Abstract
This study tested the hypothesis that coronary artery disease (CAD) alters the cortical circuitry associated with exercise. Observations of changes in heart rate (HR) and in cortical blood oxygenation level-dependent (BOLD) images were made in 23 control subjects [control; 8 women; 63 ± 11 yr; mean arterial pressure (MAP): 90 ± 9 mmHg] (mean ± SD) and 17 similarly aged CAD patients (4 women; 59 ± 9 yr; MAP: 87 ± 10 mmHg). Four repeated bouts each of 30%, 40%, and 50% of maximal voluntary contraction (MVC) force (LAB session), and seven repeated bouts of isometric handgrip (IHG) at 40% MVC force (fMRI session), were performed, with each contraction lasting 20 s and separated by 40 s of rest. There was a main effect of group (P = 0.03) on HR responses across all IHG intensities. Compared with control, CAD demonstrated less task-dependent deactivation in the posterior cingulate cortex and medial prefrontal cortex, and reduced activation in the right anterior insula, bilateral precentral cortex, and occipital lobe (P < 0.05). When correlated with HR, CAD demonstrated reduced activation in the bilateral insula and posterior cingulate cortex, and reduced deactivation in the dorsal anterior cingulate cortex, and bilateral precentral cortex (P < 0.05). The increased variability in expected autonomic regions and decrease in total cortical activation in response to the IHG task are associated with a diminished HR response to volitional effort in CAD. Therefore, relative to similarly aged and healthy individuals, CAD impairs the heart rate response and modifies the cortical patterns associated with cardiovascular control during IHG.
Keywords: cortical autonomic network, coronary artery disease, handgrip exercise, compensation hypothesis
coronary artery disease (CAD) increases risk of stroke, cognitive impairment, and autonomic dysregulation (Barekatain et al. 2014; Martins et al. 2006; Roberts et al. 2010; Zulli et al. 2008). In turn, impaired autonomic outcomes of CAD include diminished parasympathetic modulation of heart rate (HR) (Ford 1999; Mancia et al. 1991; Seals et al. 1994). Moreover, adverse outcomes in autonomic cardiovascular control may exacerbate the disease pattern through tissue damage and diminished ability to affect rapid HR adjustments in response to stress and, thereby, limit the benefits that can be derived from exercise rehabilitation.
A role for the forebrain and brain stem in cardiac autonomic function has been established in both experimental studies in rodents (Cechetto and Chen 1990; Yasui et al. 1991) and clinical studies in patients with stroke or epileptic seizures in the prefrontal cortex (Cheung and Hachinski 2000). Recently, neuroimaging techniques have enabled investigation into a network of cortical regions associated with the autonomic nervous system and cardiovascular control in conscious humans (Basnayake et al. 2012; Cechetto 2014; Critchley et al. 2000; Gianaros et al. 2004; Macey et al. 2012; Norton et al. 2013; Shoemaker et al. 2015; Williamson 2010). These regions include the bilateral insular cortexes (IC), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), thalamus, medial prefrontal cortex (MPFC), and hippocampus (HC). Importantly, the combined results indicate close homology between cortical sites identified experimentally in lower animals and those observed in humans (Cechetto 2014). These experimental studies indicate that the IC, MPFC, and HC are of particular relevance to HR control (Burns and Wyss 1985; Cechetto and Saper 1990; Fisk and Wyss 1997; Oppenheimer et al. 1992; Owens and Verberne 2001; Ruggiero et al. 1987; Verberne 1996; Yasui et al. 1991). Anatomically, the MPFC and HC have a large number of direct connections with subcortical structures (Verberne and Owens 1998) and have been linked in connectivity analyses of functional magnetic resonance imaging data in humans (Norton et al. 2013). Thus the MPFC and HC form regions of interest for the current study.
Despite the mounting clinical evidence that vagal activity is an important predictor of cardiovascular prognosis in humans (Curtis and O'Keefe Jr. 2002), data are limited regarding the impact of CAD on the brain-heart connection. Volitional isometric handgrip (IHG) contractions offer a unique opportunity to explore the cortical representation of autonomic cardiac control. More specifically, moderate-intensity IHG exercise of short duration produces a rapid tachycardia in young and healthy individuals (Mancia et al. 1978; Mark et al. 1985; Wong et al. 2007) and pharmacological evidence indicates that a decrease in parasympathetic dominance accounts for much of this rapid HR change (Fagraeus and Linnarsson 1976; Hollander and Bouman 1975; Mitchell et al. 1989). In young individuals, the magnitude of this rapid increase in HR with IHG exercise is correlated with reduced activity within the MPFC (Gianaros et al. 2004; Wong et al. 2007) and the HC (Norton et al. 2013). It follows that these regions are associated with cardiovagal control.
The purpose of this study was to test the hypothesis that CAD impairs HR responses to volitional handgrip and that such impairment is related to dysregulation of the cortical autonomic network associated with HR control, particularly emphasizing activity patterns within the MPFC and HC.
METHODS
Participants.
A total of 40 individuals participated in this study. Observations were made in 17 patients with coronary artery disease (CAD) and 23 similarly aged healthy control subjects (control). Anthropometric and baseline cardiovascular data for each group are provided in Table 1. Control subjects were nonsmokers, free of any medications, and did not have diagnosed hypertension, vascular disease, or diabetes. CAD patients were recruited from the London Health Sciences Centre for Cardiac Rehabilitation and Secondary Prevention Program following recent diagnosis of one of the following: admission for acute coronary syndrome (ST elevation or non ST elevation myocardial infarction), angina, percutaneous coronary intervention, or coronary artery bypass graft. Thirteen of the patients were considered to be in functional Class I (as described by the New York Heart Association Functional Classification of heart failure), and four in functional Class II. Drug therapy included cholesterol-lowering statins (94%), beta-blockers (94%), ACE-inhibitors/angiotensin II receptor blockers (82%), calcium channel blockers (18%), diuretics (6%), and anti-platelets including aspirin (94%). Patients were excluded if they had uncontrolled hypertension or a history of diabetes for more than 5 years. Both CAD patients and controls were free of any neurological condition or disease. Each participant provided informed, written consent before participating in the study, which was approved by The University of Western Ontario Health Sciences Ethics Review Board and adhered to the Declaration of Helsinki.
Table 1.
Anthropometric and baseline cardiovascular data during baseline and isometric handgrip exercise
| Group | Age | Sex | MAP, mmHg | MVC, mV | Resting HR, beats/min | 40% ΔHR (LAB) | 40% ΔHR (fMRI) | 40% RPE | Stress Test ΔHR (LAB) |
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 23) | 63 ± 11 | 15M, 8F | 90 ± 9 | 62 ± 28 | 58 ± 8 | 4 ± 2 | 2 ± 2 | 13 ± 2 | 105 ± 16 |
| CAD (n = 17) | 59 ± 9 | 13M, 4F | 87 ± 10 | 63 ± 31 | 59 ± 5 | 3 ± 2 | 3 ± 2 | 11 ± 2* | 78 ± 24* |
Values are means ± SD. Control, healthy older controls; CAD, coronary artery disease patients; MAP, mean arterial pressure; MVC, maximal voluntary contraction (average of LAB + fMRI sessions); HR, heart rate (beats/min); fMRI, neuroimaging session; LAB, physiological recording session; Stress test, voluntary maximal exertion. Borg rate of perceived exertion (RPE) scale: 6–20. RPE = 11, “light” exercise; 13, “somewhat hard.” There was a main effect of group such that CAD patients had less of a HR response during all conditions than control.
Different from control (P < 0.05).
Experimental design.
Participants completed two separate experimental sessions: 1) physiological recording (LAB session) and 2) a functional magnetic resonance neuroimaging session (fMRI; Robarts Research Institute Centre for Functional and Metabolic Imaging). The sessions were performed at the same time of day and separated by a minimum period of 1 wk. Participants were familiarized with the experimental procedures prior to their first test session. Participants were instructed to arrive at the laboratory following a 12-h fast and to refrain from nicotine, alcohol, caffeine, and intense physical exertion for the same duration. Each session began with a maximal voluntary contraction (MVC) handgrip calibration, in which the participant was instructed to squeeze a nonmagnetic handgrip device connected in series to a pressure transducer (Edwards Lifesciences, PX272, Irvine CA) to their maximal ability while in the supine position. This was repeated twice with the larger value calibrated as 100%. Isometric handgrip (IHG) exercise was performed with the right hand in all subjects, regardless of handedness (n = 37 right-handed). During each recording session, visual feedback was provided to the participant of their achieved force in real time. Baseline data were collected over 5 min of quiet supine rest. Four repeated bouts each of 30%, 40%, and 50% of MVC force (LAB session) and seven repeated bouts of IHG at 40% MVC force (fMRI session) were performed, with each contraction lasting 20 s and separated by 40 s of rest. The number of trials was increased in the fMRI session to increase the signal-to-noise ratio. The level of perceived exertion produced by the exercise was monitored after each trial on a scale from 6 to 20 (Borg 1982).
Cardiorespiratory fitness test.
Breath-by-breath measurements of oxygen consumption (V̇o2), HR, and blood pressure (BP) were recorded throughout the test. Maximal oxygen consumption (V̇o2 max) is an established marker of cardiorespiratory fitness and a clinically accepted surrogate marker for left ventricular function (Fletcher et al. 2001). Each subject's V̇o2 max was estimated from a graded treadmill exercise test to volitional exhaustion under standard clinical observation (ACSM 1995).
Physiological recording session.
During the LAB session, HR was monitored by standard 3-lead electrocardiogram (ECG) techniques. Arterial BP was measured continuously from the finger of the nonexercising left hand, maintained at heart level, by photoplethysmography (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands). The BP readings recorded from the Finometer were corrected against sphygmomanometrically obtained systolic (SBP) and diastolic (DBP) pressures that were made intermittently during data collection.
Physiological data analysis.
Analog signals were sampled at 1,000 Hz with an online data-acquisition and analysis system (PowerLab, ADInstruments, Mountain View, CA). HR was calculated from successive R-R intervals obtained from the ECG signal. BP from the Finometer was converted to mean arterial pressure (MAP) using the formula MAP = 1/3 SBP + 2/3 DBP. Beat-by-beat HR data were averaged over 2.5-s bins (the TR interval for functional scans) and time aligned to ensure a corresponding mean value for each functional scan obtained during the fMRI collection period. The HR response (ΔHR) to the IHG was determined by averaging the response over the last 10 s of each rest and IHG interval. HR responses for each participant were averaged over the four repeated blocks in the three separate trials (30%, 40%, and 50%).
The effect of group and IHG intensity on HR response was assessed using a two-way mixed ANOVA with an alpha level of P < 0.05. Statistical analyses were performed using SigmaPlot (version 12.5, 2011). The Shapiro-Wilk test for normal distribution, as well as the Holm-Sidak method for pairwise multiple comparisons, was used. All data are presented as means ± SD.
Neuroimaging recording session.
All imaging data were collected using a whole body 3-T imaging system (Magnetom Prisma, Siemens Medical Solutions, Erlangen, Germany) with a 32-channel head coil (Barberi et al. 2000). A high-resolution T1-weighted structural volume was acquired with a 3D MPRAGE sequence at the beginning of the scanning session (sagittal, matrix 256 × 240 mm, voxel resolution 1.0 × 1.0 × 1.0 mm, 1 mm slice thickness, no gap, flip angle 9°, TE = 2.98 ms, TI = 900 ms, TR = 2.3 ms). Transmission and detection of the blood oxygen level-dependent (BOLD) contrast signal were acquired by T2-weighted gradient echo-echo planar imaging pulse sequence with the following parameters: TE = 30 ms; FOV = 240 × 240 mm, flip angle = 90°. Forty-five interleaved axial slices (3.0 × 3.0 mm in-plane voxel resolution, TR = 2.5 s) were acquired in each volume. Five volumes were acquired in the resting participant prior to actual data collection to allow for magnetization equilibrium; these were discarded prior to data analysis. Head movement was limited during the experimental session within a head cradle packed with foam padding, and each subject was instructed to avoid head movements during the scanning period. Beat-by-beat HR was calculated from the continuous signal derived from an MRI-compatible pulse oximeter (Nonin Medical, 8600FO MRI, Plymouth, MN) placed over the index finger of the nonexercising left hand. In each session, analog signals for pulse recordings and IHG contraction force were sampled at 1,000 Hz with an online data-acquisition and analysis system (PowerLab, ADInstruments, Mountain View, CA). Respiratory frequency was monitored continuously to prevent Valsalva maneuvers during the exercise period.
Neuroimaging data analysis.
The HR response (ΔHR) to the handgrip was determined by averaging the response over the last 10 s of each rest and IHG interval. Individual HR time courses were determined using 2.5-s averages of the beat-by-beat HR measures to generate time-aligned data with the BOLD imaging acquisition. For both the ΔHR and the HR time course, responses for each participant were averaged over the seven repeated blocks at 40% MVC.
All fMRI data were analyzed using Brain Voyager QX 2.8.2 (Brain Innovation, Maastricht, Netherlands) (Goebel et al. 2006). At the first (individual) level, preprocessing included interscan slice acquisition time correction, linear trend removal, temporal high-pass filtering to remove low-frequency drifts, and rigid-body transformation of data to the first acquired image to correct for motion. Individual functional data were coregistered to their respective anatomical template, and subsequently transformed to Talairach space (Talairach and Tournoux 1988). The change in BOLD signal over the exercise period was modeled with a boxcar function convolved with a canonical hemodynamic response function and regressed with the individual movement parameters generated during preprocessing. This resulted in subject-specific contrast images containing whole brain information related to sites of both increased and decreased BOLD signal, relative to baseline, during the IHG task as a function of the task itself and the individual HR correlation. The General Linear Model was used to calculate the parameter estimates for all brain voxels (Friston et al. 1995).
To make valid population inferences, a second-level, two-group, random effects (RFX) analysis was performed both in response to the task and the HR regression to assess the consistency of effects between individuals based on the variability of the first-level estimates across subjects. Subsequently, a subtraction analysis of the group mean parameter estimates was performed to assess significant differences between control and CAD groups. Corrections for multiple comparisons were made using the false discovery rate (P < 0.05), as well as cluster level threshold estimation (Hagler Jr. et al. 2006), with 1,000 iterations of Monte Carlo simulation and a statistical threshold of P < 0.05 for the main task effects. Due to the abundance of neural activity, both corrections were performed sequentially, such that the final results represent only clusters > 10 voxels in size (unless otherwise specified). Based on earlier data in young individuals performing the same IHG protocol (Norton et al. 2013; Wong et al. 2007), an a priori region-of-interest analysis was performed for relevant cortical autonomic network regions including the bilateral insular cortices (IC), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), thalamus, medial prefrontal cortex (MPFC), and hippocampus (HC). All fMRI data are represented in radiological convention (i.e., subject's right appears on the left).
Probabilistic functional maps were created for each group to investigate the spatial consistency of activation patterns across subjects. These maps represent the relative number of subjects leading to significant task activity at each spatial location.
RESULTS
Physiological responses.
The groups were not statistically different in age, mean arterial pressure, resting HR, or maximal handgrip strength (Table 1). The heart rate response (ΔHR) to the 40% MVC contraction was the same during the physiological (LAB) and neuroimaging (fMRI) session in both groups (Table 1). There was a significant effect of group (P = 0.03) on ΔHR across all IHG tasks (Fig. 1). None of the participants reported feeling any significant degree of aversive emotional stress or forearm fatigue as indicated by the Borg scale outcomes (Table 1) (Borg 1982). Further, the ΔHR at maximal exertion (stress test) in control participants was greater than CAD patients (Table 1, P < 0.001). All patients exercised to maximum effort (control 19 ± 1, CAD 19 ± 1 on 6–20 Borg Scale), and the tests were not discontinued due to medical reasons (angina, ST depression, arrhythmia, or abnormal BP response). In addition, left ventricular ejection fraction (LVEF) was normal (≥50%) in 11/17 patients, mild (35–49%) in 3/17 patients, moderate (20–34%) in 2/17 patients, and severe (<20%) in one patient.
Fig. 1.
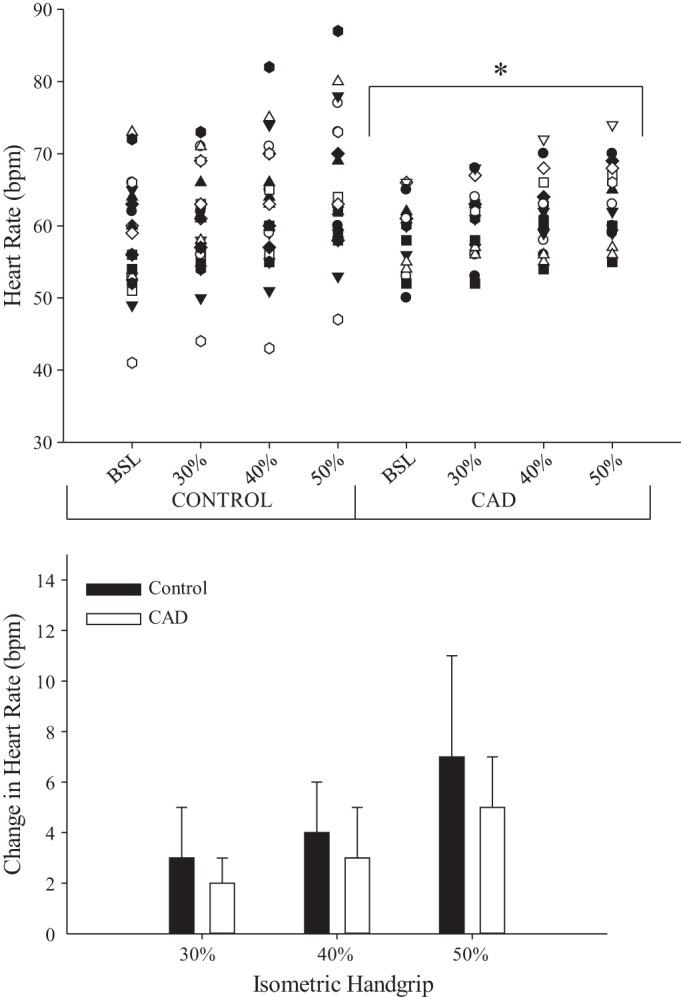
Graded heart rate response to isometric handgrip (IHG) for control and coronary artery disease patients (CAD) represented as individuals (top) and as a group (bottom). BSL, baseline. *Different from control; main effect of group (P < 0.05).
Functional (BOLD) imaging data: first-level (individual) response to 40% IHG task.
A priori region-of-interest analysis revealed high intersubject variability in both groups, with bilateral IC activation observed in 22/23 control subjects and 15/17 CAD patients, ACC deactivation observed in 17/23 control and 14/17 CAD, PCC deactivation observed in 18/23 control and 16/17 CAD, thalamus activation observed in 20/23 control and 7/17 CAD, MPFC deactivation observed in 16/23 control and 12/17 CAD, and HC deactivation observed in 8/23 control and 8/17 CAD [P < 0.05, false discovery rate (FDR)].
Functional (BOLD) imaging data: first-level (individual) response correlated with heart rate.
Bilateral IC activation was observed in 18/23 control subjects and 13/17 CAD patients, ACC deactivation was observed in 11/23 control and 5/17 CAD, PCC deactivation was observed in 5/23 control and 2/17 CAD, thalamus activation was observed in 16/23 control and 11/17 CAD, MPFC deactivation was observed in 16/23 control and 12/17 CAD, and HC deactivation was observed in 2/23 control and 2/17 CAD (P < 0.05, FDR).
Functional (BOLD) imaging data: second-level (group) response to 40% IHG task.
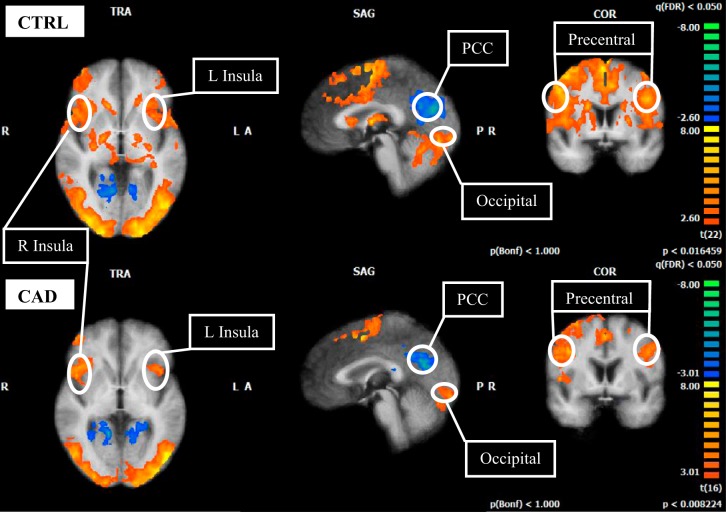
A priori region-of-interest analysis revealed common increases in BOLD signal in the primary motor cortex (precentral cortex), bilateral anterior IC and occipital lobe (Tables 2 and 3; Fig. 2). In addition, common deactivation was observed in the PCC (P < 0.05, FDR; Tables 2 and 3, Fig. 2). In control subjects, activation was observed in the ACC and thalamus, and deactivation was observed in the HC. No signal change was observed in the HC of the CAD group. No signal change was observed in the MPFC in either control or CAD patients at the group level.
Table 2.
BOLD signal changes to 40% MVC handgrip in control subjects
| Coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Location | Side | x | y | z | T-Score | Number of Voxels | |
| Insula | (↑) | L | −38 | 22 | 9 | 4.39 | 986 |
| Insula | (↑) | R | 31 | 23 | 9 | 5.44 | 1,000 |
| Dorsal ACC | (↑) | R | 5 | 40 | 14 | 2.93 | 398 |
| Mid-superior CC | (↑) | R | 1 | −1 | 30 | 3.78 | 766 |
| PCC | (↓) | R | 3 | −50 | 20 | −3.05 | 363 |
| Precentral gyrus | (↑) | L | −31 | −6 | 50 | 4.03 | 809 |
| Precentral gyrus | (↑) | R | 31 | −6 | 50 | 6.24 | 1,000 |
| Postcentral gyrus | (↑) | R | 31 | −27 | 50 | 6.61 | 1,000 |
| Thalamus | (↑) | L | −9 | −17 | 12 | 4.18 | 935 |
| Thalamus | (↑) | R | 8 | −17 | 12 | 4.61 | 896 |
| Hippocampus | (↓) | L | −28 | −27 | −3 | −3.26 | 655 |
| Occipital | (↑) | R | 0 | −87 | 9 | 4.46 | 823 |
BOLD, blood oxygen level-dependent; ACC, anterior cingulate cortex, PCC, posterior cingulate cortex. (↑), activation; (↓), deactivation. Talairach coordinates represent voxel of maximum response: x represents position in brain on horizontal axis, y represents position on vertical axis, z represents the depth position.
Table 3.
BOLD signal changes to 40% MVC handgrip in CAD subjects
| Coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Location | Side | x | y | z | T-Score | Number of Voxels | |
| Insula | (↑) | L | −33 | 18 | 11 | 4.40 | 236 |
| Insula | (↑) | R | 34 | 13 | 11 | 4.33 | 313 |
| Mid-superior CC | (↓) | L | −8 | −32 | 33 | −4.29 | 532 |
| PCC | (↓) | L | −8 | −48 | 11 | −4.22 | 362 |
| Precentral gyrus | (↑) | R | 34 | −23 | 44 | 5.29 | 525 |
| Precentral gyrus | (↑) | L | −46 | 1 | 33 | 4.04 | 750 |
| Occipital | (↑) | R | 0 | −87 | 9 | 4.06 | 792 |
See Table 2 for abbreviations.
Fig. 2.
Cortical functional response to 40% IHG task in control (CTRL; top 3 images) and CAD (bottom 3 images). L, left; R, right; PCC, posterior cingulate cortex. T-statistics (beta values) at specified regions (circled) are represented as an average for each group. FDR, P < 0.05, corrected for multiple comparisons. Color scheme identified by scale at right. Red/warm colors denote regions of activation above baseline levels; blue/cold colors denote regions of deactivation below baseline levels (exact values given in beta graphs, Fig. 3). Note the absence of deactivation at the medial prefrontal cortex and hippocampus in both groups. TRA, transversal (axial); SAG, sagittal; COR, coronal; A, anterior; P, posterior.
Contrasting BOLD responses between control and CAD to 40% IHG task.
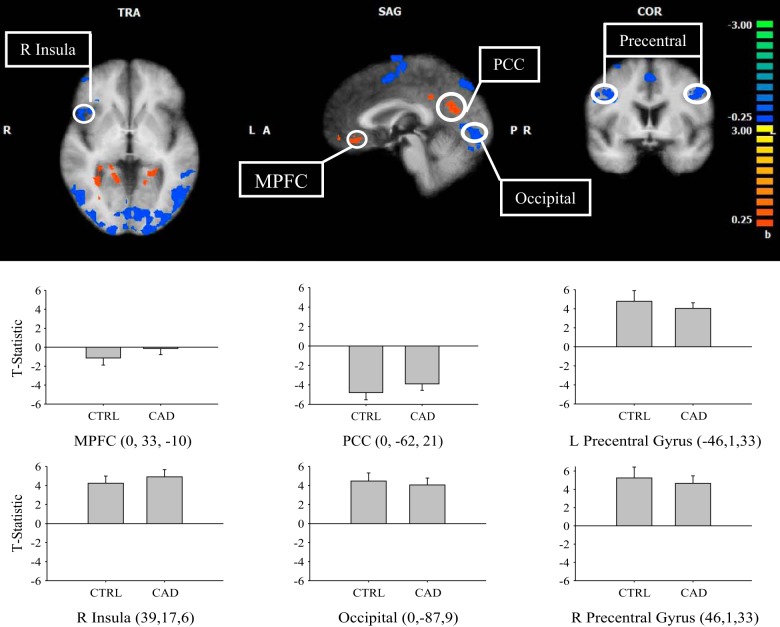
Comparisons of activated regions between control and CAD during the 40% IHG task are shown in Fig. 3. In subtraction analyses for CAD > control, greater activation (or less deactivation) was observed in the PCC and MPFC. The contrast CAD < control showed activation in the right anterior insula, bilateral precentral cortex, and occipital lobe (P < 0.05).
Fig. 3.
Subtraction result for group 1 average (CAD) vs. group 2 average (control) to 40% IHG boxcar analysis. L, left; R, right; MPFC, medial prefrontal cortex; PCC, posterior cingulate cortex. Warm colors show areas where there is a positive difference with respect to group 1 (CAD > control), and cold colors show areas where there is a negative difference with respect to group 1 (CAD < control). T-statistic (beta value) at specified regions (Talairach coordinates, circled) are represented as an average for each group and denoted by color scale at right; threshold = 2. P < 0.05. Cluster threshold = 10 voxels. Error bars represent SD.
Functional (BOLD) imaging data: second-level (group) response correlated with heart rate.
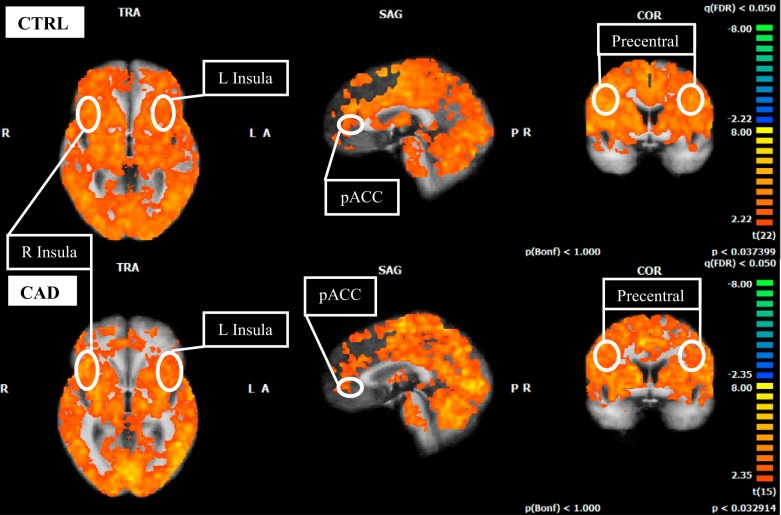
Extensive activation patterns were revealed in both control and CAD groups, but lacked significant deactivation in expected autonomic regions (Fig. 4). Specifically, the bilateral IC and precentral gyrus were activated in both control and CAD groups while deactivation in the MPFC and HC was absent (FDR P < 0.05).
Fig. 4.
Cortical functional response correlated to individual heart rate time course during 40% IHG task in control (CTRL; top 3 images) and CAD (bottom 3 images). L, left; R, right; pACC, perigenual anterior cingulate cortex. T-statistics (beta values) at specified regions (circled) are represented as an average for each group. FDR, P < 0.05, corrected for multiple comparisons. Color scheme identified by scale at right. Red/warm colors denote regions of activation above baseline levels; blue/cold colors denote regions of deactivation below baseline levels (exact values given in beta graphs, Fig. 5). Note the absence of deactivation at the medial prefrontal cortex and hippocampus in both groups.
Contrasting BOLD responses between control and CAD correlated with heart rate.
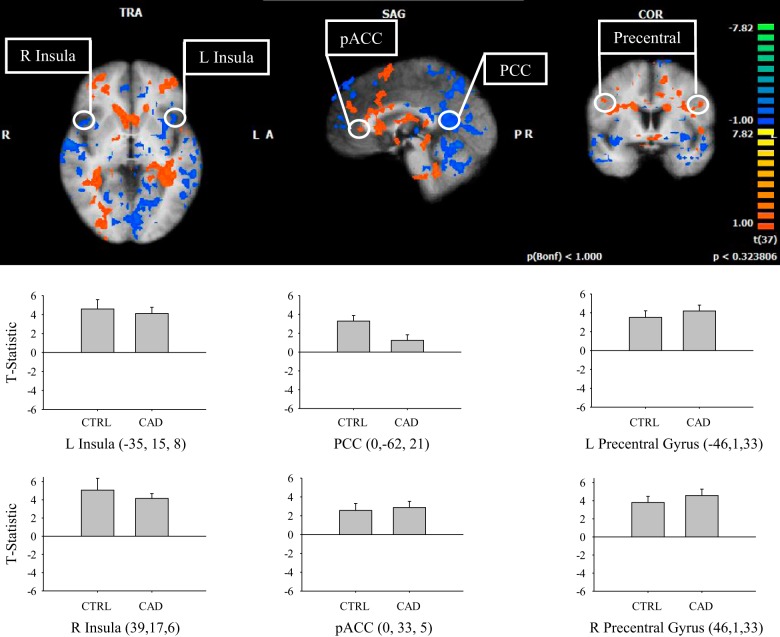
Comparisons of activated regions between control and CAD during the 40% IHG task correlated with the individual HR time courses are shown in Fig. 5. In subtraction analyses for CAD > control, greater activation was observed in the perigenual anterior cingulate cortex. The contrast CAD < control demonstrated activation in the bilateral insula and posterior cingulate cortex (P < 0.05).
Fig. 5.
Subtraction result for group 1 average (CAD) vs. group 2 average (control) correlated to individual heart rate time course during 40% IHG task. L, left; R, right; PCC, posterior cingulate cortex; pACC, perigenual anterior cingulate cortex. Warm colors show areas where there is a positive difference with respect to group 1 (CAD > control), and cold colors show areas where there is a negative difference with respect to group 1 (CAD < control). T-statistic (beta value) at specified regions (Talairach coordinates, circled) are represented as an average for each group and denoted by color scale at right; threshold = 2. P < 0.05. Cluster threshold = 10 voxels. Error bars represent SD.
Probability mapping.
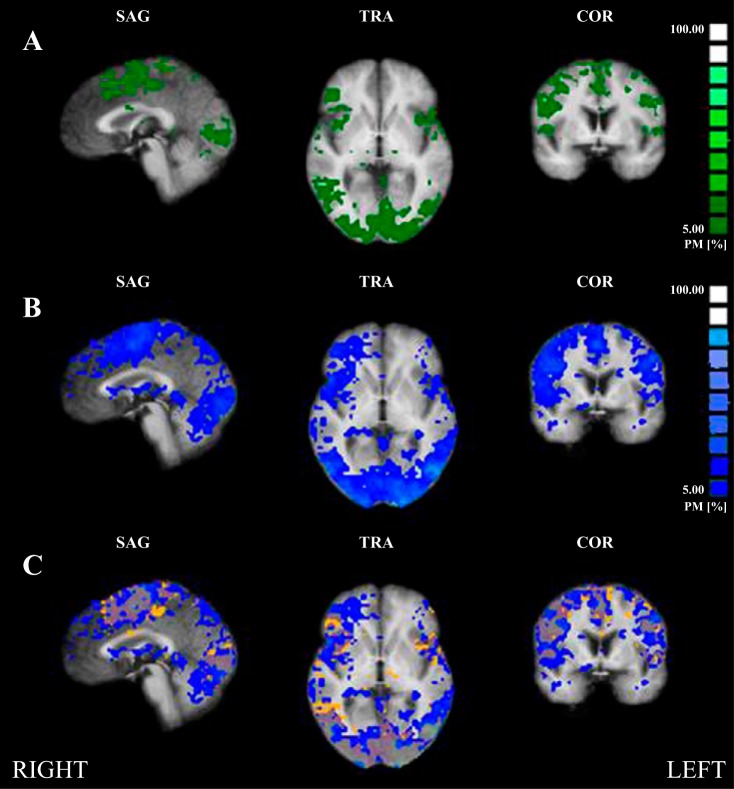
Probabilistic maps were created for control and CAD groups to provide a general means to evaluate the spatial consistency of task-specific brain activation across subjects. We plotted the cross-subject (control and CAD) overlap probability maps for 40% IHG at a range of 0–100% (Fig. 6). Control subjects (yellow/orange) displayed greater anatomical consistency compared with CAD patients (blue) who showed much greater variability in activation responses (cluster threshold = 15 voxels). Probability percent values (overlap) between control and CAD in expected CAN regions include the left anterior IC (8.43% overlap; x, y, z coordinates: −40, 14, 5), right anterior IC (11.02%; x, y, z coordinates: 34, 20, 9), PCC (0.08%; x, y, z coordinates: −8, −55, 14), precentral gyrus (31.97%; x, y, z coordinates: 34, −21, 52), and MPFC (2.81%; x, y, z coordinates: 4, 37, −3).
Fig. 6.
Probability mapping. A: control subjects (green), 0% minimum threshold. B: coronary artery disease patients (CAD; blue), 5% minimum threshold. Each colored cluster represents the relative percentage of subjects leading to significant task activity during a 40% maximal voluntary contraction (MVC) handgrip task based on the bar graph at right. C: probability map overlap of both groups. Control, orange; CAD, blue (voxel threshold = 15 voxels; 0% minimum threshold). CAD patients (blue) had much greater variability in activation responses than control, who indicated greater anatomical consistency.
DISCUSSION
In contrast to healthy controls, the CAD patient group demonstrated diminished HR responses across all exercise workloads and high variance in activation patterns among regions of the cortical autonomic network. These cortical patterns appear to be consistent with the overall suppressed HR response despite the ability to perform the IHG task adequately. Therefore, the current data support the hypothesis that CAD alters the cortical circuitry associated with exercise, and patients exhibit accelerated age-related dysregulation of the brain-heart connection.
An initial important observation of the current study was the difference in HR responses to IHG between the current participants and those of younger individuals reported earlier from our laboratory (Goswami et al. 2012; Norton et al. 2013; Wong et al. 2007). This difference was statistically significant, as determined by an independent group's t-test that contrasted the present data with those published earlier. Specifically, young individuals (25 ± 4 yr), compared with the current participants (61 ± 10 yr), generate a much larger HR response (6–15 beats/min) to a similar relative IHG tension (P < 0.0001). Mechanistically, this IHG protocol is designed to engage exercise-onset reflexive increases in HR that predominantly reflect reduced dominance of parasympathetic control (Fagraeus and Linnarsson 1976; Hollander and Bouman 1975; Mitchell et al. 1989). Therefore, the smaller HR responses in control, and smaller yet in CAD, are likely a consequence of age-related impairment of parasympathetic outflow (Monahan et al. 2001; Seals et al. 1994) that is further negatively impacted by CAD (Eckberg et al. 1971; Gribbin et al. 1969). Recently, our laboratory reported the cortical activation patterns and HR responses in healthy individuals ranging from 21–80 yr of age, with conclusions that age alone does not determine a smaller ΔHR response (Norton et al. 2013) in that several older adults still generated similar responses to young individuals. Thus interindividual variability in HR responses was augmented with increasing age. The current study further supports a depressed HR response overall, as well as enhanced variability in HR responses, as an effect of age (Fig. 1).
A second observation of the current study was the marked and unexpected differences in brain activation patterns associated with both the IHG task and the HR response in both the control and CAD patients compared with previous studies from our laboratory performed by young and healthy individuals (Goswami et al. 2011; Norton et al. 2013; Wong et al. 2007). Specifically, the current participants exhibited a large and widespread pattern of enhanced brain activation relative to baseline when correlated with both the IHG task and the HR response. This widespread activation pattern was different from the discrete pattern observed in young individuals specifically in regions related to autonomic control. Within this pattern of higher overall activation, however, there was a marked absence of deactivation within the MPFC and HC in the current participants (at least at the group level) which are consistently present in young healthy subjects (Goswami et al. 2011; Norton et al. 2013; Wong et al. 2007). Yet, group-level activation was observed in the bilateral insula, and deactivation was observed in the posterior cingulate cortex, observations that are consistent with previously published results in young subjects.
Although this apparent “overactivation” identified above is unique in the context of cardiovascular control, it has been reported earlier in the context of perceptual or cognitive tasks performed by aged individuals. The mechanism(s) of these patterns is yet unknown. They may reflect alterations in the coupling between regional blood flow and oxygen extraction. However, some hypothesize these patterns to reflect compensatory neural responses (Cabeza et al. 2004; Reuter-Lorenz 2002) where, in the aging brain, previously connected networks are disrupted such that alternative patterns emerge which must “work harder” to make up either for its own declining efficiency or for processing deficiencies elsewhere in the brain. The current observations appear to be the first to report a similar phenomenon related to volitional tasks such as IHG exercise. If this observation reflects neural compensation, the smaller response in CAD vs. control participants becomes the third important observation of the current study (Fig. 4). The degree of compensatory activation is thought to be related to a sense of effort required to perform the task (Reuter-Lorenz and Park 2010). In this context, the smaller amount of compensation in CAD patients may reflect lower perceived effort required to perform the IHG. However, the similar Borg scores of perceived effort and identical absolute and relative workloads produced (MVC, Table 1) argue that the smaller brain activation patterns in CAD patients are not related to perceived effort. Previously, our laboratory reported accelerated age-related cortical atrophy in CAD patients (Anazodo et al. 2013). Thus it may be that age-related compensatory responses to IHG are also related to accelerated brain atrophy and/or impaired local flow-metabolism coupling.
The probability mapping analysis indicated that CAD patients exhibited greater regional variability in activation responses to the 40% IHG task than control. Probability percent values, which reflect overlapping patterns of activation between control and CAD participants, were highest in the primary motor cortex (31.97%), but significant variability existed in expected CAN regions such as the left anterior IC (8.43%), right anterior IC (11.02%), PCC (0.08%), and MPFC (2.81%). Thus it appears that brain regions required for motor activity are retained in CAD, but that those regions believed to be required for explicit autonomic homeostatic functions, such as the MPFC and HC, are dysregulated more in CAD patients.
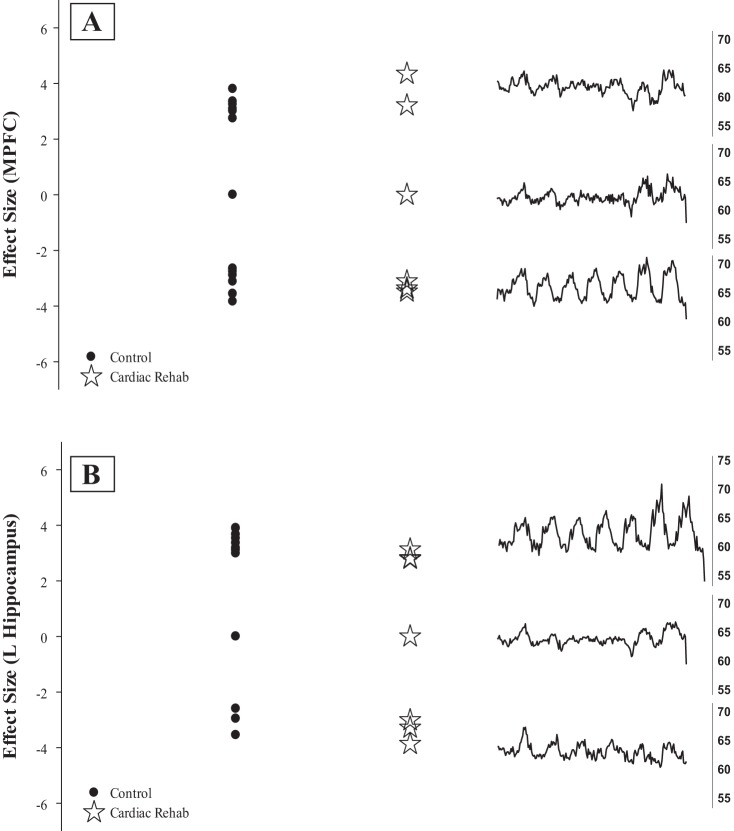
A notable outcome of the current study was the absence of deactivation associated with the HR response to IHG in the MPFC and hippocampus (HC), in both control and CAD patients when studied at the group level (Fig. 4). The MPFC region was of particular interest in the present study as it has been associated with cardiovagal control across many stimuli that elicit cardiovascular arousal (Critchley et al. 2000; Gianaros et al. 2004; Kimmerly et al. 2005; Matthews et al. 2004; Norton et al. 2013; Resstel et al. 2004; Thayer et al. 2009; Williamson et al. 2003). Previous studies have further suggested that the MPFC is involved in the processes of integrating sensory information during the resting default state and its activity is hence attenuated during goal-directed behaviors (Raichle et al. 2001). This interpretation is consistent with repeated observations of decreased MPFC activation, relative to baseline, during volitional IHG (Goswami et al. 2011; Norton et al. 2013; Wong et al. 2007). Despite high interindividual variability, the MPFC factored importantly into the subtraction analyses when correlated with the task alone (Fig. 3) illustrating that control subjects had more deactivation (or less activation) than CAD in response to the exercise task. To investigate this outcome further, a secondary analysis of individual brain activation patterns was pursued. This analysis indicated that 16/23 control and 12/17 CAD patients exhibited a reduction in MPFC activation relative to baseline during the IHG period. However, the number of participants who demonstrated a reduction in MPFC activation and produced a HR response of ≥3 beats/min to IHG was 9/23 (39%) in control and 3/17 (18%) in CAD. Thus the interindividual variability minimized group-level statistical power in both the specified MPFC regional activation and in the HR response to IHG and, consequently, reflects the reduced brain activation evident in the subtraction analysis in control subjects.
The variability in brain deactivation patterns outlined above seems to exert a dominant impact on the current results. The high cortical variability in these groups is clear in Fig. 7, which illustrates that both groups had a lack of consistent MPFC deactivation in response to the IHG task, with some subjects having activation in expected autonomic regions and many individuals having no response at all. In earlier studies from our laboratory, and reports from several other laboratories (Critchley et al. 2000; Gianaros et al. 2004), HR variations in young, healthy subjects most strongly correlate inversely with deactivation within the HC and MPFC. Moreover, HR-associated effective connectivity exists between the MPFC and HC in healthy individuals promoting a much larger HR response when both regions are deactivated in concert rather than each region alone (Norton et al. 2013). However, HR responses in the current study were much smaller suggesting an effective consequence of cortical “decoupling.” For example, a small HR response was observed in some individuals who did not express deactivation within the MPFC or HC. This pattern was dominated by control subjects (9/12). To our knowledge, no data exist to address this critical component of changes in cardiovascular control that emerge in aging and diseased individuals.
Fig. 7.
The effect size (left) in a priori regions (A: medial prefrontal cortex; B: left hippocampus) to the 40% isometric handgrip task with the average heart rate response (beats/min) of those individuals (right).
Study limitations.
All CAD patients were on a combination of drug therapies including cholesterol-lowering statins, beta-blockade, angiotensin-converting enzyme (ACE) inhibitors/angiotensin II receptor blockers, calcium channel blockers, diuretics, and anti-platelets including aspirin. ACE-inhibitors and angiotensin II receptor blockers affect vasomotor control but exert minimal effect on cardiac function and should not have impacted the current results. Beta-blockers can reduce baseline HR and interfere with sympathetically driven changes in HR. However, these effects are largely seen at maximal workloads, which is supported by our data (stress test data, Table 1) and are not expected to affect HR responses to the 40% IHG task where vagal control dominates below 100 beats/min (Rowell 1993). Furthermore, the heart rate response to the 40% IHG was not different between groups suggesting that beta-blockade did not influence HR responses at the level of the heart. Finally, as outlined above, CAD patients were capable of mounting a significant HR response during the cardiorespiratory fitness test indicating that the heart's ability to respond to volitional effortful tasks was not altered by the medication.
Conclusions.
Overall, the current results indicate that relative to similarly aged, and apparently healthy, individuals, vascular disease impairs functional outcomes in the brain in response to moderate-intensity IHG. In particular, the enhanced variability of cortical responses and diminished total cortical activation patterns in CAD are consistent with an overall lower HR response, promoting the hypothesis that CAD patients appear to exhibit dysregulation of the brain-heart connection.
GRANTS
This study was funded by the Canadian Institutes for Health Research Team Grant in Physical Activity, Mobility and Neural Health (Grant No. 217532) (J. K. Shoemaker, nominated Principal Investigator), and the Heart and Stroke Foundation of Ontario (Grant No. T6334). J. K. Shoemaker is a Tier 1 Canada Research Chair in the Integrative Physiology of Exercise and Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.N.N., N.S., and J.K.S. conception and design of research; K.N.N., M.B.B., and C.C.B. performed experiments; K.N.N. analyzed data; K.N.N. and J.K.S. interpreted results of experiments; K.N.N. prepared figures; K.N.N. drafted manuscript; K.N.N., A.H., and J.K.S. edited and revised manuscript; K.N.N., A.H., and J.K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Cortical imaging data were obtained in the Robarts Centre for Functional and Metabolic Mapping, The Univ. of Western Ontario, under the direction of O. Opalevych and J. S. Gati.
REFERENCES
- ACSM. Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Lippincott Williams and Wilkins, 1995. [Google Scholar]
- Anazodo UC, Shoemaker JK, Suskin N, St Lawrence KS. An investigation of changes in regional gray matter volume in cardiovascular disease patients, pre and post cardiovascular rehabilitation. Neuroimage Clin 3: 388–395, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberi EA, Gati JS, Rutt BK, Menon RS. A transmit-only/receive-only (TORO) RF system for high-field MRI/MRS applications. Magn Reson Med 43: 284–289, 2000. [DOI] [PubMed] [Google Scholar]
- Barekatain M, Askarpour H, Zahedian F, Walterfang M, Velakoulis D, Maracy MR, Jazi MH. The relationship between regional brain volumes and the extent of coronary artery disease in mild cognitive impairment. J Res Med Sci 19: 739–745, 2014. [PMC free article] [PubMed] [Google Scholar]
- Basnayake SD, Green AL, Paterson DJ. Mapping the central neurocircuitry that integrates the cardiovascular response to exercise in humans. Exp Physiol 97: 29–38, 2012. [DOI] [PubMed] [Google Scholar]
- Borg G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int J Sports Med 3: 153–158, 1982. [DOI] [PubMed] [Google Scholar]
- Burns SM, Wyss JM. The involvement of the anterior cingulate cortex in blood pressure control. Brain Res 340: 71–77, 1985. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex 14: 364–375, 2004. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Role of the cerebral cortex in autonomic function. In: Central Regulation of Autonomic Functions, edited by Loewy AD, Spyer KM. New York: Oxford Univ. Press, 1990, p. 208–223. [Google Scholar]
- Cechetto DF. Cortical control of the autonomic nervous system. Exp Physiol 99: 326–331, 2014. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Chen SJ. Subcortical sites mediating sympathetic responses from insular cortex in rats. Am J Physiol Regul Integr Comp Physiol 258: R245–R255, 1990. [DOI] [PubMed] [Google Scholar]
- Cheung RT, Hachinski V. The insula and cerebrogenic sudden death. Arch Neurol 57: 1685–1688, 2000. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol 523: 259–270, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis BM, O'Keefe JH Jr. Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clin Proc 77: 45–54, 2002. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med 285: 877–883, 1971. [DOI] [PubMed] [Google Scholar]
- Fagraeus L, Linnarsson D. Autonomic origin of heart rate fluctuations at the onset of muscular exercise. J Appl Physiol 40: 679–682, 1976. [DOI] [PubMed] [Google Scholar]
- Fisk GD, Wyss JM. Pressor and depressor sites are intermingled in the cingulate cortex of the rat. Brain Res 754: 204–212, 1997. [DOI] [PubMed] [Google Scholar]
- Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, Froelicher VF, Leon AS, Pina IL, Rodney R, Simons-Morton DA, Williams MA, Bazzarre T. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation 104: 1694–1740, 2001. [DOI] [PubMed] [Google Scholar]
- Ford GA. Ageing and the baroreflex. Age Ageing 28: 337–338, 1999. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage 2: 45–53, 1995. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Van Der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology 41: 521–530, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp 27: 392–401, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami R, Frances MF, Shoemaker JK. Representation of somatosensory inputs within the cortical autonomic network. Neuroimage 54: 1211–1220, 2011. [DOI] [PubMed] [Google Scholar]
- Goswami R, Frances MF, Steinback CD, Shoemaker JK. Forebrain organization representing baroreceptor gating of somatosensory afferents within the cortical autonomic network. J Neurophysiol 108: 453–466, 2012. [DOI] [PubMed] [Google Scholar]
- Gribbin B, Pickering TG, Sleight P. Decrease in baroreflex sensitivity with increasing arterial pressure and with increasing age. Br Heart J 31: 791–798, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage 33: 1093–1103, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander AP, Bouman LN. Cardiac acceleration in man elicited by a muscle-heart reflex. J Appl Physiol 38: 272–278, 1975. [DOI] [PubMed] [Google Scholar]
- Kimmerly DS, O'Leary DD, Menon RS, Gati JS, Shoemaker JK. Cortical regions associated with autonomic cardiovascular regulation during lower body negative pressure in humans. J Physiol 569: 331–345, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Wu P, Kumar R, Ogren JA, Richardson HL, Woo MA, Harper RM. Differential responses of the insular cortex gyri to autonomic challenges. Auton Neurosci 168: 72–81, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia G, Cleroux J, Daffonchio A, Ferrari AU, Giannattasio C, Grassi G. Reflex control of circulation in the elderly. Cardiovasc Drugs Ther 4, Suppl 6: 1223–1228, 1991. [DOI] [PubMed] [Google Scholar]
- Mancia G, Iannos J, Jamieson GG, Lawrence RH, Sharman PR, Ludbrook J. Effect of isometric hand-grip exercise on the carotid sinus baroreceptor reflex in man. Clin Sci Mol Med 54: 33–37, 1978. [DOI] [PubMed] [Google Scholar]
- Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57: 461–469, 1985. [DOI] [PubMed] [Google Scholar]
- Martins IJ, Hone E, Foster JK, Sunram-Lea SI, Gnjec A, Fuller SJ, Nolan D, Gandy SE, Martins RN. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer's disease and cardiovascular disease. Mol Psychiatry 11: 721–736, 2006. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. Neuroimage 22: 1151–1156, 2004. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Reeves DR Jr, Rogers HB, Secher NH, Victor RG. Autonomic blockade and cardiovascular responses to static exercise in partially curarized man. J Physiol 413: 433–445, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan KD, Dinenno FA, Seals DR, Clevenger CM, DeSouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol 281: H284–H289, 2001. [DOI] [PubMed] [Google Scholar]
- Norton KN, Luchyshyn TA, Kevin SJ. Evidence for a medial prefrontal cortex-hippocampal axis associated with heart rate control in conscious humans. Brain Res 1538: 104–115, 2013. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology 42: 1727–1732, 1992. [DOI] [PubMed] [Google Scholar]
- Owens NC, Verberne AJ. Regional haemodynamic responses to activation of the medial prefrontal cortex depressor region. Brain Res 919: 221–231, 2001. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Fernandes KB, Correa FM. Medial prefrontal cortex modulation of the baroreflex parasympathetic component in the rat. Brain Res 1015: 136–144, 2004. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P. New visions of the aging mind and brain. Trends Cogn Sci 6: 394, 2002. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Park DC. Human neuroscience and the aging mind: a new look at old problems. J Gerontol B Psychol Sci Soc Sci 65: 405–415, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RO, Knopman DS, Geda YE, Cha RH, Roger VL, Petersen RC. Coronary heart disease is associated with non-amnestic mild cognitive impairment. Neurobiol Aging 31: 1894–1902, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York: Oxford Univ. Press, 1993. [Google Scholar]
- Ruggiero DA, Mraovitch S, Granata AR, Anwar M, Reis DJ. A role of insular cortex in cardiovascular function. J Comp Neurol 257: 189–207, 1987. [DOI] [PubMed] [Google Scholar]
- Seals DR, Taylor JA, Ng AV, Esler MD. Exercise and aging: autonomic control of the circulation. Med Sci Sports Exerc 26: 568–576, 1994. [PubMed] [Google Scholar]
- Shoemaker JK, Norton KN, Baker J, Luchyshyn T. Forebrain organization for autonomic cardiovascular control. Auton Neurosci 188: 5–9, 2015. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical, 1988. [Google Scholar]
- Thayer JF, Sollers JJ 3rd, Labiner DM, Weinand M, Herring AM, Lane RD, Ahern GL. Age-related differences in prefrontal control of heart rate in humans: a pharmacological blockade study. Int J Psychophysiol 72: 81–88, 2009. [DOI] [PubMed] [Google Scholar]
- Verberne AJ. Medullary sympathoexcitatory neurons are inhibited by activation of the medial prefrontal cortex in the rat. Am J Physiol Regul Integr Comp Physiol 270: R713–R719, 1996. [DOI] [PubMed] [Google Scholar]
- Verberne AJM, Owens NC. Cortical modulation of the cardiovascular system. Prog Neurobiol 54: 149–168, 1998. [DOI] [PubMed] [Google Scholar]
- Williamson JW. The relevance of central command for the neural cardiovascular control of exercise. Exp Physiol 95: 1043–1048, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JW, McColl R, Mathews D. Evidence for central command activation of the human insular cortex during exercise. J Appl Physiol (1985) 94: 1726–1734, 2003. [DOI] [PubMed] [Google Scholar]
- Wong SW, Masse N, Kimmerly DS, Menon RS, Shoemaker JK. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage 35: 698–708, 2007. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol 303: 355–374, 1991. [DOI] [PubMed] [Google Scholar]
- Zulli R, Nicosia F, Borroni B, Agosti C, Prometti P, Donati P, De VM, Turini D, Romanelli G, Grassi V, Padovani A. Increased prevalence of silent myocardial ischaemia and severe ventricular arrhythmias in untreated patients with Alzheimer's disease and mild cognitive impairment without overt coronary artery disease. Clin Neurol Neurosurg 110: 791–796, 2008. [DOI] [PubMed] [Google Scholar]