Abstract
AIM: To evaluate the diagnostic effectiveness of white light endoscopy, magnifying endoscopy (ME), and magnifying narrow-band imaging endoscopy (ME-NBI) in detecting early gastric cancer (EGC).
METHODS: From March 2010 to June 2012, a total of 3616 patients received screening for gastric cancer by magnifying endoscopy. There were 3675 focal gastric lesions detected using conventional high definition white light endoscopy (HD-WLE) in four different referential hospitals that were recruited for further investigation using ME and ME-NBI. The images obtained from HD-WLE, ME, and ME-NBI were reviewed by four experienced endoscopists to evaluate their diagnostic effectiveness for EGC. The diagnosis of cancerous and non-cancerous lesions was conducted by evaluating the microvascular and microsurface patterns using the VS classification system. The final endoscopic diagnosis of each lesion was determined by consultation when a disagreement occurred. We used histopathological results as the gold standard for the diagnosis of EGC.
RESULTS: Among the 3675 lesions found, 1508 were validated by pathological findings as chronic gastritis, 1279 as chronic gastritis with intestinal metaplasia, 631 as low-grade neoplasia, and 257 as EGC. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of HD-WLE for the diagnosis of EGC were 71.2%, 99.1%, 85.5%, 97.9% and 97.1%, respectively. The results of ME for diagnosing EGC were 81.3%, 98.8%, 83.3%, 98.6% and 97.6%, respectively. The results of ME-NBI for the diagnosis of EGC were 87.2%, 98.6%, 82.1%, 99.0% and 97.8%, respectively. The diagnostic sensitivity and accuracy of paired ME and ME-NBI were significantly better than those of HD-WLE (P < 0.05).
CONCLUSION: HD-WLE has a relatively high accuracy for diagnosing EGC and is an effective screening tool. Further investigations of ME and ME-NBI are required to achieve superior accuracy.
Keywords: Early diagnosis, Gastric cancer, Gastric mucosa, Magnifying endoscopy, Narrow-band imaging
Core tip: The early detection of gastric cancer is critical to improving prognosis. A variety of techniques, including magnifying endoscopy (ME) and magnifying narrow-band imaging endoscopy (ME-NBI), are used to identify early gastric cancer (EGC). Conventional white light endoscopy shows a relatively high specificity and accuracy in diagnosing EGC, which suggests that it should be a first-line endoscopic screening modality. However, further investigations using ME and ME-NBI are needed to achieve higher sensitivity and accuracy. Additionally, the ability to visualize microstructures in suspected gastric lesions could be significantly improved.
INTRODUCTION
Gastric cancer is the second leading cause of cancer related mortality and is the fourth most common malignancy worldwide[1]. Patients who are diagnosed at an early stage and treated properly by endoscopic dissection or surgical resection have a 5-year survival rate of 90%-97%. However, for patients with advanced gastric carcinoma, the 5-year survival rate is less than 20%[2-7]. Thus, the early detection of gastric cancer improves prognosis[8]. However, patients with early stage gastric cancer experience only vague epiabdominal symptoms, and there are no serum tumor markers with satisfactory sensitivity. As a result, endoscopic screening has been recognized as one of the most effective methods for the detection of early gastric cancer (EGC) in endemic countries. In Japan, the rapid development and use of new endoscopic screening modalities have improved the detection rate of EGC from 15% to 57%[9]. The incidence of EGC is less than 20% in the Chinese population, which prompted us to investigate better strategies for the early detection of gastric cancer.
Conventional high definition white light endoscopy (HD-WLE) is one of the most widely used endoscopic modalities. HD-WLE cannot detect and delineate the margin of small EGC lesions with subtle mucosal changes, which further limits a thorough elimination of early gastric lesions through endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). In contrast to HD-WLE, magnifying endoscopy (ME) can clearly show the microsurface structure of the gastric mucosa. Furthermore, the microvascular structure of gastric mucosal lesions can be better visualized with the help of narrow-band imaging (NBI)[10]. The combination of ME and NBI can enhance the contrast between gastric lesions and normal tissues and provide a better understanding of the microstructure of suspicious lesions[11,12]. The current evidence suggests that magnifying NBI endoscopy (ME-NBI) is superior to conventional endoscopy. However, the exact sensitivity, specificity and accuracy data of ME-NBI compared with HD-WLE and ME in large-scale populations is less clear.
We conducted the present multicenter prospective study to evaluate the exact diagnostic value of conventional endoscopy and ME-NBI in delineating EGC. We also validated the usefulness of ME-NBI for the early detection of gastric cancer in a Chinese population.
MATERIALS AND METHODS
Patients
This study was conducted in patients with focal gastric mucosal lesions that were detected by conventional white light endoscopy from March 2010 to June 2012 in four different referential medical centers in China. Patients who were more than 40 years old without any obvious epiabdominal symptoms, patients with gastrointestinal symptoms such as anorexia or dyspepsia, and patients seeking a follow-up endoscopy subsequent to a previous pathological diagnosis of intestinal metaplasia or chronic atrophic gastritis were eligible for inclusion. Patients who had an established diagnosis of advanced gastric cancer and those who had been treated with prior gastric surgery were excluded from the study. The study protocol was approved by the Ethics Committee of the medical centers. All of the participants, or their legal guardian, provided written informed consent before being enrolled in the study.
Endoscopic procedure
The patients were administered a mixture of following medications to eliminate the mucus adhering to mucosa approximately 30 min before endoscopic procedures: 8000 units of chymotrypsin (Shanghai No. 1 Biochemical and Pharmaceutical, Shanghai, China), 8 mL of dimeticone (Berlin-Chemie AG, Berlin, Germany), 25 mL of sodium bicarbonate (CR Double-Crane, Beijing, China), 50 mL of sodium chloride (CR Double-Crane, Beijing, China). The patients were asked to roll on the examination table for 10-15 min. The patients were then injected with 20 mg of scopolamine butylbromide (ZiZhu Pharmaceutical, Beijing, China) to reduce stomach spasms during the procedure.
All of the endoscopic examinations were performed using an upper gastrointestinal zoom endoscope (GIF-H260Z; Olympus, Tokyo, Japan). A soft black hook was mounted on the tip of the endoscope. The hook helps fix the focal distance between the tip of the endoscope and the target mucosa at 3 mm and allows the endoscopist to clearly visualize the gastric lesions. HD-WLE was first performed in eligible patients to determine the appearance of the suspicious gastric mucosa as a whole. ME was then used to reveal the microstructure of the targeted mucosa. The endoscope was then changed to magnifying NBI mode for further observations of the gastric lesions. The biopsy specimens were collected using standard forceps after observing the target gastric lesions. The images of the lesions obtained by each endoscopic modality were recorded and saved in the digital filling system for subsequent evaluation.
Analysis of endoscopic images
Four experienced endoscopists diagnosed the lesions based on the images recorded in one of the endoscopic modalities regardless of the images of the other two modalities. This strategy was used to avoid potential subjective deviation and guarantee the quality of the study. The endoscopists had no access to patients’ clinical or pathological data. A conclusion for each image was made after discussion among the four endoscopists. In the HD-WLE mode, cancerous lesions were defined based on the color and appearance of the mucosa. We concluded that the lesion was cancerous if we visualized the following endoscopic characteristics: color change (such as redness, whiteness or obvious color contrast), uneven mucosal surface, and irregular lesion margins. For images obtained in the ME and ME-NBI mode, the diagnosis of cancerous and non-cancerous lesions was made by evaluating the microsurface and microvascular pattern using the VS classification system developed by Yao et al[13]. The microstructure (including microsurface and microvascular patterns) was classified as regular, irregular or absent. In addition, an assessment of the margin between the lesion and the surrounding mucosa was made and recorded as present or absent (Figure 1). Gastric mucosa with an irregular microvascular pattern and a demarcation line and/or an irregular microsurface pattern with a demarcation line was defined as a cancerous lesion[13].
Figure 1.
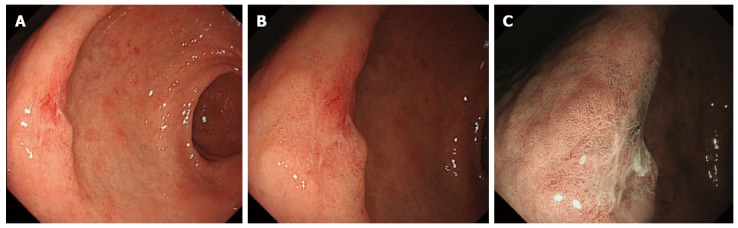
Assessment of the margin between the lesion and the surrounding mucosa was made and recorded as present or absent. A: High definition white light endoscopy finding of a focal lesion 15 mm x 10 mm in diameter, IIa + IIc type in the gastric antrum; B: Magnifying endoscopy shows irregular pattern of microsurface, with a relatively clear demarcation line; C: Magnifying narrow-band imaging endoscopy shows irregular and absent microsurface pattern, and microvascular dilation, with a clear demarcation line. The histopathological diagnosis of the surgery specimen is early gastric cancer (revised Vienna classification C5).
Histopathology
The forceps biopsy specimens taken from the target lesion were immediately placed in 10% buffered formalin. The histopathological diagnoses of biopsy specimens were made by an experienced gastrointestinal pathologist who had access to the clinical patient information. In the present study, lesions were diagnosed pathologically as chronic gastritis, chronic gastritis with intestinal metaplasia, and low-grade neoplasia (category 3) according to the revised Vienna classification[14] and were designated as non-cancerous lesions. High-grade neoplasias and noninvasive cancerous lesions (category 4 and category 5) were designated as EGC. The histopathology of biopsy specimens was used as the gold standard for the diagnosis of EGC.
Statistical analysis
The statistical data were analyzed by Statistical Program for Social Sciences (SPSS), Version 22.0 software (IBM, United States). The accuracy, sensitivity, specificity, positive predictive value, and negative predictive value of the diagnoses were evaluated for HD-WLE, ME and ME-NBI. The values were calculated with reference to the pathological diagnosis. The Wilson score method was used to measure the 95%CI. The comparison of accuracy and sensitivity among the three different endoscopic modalities was performed using the McNemar test, and P < 0.05 was considered significant. All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
Clinical characteristics of patients and lesions
Overall, 3616 patients were enrolled in the study, and the median age was 56 years (range: 40-90 years). There were 1910 male patients and 1706 female patients. There were 3675 lesions detected. The pathological diagnoses revealed that there were 257 cases of EGC (including category 4 and category 5), 631 cases of low-grade neoplasia (category 3), and 1279 cases of chronic gastritis with intestinal metaplasia. There were 1508 cases defined as chronic gastritis. The patients’ clinical characteristics and lesion types are summarized in Table 1.
Table 1.
Clinical characteristics of eligible patients with gastric lesions
| Characteristic | Number |
| Patients | 3616 |
| Gender (male/female) | 1910/1706 |
| Gastric mucosal lesions | 3675 |
| Location of lesions | |
| Upper third | 182 (5.0) |
| Middle third | 206 (5.6) |
| Lower third | 3287 (89.4) |
| Pathological diagnosis | |
| Chronic gastritis with/without intestinal metaplasia | 2787 (75.8) |
| Low-grade neoplasia (C3)1 | 631 (17.2) |
| EGC (including C4 and C5)1 | 257 (7.0) |
According to revised Vienna classification[14].
Relationship between endoscopic and pathological diagnosis
We analyzed the diagnostic value of HD-WLE, ME and ME-NBI and found that all three modalities had a high diagnostic accuracy (> 97%) for EGC compared with pathology. The sensitivity of HD-WLE was 71.2%. The sensitivity was 81.3% and 87.2% for ME and ME-NBI, respectively. The data for sensitivity, specificity, positive predictive value, negative predictive value, and accuracy used to evaluate the diagnostic value of each method for EGC are shown in Table 2. The diagnostic sensitivity and accuracy of both ME and ME-NBI were significantly better at detecting ECG than those of HD-WLE based on the VS classification system (P < 0.05). Thus, the use of ME and ME-NBI improved the endoscopic recognition of EGC.
Table 2.
Comparison of the diagnostic value for early gastric cancer among different modalities of endoscopy
| Procedure | Number |
Pathological diagnosis |
Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy | |
| 0 | 1 | (95%CI) | (95%CI) | (95%CI) | (95%CI) | (95%CI) | ||
| HD-WLE | ||||||||
| 0 | 3461 | 3387 | 74 | 71.2% | 99.1% | 85.5% | 97.9% | 97.1% |
| 1 | 214 | 31 | 183 | (65.4-76.4) | (98.7-99.4) | (80.2-89.6) | (97.3-98.3) | (96.6-97.6) |
| ME | ||||||||
| 0 | 3424 | 3376 | 48 | 81.3%a | 98.8% | 83.3% | 98.6% | 97.6%a |
| 1 | 251 | 42 | 209 | (76.1-85.6) | (98.3-99.1) | (78.2-87.4) | (98.2-98.9) | (97.0-98.0) |
| ME-NBI | ||||||||
| 0 | 3402 | 3369 | 33 | 87.2%a | 98.6% | 82.1% | 99.0% | 97.8%a |
| 1 | 273 | 49 | 224 | (82.5-90.1) | (98.1-98.9) | (77.1-86.2) | (98.6-99.3) | (97.2-98.2) |
P < 0.05 vs WLE. 0: Noncancerous lesions (chronic gastritis, chronic gastritis with intestinal metaplasia and low-grade neoplasia); 1: Cancerous lesions (EGC including high-grade neoplasia); HD-WLE: High definition white light endoscopy; ME: Magnifying endoscopy; ME-NBI: Magnifying narrow-band imaging endoscopy.
DISCUSSION
The early detection and proper treatment of gastric cancer are closely associated with its cure rate. Gastric endoscopy is globally recognized as the main method for the early diagnosis of gastric cancer. However, it should be noted that early pathological changes such as altered arrangement of the gastric pit are not easily detected by conventional HD-WLE before the cancer progresses into advanced stages. The administration of intravital dyes such as acetic acid or indigo carmine during chromo-endoscopy facilitates the detection of small neoplastic lesions[15,16]. However, chromo-endoscopy is a relatively time-consuming process and is inconvenient for endoscopists. Recent progress in ME and NBI technology has allowed endoscopists to visualize the microsurface pattern and the microvascular architecture of gastric lesions without the administration of dyes[13]. The maximal resolution of ME is 6-9 micrometers. Thus, ME can reveal the configuration of the gastric pit clearly and can identify superficial lesions[13,17]. The NBI technology uses special light filters and reduces white light to two 30-nm-wide spectra corresponding to blue and green light, which creates a pseudo-colored image[18]. This image has enhanced blood vessel contrast relative to the surrounding mucosa[19]. As a result, the microvascular architecture of gastric lesions is clearly revealed and a subsequent target biopsy of the suspected mucosa could be performed with higher accuracy[20]. There are several studies on the diagnostic value of HD-WLE and ME-NBI in EGC screening[17,21-26]. However, the sample size of these studies was small and a comparison between HD-WLE and ME not presented. The present study enrolled a large number of patients from four medical centers and analyzed the statistical data to evaluate the exact diagnostic value of HD-WLE, ME and ME-NBI for EGC.
As mentioned above, progress in the detection of EGC is largely attributed to the development of enhanced endoscopy (including ME, ME-NBI), which enables the endoscopists to visualize the microstructure within superficial gastric lesions. Based on the microsurface and microvascular characteristics and the presence of a demarcation line, Yao et al[13] proposed the vs classification system. In this system, gastric mucosa with an irregular microvascular pattern and a demarcation line and/or an irregular microsurface pattern with a demarcation line could be considered cancer. In the present study, we evaluated the diagnostic effectiveness of different endoscopic modalities according to this criterion.
Our study showed that the specificity and negative predictive value of HD-WLE (both > 97%) are high, and we believe that a further examination is not required if no abnormal mucosa is detected by HD-WLE because such a mucosa is likely to be a noncancerous lesion. HD-WLE also showed a high diagnostic accuracy (97.1%), which means that the endoscopic diagnosis from HD-WLE images is already consistent with the pathological diagnosis. Additionally, HD-WLE is convenient and does not require other special equipment. Thus, we recommend HD-WLE as the first choice for EGC screening.
ME and ME-NBI showed superior diagnostic sensitivity and accuracy to HD-WLE in our study, and the differences are statistically significant. ME and NBI can reveal the morphology of microsurfaces and microvasculature. When used together, these two technologies further facilitate the observation of the microstructures of suspected lesions[27-29]. Moreover, it is known that pathological results instead of endoscopic findings are the gold standard for the diagnosis of gastric carcinoma. As a result, determining which area of the gastric mucosa is most likely to be cancerous and how to perform a subsequent biopsy at the exact target mucosa is critical to the diagnosis of EGC. Therefore, using ME and ME-NBI may increase both the accuracy of locating suspicious mucosa and the positive biopsy rate. Furthermore, early diagnosis of gastric cancer is not the ultimate goal for clinicians. The long-term prognosis of gastric cancer could be improved only if proper and successful endoscopic or surgical resection of the cancerous mucosa is performed. Therefore, it is crucial for endoscopists to delineate the exact margin of gastric lesions. ME-NBI is superior to other endoscopic modalities because it distinguishes the cancerous mucosa from surrounding tissues by revealing the irregular microstructure of gastric lesions[30]. It is then obvious that although conventional HD-WLE could be used as a screening tool for EGC, ME and ME-NBI show great superiority in delineating gastric lesions for subsequent targeted biopsy and endoscopic resection.
There are some limitations of the present study: (1) the data of our study can not be directly applied to real life clinical practice, since the diagnosis of the endoscopic images was obtained by four experienced endoscopists instead of real time evaluation during the process. Moreover, the participants in our study were administered a mixture of medication to eliminate mucus adhering to mucosa, thus achieving perfect conditions for endoscopic observations, which was not commonly used in clinical practice. As a result, the data of our study should be further confirmed in more clinical trials where the differences of HD-WLE, ME and ME-NBI in detecting EGC may appear less relevant or even more significant; (2) since there are no well-recognized diagnostic criteria for precancerous lesions such as low-grade neoplasia, we only analyzed the differences of three endoscopic modalities in recognizing cancerous lesions including high-grade neoplasia and noninvasive gastric cancer, and no data involving precancerous lesions were present; and (3) in the present study, we analyzed the overall diagnostic value for gastric lesions regardless of the specific shape, type and location of these lesions. In order to obtain more statistics as to which subtype of lesions is more easily recognized by some endoscopic modality, further clinical trials are needed.
In conclusion, HD-WLE can remain the first choice of doctors for screening EGC because it is a convenient and effective method of examination. Additionally, HD-WLE has a high diagnostic accuracy compared with pathology. However, ME and ME-NBI show superior sensitivity and accuracy for diagnosing gastric mucosal lesions in early stages. These two endoscopic technologies could enable endoscopists to observe gastric lesions more clearly and identify suspected lesions more accurately. Furthermore, proper identification could facilitate the subsequent endoscopic resection of gastric mucosa if a cancerous lesion is validated by histopathological results.
COMMENTS
Background
Early detection of gastric cancer is crucial to better prognosis. The development of endoscopic modalities such as magnifying endoscopy and magnifying narrow-band imaging endoscopy has made it possible for clinicians to delineate early gastric lesions precisely and thus facilitated further endoscopic resection.
Research frontiers
The current study demonstrated that magnifying endoscopy and magnifying narrow-band imaging endoscopy, which could clearly reveal the microstructure of the gastric mucosa, show superiority to conventional white light endoscopy in recognizing early gastric cancer.
Innovations and breakthroughs
The present study analyzed the recognition for early gastric cancer with a large sample size. Furthermore, the study highly precisely described the diagnostic value of the three endoscopic modalities in Chinese population.
Applications
The study illustrated that enhanced endoscopy exhibits higher sensitivity and accuracy in recognizing early gastric cancer compared with conventional endoscopy. Thus, for patients suspicious of gastric cancer, magnifying endoscopy or magnifying narrow-band imaging endoscopy could better delineate the lesion and facilitate further targeted biopsy and endoscopic resection.
Terminology
Narrow-band imaging is a kind of technology that uses special light filters to create a pseudo-colored image, which enhances blood vessel contrast relative to the surrounding mucosa and clearly reveals the microvascular architecture of gastric lesions.
Peer-review
The field of investigation represents an interesting topic, the manuscript is clear and the authors critically discussed most of the open issues in this field. Moreover, the study population is quite relevant.
Footnotes
Supported by Profession Specific Funded Projects in Standardization of Targeted Therapy and Cell Therapy and Applied Research of Early Diagnosis and Treatment for Cancer from Chinese Ministry of Health, No. 200902002.
Institutional review board statement: The study was reviewed and approved by the Peking Union Medical College Hospital Institutional Review Board.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors report no financial disclosures or conflict of interest.
Data sharing statement: Statistical code and dataset available from the corresponding author at yangaiming@medmail.com.cn. Consent of data sharing for participants was not obtained but the presented data are anonymized and risk of identification is low. No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 13, 2015
First decision: March 26, 2015
Article in press: May 27, 2015
P- Reviewer: Huang CM, Neumann H, Paydas S, Zhang J S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J. 2014;55:621–628. doi: 10.11622/smedj.2014174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oda I, Oyama T, Abe S, Ohnita K, Kosaka T, Hirasawa K, Ishido K, Nakagawa M, Takahashi S. Preliminary results of multicenter questionnaire study on long-term outcomes of curative endoscopic submucosal dissection for early gastric cancer. Dig Endosc. 2014;26:214–219. doi: 10.1111/den.12141. [DOI] [PubMed] [Google Scholar]
- 3.Tanabe S, Ishido K, Higuchi K, Sasaki T, Katada C, Azuma M, Naruke A, Kim M, Koizumi W. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center. Gastric Cancer. 2014;17:130–136. doi: 10.1007/s10120-013-0241-2. [DOI] [PubMed] [Google Scholar]
- 4.Kim YI, Kim YW, Choi IJ, Kim CG, Lee JY, Cho SJ, Eom BW, Yoon HM, Ryu KW, Kook MC. Long-term survival after endoscopic resection versus surgery in early gastric cancers. Endoscopy. 2015;47:293–301. doi: 10.1055/s-0034-1391284. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Oda I, Abe S, Sekiguchi M, Mori G, Nonaka S, Yoshinaga S, Saito Y. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer. 2015:Epub ahead of print. doi: 10.1007/s10120-015-0469-0. [DOI] [PubMed] [Google Scholar]
- 6.Lazăr D, Tăban S, Dema A, Cornianu M, Goldiş A, Raţiu I, Sporea I. Gastric cancer: the correlation between the clinicopathological factors and patients’ survival (I) Rom J Morphol Embryol. 2009;50:41–50. [PubMed] [Google Scholar]
- 7.Choi IJ. Endoscopic gastric cancer screening and surveillance in high-risk groups. Clin Endosc. 2014;47:497–503. doi: 10.5946/ce.2014.47.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14:e535–e547. doi: 10.1016/S1470-2045(13)70436-4. [DOI] [PubMed] [Google Scholar]
- 9.Gotoda T. Endoscopic resection of early gastric cancer: the Japanese perspective. Curr Opin Gastroenterol. 2006;22:561–569. doi: 10.1097/01.mog.0000239873.06243.00. [DOI] [PubMed] [Google Scholar]
- 10.Boeriu A, Boeriu C, Drasovean S, Pascarenco O, Mocan S, Stoian M, Dobru D. Narrow-band imaging with magnifying endoscopy for the evaluation of gastrointestinal lesions. World J Gastrointest Endosc. 2015;7:110–120. doi: 10.4253/wjge.v7.i2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo T, Qian JM, Yang AM. Lu XH. Application of Narrow-Band Imaging (NBI) in gastrointestinal endoscopy. Zhonghua Xiaohua Neijing Zazhi. 2007;24:234–236. [Google Scholar]
- 12.Horiuchi Y, Fujisaki J, Yamamoto N, Shimizu T, Miyamoto Y, Tomida H, Omae M, Ishiyama A, Yoshio T, Hirasawa T, et al. Accuracy of diagnostic demarcation of undifferentiated-type early gastric cancers for magnifying endoscopy with narrow-band imaging: endoscopic submucosal dissection cases. Gastric Cancer. 2015:Epub ahead of print. doi: 10.1007/s10120-015-0488-x. [DOI] [PubMed] [Google Scholar]
- 13.Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009;41:462–467. doi: 10.1055/s-0029-1214594. [DOI] [PubMed] [Google Scholar]
- 14.Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130–131. doi: 10.1136/gut.51.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujiwara S, Yao K, Nagahama T, Uchita K, Kanemitsu T, Tsurumi K, Takatsu N, Hisabe T, Tanabe H, Iwashita A, et al. Can we accurately diagnose minute gastric cancers (≤ 5 mm)? Chromoendoscopy (CE) vs magnifying endoscopy with narrow band imaging (M-NBI) Gastric Cancer. 2015;18:590–596. doi: 10.1007/s10120-014-0399-2. [DOI] [PubMed] [Google Scholar]
- 16.Kono Y, Takenaka R, Kawahara Y, Okada H, Hori K, Kawano S, Yamasaki Y, Takemoto K, Miyake T, Fujiki S, et al. Chromoendoscopy of gastric adenoma using an acetic acid indigocarmine mixture. World J Gastroenterol. 2014;20:5092–5097. doi: 10.3748/wjg.v20.i17.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen WF, Li QL, Zhou PH, Xu MD, Zhang YQ, Zhong YS, Ma LL, Qin WZ, Hu JW, Cai MY, et al. [Clinical value of different magnifying chromoendoscopy methods in screening gastric precancerous lesions and early cancers] Zhonghua Weichang Waike Zazhi. 2012;15:662–667. [PubMed] [Google Scholar]
- 18.Matsuo K, Takedatsu H, Mukasa M, Sumie H, Yoshida H, Watanabe Y, Akiba J, Nakahara K, Tsuruta O, Torimura T. Diagnosis of early gastric cancer using narrow band imaging and acetic acid. World J Gastroenterol. 2015;21:1268–1274. doi: 10.3748/wjg.v21.i4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T, Zheng H, Gong W, Chen C, Jiang B. The accuracy of confocal laser endomicroscopy, narrow band imaging, and chromoendoscopy for the detection of atrophic gastritis. J Clin Gastroenterol. 2015;49:379–386. doi: 10.1097/MCG.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 20.Gheorghe C. Narrow-band imaging endoscopy for diagnosis of malignant and premalignant gastrointestinal lesions. J Gastrointestin Liver Dis. 2006;15:77–82. [PubMed] [Google Scholar]
- 21.Song JY, Li HY, Zhu LY, Chen XY, Ge ZZ. Li XB. The value of target biopsy using magnifying endoscopy combined with narrow band imaging for early gastric malignancy. Zhonghua Xiaohua Neijing Zazhi. 2014;31:455–458. [Google Scholar]
- 22.Gao XZ, Chu YL, Qiao XL, Wang XF, Liu F. Liu J. Narrow band imaging endoscopy in diagnosis of early gastric cancer and dysplasia. Zhonghua Xiaohua Neijing Zazhi. 2009;26:134–137. [Google Scholar]
- 23.Xu L, Liu JY. Combination of magnifying endoscopy and narrow band imaging in diagnosis of early gastric cancer. Zhonghua Xiaohua Neijing Zazhi. 2009;26:415–418. [Google Scholar]
- 24.Li HY, Dai J, Xue HB, Zhao YJ, Chen XY, Gao YJ, Song Y, Ge ZZ, Li XB. Application of magnifying endoscopy with narrow-band imaging in diagnosing gastric lesions: a prospective study. Gastrointest Endosc. 2012;76:1124–1132. doi: 10.1016/j.gie.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Guo T, Lu XH, Zhou WX, Yang AM, Yao F, Wu X, Li Y, Wang LY. Qian JM. Magnifying endoscopy with narrow-band imaging for early gastric cancer diagnosis. Zhonghua Xiaohua Neijing Zazhi. 2011;28:375–379. [Google Scholar]
- 26.Tao G, Xing-Hua L, Ai-Ming Y, Wei-Xun Z, Fang Y, Xi W, Li-Yin W, Chong-Mei L, Gui-Jun F, Hui-Jun S, et al. Enhanced magnifying endoscopy for differential diagnosis of superficial gastric lesions identified with white-light endoscopy. Gastric Cancer. 2014;17:122–129. doi: 10.1007/s10120-013-0250-1. [DOI] [PubMed] [Google Scholar]
- 27.Yamada S, Doyama H, Yao K, Uedo N, Ezoe Y, Oda I, Kaneko K, Kawahara Y, Yokoi C, Sugiura Y, et al. An efficient diagnostic strategy for small, depressed early gastric cancer with magnifying narrow-band imaging: a post-hoc analysis of a prospective randomized controlled trial. Gastrointest Endosc. 2014;79:55–63. doi: 10.1016/j.gie.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video) Endoscopy. 2004;36:1080–1084. doi: 10.1055/s-2004-825961. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Wu J, Lin XC, Wei N, Lin W, Chang H, Du XM. Evaluating the diagnoses of gastric antral lesions using magnifying endoscopy with narrow-band imaging in a Chinese population. Dig Dis Sci. 2014;59:1513–1519. doi: 10.1007/s10620-014-3027-4. [DOI] [PubMed] [Google Scholar]
- 30.Qin X. To enhance the diagnosis of early gastric cancer in China. Zhonghua Xiaohua Neijing Zazhi. 2010;13:9–10. [Google Scholar]


