Abstract
AIM: To evaluate the impact of enhanced recovery after surgery (ERAS) programs in comparison with traditional care on liver surgery outcomes.
METHODS: The PubMed, EMBASE, CNKI and Cochrane Central Register of Controlled Trials databases were searched for randomized controlled trials (RCTs) comparing the ERAS program with traditional care in patients undergoing liver surgery. Studies selected for the meta-analysis met all of the following inclusion criteria: (1) evaluation of ERAS in comparison to traditional care in adult patients undergoing elective open or laparoscopic liver surgery; (2) outcome measures including complications, recovery of bowel function, and hospital length of stay; and (3) RCTs. The following exclusion criteria were applied: (1) the study was not an RCT; (2) the study did not compare ERAS with traditional care; (3) the study reported on emergency, non-elective or transplantation surgery; and (4) the study consisted of unpublished studies with only the abstract presented at a national or international meeting. The primary outcomes were complications. Secondary outcomes were length of hospital stay and time to first flatus.
RESULTS: Five RCTs containing 723 patients were included in the meta-analysis. In 10/723 cases, patients presented with benign diseases, while the remaining 713 cases had liver cancer. Of the five studies, three were published in English and two were published in Chinese. Three hundred and fifty-four patients were in the ERAS group, while 369 patients were in the traditional care group. Compared with traditional care, ERAS programs were associated with significantly decreased overall complications (RR = 0.66; 95%CI: 0.49-0.88; P = 0.005), grade I complications (RR = 0.51; 95%CI: 0.33-0.79; P = 0.003), and hospital length of stay [WMD = -2.77 d, 95%CI: -3.87-(-1.66); P < 0.00001]. Similarly, ERAS programs were associated with decreased time to first flatus [WMD = -19.69 h, 95%CI: -34.63-(-4.74); P < 0.0001]. There was no statistically significant difference in grade II-V complications between the two groups.
CONCLUSION: ERAS is a safe and effective program in liver surgery. Future studies should define the active elements to optimize postoperative outcomes for liver surgery.
Keywords: Enhanced recovery after surgery, Liver surgery, Complications, Hospital length of stay, Meta-analysis
Core tip: To the best of our knowledge, this is the first meta-analysis of randomized controlled trails that have investigated the impact of enhanced recovery after surgery (ERAS) programs on surgical outcomes in liver surgery patients. The implementation of ERAS programs is safe and effective for liver surgery. However, we found some problems, which involved inconsistent outcome measures and ERAS criteria that were not specific to liver surgery. Future research in this field should develop liver surgery-specific ERAS programs.
INTRODUCTION
The enhanced recovery after surgery (ERAS) program, or fast-track surgery (FTS), was first initiated by Kehlet et al[1] in colorectal surgery during the 1990s. In the first ERAS study, the authors demonstrated accelerated recovery, shorter hospital length of stay (LoS) and reduced postoperative morbidity in the ERAS group[2]. Since that study was published, ERAS has been strongly promoted worldwide and has revolutionized the traditional thinking and principles of behavior in the perioperative process developed over the past 100 years. Consequently, the clinical pattern of many diseases has changed. ERAS is characterized by a series of optimization measures grounded in evidence-based medicine during the perioperative period to attenuate the physical and psychological stress responses and complications, and to potentiate postoperative rehabilitation for patients following a variety of surgical procedures[3]. Preoperative education, epidural or regional anesthesia, perioperative fluid management, minimally invasive techniques, optimal pain control, early initiation of oral feeding, and mobilization are some of the hallmarks of ERAS programs[4,5]. In recent years, ERAS protocols have been applied to different types of surgery, including colorectal[6], gastric[7], vascular[8], urologic[9] and gynecologic[10] procedures.
ERAS programs have also been used during hepatic surgery[11,12]. With the improvement of operative techniques and perioperative management, mortality after liver resection surgery has decreased to its current level of 5%. Morbidity rates, however, remain high and range from 15% to 50%[13]. Although a number of studies have evaluated ERAS programs in relation to liver surgery, limited data have precluded proper analysis of their effectiveness. Recently, randomized controlled trials (RCTs) comparing ERAS programs with traditional care in liver surgery patients have been published. We performed a meta-analysis of the published literature to assess the safety and efficacy of ERAS programs in comparison with traditional care in patients undergoing liver surgery for liver cancer. This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[14].
MATERIALS AND METHODS
Publication search
Two reviewers (Han-Teng Yang and Hao Zhang) independently performed a literature search in the PubMed, EMBASE, China National Knowledge Infrastructure (CNKI) and Cochrane Central Register of Controlled Trials databases for all RCTs that assessed the impact of ERAS programs on patients following hepatectomy in comparison to traditional care, which had been published in 1966 through November 8, 2014. We did not apply language restrictions. The following search terms were used: fast track, fast-track, enhanced recovery, liver, hepatic, hepatocellular, hepatectomy, resection, surgery, surgical, randomized controlled trial, randomized, randomly and clinical trial. Synonyms of each of the terms were also used in the search. Abstracts from each of the studies were reviewed. In addition, the references from each of the retrieved articles were manually screened to identify other potential eligible RCTs.
Inclusion and exclusion criteria
Studies selected for the meta-analysis met all of the following inclusion criteria: (1) the study evaluated ERAS in comparison to traditional care in adult patients undergoing elective open or laparoscopic liver surgery; (2) the outcome measures included complications, recovery of bowel function, and hospital LoS; and (3) the study must be an RCT. The following exclusion criteria were applied: (1) the study was not an RCT; (2) the study did not compare ERAS with traditional care; (3) the study reported on emergency, non-elective or transplantation surgery; and (4) the study consisted of unpublished data with only the abstract presented at a national or international meeting. For different publications with overlapping data, the most complete publication was selected. Two authors (Tian-Gen Ni and Bo L) independently assessed the articles for compliance with the inclusion/exclusion criteria, resolved disagreements, and reached a unified decision.
Data extraction
Two researchers (Han-Teng Yang and Hao Zhang) independently reviewed and extracted the following information from each of the studies: first author’s name, publication year, country, total number of cases and controls, age, sex, type of surgery, outcome measures, and number of ERAS program items according to the guidelines established by the ERAS group[15]. The core elements of ERAS include preadmission information and counseling, preoperative bowel preparation, preoperative fasting and carbohydrate loading, preanesthetic medication, prophylaxis against thromboembolism, antimicrobial prophylaxis, standard anesthetic protocol, preventing and treating postoperative nausea and vomiting, laparoscopy-assisted surgery, surgical incisions, nasogastric intubation, preventing intraoperative hypothermia, perioperative fluid management, drainage of the peritoneal cavity, urinary drainage, preventing postoperative ileus, postoperative analgesia, postoperative nutritional care and early mobilization. If there were any disagreements between the two reviewers, a third reviewer (Hai-Peng Meng) was recruited and the issue was discussed until a consensus was achieved. When multiple publications reported on the same or overlapping data, we selected the study with the most complete dataset.
Assessment of risk of bias
The methodology for each RCT was assessed independently by two reviewers (Tian-Gen Ni and Han-Teng Yang) according to the Cochrane Collaboration’s risk of bias tool[16,17], which analyzes the following criteria: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting; and (7) other bias. For each entry based on the risk of bias assessment guidelines, we made a judgment (low risk of bias, high risk of bias, or uncertain). All disagreements were resolved by discussion until a consensus was achieved.
Outcome measures
Complications (defined according to the Dindo-Clavien classification[18]) were the primary outcome measure. Secondary outcome measures included: (1) hospital LoS (defined as the number of days in the hospital after surgery until discharge); and (2) time to first flatus.
Statistical analysis
Meta-analyses were performed by using risk ratios (RRs) for dichotomous outcomes, and weighted mean differences (WMDs) were used for continuous outcomes. Pooled estimates were presented with 95%CIs. If the included studies provided medians and interquartile ranges, we calculated the mean ± SD according to the methods outlined by Hozo et al[19]. When heterogeneity was found to be statistically significant (P < 0.05 or I2 > 50%[20,21]), a random effects model was applied. Otherwise, a fixed effects model was adopted to calculate the pooled RRs or WMDs. Funnel plots were generated to determine the presence of publication bias. When a study presented with significant heterogeneity, sensitivity analyses were performed to assess how inferring standard deviations from medians and interquartile ranges from poor quality studies affected the overall results. We further identified sources of heterogeneity and assessed the robustness and consistency of statistical techniques used. For all other comparisons, statistical significance was defined by P < 0.05 and all tests were two-tailed. All statistical analyses were performed using the Review Manager (RevMan) software, version 5.3 from the Cochrane Collaboration (http://tech.cochrane.org/revman). Some outcomes were not analyzed but instead were presented as descriptive information.
RESULTS
Study characteristics and assessment of bias risk
After the initial literature search, a total of 101 potentially relevant studies were identified. The final meta-analysis, after application of inclusion and exclusion criteria, included five RCTs with a total of 723 patients[22-26]. In 10/723 cases, patients presented with benign diseases, while the remaining 713 cases had liver cancer. Of the five studies, three were published in English and two were published in Chinese. The PRISMA flow diagram is shown in Figure 1. Three hundred and fifty-four patients were in the ERAS group, while 369 patients were in the traditional care group. The sample size for the included studies ranged from 60 to 297 patients. The included RCTs were published between 2012 and 2014 and were conducted solely in adult patients. There were no multicenter trials. Characteristics of each included RCT are presented in Table 1. Each reviewer performed an assessment of risk of bias of each methodological component. The risk of bias summary for the included RCTs is presented in Figure 2.
Figure 1.
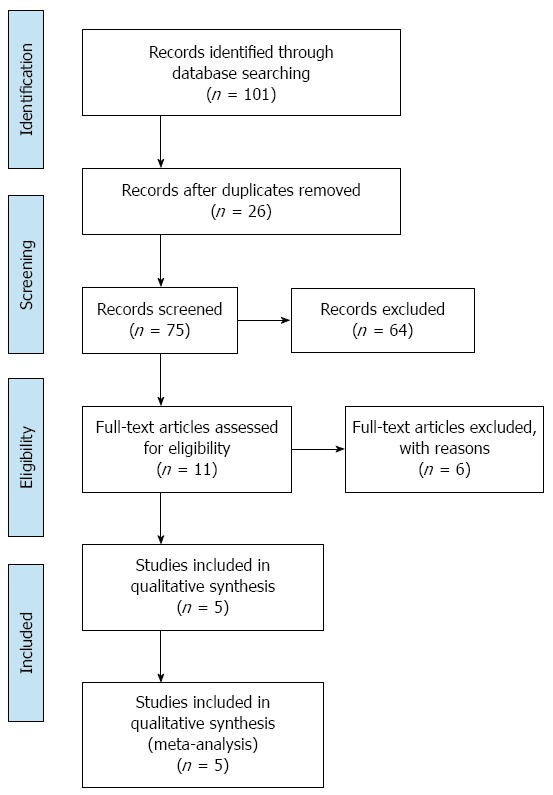
Preferred reporting items for systematic reviews and meta-analyses flow diagram.
Table 1.
Characteristics of included trials in this meta-analysis
| Trials | Year | Country |
No. of patients |
Age in years |
Sex, M/F |
Type of surgery | ERAS items | |||
| ERAS | TC | ERAS | TC | ERAS | TC | |||||
| Jones | 2013 | Britain | 46 | 45 | 64 (27-83) | 67 (27-84) | 31/15 | 23/22 | Open, MR/mR | 19 |
| Ni | 2013 | China | 80 | 80 | 48.4 ± 15.6 | 50.1 ± 21.8 | 66/14 | 59/21 | Open, PH | 12 |
| Chi | 2012 | China | 63 | 52 | Total: 46.5 ± 5.8 | 80/35 | Open, PH/HH | 10 | ||
| Huang | 2013 | China | 30 | 30 | NR | NR | LLR | 14 | ||
| Lu | 2014 | China | 135 | 162 | 54.03 ± 11.4 | 52.55 ± 11.3 | 111/24 | 133/29 | Open, PH | 13 |
ERAS: Enhanced recovery after surgery; HH: Hemihepatectomy; LLR: Laparoscopic liver resection; MR: Major resection (≥ 3 segments); mR: Minor resection (< 3 segments); NR: Not reported; Open: Open surgery; PH: Partial hepatectomy; TC: Traditional care.
Figure 2.
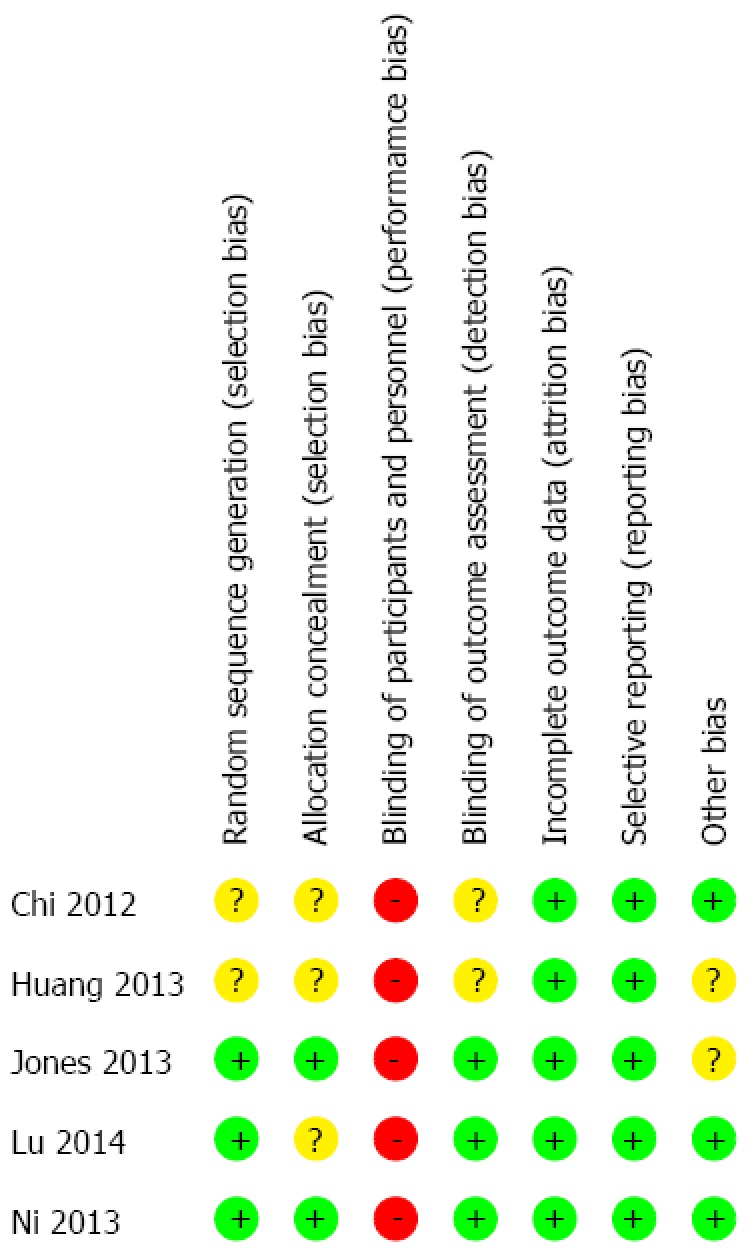
Risk of bias summary in included studies. The symbol of (-) red indicates that there is a high risk of bias, of (+) green indicates a low risk of bias and of (?) yellow indicates uncertainty.
Primary outcome measures
Overall complications were reported in all included studies. Because we did not identify significant heterogeneity among the trials (I2 = 0%, P = 0.93), a fixed-effects model was applied to this meta-analysis. In the ERAS group, there was a significant reduction in overall complications (RR = 0.66, 95%CI: 0.49-0.88; P = 0.005) (Figure 3). Using the Dindo-Clavien classification[18], information regarding grade I complications and grade II-V complications was available from the five studies. In the fixed-effects model, the ERAS group had significantly fewer grade I complications (RR = 0.51, 95%CI: 0.33-0.79; P = 0.003), without heterogeneity among the trials (I2 = 0%, P = 0.44) (Figure 3). However, no differences in grade II-V complications were found between the ERAS and traditional care groups (RR = 0.94, 95%CI: 0.58-1.52; P = 0.80), without heterogeneity among the trials (I2 = 0%, P = 0.58) (Figure 3).
Figure 3.
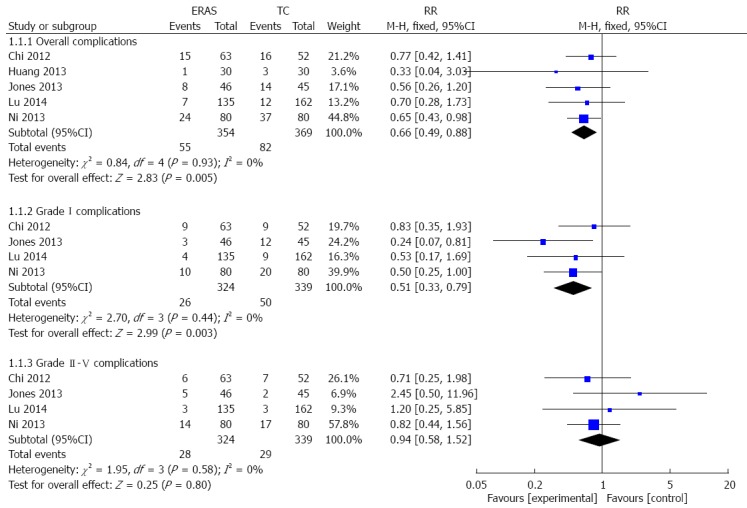
Forest plot of enhanced recovery after surgery programs vs traditional care for patient complications. ERAS: Enhanced recovery after surgery; TC: Traditional care.
Secondary outcome measures
In all included RCTs, hospital LoS was reported and was significantly shorter for the ERAS group than the traditional care group [WMD = -2.97 d, 95%CI: -3.18-(-2.76); P < 0.00001]. There was, however, significant heterogeneity among the trials (I2 = 91%, P < 0.00001). Due to this heterogeneity, a random effects model was applied to the studies. This analysis demonstrated that the ERAS group had a shorter hospital LoS [WMD = -2.77 d, 95%CI: -3.87-(-1.66); P < 0.00001] (Figure 4A). After one study was excluded[24] due to standard deviations from medians and interquartile ranges, a sensitivity analysis did not substantially change the results of the original analysis.
Figure 4.
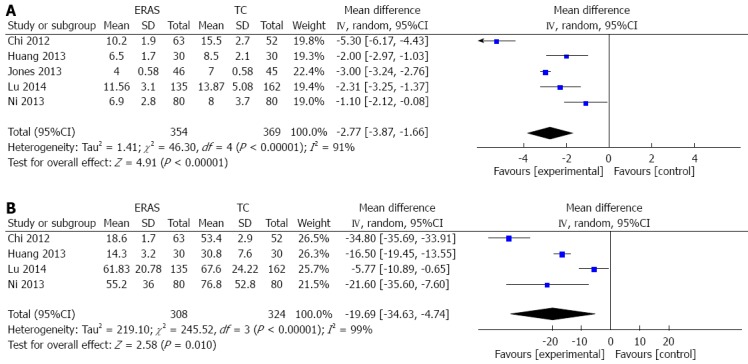
Forest plots of enhanced recovery after surgery programs vs traditional care for hospital length of stay (A) and time to first flatus (B). ERAS: Enhanced recovery after surgery; TC: Traditional care.
Time to first flatus was reported in 4/5 studies. The heterogeneity among the trials was significant (I2 = 99%, P < 0.00001), thus a random effects model was applied. In the ERAS group, time to first flatus was significantly reduced in comparison to those undergoing traditional care [WMD = -19.69 h, 95%CI: -34.63-(-4.74); P < 0.0001] (Figure 4B).
Publication bias
Because less than 10 studies were included in this meta-analysis, we did not evaluate the publication bias for ERAS programs in patients undergoing liver surgery. In accordance with the guidelines established by the Cochrane Handbook for Systematic Reviews, the test for publication bias is unreliable when less than 10 studies are included in a meta-analysis[27].
DISCUSSION
The aim of this meta-analysis was to evaluate the effects of ERAS programs in comparison to traditional care on patient recovery after liver surgery. Although two reviews previously concluded that ERAS programs showed lower complication rates and shorter hospital LoS in patients undergoing liver surgery[28,29], these reviews predominantly included controlled clinical trials or case-control studies. Therefore, the results of the previous analyses may not be strong enough due to a lack of sufficient data and/or the limited quality of the clinical trials. Because additional RCTs comparing ERAS to traditional care have been published since the previous two studies, the present study was warranted. This meta-analysis was performed according to the PRISMA statement[14] and the results suggest that implementation of ERAS programs is safe and effective in liver surgery.
Compared with traditional care, ERAS programs result in a significant reduction in overall complications, grade I complications and time to first flatus. Moreover, the hospital LoS was shortened, which likely indicates a reduction in associated hospital costs.
ERAS programs in colon surgery were first initiated in 1997 by Kehlet[3]. Over the past 17 years, ERAS programs have received worldwide attention in patient care, particularly after successful implementation and promotion in the field of colorectal surgery, which demonstrated its feasibility and superiority in clinical applications. Several studies have reported that ERAS programs significantly reduce both postoperative hospital LoS and hospital cost, without increasing the readmission rate, recurrence rate or mortality[30,31]. In 2005 and 2009, consensus guidelines of ERAS programs were developed and modified by a collective of colorectal surgeons[15,32]. Despite the development of these guidelines, ERAS programs have not been implemented as a standard care in many other surgical fields. After MacKay et al[33] published their initial ERAS protocol for liver resection, the majority of more recent studies were either observational[12,34] or contained limited RCTs[11]. Few studies have compared ERAS programs with traditional care in liver surgery patients. Moreover, principles of perioperative ERAS and outcome measures have primarily been informed by literature on colorectal surgery. Because features like the patient’s physical condition, liver background, surgical complexity and postoperative residual liver function, are unique to liver surgery patients, liver surgery-specific programs should be developed to optimize ERAS protocols and outcome measures.
Although this meta-analysis included only RCTs and resulted from a rigorous search strategy with detailed inclusion and exclusion criteria, there are still limitations. For example, some studies[22,23,25] did not provide adequate statements regarding their random sequence generation methods and allocation concealment, which could lead to selection bias. Second, the nature of the surgical research often precludes blinding of personnel and participants in the RCT, which leads to an increased risk for both performance and measurement bias. Factors such as differences in basic patient characteristics, each study’s inclusion and exclusion criteria, and the personal experience of the surgeon may also affect, to a certain extent, the stability of results. Finally, the subjective nature of the chosen endpoint and variation in data reported for outcome measures (e.g., “time to flatus” and “length of stay”) suggest an underlying imprecision in the reporting of both of these outcomes, which can result in heterogeneity.
In conclusion, the results from our meta-analysis confirm that the implementation of ERAS programs is both safe and effective in hepatectomy performed for liver cancer. ERAS reduces overall complication rates, accelerates postoperative recovery and shortens hospital LoS without increasing surgical complication rates. Future studies should determine which components of ERAS are most effective for improving outcomes in liver surgery patients.
COMMENTS
Background
Enhanced recovery after surgery (ERAS) programs have been successfully implemented in different surgical fields to improve postoperative outcomes. Despite a number of studies evaluating ERAS programs in liver surgery, their safety and effectiveness have not been systematically evaluated.
Research frontiers
In recent years, ERAS programs have been successfully implemented in colorectal, gastric and gynecologic surgical procedures. ERAS programs have also been used for hepatic surgery. This meta-analysis was performed to evaluate the safety and effectiveness of ERAS programs on liver surgery outcomes. The outcome measures in this study included complications, hospital length of stay, and the time to first flatus.
Innovations and breakthroughs
The present meta-analysis indicates that ERAS programs are safe and effective in liver surgery. ERAS reduces overall complication rates, accelerates postoperative recovery, and shortens hospital length of stay, without increasing surgical complication rates. Inconsistent and subjective outcome measures and ERAS programs that were not specific to the livery surgery field still represent limitations. Future studies should develop ERAS protocols and outcome measures optimized for the liver surgery field.
Applications
The results of this meta-analysis demonstrated the enhanced clinical effectiveness of ERAS programs in comparison to traditional care in liver surgery. ERAS programs result in a significant reduction in complications and recovery of intestinal function. Because the hospital length of stay is shortened after ERAS implementation, the authors infer that there is also a simultaneous reduction in associated hospital costs. Thus, the implementation of ERAS programs contributes to rapid postoperative recovery in liver surgery patients.
Peer-review
In this meta-analysis of randomized controlled trials, the authors found a positive impact of ERAS programs on liver surgery outcomes when compared with traditional care. This study was well conducted and meets all of the standard requirements for a meta-analysis. The study results are clear, reliable and clinically relevant. The findings from this study are novel and applicable to a wide readership audience.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interest.
Data sharing statement: Technical appendix, statistical code, and datasets are available from the corresponding author at libo14@126.com.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 22, 2015
First decision: April 23, 2015
Article in press: June 10, 2015
P- Reviewer: Kadusevicius E, Midha T, Zhang Q S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Kehlet H, Slim K. The future of fast-track surgery. Br J Surg. 2012;99:1025–1026. doi: 10.1002/bjs.8832. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189–198. doi: 10.1097/SLA.0b013e31817f2c1a. [DOI] [PubMed] [Google Scholar]
- 3.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606–617. doi: 10.1093/bja/78.5.606. [DOI] [PubMed] [Google Scholar]
- 4.Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ. 2001;322:473–476. doi: 10.1136/bmj.322.7284.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grade M, Quintel M, Ghadimi BM. Standard perioperative management in gastrointestinal surgery. Langenbecks Arch Surg. 2011;396:591–606. doi: 10.1007/s00423-011-0782-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29:434–440. doi: 10.1016/j.clnu.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Kim JW, Kim WS, Cheong JH, Hyung WJ, Choi SH, Noh SH. Safety and efficacy of fast-track surgery in laparoscopic distal gastrectomy for gastric cancer: a randomized clinical trial. World J Surg. 2012;36:2879–2887. doi: 10.1007/s00268-012-1741-7. [DOI] [PubMed] [Google Scholar]
- 8.Muehling B, Schelzig H, Steffen P, Meierhenrich R, Sunder-Plassmann L, Orend KH. A prospective randomized trial comparing traditional and fast-track patient care in elective open infrarenal aneurysm repair. World J Surg. 2009;33:577–585. doi: 10.1007/s00268-008-9892-2. [DOI] [PubMed] [Google Scholar]
- 9.Kirsh EJ, Worwag EM, Sinner M, Chodak GW. Using outcome data and patient satisfaction surveys to develop policies regarding minimum length of hospitalization after radical prostatectomy. Urology. 2000;56:101–106; discussion 106-107. doi: 10.1016/s0090-4295(00)00594-x. [DOI] [PubMed] [Google Scholar]
- 10.Santillan A, Govan L, Zahurak ML, Diaz-Montes TP, Giuntoli RL, Bristow RE. Feasibility and economic impact of a clinical pathway for pap test utilization in Gynecologic Oncology practice. Gynecol Oncol. 2008;109:388–393. doi: 10.1016/j.ygyno.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Hendry PO, van Dam RM, Bukkems SF, McKeown DW, Parks RW, Preston T, Dejong CH, Garden OJ, Fearon KC. Randomized clinical trial of laxatives and oral nutritional supplements within an enhanced recovery after surgery protocol following liver resection. Br J Surg. 2010;97:1198–1206. doi: 10.1002/bjs.7120. [DOI] [PubMed] [Google Scholar]
- 12.van Dam RM, Hendry PO, Coolsen MM, Bemelmans MH, Lassen K, Revhaug A, Fearon KC, Garden OJ, Dejong CH. Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg. 2008;95:969–975. doi: 10.1002/bjs.6227. [DOI] [PubMed] [Google Scholar]
- 13.Virani S, Michaelson JS, Hutter MM, Lancaster RT, Warshaw AL, Henderson WG, Khuri SF, Tanabe KK. Morbidity and mortality after liver resection: results of the patient safety in surgery study. J Am Coll Surg. 2007;204:1284–1292. doi: 10.1016/j.jamcollsurg.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, von Meyenfeldt MF, Fearon KC, Revhaug A, Norderval S, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144:961–969. doi: 10.1001/archsurg.2009.170. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J, Green S. The Cochrane Collaboration; 2011. Cochrane Collaboration: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. 0 [updated March 2011]. [Google Scholar]
- 17.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37:256–266. [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chi MH, YY Ceng, JF Liu. Fast-track Surgical Treatment of Liver Cancer Patients through Liver Resection with in the Perioperative Period. Linchuang Zhongliuxue Zazhi. 2012;39:1939–1942. [Google Scholar]
- 23.Huang H, HC Zhang, SF Mo, KJ Wang, Q Li, XH Tan. The application of fast track surgery in laparoscopic precise liver resection. Zhongguo Neijing Zazhi. 2013;19:603–606. [Google Scholar]
- 24.Jones C, Kelliher L, Dickinson M, Riga A, Worthington T, Scott MJ, Vandrevala T, Fry CH, Karanjia N, Quiney N. Randomized clinical trial on enhanced recovery versus standard care following open liver resection. Br J Surg. 2013;100:1015–1024. doi: 10.1002/bjs.9165. [DOI] [PubMed] [Google Scholar]
- 25.Lu H, Fan Y, Zhang F, Li G, Zhang C, Lu L. Fast-track surgery improves postoperative outcomes after hepatectomy. Hepatogastroenterology. 2014;61:168–172. [PubMed] [Google Scholar]
- 26.Ni CY, Yang Y, Chang YQ, Cai H, Xu B, Yang F, Lau WY, Wang ZH, Zhou WP. Fast-track surgery improves postoperative recovery in patients undergoing partial hepatectomy for primary liver cancer: A prospective randomized controlled trial. Eur J Surg Oncol. 2013;39:542–547. doi: 10.1016/j.ejso.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and related biases. Health Technol Assess. 2000;4:1–115. [PubMed] [Google Scholar]
- 28.Coolsen MM, Wong-Lun-Hing EM, van Dam RM, van der Wilt AA, Slim K, Lassen K, Dejong CH. A systematic review of outcomes in patients undergoing liver surgery in an enhanced recovery after surgery pathways. HPB (Oxford) 2013;15:245–251. doi: 10.1111/j.1477-2574.2012.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes MJ, McNally S, Wigmore SJ. Enhanced recovery following liver surgery: a systematic review and meta-analysis. HPB (Oxford) 2014;16:699–706. doi: 10.1111/hpb.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li MZ, Xiao LB, Wu WH, Yang SB, Li SZ. Meta-analysis of laparoscopic versus open colorectal surgery within fast-track perioperative care. Dis Colon Rectum. 2012;55:821–827. doi: 10.1097/DCR.0b013e31824bd31e. [DOI] [PubMed] [Google Scholar]
- 31.Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, Gerhards MF, van Wagensveld BA, van der Zaag ES, van Geloven AA, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study) Ann Surg. 2011;254:868–875. doi: 10.1097/SLA.0b013e31821fd1ce. [DOI] [PubMed] [Google Scholar]
- 32.Fearon KC, Ljungqvist O, Von Meyenfeldt M, Revhaug A, Dejong CH, Lassen K, Nygren J, Hausel J, Soop M, Andersen J, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24:466–477. doi: 10.1016/j.clnu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 33.MacKay G, O’Dwyer PJ. Early discharge following liver resection for colorectal metastases. Scott Med J. 2008;53:22–24. doi: 10.1258/rsmsmj.53.2.22. [DOI] [PubMed] [Google Scholar]
- 34.Lin DX, Li X, Ye QW, Lin F, Li LL, Zhang QY. Implementation of a fast-track clinical pathway decreases postoperative length of stay and hospital charges for liver resection. Cell Biochem Biophys. 2011;61:413–419. doi: 10.1007/s12013-011-9203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


