Abstract
AIM: To investigate the value of elevated drain amylase concentrations for detecting anastomotic leakage (AL) after minimally invasive Ivor-Lewis esophagectomy (MI-ILE).
METHODS: This was a retrospective analysis of prospectively collected data in two hospitals in the Netherlands. Consecutive patients undergoing MI-ILE were included. A Jackson-Pratt drain next to the dorsal side of the anastomosis and bilateral chest drains were placed at the end of the thoracoscopic procedure. Amylase levels in drain fluid were determined in all patients during at least the first four postoperative days. Contrast computed tomography scans and/or endoscopic imaging were performed in cases of a clinically suspected AL. Anastomotic leakage was defined as any sign of leakage of the esophago-gastric anastomosis on endoscopy, re-operation, radiographic investigations, post mortal examination or when gastro-intestinal contents were found in drain fluid. Receiver operator characteristic curves were used to determine the cut-off values. Sensitivity, specificity, positive predictive value, negative predictive value, risk ratio and overall test accuracy were calculated for elevated drain amylase concentrations.
RESULTS: A total of 89 patients were included between March 2013 and August 2014. No differences in group characteristics were observed between patients with and without AL, except for age. Patients with AL were older than were patients without AL (P = 0.01). One patient (1.1%) without AL died within 30 d after surgery due to pneumonia and acute respiratory distress syndrome. Anastomotic leakage that required any intervention occurred in 15 patients (16.9%). Patients with proven anastomotic leakage had higher drain amylase levels than patients without anastomotic leakage [median 384 IU/L (IQR 34-6263) vs median 37 IU/L (IQR 26-66), P = 0.003]. Optimal cut-off values on postoperative days 1, 2, and 3 were 350 IU/L, 200 IU/L and 160 IU/L, respectively. An elevated amylase level was found in 9 of the 15 patients with AL. Five of these 9 patients had early elevations of their amylase levels, with a median of 2 d (IQR 2-5) before signs and symptoms occurred.
CONCLUSION: Measurement of drain amylase levels is an inexpensive and easy tool that may be used to screen for anastomotic leakage soon after MI-ILE. However, clinical validation of this marker is necessary.
Keywords: Esophageal cancer, Esophageal surgery, Anastomotic leakage, Amylase, Drain fluid
Core tip: Intrathoracic leakage following esophagectomy is a dreaded complication that requires prompt diagnosis. However, early recognition remains difficult. Elevated drain amylase levels following other types of upper gastrointestinal surgery suggest that the amylase levels may be useful as an early marker for anastomotic leakage following esophagectomy. This study found that the drain amylase levels were higher in patients with proven anastomotic leakage than in patients without anastomotic leakage. This study demonstrates that amylase measurements in drain fluid may be a potential marker for detecting anastomotic leakage after an Ivor-Lewis esophagectomy.
INTRODUCTION
Anastomotic leakage (AL) following esophagectomy is a dreaded complication that occurs in 4.0%-28.6% of all patients who undergo minimally invasive Ivor-Lewis esophagectomy (MI-ILE)[1,2]. This large variation in incidence is partially explained by the use of different definitions of AL in the literature[3]. Improving surgical techniques and postoperative care have decreased the mortality and morbidity of AL over the past decades[4,5]. Nevertheless, this complication remains associated with an increased length of hospital stay, higher mortality rates[4,6], and a worse oncological outcome[7]. Early diagnosis and treatment of postoperative complications is critical to minimize the sequelae (e.g., sepsis)[8].
Several diagnostic modalities are available to detect AL, such as computed tomography (CT) scan with contrast, esophagography and endoscopy. Routine contrast esophagography minimally impacts postoperative management because of its low sensitivity for AL[9]. A CT scan with intravenous and oral contrast has a higher sensitivity for the detection of anastomotic leaks, particularly in combination with esophagography. However, the specificity of CT to detect AL is low, which leads to a low positive predictive value[10]. Routine endoscopy to detect AL after esophagectomy is 100% accurate[11,12], but endoscopy is an invasive technique with its own morbidities. Therefore, routine application of these diagnostic modalities is not frequently applied.
Multiple studies reported the diagnostic value of drain amylase to facilitate the detection of leaks following and pancreatojejunostomy[13-15]. Amylase measurements in drain fluid were used in these studies for the past several years as a simple adjunct to detect AL in an early stage. Maher et al[16] described drain amylase analysis as a potential diagnostic marker following gastro-jejunostomy. However, drain amylase levels cannot be used as a stand-alone diagnostic tool for AL in pancreatic and gastric surgeries. AL measurement following esophageal surgery has only been described in one small series (n = 12) to detect cervical esophagogastric leakage[17].
This study explored whether amylase concentrations in drain fluid correlated with the occurrence of anastomotic leakage after Ivor-Lewis esophagectomy.
MATERIALS AND METHODS
Patients and design
This study was a multicenter retrospective analysis of prospectively collected data that included all patients undergoing MI-ILE between March 2013 and August 2014 in two hospitals (Catharina Hospital Eindhoven and ZGT Hospital Group Twente) in the Netherlands. Patients were identified from an existing prospectively collected surgical database. Included patients underwent a MI-ILE and were at least 18 years old. Patients with acute pancreatitis were excluded.
Surgical technique
Specialized surgeons conducted the MI-ILE, and each surgeon performed at least 30 esophagectomies or more annually. Our surgical MI-ILE technique was adapted from a technique described elsewhere[18], but we used a different patient position during the thoracic phase and a different type of anastomosis. The first stage was a laparoscopic approach in which lymph node dissection and gastric conduit construction were performed. A pyloroplasty was not routinely performed. The thoracoscopic phase was performed in a prone position with single-lumen ventilation of both lungs[19]. The esophageal resection was completed in this stage, and a side-to-side intrathoracic anastomosis was constructed using a linear stapler (Endogia 30/45; Covidien, Minneapolis, Minnesota, United States)[20]. An end-to-side anastomosis was constructed in some cases using a hand-sutured technique. An omental wrap was draped around the anastomosis in all patients. A Jackson-Pratt drain (JP-drain) next to the dorsal side of the anastomosis and bilateral chest drains were placed at the end of the procedure. The nasogastric tube was removed at the end of the surgical procedure.
Postoperative care
Patients were transferred to the intensive care unit (ICU) postoperatively and were monitored for at least one day. Analgesia was provided via an epidural catheter or intravenous morphine using a patient-controlled system. Enteral nutrition was started on postoperative day (POD) 1 in all patients via early oral intake or a feeding jejunostomy. Nasojejunal tube feeding or total parenteral nutrition was initiated in cases of complications that prohibited oral intake in patients without a feeding jejunostomy.
The JP drain was removed after the fourth postoperative day in the Catharina Hospital Eindhoven if the daily production of drain fluid was less than 150cc. The JP drain was removed on the day of discharge in the ZGT Hospital Group Twente. Pleural drains were removed on POD 1 (left) and POD 2 (right).
Contrast CT scans and/or endoscopic imaging were performed in cases of a clinically suspected AL. Anastomotic leakage was defined as any sign of leakage of the esophago-gastric anastomosis on endoscopy, re-operation, radiographic investigations, post mortal examination or when gastro-intestinal contents were found in drain fluid. The severity of AL was recorded according to the Clavien-Dindo classification[21] and the Esophagectomy Complications Consensus Group (ECCG)[22].
Patients with a proven AL were treated using a nasogastric decompression tube, nil by mouth, antibiotics, or endoscopic stent, or they underwent thoracoscopic drainage of a pleural empyema or leak closure.
Amylase measurements
The amylase concentration in JP drain fluid was measured daily from POD 1 until drain removal. Drain amylase data were collected until POD 6. If AL occurred after POD 6 and the JP drain was still in situ, an additional amylase measurement on the day of diagnosis was added to the database. Fluid from the JP drain was sent to the clinical laboratory and analyzed using a COBAS 6000/8000 Analyzer (F. Hoffmann-La Roche Ltd., Basel, Switzerland). The results were obtained within 4 h after the sample was taken from the drain fluid bag. The analysis costs €2.43 ($2.77) per measurement in the Catharina Hospital. Serum amylase concentrations were analyzed, if available, to compare the drain fluid concentration with serum concentration and distinguish between pancreatitis and anastomotic leakage. Elevation of serum and drain fluid amylase indicated pancreatitis, and these patients were excluded from this study.
Patient demographics, neoadjuvant therapy, co-morbidities, type of surgery, time to resumption of oral intake, anastomotic leakage and 30-d mortality were recorded. Signs and symptoms of AL were recorded, including fever, elevated inflammation markers, atrial fibrillation[23], delirium[24], pericarditis, mediastinitis, empyema, acute respiratory insufficiency and/or chest pain.
Statistical analysis
Statistical analysis was performed using SPSS 22.0. Normality of all data was evaluated using a Shapiro-Wilk test. χ2, Fisher’s exact and Mann-Whitney U tests were used to compare the amylase levels between patients with and without AL. Receiver operator characteristic (ROC) curves were used to determine the cut-off values. Sensitivity (SENS), specificity (SPEC), positive predictive value (PPV), negative predictive value (NPV), risk ratio and overall test accuracy were calculated for elevated drain amylase concentrations. Continuous data are presented as medians with corresponding interquartile ranges, and dichotomous results are presented as frequencies. A P value < 0.05 was considered statistically significant.
The statistical methods of this study were reviewed by epidemiologist/statistician S. Houterman, PhD from the Catharina Hospital Eindhoven.
RESULTS
Patient characteristics
Eighty-nine patients were included from March 2013 until August 2014. One patient was excluded due to postoperative pancreatitis (diagnosed with clinical symptoms, elevated serum and drain amylase, and CT scan confirmation). The median patient age was 66 years (range: 43-80), and 79.8% were male. Eighty-eight percent of the patients received neoadjuvant chemoradiation. All patients received a MI-ILE. Thirty (33.7%) patients received a jejunostomy catheter during surgery according to the local protocol, and enteral feeding was initiated on POD 1 as an addition to oral intake. The median time to begin oral intake was POD 1. Table 1 shows the group characteristics. No differences in group characteristics were observed between patients with and without AL, except for age. Patients with AL were older than were patients without AL (P = 0.01).
Table 1.
Patient characteristics
| Anastomotic leakage (n = 15) | No anastomotic leakage (n = 74) | P value | |
| Sex | 1.001 | ||
| Male | 12 (80) | 59 (80) | |
| Female | 3 (20) | 15 (20) | |
| Age | 71 (64-76) | 65 (57-69) | 0.012 |
| Steroid use | 1 (7) | 2 (3) | 0.431 |
| Comorbidity | 11 (73) | 51 (69) | 1.001 |
| Cardiac | 2 (13) | 18 (24) | 0.511 |
| Vascular | 4 (27) | 34 (46) | 0.251 |
| Diabetes | 4 (27) | 18 (24) | 1.001 |
| Pulmonary | 5 (33) | 17 (23) | 0.403 |
| Renal | 0 (0) | 1 (1) | 1.001 |
| BMI > 30 kg/m2 | 3 (20) | 13 (18) | 0.731 |
| ASA score | 0.462 | ||
| I | 1 (7) | 5 (7) | |
| II | 11 (73) | 46 (62) | |
| III | 3 (20) | 23 (31) | |
| Neoadjuvant therapy | 0.762 | ||
| None | 0 (0) | 6 (8) | |
| Chemotherapy | 0 (0) | 4 (5) | |
| Chemoradiotherapy | 15 (100) | 65 (87) | |
| Jejunostomy | 6 (40) | 24 (32) | 0.573 |
| Start of oral feeding (POD) | 1 (1-1) | 1 (1-1) | 0.462 |
Fisher’s exact test;
Mann Whitney U test;
χ2 test. Values are presented as absolute numbers (percentage) or medians (lower quartile-upper quartile). POD: Postoperative day.
Anastomotic leakage and overall mortality
Fifteen patients (16.9%) developed AL during postoperative admission, and all AL cases were confirmed using endoscopic imaging. The median time to diagnosis was 7 d (IQR 3-12). Treatment with antibiotics and nil-by-mouth was sufficient in 8 patients (9.0%). Four patients (4.5%) received surgical reintervention. Table 2 shows the severity of anastomotic leakage according to the Clavien-Dindo classification and the ECCG. One patient (1.1%) without AL died within 30 d after surgery due to pneumonia and acute respiratory distress syndrome (ARDS).
Table 2.
Intrathoracic anastomotic leakage n (%)
| n = 15/89 (16.9) | |
| Intervention type | |
| Antibiotics and NPO | 8 (9.0) |
| Endoscopic stent | 3 (3.4) |
| Surgical reintervention | 4 (4.5) |
| According to Clavien-Dindo | |
| Grade II | 0 (0) |
| Grade IIIa | 9 (10.1) |
| Grade IIIb | 0 (0) |
| Grade IVa | 6 (6.7) |
NPO: Nil-by-mouth.
Drain placement
The Jackson-Pratt drain in patients with AL was retrospectively evaluated at its closest distance to the esophago-gastric anastomosis on CT imaging. The median distance for patients with elevated drain amylase was 11.0 mm compared with a median distance of 34.5 mm for patients with normal amylase levels (P = 0.06).
Amylase concentration comparison
The combination of all amylase measurements per patient (Figure 1) revealed that drain amylase levels were higher in patients with AL than in patients without AL [median 384 IU/L (IQR 34-6263) vs median 37 IU/L (IQR 26-66) P = 0.003].
Figure 1.
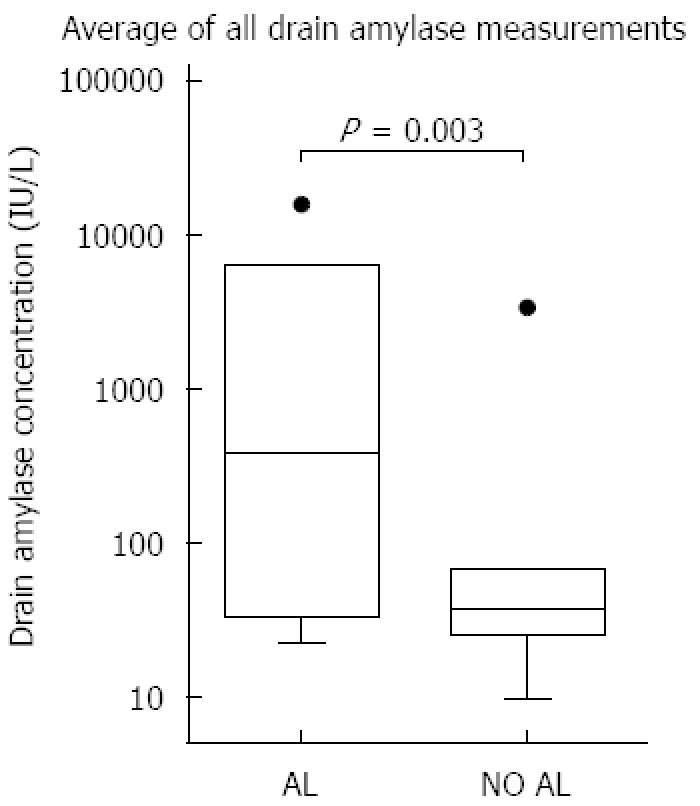
Whiskers-Tukey boxplot. Comparison of median drain amylase levels using the average of all measurements. Dots in this figure are outliers. Al: Anastomotic leakage.
Differences in daily drain amylase levels were also found between patients with AL and patients without AL on POD 1-3. The median drain amylase levels in patients with AL on POD 1, 2 and 3 were 121 IU/L, 59 IU/L and 50 IU/L, respectively. The median levels in patients without AL were 59 IU/L, 39 IU/L and 28 IU/L, respectively.
Figure 2 shows the course of the daily median drain amylase levels for patients with and without AL. Patients without AL showed a gradual but steady decrease in drain amylase concentrations over time. The daily drain amylase levels in patients with AL also declined, but the interquartile range of these values increased daily.
Figure 2.
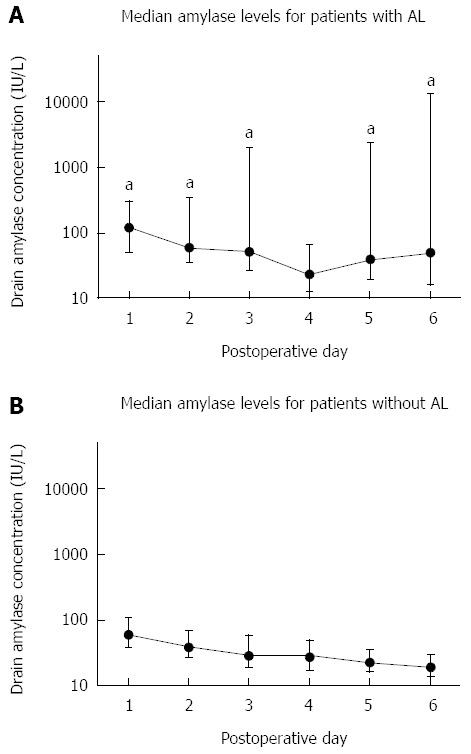
Daily median drain amylase levels in patients with (A) and without (B) anastomotic leakage. Error bars represent interquartile range. aP < 0.05. Al: Anastomotic leakage.
Accuracy of amylase concentration
Table 3 presents different cut-off values. Optimal cut-off values were determined using ROC curves. The area under the curve for cut-off values on POD 1, 2 and 3 was approximately 0.68. Cut-off values for POD 1, 2 and 3 were 350 IU/L, 200 IU/L and 160 IU/L, respectively. The specificity of these values ranged from 94.0% to 95.7%, and the sensitivity ranged from 21.4% to 35.7%.
Table 3.
Diagnostic value of drain amylase
| Time of measurement | AUC | Cut-off (IU/L) | Sensitivity | Specificity | RR |
| Per postoperative day | |||||
| POD 1 | 0.672 | 350 | 21.4 | 95.6 | 3.5a |
| POD 2 | 0.676 | 200 | 26.4 | 95.7 | 4.0a |
| POD 3 | 0.685 | 160 | 35.7 | 94.0 | 4.4a |
| Any POD | |||||
| Same cut-off as Machens et al[17] | 600 | 53.3 | 97.3 | 9.0a | |
| Most accurate in our cohort | 1900 | 53.3 | 98.7 | 10.2a |
Values are presented as international units per liter or as percentages.
P < 0.05 vs control. POD: Postoperative day; AUC: Area under the curve; RR: Risk ratio.
Machens et al[17] used a cut-off value of 600 IU/L independently of the day of postoperative measurement. A cut-off value of 600 IU/L in this trial revealed a sensitivity of 53.3% and a specificity of 97.3%. Furthermore, this cut-off value correlated with a positive predictive value of 80.0% and a negative predictive value of 92.3%. A cut-off value of 1900 IU/L was the most accurate in our cohort, independent of postoperative day measurement. We achieved a sensitivity of 53.3% and a specificity of 98.7% using this cut-off value.
Amylase concentration in relation to signs and symptoms
An elevated amylase level (i.e., higher than the specific cut-off values for each postoperative day) was found in 9 of the 15 patients (60%) with AL. Patients with AL developed signs and symptoms (e.g., atrial fibrillation, acute respiratory insufficiency, elevation of inflammation markers, fever) on median POD 4 (IQR 3-6). Five of these 9 patients had early elevations of their amylase levels, with a median of 2 d (IQR 2-5) before signs and symptoms occurred. In 4 of these 9 patients, the elevation of amylase levels occurred after signs and symptoms were observed.
DISCUSSION
Anastomotic leakage following MI-ILE is a dreaded complication that is associated with a significant increase in morbidity and mortality[4,6], which emphasizes the need for early diagnosis and treatment[8]. The anastomotic leakage rate in our study (4.5% required surgical re-intervention, 16.9% overall) is comparable to the published data of patients undergoing Ivor-Lewis esophagectomy. A large published series of patients undergoing MI-ILE included 530 patients, and 4% had an anastomotic leakage that required reoperation, and 2% showed necrosis of the gastric conduit[1]. Rutegård et al[25] described a 5% rate of AL that required surgery in a nationwide prospective study. The incidence of AL varies between 6 and 28% when AL was defined as requiring any type of intervention[2,5].
Little is known about the value of drain amylase for detecting AL in esophageal surgery. Amylase in drain fluid following gastric or pancreatic surgery was described previously as a potential adjunct in the diagnosis of AL. However, there is no consensus on its clinical potential in the diagnosis of AL following gastric bypass surgery or pancreaticoduodenectomy because of the variable results presented in the current literature[13-16,26-28].
Machens et al[17] described the use of amylase in drain fluid to detect AL in a small series of 12 patients with cervical anastomosis following esophagectomy. The results obtained in this study indicated that AL was chemically detectable in drain fluid by a mean of 2.1 d before clinical evidence. However, no definitive conclusions could be drawn because few patients were included and because no comparisons were performed with a group without AL. No work published the value of drain amylase as an early indicator for AL following esophageal surgery since this study was conducted in 1996.
Our data demonstrated that the median drain amylase levels in patients with AL were higher than patients without AL on POD 1-3 and POD 6. This result might reflect an early stage of AL. However, the drain amylase levels are also slightly elevated in patients without AL in the first postoperative days. This elevation may be due to spilled saliva during surgery or to an early, but transient, saliva leak between the sutures or staples. This observation is consistent with elevated drain amylase on POD 1-3 in patients following pancreaticojejunostomy[15,28]. Nevertheless, an increase in drain amylase was observed as early as the first POD in patients with AL in our study.
Our data show that a drain amylase level > 350 IU/L on POD 1, > 200 IU/L on POD 2 and > 160 IU/L on POD 3 and cut-off values of 600 and 1900 IU/L were very specific for AL. However, sensitivity was limited, and low amylase levels in drain fluid do not exclude AL. This result may be explained in several ways. An omental wrap is draped around the anastomosis at the end of the surgical procedure. This omental wrap may act as a physical barrier between the leakage site and the drain, which leads to decreased detection in drain fluid. Another explanation is the positioning of the JP drain. The drain is routinely positioned in the right thorax, but dislocation may occur. Dislocation would cause inadequate drainage and lead to decreased sensitivity.
The position of the drain in our study was re-evaluated in patients who underwent a CT scan and were diagnosed with AL. The median distance between the anastomosis and the drain was shorter in patients with elevated drain amylase levels than in patients without elevated drain amylase levels, but this difference was not significant. Therefore, the distance of anastomosis to the drain may influence the analysis of the detection potential of drain amylase. Another explanation for the observed low sensitivity may be early drain removal. Drain removal before AL was diagnosed may contribute to lower sensitivity. These items may partially account for the low sensitivity, which could be addressed in future prospective studies to improve sensitivity. Two patients were diagnosed with AL after drain removal. These patients had normal postoperative amylase levels, and the diagnostic value would increase slightly if these patients were excluded. The sensitivity increased to 61.5% when the cut-off value of 1900 IU/L was used.
We chose not to exclude these patients because their exclusion could lead to a suspicion of selection bias.
We’ve observed elevation of drain amylase levels in patients after symptoms of anastomotic leakage occurred. These patients were treated when diagnosis was confirmed, possibly elevation of amylase occurred by manipulation of the anastomosis in gastroscopy and/or surgical intervention.
It is possible that clinical decisions were made based on the elevation of amylase during the postoperative course, which potentially hampered our data. The retrospective aspect of this study could not prevent this clinical decision-making. This study only included patients undergoing MI-ILE, and the results of this study may not be applicable to different types of esophageal surgery.
A prospective study is needed to ensure uniformity per patient, i.e., standardized drain positioning and time of drain removal. Future research including high patient numbers and fewer missing values may lead to more reliable cut-off values for drain amylase levels that are predictive of AL. The influence of early AL detection on patients’ outcome can be studied in prospective studies.
In conclusion, the daily measurement of amylase levels in drain fluid of a drain close to the anastomosis is a simple, inexpensive and easy tool that may be used as a marker for AL early after MI-ILE. Patients with AL have significantly higher drain amylase levels in the postoperative period. Elevated drain amylase may be used to suggest the use of diagnostic tools, such as endoscopy. However, clinical validation of this marker is necessary to confirm its clinical usefulness.
ACKNOWLEDGMENTS
We thank R. van Eck for his contribution in data collection. Gijs HK Berkelmans received the “Van Walree” scholarship from the “Royal Dutch Academies for Sciences” to present his abstract during the 14th ISDE congress in Vancouver, Canada.
COMMENTS
Background
Anastomotic leakage (AL) following an esophagectomy is a dreaded complication that is associated with an increased length of stay, higher mortality rates, and a worse oncological outcome. Several diagnostic modalities are available to detect AL, such as computed tomography scan with contrast, esophagography and endoscopy. However, each modality has its own drawback because they either are invasive or require radiation. Amylase in drain fluid is documented as a marker for AL after pancreatic surgery, but only one study reported the use of this marker in esophageal surgery. An inexpensive and non-invasive marker could identify patients who require further invasive diagnostics.
Research frontiers
Multiple studies reported the diagnostic value of drain amylase to facilitate the detection of leaks following and pancreatojejunostomy. The measurement of amylase in drain fluid was used for several years in these studies as a simple adjunct to detect AL in an early stage. The current hotspot is to identify the usefulness of this marker in other types of surgery in the upper gastrointestinal tract.
Innovations and breakthroughs
Esophagectomy is primarily performed because of esophageal cancer. This major procedure is accompanied with high postoperative morbidity and mortality, and it majorly impacts the patient’s quality of life. The introduction of chemoradiotherapy as neoadjuvant treatment and improvements in surgical techniques significantly improved overall survival. However, postoperative morbidity from anastomotic leakage remains high following an esophagectomy. Early recognition of AL can reduce hospital stay and mortality. Further reductions may be achieved if amylase in drain fluid is detected before clinical symptoms occur.
Applications
This study suggests the possible usefulness of amylase in drain fluid as an inexpensive and non-invasive marker for intrathoracic anastomotic leakage after an esophagectomy.
Terminology
Anastomotic leakage is leakage from the new anastomosis of the esophagus and the stomach in the thoracic cavity. This complication frequently occurs after an esophageal resection. Amylase is an enzyme that is naturally present in saliva and pancreatic fluid. Saliva runs down the esophagus, and saliva can drip into the thoracic cavity when leakage is present.
Peer-review
The paper is an interesting analysis of the ability to detect anastomotic leakages early in patients undergoing Ivor-Lewis esophagectomy with the use of an easy, inexpensive test. Anastomotic leakage is an important complication after esophagectomy, and the early detection of AL is crucial to establish adequate treatments that influence the prognosis.
Footnotes
Institutional review board statement: The Medical Ethics Committees United of the Catharina Hospital Eindhoven reviewed and approved this study.
Informed consent statement: Informed consent is given by patients preoperatively and registered in the electronic patient file. All included patients accepted the possibility to collect their patient data.
Conflict-of-interest statement: The authors declare no conflicts of interest.
Data sharing statement: Technical appendix, statistical codes, and the dataset are available from the corresponding author at misha.luyer@cze.nl. Informed consent was obtained as described above.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 25, 2015
First decision: April 16, 2015
Article in press: June 10, 2015
P- Reviewer: Bordas JM, Chen Z, Kuo SM S- Editor: Yu J L- Editor: A E- Editor: Liu XM
References
- 1.Luketich JD, Pennathur A, Awais O, Levy RM, Keeley S, Shende M, Christie NA, Weksler B, Landreneau RJ, Abbas G, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg. 2012;256:95–103. doi: 10.1097/SLA.0b013e3182590603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trugeda S, Fernández-Díaz MJ, Rodríguez-Sanjuán JC, Palazuelos CM, Fernández-Escalante C, Gómez-Fleitas M. Initial results of robot-assisted Ivor-Lewis oesophagectomy with intrathoracic hand-sewn anastomosis in the prone position. Int J Med Robot. 2014;10:397–403. doi: 10.1002/rcs.1587. [DOI] [PubMed] [Google Scholar]
- 3.Bruce J, Krukowski ZH, Al-Khairy G, Russell EM, Park KG. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg. 2001;88:1157–1168. doi: 10.1046/j.0007-1323.2001.01829.x. [DOI] [PubMed] [Google Scholar]
- 4.Kassis ES, Kosinski AS, Ross P, Koppes KE, Donahue JM, Daniel VC. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg. 2013;96:1919–1926. doi: 10.1016/j.athoracsur.2013.07.119. [DOI] [PubMed] [Google Scholar]
- 5.Martin LW, Swisher SG, Hofstetter W, Correa AM, Mehran RJ, Rice DC, Vaporciyan AA, Walsh GL, Roth JA. Intrathoracic leaks following esophagectomy are no longer associated with increased mortality. Ann Surg. 2005;242:392–399; discussion 399-402. doi: 10.1097/01.sla.0000179645.17384.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizk NP, Bach PB, Schrag D, Bains MS, Turnbull AD, Karpeh M, Brennan MF, Rusch VW. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. J Am Coll Surg. 2004;198:42–50. doi: 10.1016/j.jamcollsurg.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Lerut T, Moons J, Coosemans W, Van Raemdonck D, De Leyn P, Decaluwé H, Decker G, Nafteux P. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg. 2009;250:798–807. doi: 10.1097/SLA.0b013e3181bdd5a8. [DOI] [PubMed] [Google Scholar]
- 8.Hölscher AH, Vallböhmer D, Brabender J. The prevention and management of perioperative complications. Best Pract Res Clin Gastroenterol. 2006;20:907–923. doi: 10.1016/j.bpg.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Cools-Lartigue J, Andalib A, Abo-Alsaud A, Gowing S, Nguyen M, Mulder D, Ferri L. Routine contrast esophagram has minimal impact on the postoperative management of patients undergoing esophagectomy for esophageal cancer. Ann Surg Oncol. 2014;21:2573–2579. doi: 10.1245/s10434-014-3654-1. [DOI] [PubMed] [Google Scholar]
- 10.Lantos JE, Levine MS, Rubesin SE, Lau CT, Torigian DA. Comparison between esophagography and chest computed tomography for evaluation of leaks after esophagectomy and gastric pull-through. J Thorac Imaging. 2013;28:121–128. doi: 10.1097/RTI.0b013e31826ff062. [DOI] [PubMed] [Google Scholar]
- 11.Page RD, Asmat A, McShane J, Russell GN, Pennefather SH. Routine endoscopy to detect anastomotic leakage after esophagectomy. Ann Thorac Surg. 2013;95:292–298. doi: 10.1016/j.athoracsur.2012.09.048. [DOI] [PubMed] [Google Scholar]
- 12.Schaible A, Sauer P, Hartwig W, Hackert T, Hinz U, Radeleff B, Büchler MW, Werner J. Radiologic versus endoscopic evaluation of the conduit after esophageal resection: a prospective, blinded, intraindividually controlled diagnostic study. Surg Endosc. 2014;28:2078–2085. doi: 10.1007/s00464-014-3435-8. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen JH. Distinguishing between parenchymal and anastomotic leakage at duct-to-mucosa pancreatic reconstruction in pancreaticoduodenectomy. World J Gastroenterol. 2008;14:6648–6654. doi: 10.3748/wjg.14.6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid-Lombardo KM, Farnell MB, Crippa S, Barnett M, Maupin G, Bassi C, Traverso LW. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1,507 patients: a report from the Pancreatic Anastomotic Leak Study Group. J Gastrointest Surg. 2007;11:1451–1458; discussion 1459. doi: 10.1007/s11605-007-0270-4. [DOI] [PubMed] [Google Scholar]
- 15.Shinchi H, Wada K, Traverso LW. The usefulness of drain data to identify a clinically relevant pancreatic anastomotic leak after pancreaticoduodenectomy? J Gastrointest Surg. 2006;10:490–498. doi: 10.1016/j.gassur.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Maher JW, Bakhos W, Nahmias N, Wolfe LG, Meador JG, Baugh N, Kellum JM. Drain amylase levels are an adjunct in detection of gastrojejunostomy leaks after Roux-en-Y gastric bypass. J Am Coll Surg. 2009;208:881–884; discussion 885-886. doi: 10.1016/j.jamcollsurg.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Machens A, Busch C, Bause H, Izbicki JR. Gastric tonometry and drain amylase analysis in the detection of cervical oesophagogastric leakage. Br J Surg. 1996;83:1614–1615. doi: 10.1002/bjs.1800831139. [DOI] [PubMed] [Google Scholar]
- 18.Pennathur A, Awais O, Luketich JD. Technique of minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2010;89:S2159–S2162. doi: 10.1016/j.athoracsur.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 19.Palanivelu C, Prakash A, Senthilkumar R, Senthilnathan P, Parthasarathi R, Rajan PS, Venkatachlam S. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position--experience of 130 patients. J Am Coll Surg. 2006;203:7–16. doi: 10.1016/j.jamcollsurg.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Okabe H, Tanaka E, Tsunoda S, Obama K, Sakai Y. Intrathoracic esophagogastric anastomosis using a linear stapler following minimally invasive esophagectomy in the prone position. J Gastrointest Surg. 2013;17:397–402. doi: 10.1007/s11605-012-2009-0. [DOI] [PubMed] [Google Scholar]
- 21.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 22.Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, D’Journo XB, Griffin SM, Hölscher AH, Hofstetter WL, Jobe BA, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG) Ann Surg. 2015;262:286–294. doi: 10.1097/SLA.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 23.Stawicki SP, Prosciak MP, Gerlach AT, Bloomston M, Davido HT, Lindsey DE, Dillhoff ME, Evans DC, Steinberg SM, Cook CH. Atrial fibrillation after esophagectomy: an indicator of postoperative morbidity. Gen Thorac Cardiovasc Surg. 2011;59:399–405. doi: 10.1007/s11748-010-0713-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi CM, Wang DX, Chen KS, Gu XE. Incidence and risk factors of delirium in critically ill patients after non-cardiac surgery. Chin Med J (Engl) 2010;123:993–999. [PubMed] [Google Scholar]
- 25.Rutegård M, Lagergren P, Rouvelas I, Lagergren J. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: a population-based study. Ann Surg Oncol. 2012;19:99–103. doi: 10.1245/s10434-011-1926-6. [DOI] [PubMed] [Google Scholar]
- 26.Kong J, Gananadha S, Hugh TJ, Samra JS. Pancreatoduodenectomy: role of drain fluid analysis in the management of pancreatic fistula. ANZ J Surg. 2008;78:240–244. doi: 10.1111/j.1445-2197.2008.04428.x. [DOI] [PubMed] [Google Scholar]
- 27.Moskovic DJ, Hodges SE, Wu MF, Brunicardi FC, Hilsenbeck SG, Fisher WE. Drain data to predict clinically relevant pancreatic fistula. HPB (Oxford) 2010;12:472–481. doi: 10.1111/j.1477-2574.2010.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shyr YM, Su CH, Wu CW, Lui WY. Does drainage fluid amylase reflect pancreatic leakage after pancreaticoduodenectomy? World J Surg. 2003;27:606–610. doi: 10.1007/s00268-003-6841-y. [DOI] [PubMed] [Google Scholar]


