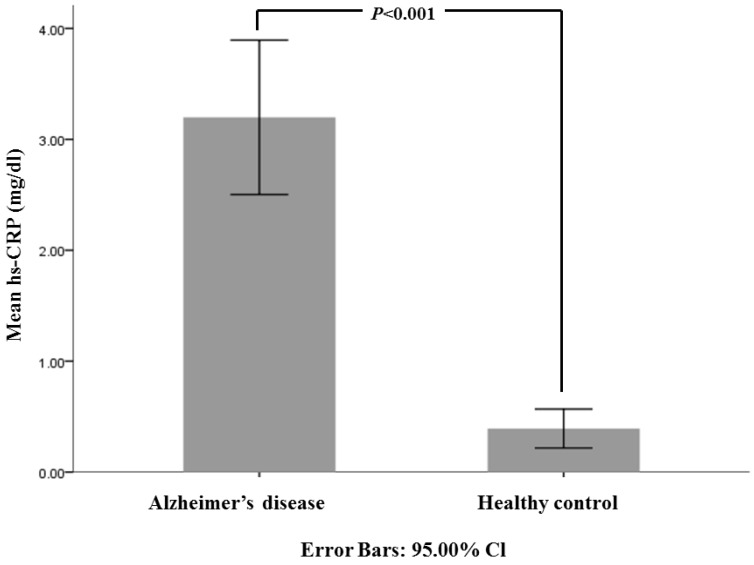
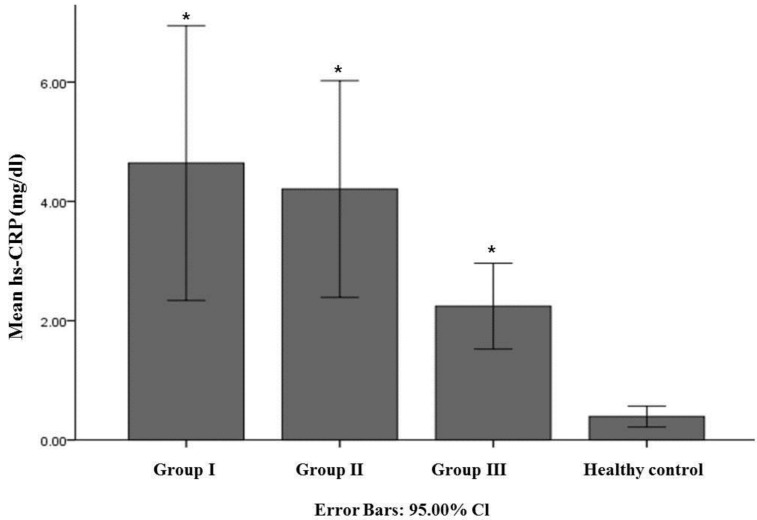
The demographic and baseline data of 532 patients with newly diagnosed AD and 319 healthy controls are presented in Table 1. The mean MMSE score of AD and healthy controls were 17.63±5.68 and 28.86±1.19, respectively (P<0.001). Mean serum hs-CRP values in patients with AD were significantly higher than those in the normal control group (Fig.1). There were 85 patients in group I, 275 patients in group II and 168 patients in group III. The post hoc analysis and co-varance analysis among these 3 AD subgroups did not show any significant differences in mean hs-CRP value but showed higher hs-CRP levels compared to healthy controls group (Table 2, Fig 2). The MMSE in general neuropsychological tests showed significant difference related to age at newly diagnosed dementia because there were significant difference between each groups as follow; group I = 19.29±5.24, group II= 17.91±5.53 and group III = 16.32±5.87 (Table 2). The CDR with SOB and GDS scores showed significant higher score in group III as compared with other groups. And all neuropsychological tests in each 3 AD subgroup revealed significant difference compared to healthy controls (Table 2).
Table 1.
Baseline Characteristics of the study group
| AD | HC | P-value | |
|---|---|---|---|
| Number of subjects | 532 | 319 | - |
| Gender, male | 201 | 137 | 0.098 |
| Age (year) | 75.86±7.94 | 74.47±9.4 | 0.103 |
| hs-CRP (mg/dl) | 3.20±8.18 | 0.39±0.97 | <0.001 |
| MMSE | 17.63±5.68 | 28.86±1.19 | <0.001 |
| CDR | 0.89±0.64 | - | - |
| SOB | 4.62±4.06 | - | - |
| GDS | 3.94±1.06 | - | - |
AD: Alzheimer's disease
HC; Healthy control group, hs-CRP; high-sensitivity C-reactive protein, MMSE: Mini-mental State Examination, CDR: Clinical Dementia Scale, SOB: the Sum of the Box score of the CDR, GDS: Global Deterioration Scale
Values was expressed as mean ± standard deviation
Gender was analyzed by Pearson chi-square test
P-value was calculated by independent T-test
Figure 1.
Comparison of mean hs-CRP (mg/dl) among all patients with Alzheimer's disease and healthy controls. The bar graph with error bars presents the mean hs-CRP level associated with a 95% CI for the mean. The independent t-test compared all patients with AD to healthy controls (P<0.001).
Table 2.
Comparison of characteristics and hs-CRP in 3 subgroups of AD and healthy controls
| Group I | Group II | Group III | HC | P-value | Post-hoc analysis | |
|---|---|---|---|---|---|---|
| Number of subjects | 85 | 275 | 168 | 319 | - | - |
| Age | 63.28±5.93 | 74.77±2.86 | 84.04±4.42 | 74.47±9.4 | <0.001 | I<II=HC<III |
| Gender, male | 32 | 103 | 66 | 137 | 0.184 | I=II=III=HC |
| hs-CRP (mg/dl)* | 4.67±11.96 | 4.19±14.81 | 2.25±4.70 | 0.39±0.97 | <0.001 | I=II=III>HC |
| MMSE | 19.29±5.24 | 17.91±5.53 | 16.32±5.87 | 28.86±1.19 | <0.001 | I<II<III<HC |
| CDR | 0.86±0.61 | 0.81±0.58 | 1.02±0.73 | - | <0.001 | I=II<III |
| SOB | 4.29±3.81 | 4.14±3.63 | 5.61±4.66 | - | <0.001 | I=II<III |
| GDS | 3.81±0.93 | 3.83±0.99 | 4.18±1.19 | - | <0.001 | I=II<III |
AD: Alzheimer's disease, HC; Healthy control group, hs-CRP; high-sensitivity C-reactive protein, Group I, less than 70-year-old; Group II, 70-year-old to 79-year-old; Group III, more than 80-year-old. MMSE: Mini-mental State Examination, CDR: Clinical Dementia Scale, SOB: the Sum of the Box score of the CDR, GDS:Global Deterioration Scale
Values was expressed as mean ± standard deviation
Comparison of Gender was analyzed by Pearson chi-square test among 3 AD subgroups and healthy controls
* P-value was calculated by using covariance analysis for MMSE and Age
Figure 2.
Comparison of mean hs-CRP (mg/dl) among 3 AD subgroups. The bar graph with error bars presents the mean hs-CRP level associated with a 95% CI for the mean. The analysis of variance (ANOVA) with post-hoc tests, co-variance analysis for age and MMSE, compared mean serum hs-CRP levels in each of 3 AD subgroups and there was no significant difference in hs-CRP level among 3 AD subgroups. Group I, less than 70-year-old; Group II, 70-year-old to 79-year-old; Group III, more than 80-year. * P-value between group and healthy control <0.001.
Discussion
Previous studies indicate that microglia are capable of both neurotrophic and neurotoxic effects depending on the specific stimulus, injury severity and environment 10,15,16. Nevertheless, it have been well-known that microglia-mediated neuroinflammation has been hypothesized to play an important role in the pathogenesis of neurodegenerative diseases such as PD and AD1,17. The present study also supports the hypothesis that neuroinflammation contributes to the pathogenesis of AD because a significantly increased mean hs-CRP value was found in patients with AD compared to normal subjects. On the other hand, it is not clear whether clinical effects of microglia-mediated neuroinflammation in patients with AD are related with the aging process of AD patients or not. Previous studies have suggested that microglia senescence appears to underlie the microglia switch from neuroprotective in young brains to neurotoxic in aged brains. In other word, these previous studies hypothesized that activated microglia in young healthy brain exert a neuroprotective role by shielding injured sites and phagocytosing damaged tissue but reversely activated microglia in the aged brain play important detrimental roles as neurotoxic effect8-10. In case of the present study, there is no significant correlation between mean serum hs-CRP value, a sensitive marker of systemic inflammation, and aging of patients with AD. Namely, there were no significant changes or differences in serum hs-CRP levels between the 3 subgroups according to aging process. Therefore, we could cautiously speculate that there is no difference of hs-CRP level between each subgroup of AD according to aging process, because patients with AD maybe undergo an earlier and faster cellular aging process in the brain compared to healthy people. Moreover, the present study significantly showed more severe cognitive decline in group III, which is the oldest AD group, as compared with other groups. On the basis of this finding, we could assume that the older the patients with AD gets, the more they is affected by multiple factors except for neuroinflammation, such as cerebral atrophy.
The limitation of this study is that we cannot be certain about the accuracy of our clinical diagnoses because of the lack of neuropathologic confirmation, because the patients were still alive. However, we attempted to reduce these confounders by including AD patients who fulfilled two sets of diagnostic criteria by experienced experts.
In conclusion, to the best knowledge, there is no study regarding relationship between neuroinflammation and aging process in patients with AD. The results of the present study support the hypothesis that neuroinflammatory reactions play an important role in the pathogenesis of AD, as significantly increased serum hs-CRP levels were found in patients with AD compared to healthy subjects. However, no clinical evidence was found indicating that, unlike previous papers, microglia-mediated neuroinflammation does not contribute to the pathogenesis of AD in young brains. Namely, this study could not clinically support the suggestion that microglia senescence underlies the microglia switch from neuroprotective in young brains to neurotoxic in aged brains. Therefore, longitudinal and clinicopathological studies involving a large cohort of patients with AD are required in the future , to clarify whether microglia-mediate neuroinflammation plays an important role in the pathogenesis of AD in keeping with aging process.
References
- 1.Song IU, Kim JS, Chung SW. et al. Is there an association between the level of high-sensitivity C-reactive protein and idiopathic Parkinson's disease? A comparison of Parkinson's disease patients, disease controls and healthy individuals. Eur Neurol. 2009;62:99–104. doi: 10.1159/000222780. [DOI] [PubMed] [Google Scholar]
- 2.Windgassen EB, Funtowicz L, Lunsford TN. et al. C-reactive protein and high-sensitivity C-reactive protein: an update for clinicians. Postgrad Med. 2011;123:114–9. doi: 10.3810/pgm.2011.01.2252. [DOI] [PubMed] [Google Scholar]
- 3.Hsuchou H, Kastin AJ, Mishra PK. et al. C-reactive protein increases BBB permeability: implications for obesity and neuroinflammation. Cell Physiol Biochem. 2012;30:1109–19. doi: 10.1159/000343302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song IU, Kim YD, Cho HJ. et al. Is neuroinflammation involved in the development of dementia in patients with Parkinson's disease? Intern Med. 2013;52:1787–92. doi: 10.2169/internalmedicine.52.0474. [DOI] [PubMed] [Google Scholar]
- 5.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–8. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 6.Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31:578–85. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- 7.Ginaldi L, De Martinis M, D'Ostilio A. et al. Immunological changes in the elderly. Aging (Milano) 1999;11:281–6. doi: 10.1007/BF03339801. [DOI] [PubMed] [Google Scholar]
- 8.Sawada M, Imamura K, Nagatsu T. Role of cytokines in inflammatory process in Parkinson's disease. J Neural Transm Suppl; 2006. pp. 373–81. [DOI] [PubMed] [Google Scholar]
- 9.Sawada M, Sawada H, Nagatsu T. Effects of aging on neuroprotective and neurotoxic properties of microglia in neurodegenerative diseases. Neurodegener Dis. 2008;5:254–6. doi: 10.1159/000113717. [DOI] [PubMed] [Google Scholar]
- 10.Luo XG, Ding JQ, Chen SD. Microglia in the aging brain: relevance to neurodegeneration. Mol Neurodegener. 2010;5:12. doi: 10.1186/1750-1326-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–92. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- 12.Ogura K, Ogawa M, Yoshida M. Effects of ageing on microglia in the normal rat brain: immunohistochemical observations. Neuroreport. 1994;5:1224–6. doi: 10.1097/00001756-199406020-00016. [DOI] [PubMed] [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M. et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 14.Roman GC, Tatemichi TK, Erkinjuntti T. et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–60. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 15.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Kumagai N, Chiba Y, Hosono M. et al. Involvement of pro-inflammatory cytokines and microglia in an age-associated neurodegeneration model, the SAMP10 mouse. Brain Res. 2007;1185:75–85. doi: 10.1016/j.brainres.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 17.McGeer PL, McGeer EG, Yasojima K. Alzheimer disease and neuroinflammation. J Neural Transm Suppl. 2000;59:53–7. doi: 10.1007/978-3-7091-6781-6_8. [DOI] [PubMed] [Google Scholar]