Abstract
Neural circuits controlling defensive behavior were investigated by recording single units in medial prefrontal cortex (mPFC) and dorsolateral periaqueductal gray (dlPAG) while rats expressed conditioned fear responses to an auditory conditioned stimulus (CS; 20-s train of white noise pips) previously paired with an aversive unconditioned stimulus (US; 2-s train of periorbital shocks). The CS elicited conditioned movement inhibition (CMI; characterized by decreased movement speed and freezing) when rats had not recently encountered the US, whereas the CS elicited conditioned movement excitation (CME; characterized by increased movement speed and flight behavior) after recent US encounters. Many mPFC neurons were “strategy-selective” cells that changed their firing rates only when the CS elicited CME (15/71) or CMI (13/71) responses, whereas few mPFC cells (4/71) responded nonselectively to the CS during either response. By contrast, many dlPAG neurons (20/74) responded nonselectively to the CS, but most (40/74) were excited by the CS selectively during CME trials (and none during CMI trials). CME-selective neurons in dlPAG responded phasically after CS pips that elicited CME responses, whereas CME-selective neurons in mPFC showed tonically elevated activity before and after pips that evoked CME responses. These findings suggest that, at the time when the CS occurs, tonic firing rates of CME- and CMI-selective mPFC neurons may bias the rat's choice of whether to express CME vs. CMI responses, perhaps via projections to downstream structures (such as amygdala and PAG) that influence how sensory stimuli are mapped onto motor circuits that drive the expression of competing behaviors.
Keywords: medial prefrontal cortex, periaqueductal gray, fear conditioning, prelimbic, infralimbic
single-unit recording studies have shown that firing rates of neurons in medial prefrontal cortex (mPFC) are correlated with expression and extinction of conditioned fear in rodents (Baeg et al. 2001; Burgos-Robles et al. 2009; Herry et al. 2008; McGinty and Grace 2008; Senn et al. 2014). The prelimbic (PL) subregion of mPFC contains neurons that increase their firing rates during conditioned freezing responses (Burgos-Robles et al. 2009; Senn et al. 2014), and disruption of PL can impair conditioned freezing (Corcoran and Quirk 2007; Laurent and Westbrook 2009; but see also Bravo-Rivera et al. 2014). The infralimbic (IL) subregion of mPFC contains neurons that respond to a fear-conditioned conditioned stimulus (CS) after it has been extinguished (Burgos-Robles et al. 2007; Knapska and Maren 2009; Santini et al. 2008), and disruption of IL can impair fear extinction (Laurent and Westbrook 2009; Quirk et al. 2000; Sierra-Mercado et al. 2011; Sotres-Bayon et al. 2009; but see also Chang and Maren 2010; Garcia et al. 2006). Moreover, properly timed electrical stimulation of IL can disrupt freezing responses elicited by a fear-conditioned CS (Milad and Quirk 2002; Milad et al. 2004), suggesting that IL neurons can block freezing expression.
Taken together, these findings provide compelling evidence that mPFC plays a key role in regulating the expression of conditioned fear, possibly via its reciprocal connections to the amygdala (Duvarci and Pare 2014; Gilmartin et al. 2014; Maren et al. 2013; Sotres-Bayon and Quirk 2010). However, because most of these findings come from studies where freezing served as the primary behavioral index of fear, it remains unclear whether mPFC neurons regulate CS-evoked expectations of the unconditioned stimulus (US) (which are antecedent to “fear”) or whether they instead regulate the performance of specific defensive responses to the CS, such as freezing. On the basis of current evidence, PL neurons that respond to a fear-conditioned CS might either signal the animal's anticipation of danger during the CS or instead promote the expression of freezing responses to the CS, without influencing the animal's expectation of threat. Likewise, IL neurons that respond to an extinguished CS might suppress the animal's learned expectation of the US (thus inhibiting fear), or they might instead suppress freezing responses to the CS (and thereby disinhibit other behaviors) without inhibiting the animal's expectation of danger.
To further investigate which aspects of fear and defensive behavior are correlated with neural activity in mPFC, we recorded responses of mPFC neurons to a fear-conditioned auditory CS under conditions where it evoked two distinct defensive responses from rats: conditioned movement inhibition (CMI) vs. conditioned movement excitation (CME) behaviors. We reasoned that, if mPFC neurons excite or inhibit the rat's fear of an aversive US, then they should respond similarly to a CS that predicts an aversive US, regardless of which defensive response is evoked by that CS. On the other hand, if mPFC neurons drive specific defensive responses, then they should respond differently to the CS depending on whether it evokes CMI or CME behaviors, even though both are conditioned fear responses. For comparison, we also recorded neurons in dorsolateral periaqueductal gray (dlPAG), a structure that receives afferent projections from mPFC and is thought to drive CME behaviors such as escape and avoidance (Bandler and DePaulis 1988; De Oca et al. 1998; Fanselow 1991; Vianna et al. 2001; Walker et al. 1997).
We found that more than half of neurons recorded in dlPAG responded preferentially to the CS when it elicited CME responses, and, in addition, more than a quarter of dlPAG neurons responded nonselectively to the CS regardless of whether it elicited CME or CMI responses. By contrast, very few (<6%) mPFC neurons responded nonselectively to the CS, and >40% of mPFC neurons were “strategy-selective” cells that responded preferentially to the CS either during CMI responses (∼20% of cells) or CME responses (∼20% of cells). These findings suggest that, rather than exciting or inhibiting the rat's fear of the aversive US, mPFC neurons may instead coordinate the selection of specific defensive responses to anticipated threats, in accordance with existing theories that mPFC regulates the selection of behavioral response strategies by adjusting the manner in which sensory stimuli are mapped onto behavioral responses (Miller and Cohen 2001; Shackman et al. 2011).
MATERIALS AND METHODS
Subjects and Surgery
Adult male Long-Evans rats weighing 350–400 g were housed singly and reduced to 85% of ad libitum weight by daily limited feeding. All rats were deeply anaesthetized with isofluorane and surgically implanted with a pair of insulated stainless steel wires (75-μm diameter) threaded into the skin of each eyelid for delivering the periorbital shock US. Rats (n = 5, of which 4 were included in the study and 1 was excluded because of failure to obtain recordings during the expression of conditioned fear) were implanted with 16 individually moveable tetrodes, which targeted mPFC and dlPAG. Four tetrodes per hemisphere were implanted in each structure of each rat. In mPFC, the four tetrodes were arrayed from 1.7 to 2.7 mm anterior to bregma at a lateral offset of 0.5–1.0 mm from the midline, and the tips were advanced over the course of the experiment from a depth of 2.0–5.0 mm dorsal to bregma. In dlPAG, the four tetrodes were arrayed from 6.8–7.8 mm posterior to bregma at a lateral offset of 0.5–1.0 mm from the midline, and the tips were advanced over the course of the experiment from a depth of 4.0–6.0 mm dorsal to bregma. Rats were also implanted with a pair of 22-gauge microinjection cannula targeted bilaterally in the basolateral amygdala (3.0 mm posterior, 5.3 mm lateral, and 8.0 mm ventral to bregma), which were used to inactivate the amygdala with muscimol during neural recordings on some experiment days. We did not obtain a sufficiently large sample of recorded neurons during amygdala inactivations to report conclusive results on the effects of amygdala disruption, so inactivation sessions are excluded from the present analyses; all data reported here were obtained during drug-free recording sessions conducted at least 48 h after the most recent amygdala inactivation (in all graphs where data is plotted over sessions, the session number tabulates drug-free sessions only). All experimental procedures were approved by the UCLA Animal Research Committee and were conducted in accordance with USA federal guidelines.
Fear Conditioning
After recovery from surgery, rats were preexposed for 5 days (20 min/day) to the experimental context (80-cm diameter cylindrical enclosure) before any fear-conditioning sessions were conducted. To provide a baseline of motor activity against which stimulus-evoked movement and turning behavior could be measured, rats constantly foraged for 20 mg purified food pellets (Bioserv, Frenchtown, NJ) dropped from an overhead dispenser at ∼30-s intervals throughout all preexposure and fear-conditioning sessions. On every day following preexposure, rats received an identical regimen of fear conditioning trials: 6 CS-alone presentations (test trials) followed by 16 CS-US pairings (training trials). The present experiments were designed to investigate expression (not acquisition) of conditioned fear responses; on the basis of prior behavioral findings (Tarpley et al. 2010), all rats were first trained to near-asymptotic levels of CS-evoked fear behavior over 5 consecutive days before neurophysiological recordings. The CS used for fear conditioning and testing was a train of 70-dB white noise pips, each lasting 250 ms, delivered at 1 Hz for 20 s through an overhead speaker. The US was a train of 2.0-mA shock pulses, each lasting 2.0 ms, delivered to one eyelid at a rate of 6.66 Hz for 2 s. During CS-US pairing trials, the first shock pulse was always delivered 300 ms after the offset of the final (20th) CS pip. The intertrial interval was uniformly random between 180 and 240 s.
The rat's position on the experimental platform was sampled at 30 Hz by an overhead video tracking system (Neuralynx, Bozeman, MT), which monitored the location of three light-emitting diodes of different colors (red, blue, and green) attached to the animal's headstage for automated measurement of movement speed and turning behavior (Halladay and Blair 2012; Tarpley et al. 2010). Conditioned fear responses were assessed by comparing the animal's behavior during the context (CX) period (20 s immediately preceding CS onset) vs. CS period (19 s immediately following CS onset; the 20th pip was omitted from CS period because the first shock pulse was delivered <1 s after the offset of this pip) of each trial.
Each trial was classified as a CMI, CME, or no conditioned response (NCR) trial based on the rat's behavior during the trial. The rat's movement speed was averaged during consecutive 1-s bins throughout the trial, and a t-test was then performed to compare mean movement speed during bins from the CX period (n = 20) vs. CS period (n = 19). A one-tailed cutoff of P = 0.05 was used to classify the type of trial as either CMI (CX > CS), CME (CX < CS), or NCR (CX not different from CS).
Neurophysiological Data Acquisition and Analysis
Single units in mPFC and dlPAG were recorded using a DigitalLynx S-series acquisition system (Neuralynx). Waveforms were isolated manually using Spikesort3D (Neuralynx) software. To be included in data analyses, spikes had to exceed a minimum amplitude threshold of 70 μV peak to peak (against a mean noise floor of ∼25 μV peak to peak) and exhibit a refractory period of at least 1-ms base upon analysis of interspike interval histograms. Spike trains recorded on different experiment days were considered to be from the same cell if the following conditions were met: 1) spikes were recorded from the same tetrode, 2) the tetrode had been advanced <80 μm between recordings, 3) cluster boundaries and waveform shapes were visually similar on all tetrode channels for each session, and 4) the effect of the CS upon the firing rate of the cell did not differ significantly across any pair of consecutive experiment days (see below, Classification of cell types). Neurophysiological data analysis only included cells that were recorded during at least three CMI and three CME trials across all sessions during which the cell was isolated. Post hoc comparisons for all ANOVAs were made using the Newman-Keuls method, unless otherwise noted.
Classification of cell types.
Single units were classified according to how their mean firing rates changed between the CX vs. CS periods during CMI and CME trials. For each recording session, spike data of a cell from trials of a given type (CMI or CME) were used to generate a peristimulus time histogram (PSTH; bin size = 1 s) aligned to the onset of the first CS pip of each trial; the CX period spanned 20 bins before the first CS onset, and the CS period spanned 19 bins after the first CS onset. If a cell was recorded across more than one session, then spike data from each pair of consecutive sessions were analyzed by performing a 2 × 2 × 2 independent ANOVA with stimulus (CX vs. CS), trial type (CMI vs. CME), and session (1st vs. 2nd day of the consecutive pair) on spike counts from each 1-s bin of the PSTH of the cell. If the three-way interaction was significant for any pair of consecutive days, then data from the second day and all subsequent days were discarded from further analysis, so that only data from days across which the firing properties of the cell remained consistent were included in the analysis. The remaining spike data were collapsed across all included days, and an independent t-test compared spike counts in PSTH bins from all included days for the CX vs. CS period of each trial type (CMI or CME); a one-tailed cutoff of P = 0.05 was used to classify cells into nine categories based on the outcome of this comparison (see Classification of Cell Types in results).
Spike waveform parameters.
To classify neurons as principal cells vs. interneurons, a 3D scatterplot was generated in which each cell was plotted as a single point in a space with three dimensions: baseline firing rate (mean spike count per 1-s bin of the CX period), spike width (mean time interval between the initial departure of the cell from and subsequent return to voltage baseline), and spike area (cumulative sum of voltage across the spike width interval). A Gaussian mixture model with two components (1 for principal cells, 1 for interneurons) was then fit to the 3D point cloud from each region (PAG or mPFC), using MATLAB “fitgmdist” command. The resulting model was then used to classify each neuron into one of the two categories with MATLAB “cluster” command. The validity of the classification was tested by comparing the mean distance separating pairs of points within vs. between the two categories (see results).
Population-averaged PSTH.
To generate population-averaged PSTHs for CMI or CME trials, the PSTH of each cell was first normalized by converting its firing rate axis to a Z score. This was done by subtracting the mean and dividing by standard deviation of the firing rate during 20 bins from a baseline period, which was defined as the CX period of CMI trials (note that the CX period of CMI trials was used as the baseline for normalizing both CMI and CME histograms, so that population firing rates during the CX period of CMI vs. CME trials could be compared). Normalized histograms were then averaged across cells to generate the population PSTH.
Responses to pips and shocks.
Responses to auditory pips were analyzed on a short time scale by plotting PSTHs aligned to the onset of each CS pip using a bin size of 2 ms. Confidence intervals on the spike counts in each bin were computed from a baseline period spanning 500 ms (250 bins) before the onset of each pip, based on an assumption of Poisson spiking (Abeles 1982). A neuron was considered to exhibit auditory evoked responses if there were one or more bins exceeding 99% confidence or two or more bins exceeding 95% confidence, in the 250-ms (125 bins) time window spanning pip onset to offset. The response latency of the cell was computed as the time delay from pip onset to the first bin exceeding the 95% confidence threshold. Responses to shock pulses were analyzed in a similar manner, except that the baseline period for computing confidence intervals spanned 50 ms (25 bins) preceding each shock onset, and a neuron was considered to be shock responsive if there were one or more bins exceeding 99% confidence or two or more bins exceeding 95% confidence, in the time window spanning 6–100 ms (47 bins) following pip onset (the 0–6-ms time period was omitted because each 2-ms shock pulse generated a brief stimulus artifact that occluded recording at the time of the pulse). The latency of shock-evoked responses was computed as the time delay from shock onset to the first bin in the analysis window exceeding the 95% confidence threshold.
Freezing vs. flight pips.
To classify pips based on motor behavior, movement speed was measured in 1/30th-s time bins (the sampling period of the video tracker) during two 500-ms time windows, one before and one after the onset of each pip. If movement speed was significantly greater during the post-pip time window (P < 0.05 by an unpaired t-test, 1-tailed), then the pip was classified as a “flight” pip. If there was no change in the movement speed (P ≥ 0.05), and if the mean speed for all bins from the pre- and post-pip time windows combined (1 s total) was <0.01 standard deviation above 0, then the pip was classified as a “freezing” pip. Pips that did not meet criterion for classification as flight or freezing pips were discarded from analysis. To analyze neural population activity during freezing vs. flight pips, two PSTHs (bin size = 20 ms) were computed for each cell, one triggered by freezing pips and the other by flight pips. Both PSTHs were normalized by converting their firing rate bins to Z scores using the mean and standard deviation of spike counts from a baseline period defined as the 500-ms time window preceding the onset of freezing pips (note that the pre-pip period of freezing pips was used as the baseline for normalizing both freezing and flight PSTHs, so that population firing rates during the pre-pip period of freezing vs. flight pips could be compared). Statistical analyses compared Z scores at each time point, as described in results.
Histological Procedures
At the end of the experiment, rats were intraperitoneally injected with an overdose of pentobarbital (100 mg/kg) and perfused intracardially. Brains were extracted and fixed in a formalin sucrose solution. Tissue was later sectioned into 40-μm slices and mounted on slides for electrode placement verification.
RESULTS
Single-unit activity was recorded in dlPAG and mPFC while rats (n = 4) expressed conditioned fear responses to an auditory CS. A total of 74 neurons were recorded from dlPAG, with a mean baseline firing rate of 2.96 ± 0.36 Hz and a mean peak-to-peak spike height of 93.6 ± 1.0 μV. Most dlPAG cells (n = 57) were recorded from the dorsal PAG (dPAG), which is comprised of the dorsomedial and dorsolateral columns, and the remaining cells (n = 17) were recorded from the lateral column (lPAG). Reconstructions of dPAG and lPAG recording sites are shown in Fig. 1A. A total of 71 neurons were recorded from mPFC, with a mean baseline firing rate of 2.93 ± 0.32 Hz and a mean peak-to-peak spike height of 108.8 ± 3.1 μV. These cells were distributed among three mPFC subregions: anterior cingulate (ACC; n = 31), PL (n = 29), and IL (n = 11) subdivisions of mPFC (Fig. 1B).
Fig. 1.
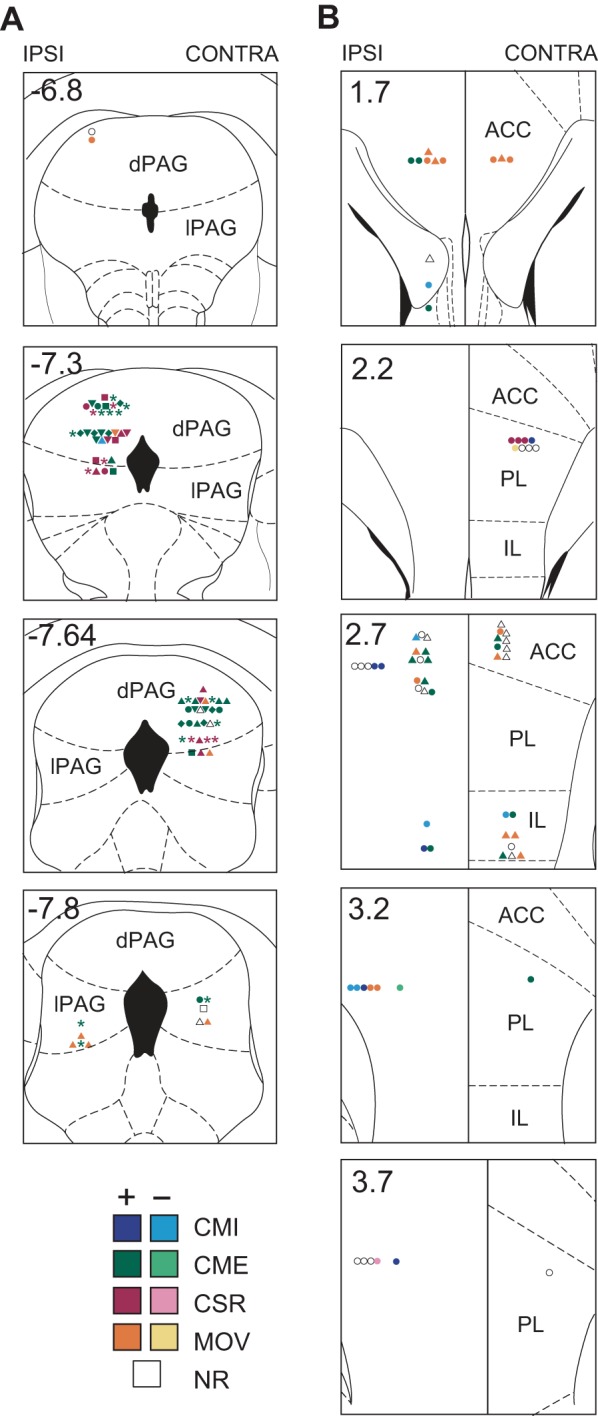
Histological reconstruction of recording sites in dorsolateral periaqueductal gray (dlPAG) and medial prefrontal cortex (mPFC). Reconstructed recording sites of dlPAG neurons (A; n = 74) and mPFC neurons (B; n = 71) are overlaid on coronal templates (with coordinates in mm relative to bregma) from the atlas of Paxinos and Watson (1997). Symbol colors indicate the type classification of each cell (see results), and symbol shapes indicate how the cell responded to conditioned stimulus (CS) pips or shock pulses (*, excited by pip and shocks; ▲, excited by shocks but not pips; ■, excited by pips but not shocks; ⧫, excited by pips and inhibited by shocks, ▼, no pip response and inhibited by shocks; ●, no response to pips or shocks). Left and right sides of the midline correspond to hemispheres ipsilateral (IPSI) and contralateral (CONTRA) to the eyelid where periorbital shocks were delivered. dPAG, dorsal PAG; lPAG, lateral PAG; ACC, anterior cingulate; PL, prelimbic; IL, infralimbic; CMI, conditioned movement inhibition; CME, conditioned movement excitation; CSR, CS responsive; MOV, movement cell; NR, nonresponsive.
Classification of Trial Types
Each experimental session consisted of 6 presentations of the CS alone followed by 16 CS-US pairings (Fig. 2A). Rats were trained on this regimen for 5 consecutive days before their first recording session, so that CS-evoked CMI and CME responses would be at or near asymptote when recordings began (confirmed by data analysis presented below). The same daily regimen of 6 CS-alone trials and 16 CS-US pairing trials then continued throughout the duration of the neural recording experiments, for up to 15 recording sessions. After the initial 5-day training period, fear-conditioning sessions were only conducted on days when well-isolated neurons were observed on the electrodes. Consequently, the number of recording sessions differed for each rat because of variability in cell yields: rat 1 was run for 15 sessions, rat 2 was run for 14 sessions, rat 3 was run for 10 sessions, and rat 4 was run for 6 sessions.
Fig. 2.
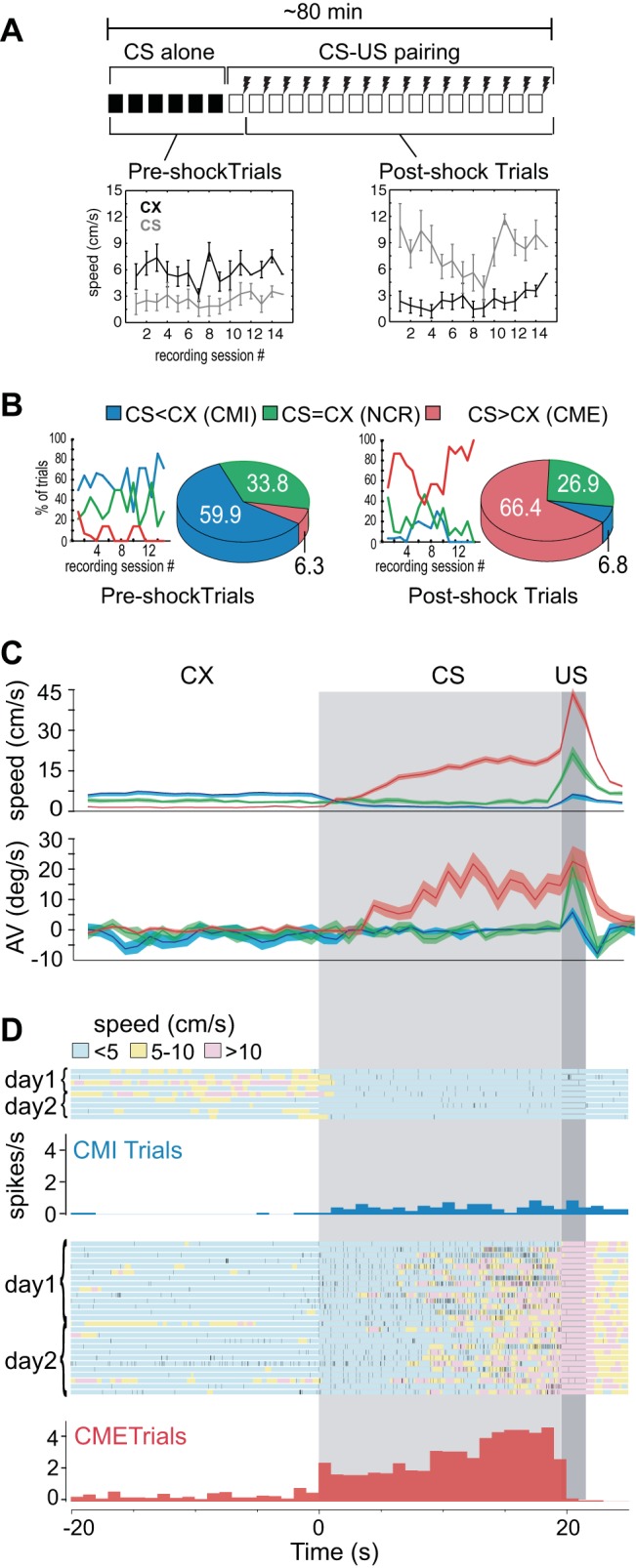
Behavioral and neural activity during fear-conditioning trials. A: each session consisted of 6 CS-alone trials followed by 16 CS-unconditioned stimulus (US) pairings, so there were 7 pre-shock and 15 post-shock trials per recording session; line graphs show mean movement speeds during the context (CX) and CS periods of pre- vs. post-shock trials. B: line graphs show percentage of trials (for all rats combined) that were classified as CMI, no conditioned response (NCR), or CME per session (session 1 is the first session after 5 days of initial training); pie charts show proportions of trial types from all sessions combined. C: movement speed (top) and turning bias (bottom) during the CX (unshaded area), CS (light gray shaded area), and US (dark gray shaded area) periods of each trial; data are averaged (AV) separately for CMI, NCR, and CME trials over all sessions. D: example of spike data from a dlPAG neuron recorded over 2 days; color of shading beneath spike rasters indicates the rat's movement speed during each trial, and rate histograms (1-s bins) show the mean firing rate of the cell averaged over all CMI and CME trials.
Within each trial, we define the CX period as the 20-s time window preceding onset of the first CS pip, the CS period as the 19.6-s time window between CS and US onset, and the US period as the 2-s period following US onset. On each experiment day, “pre-shock trials” were defined as the seven trials during which both the CX and CS preceded the first US presentation of the day (all 6 CS-alone trials, plus the first CS-US pairing trial), whereas “post-shock trials” were defined as the remaining 15 CS-US pairing trials. Figure 2A shows line graphs plotting the rats' mean movement speed (averaged first over trials, then over rats) during pre- and post-shock trials across recording sessions, up to the maximum of 15 sessions. Because each of the four rats was run for a different number of sessions (as explained above), the number of included rats decreased as the session number increased: n = 4 for sessions 1–6, n = 3 for sessions 7–10, n = 2 for sessions 11–14, and n = 1 for session 15.
The rats' movement speed was suppressed by the CS during pre-shock trials but enhanced by the CS during post-shock trials, in agreement with prior studies showing that a fear-conditioned CS evokes freezing responses in rats that have not recently been shocked and flight responses in rats that have recently been shocked (Halladay and Blair 2012; Tarpley et al. 2010). Confirming this, a 6 × 2 × 2 repeated-measures ANOVA with session (1st 6 sessions only because these were the only sessions that included all 4 rats), phase (pre- vs. post-shock), and stimulus (CX vs. CS) as factors yielded a significant two-way interaction between phase and stimulus (F1,3 = 14.6, P = 0.032). There was no main effect of session (F5,15 = 0.74, P = 0.60), nor was there any significant two-way or three-way interaction between session and any other variable (P > 0.1 for all interactions), supporting the conclusion that rats had reached behavioral asymptote during the pretraining phase before the first recording session so that their conditioned behavior did not change significantly across recording sessions.
Movement speed data in Fig. 2A indicates that, during recording sessions, rats exhibit two distinct modes of defensive responding to the same CS, CMI responses during pre-shock trials and CME responses during post-shock trials. To analyze relations between neural activity and defensive behavior, each experimental trial was classified into one of three types, depending on the rat's behavior during that trial (see materials and methods): 1) CMI trials were those during which the rat's movement speed was suppressed during the CS when compared against CX; 2) CME trials were those during which the rat's movement speed was enhanced during the CS when compared against CX; and 3) NCR trials were defined as those during which the rat's movement speed did not differ during the CS vs. CX. Pie graphs in Fig. 2B show that, across all sessions and rats, 59.9% of the pre-shock trials were CMI trials, and only 6.3% were CME trials (the rest were NCR trials). Conversely, 66.4% of the post-shock trials were CME trials, and only 6.8% were CMI trials (again, the rest were NCR trials). Line graphs in Fig. 2B show that this differential distribution of pre- vs. post-shock trial types persisted across all recording sessions.
Figure 2C (top) shows mean linear movement speeds during CMI, CME, and NCR trials, averaged over all trials of each type during which at least one neuron was recorded (total of 990 trials from 45 recording sessions in 4 rats). Figure 2C (bottom) shows the rats' mean turning velocity toward vs. away from the shocked eyelid during each trial type. A 3 × 2 ANOVA of turning velocity with trial type (CMI, CME, NCR) and stimulus (CX, CS) as independent factors yielded a significant interaction effect (F2,896 = 21.48; P < 0.00001), and Scheffe corrected post hoc comparisons revealed that turning during the CS for CME trials was greater than all other conditions (P < 0.00001 for all comparisons), but other conditions did not differ significantly from one another (P > 0.66 for all comparisons). These results show that, during the CS period of CME trials, rats turned away from the eyelid where shock delivery was anticipated, in accordance with prior work showing that these CS-evoked movements are flight CRs directed away from the eyelid where shock is expected (Halladay and Blair 2012; Tarpley et al. 2010).
Classification of Cell Types
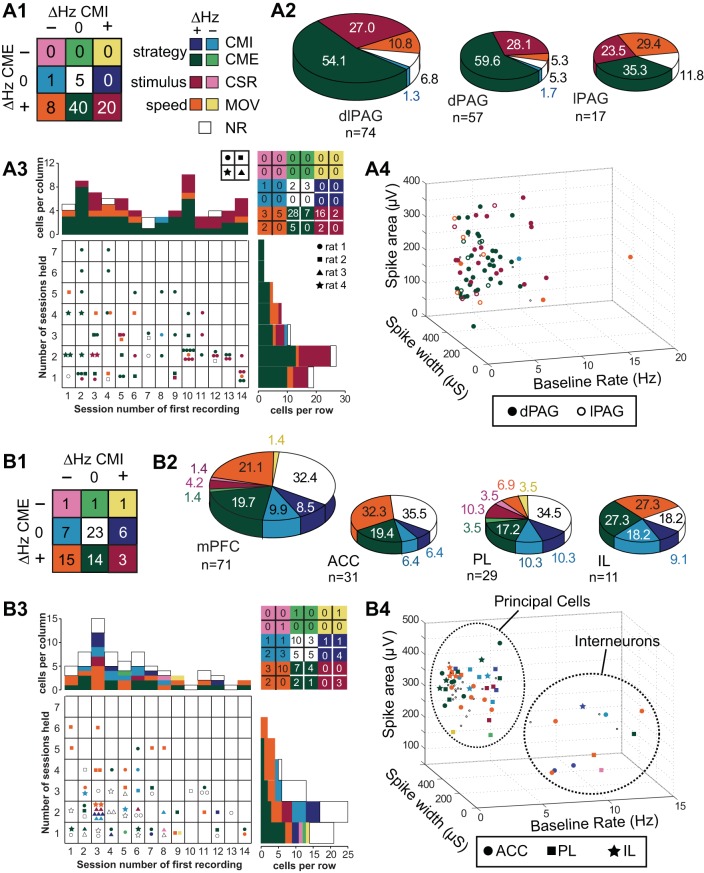
Neurons were classified into nine types, with each type corresponding to an entry in a 3 × 3 table (Fig. 3, A1 and B1), where rows denote the sign of the firing rate difference of the cell between the CX vs. CS periods of CME trials (ΔHz CME), and columns denote the sign of the firing rate difference of the cell between the CX vs. CS periods of CMI trials (ΔHz CMI). When a single cell was recorded during more than one session (see example, Fig. 2D), its type was classified based on spike data collapsed across consecutive sessions during which the cell met criteria for inclusion in the study (see materials and methods). The nine cell types fell into four functional categories: strategy-selective cells that responded to the CS differently depending on the rat's behavioral response to the CS, stimulus-selective cells that responded to the CS in the same way regardless of the rat's behavior, speed-selective cells that fired in correlation with the rat's movement speed regardless of whether the CS was present, and nonresponsive (NR) cells that did not exhibit any clear task-related activity.
Fig. 3.
Cell type classifications in dlPAG (A) and mPFC (B). A1 and B1: summary table provides a color-coded key for the number of cells of each type; labels denote which cell types belong to the strategy-, stimulus-, and speed-selective categories. A2 and B2: large pie chart shows proportion of cells from each region that was classified in each type category; small pie charts show proportions of cell classifications by subregion. A3 and B3: table shows distribution of recording days across which cells were held (vertical axis) and of which session cells were first encountered in (horizontal axis, session 1 is the first session after 5 days of initial training); type category is indicated by symbol color, and rat is indicated by symbol shape (table at upper right summarizes total number of cells in each category by rat). A4 and B4: distributions of waveform parameters for all cells, with symbol shapes denoting which subregion the cell was recorded from (NR cells are plotted as small dots).
Strategy-selective cell types.
Four cell types were classified as strategy-selective neurons because their firing rates during the CS depended on the rat's defensive response strategy during the CS. If the firing rate of a neuron changed during the CS period of CMI but not CME trials, then the neuron was classified as a CMI+ cell if its firing rate increased (CS > CX) during the CS period or as a CMI- cell if its firing rate decreased (CS < CX) during the CS period of CMI trials. Conversely, if the firing rate of a neuron changed during the CS period of CME but not CMI trials, then the neuron was classified as either a CME+ or CME- cell, depending on whether its firing rate increased or decreased, respectively, during the CS period of CME trials.
Stimulus-selective cell types.
Two cell types were classified as stimulus-selective neurons because these neurons always responded to the auditory CS in the same way, regardless of the rat's defensive response strategy during the CS. A neuron was classified as a CS-responsive (CSR) cell if CS presentations evoked firing rate changes of the same sign during both CMI and CME trials; CSR+ cells always increased their firing rate during the CS, and CSR- cells always decreased their firing rate during the CS. Because the CS predicted the same threat (the eyelid shock US) during both CMI and CME trials, it was not possible to dissociate whether stimulus-selective neurons were selective for the auditory sensory properties of the CS, motivational valance properties, or some other uncontrolled aspect of the CS.
Speed-selective cell types.
Two cell types were classified as speed-selective neurons because they were always modulated by the rat's movement speed in the same way, regardless of other factors. As shown in Fig. 2A, the CS evoked decreases in movement speed during CMI trials and evoked increases in movement speed during CME trials. A neuron was classified as a movement cell (MOV) if CS presentations likewise evoked firing rate changes of the opposite sign during CMI vs. CME trials. MOV+ cells changed their firing rate in the same direction as the change in the rat's movement speed (decreased firing during CMI trials and increased firing during CME trials), whereas MOV- cells change their firing rate in the opposite direction from the rat's movement speed (increased firing during CMI trials and decreased firing during CME trials).
NR neurons.
NR cells were neurons that did not change their firing rate between the CX vs. CS period of either CMI or CME trials. The firing rates of these neurons were not related in any clear way to the rat's behavior during the task.
Distribution of Cell Types in PAG
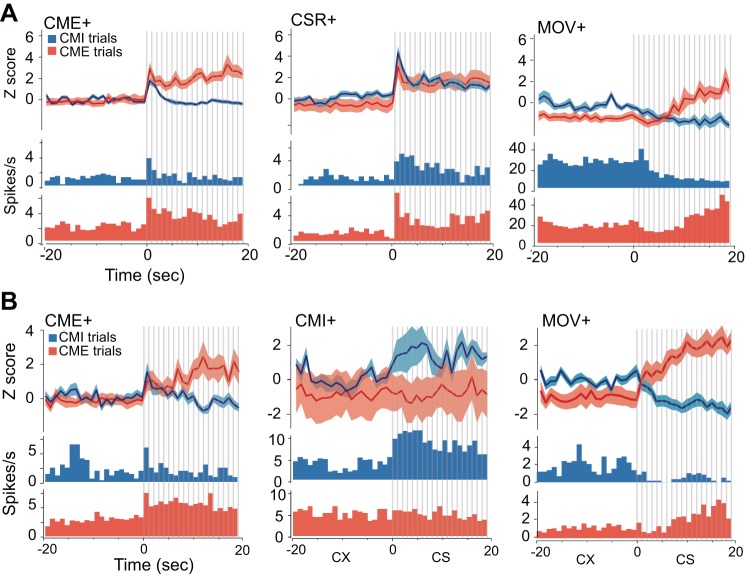
Out of 74 neurons recorded in dlPAG, 40 cells (54%) were classified as CME+ cells, 20 cells (27%) were classified as CSR+ cells, 8 cells (11%) were classified as MOV+ cells, 5 cells (8%) were classified as NR cells, and 1 cell was classified as a CMI- cell (Fig. 3, A1 and A2). Population averages and example neurons for three prevalent dlPAG cell types (CME+, CSR+, and MOV+) are shown in Fig. 4A. Notice that CME+ cells exhibited sustained firing throughout the CS period of CME trials but not CMI trials (which is why they were classified as CME+ cells), but CME+ cells responded to the initial onset of the CS during both CME and CMI trials; possible explanations for this will be addressed in discussion.
Fig. 4.
Dominant cell types in dlPAG and mPFC neurons. A: each panel shows population-averaged firing rates (top) and a peristimulus time histogram (PSTH) for 1 example cell (bottom) for the 3 most prevalent cell types recorded in dlPAG: CME+ cells (n = 40), CSR+ cells (n = 20), and MOV+ cells (n = 8). B: population-averaged firing rates (top) and a PSTH for 1 example cell (bottom) for 3 prevalent cell types recorded in mPFC: CME+ cells (n = 14), CMI+ cells (n = 6), and MOV+ cells (n = 15). For all graphs, bin size is 1 s, and vertical gray bars indicate individual CS pips.
Baseline firing rates of dlPAG neurons during the CX period were analyzed using a 4 × 2 ANOVA with cell type (CME+, CSR+, MOV+, and NR) as an independent factor and trial type (CMI and CME) as a repeated factor (Table 1); there was a significant main effect of trial type (F1,70 = 5.28, P = 0.02) but not cell type (F1,70 = 1.09, P = 0.36) with no interaction (F3,69 = 1.53, P = 0.22). Post hoc comparisons revealed that only cells belonging to the MOV+ category showed significantly lower baseline firing rates during the CX period of CME trials vs. CMI trials (P = 0.047). This supports the conclusion that speed was the primary determinant of firing rates for MOV+ cells because movement speed was also lower during the CX period of CME than CMI trials (see Fig. 2C). All other cell types exhibited similar baseline firing rates during the CX periods of CME vs. CMI trials.
Table 1.
Trial type columns show mean CX firing rates during CMI vs. CME trials for main cell types recorded in dlPAG and mPFC
| Trial Type |
HEMI% |
|||||
|---|---|---|---|---|---|---|
| Site | Cell Type | N | CMI | CME | IPSI | CONTRA |
| dlPAG | CME+ | 40 | 2.3 ± 0.3 | 2.2 ± 0.3 | 51.2 | 57.6 |
| dlPAG | CSR+ | 20 | 4.4 ± 2.8 | 2.9 ± 1.7 | 31.7 | 21.2 |
| dlPAG | MOV+ | 8 | 3.6 ± 0.7 | 3.1 ± 0.7* | 12.2 | 9.1 |
| dlPAG | NR | 5 | 3.1 ± 1.3 | 2.9 ± 1.2 | 2.4 | 12.1 |
| mPFC | NR | 23 | 2.0 ± 0.7 | 1.7 ± 0.5 | 29.2 | 36.7 |
| mPFC | MOV+ | 15 | 3.5 ± 1.0 | 2.5 ± 0.8† | 17.0 | 26.7 |
| mPFC | CME+ | 14 | 1.9 ± 1.0 | 2.0 ± 1.0 | 22.0 | 16.7 |
| mPFC | CMI+ | 6 | 3.3 ± 1.4 | 2.4 ± 0.9 | 12.2 | 3.3 |
| mPFC | CMI- | 7 | 5.7 ± 1.5 | 5.7 ± 2.6 | 14.6 | 3.3 |
| mPFC | CSR+ | 3 | 5.1 ± 3.8 | 6.5 ± 5.7 | 0.0 | 10.0 |
Values are means ± SD.
P < 0.05 and
P <0.10 for 2-tail paired t-test comparing conditioned movement inhibition (CMI) vs. conditioned movement excitation (CME) trials. Hemi% columns show the percentage of cells that were classified as a given type in the brain hemisphere ipsilateral (IPSI) vs. contralateral (CONTRA) from the shocked eyelid [41 IPSI and 33 CONTRA cells were recorded in dorsolateral periaqueductal gray (dlPAG); 41 IPSI and 30 CONTRA cells were recorded in medial prefrontal cortex (mPFC)]. CX, context; CSR, conditioned stimulus responsive; MOV, movement cell; NR, nonresponsive.
We performed a series of 2 × 2 χ2 tests to examine whether specific types of cells were more common in the lPAG vs. dPAG subregions. Only MOV+ cells were unevenly distributed, constituting 30% of lPAG cells but only 5% of dPAG cells (χ21,74 = 7.92, P = 0.005, uncorrected). To examine whether certain cell types became more or less prevalent over the course of the experiment, we analyzed whether the type classification of a cell was dependent on the session number of the first day on which it was recorded (columns in Fig. 3A3). About half of the PAG neurons were first recorded during or before session 7 (early cells), whereas the remaining half were first recorded during or after session 8 (late cells). CSR+ cells constituted 18% of cells recorded early in the study and 38% of cells recorded late in the study (χ21,74 = 4.01, P = 0.045, uncorrected), but this difference was not significant after correction for multiple comparisons; no other cell type was differentially distributed across early vs. late recording sessions (P > 0.05 for all uncorrected χ2 tests). To examine the influence of data sampling on cell types, we tested whether the type classification of a cell was contingent on the number of days across which is was held (rows in Fig. 3A3). PAG neurons were held across a median of two sessions per neuron; no cell type was more or less prevalent among neurons that had been recorded for more vs. fewer than the median number of sessions (P > 0.05 for all χ2 tests). We also examined whether PAG cell type classifications were contingent on whether the cell was recorded in the hemisphere ipsilateral vs. contralateral from the shocked eyelid (see Table 1) and found no evidence for such a contingency in any cell type. Finally, we tested whether any cell type was more common in specific rats. PAG neurons were recorded from three of the four rats in the study, and a series of 3 × 2 χ2 tests yielded no evidence that any cell type was significantly more or less common in specific rats (P > 0.05 for all χ2 tests).
To test whether PAG cells could be subdivided into principal cells vs. interneurons, a 3D scatter plot was generated from their spike area, spike width, and baseline firing rate parameters (Fig. 3 A4); then a two-component Gaussian mixture model was used to cluster spikes into two categories (see materials and methods). The mean between-category distance was only 8% larger than the mean within-category distance separating points in the scatterplot, so the spike parameters did not separate into two well-distinguished clusters. Hence, PAG neurons were not easily classifiable into categories corresponding to principal cells vs. interneurons.
Distribution of Cell Types in mPFC
Neurons recorded in mPFC exhibited a broader diversity of type classifications than those in dlPAG. Figure 3, B1 and B2, shows that, out of 71 neurons recorded in mPFC, 23 cells (32%) were classified as NR cells, 15 (23%) cells were classified as MOV+ cells (with 1 additional neuron classified as a MOV- cell), 14 (20%) cells were classified as CME+ cells (with 1 additional neuron classified as a CME- cell), 6 cells (8%) were classified as CMI+ cells, 7 cells (10%) were classified as CMI- cells, and 3 cells (4%) were classified as CSR+ cells (with 1 additional neuron classified as a CSR- cell). Figure 4B shows population averages and example neurons for prevalent mPFC cell types (CME+, CMI+, and MOV+). Notice that, like CME+ cells in PAG, CME+ cells in mPFC tended to respond to the initial onset of the CS during both CMI and CME trials (see discussion).
A 5 × 2 ANOVA of baseline firing rates for the most prevalent cell types recorded in mPFC (NR, CME+, CMI+, CMI-, and MOV+) revealed a significant main effect of trial type (F1,68 = 5.44, P = 0.02) but not of cell type (F1,68 = 0.54, P = 0.66) with no interaction (F4,68 = 1.8, P = 0.16). Post hoc comparisons revealed that, as in PAG (see above), only cells belonging to the MOV+ category trended toward lower baseline firing rates during the CX period of CME vs. CMI trials (P = 0.054), again reinforcing the conclusion that movement speed was the primary determinant of firing rates for MOV+ cells. All other cell types exhibited stable baseline firing rates during the CX periods of CME vs. CMI trials.
We performed a series of 3 × 2 χ2 tests to investigate whether cells in ACC, PL, or IL were more likely to be of a specific type. As in PAG, only MOV+ cells were unevenly distributed across subregions, constituting 32% of ACC cells and 27% of IL cells but only 7% of PL cells (χ22,71 = 6.08, P = 0.048, uncorrected). All CSR+ and CSR- cells were recorded in PL, with none in ACC or IL, but the small sample of such cells (n = 4) did not supply sufficient statistical power to test whether CSR neurons were more concentrated in PL than other subregions. About half of the mPFC cells were first recorded during or before session 4 (early sessions), and the remainder were recorded during or after session 5 (late sessions). No cell type in mPFC was differentially distributed across early vs. late recording sessions (P > 0.05 for all uncorrected χ2 tests). Individual mPFC neurons were held for a median of two recording sessions (Fig. 3B3), and a 2 × 2 χ2 test indicated that MOV+ cells constituted 40% of neurons that had been held for more than the median number of sessions but only 11% of neurons that had been held for less than or equal to the median number of sessions (χ22,71 = 8.25, P = 0.004, uncorrected). The prevalence of no other cell type was contingent on the number of sessions over which the cell was held (P > 0.05 for all χ2 tests). We examined whether mPFC cell type classifications were contingent on which hemisphere the cell was recorded in (ipsilateral vs. contralateral from the shocked eyelid, see Table 1) and found no evidence for lateralization of any cell type. We also tested whether any cell type was more common in specific rats; mPFC neurons were recorded from all four rats in the study, and a series of 4 × 2 χ2 tests indicated that MOV+ cells were more prevalent in rat 2 than in the other three rats (χ23,71 = 15.5, P = 0.001, uncorrected). No other cell type was significantly more common in any given rat, but it should be noted that small sample sizes made this difficult to assess for some cell types.
To test whether mPFC cells were separable into principal cells vs. interneurons, a 3D scatter plot was generated from their spike area, spike width, and baseline firing rate parameters (Fig. 3B4); then a two-component Gaussian mixture model was used to cluster spikes into two categories (see materials and methods). The mean between-category distance was 90% larger than the mean within-category distance separating points in the scatterplot, so mPFC spikes were readily classifiable into categories corresponding to principal cells (with low firing rates and large spike widths) vs. interneurons (with high firing rates and narrow spike widths). Of the 71 mPFC cells we recorded, 58 were classified as principal cells, and 13 were classified as interneurons (Fig. 3B4). We examined whether mPFC cell type classifications were contingent on whether the cell was a principal cell or interneuron and found that CMI+ cells constituted 23% of interneurons but only 5% of principal cells (χ21,71 = 5.54, P = 0.019, uncorrected). Moreover, the few CMI+ principal cells (n = 3) we did observe were all found in the PL subregion, which is consistent with prior evidence (Burgos-Robles et al. 2009; Sotres-Bayon et al. 2012) suggesting that PL principal cells may promote freezing behavior (see discussion). No other cell type differed in prevalence among principal cells vs. interneurons.
Comparison of Cell Types in PAG vs. mPFC
A 4 × 2 χ2 test indicated that cell types in different functional categories (strategy-selective, stimulus-selective, speed-selective, or NR) were differentially distributed between dlPAG vs. mPFC (χ23,145 = 27.3, P < 0.00001). This remained true even when NR cells were omitted (χ22,117 = 12.4, P = 0.002), so the effect was not wholly attributable to the larger number of NR cells in mPFC. Stimulus-selective neurons were more prevalent in dlPAG (27% of cells, all of which were CSR+ cells) than mPFC (6% of cells), whereas speed-selective neurons (all but 1 of which were MOV+ cells) were more prevalent in mPFC (22% of cells) than dlPAG (10% of cells).
Strategy-selective neurons constituted a large proportion of cells in both dlPAG (55% of cells) and mPFC (39% of cells), but different types of strategy-selective neurons were observed in each area (χ23,69 = 22.4, P = 0.00006). Almost all (97.5%) of the strategy-selective neurons in dlPAG were CME+ cells, whereas only half (50%) of the strategy-selective neurons in mPFC were CME+ cells; another quarter (25%) were CMI- cells and fifth (21%) were CMI+ cells. This suggests that dlPAG may be specifically involved in the execution of CME responses, in accordance with prior evidence that this area orchestrates escape and avoidance behavior (Bandler and DePaulis 1988; De Oca et al. 1998; Fanselow 1991). By contrast, the diversity of strategy-selective neurons in mPFC suggests involvement in selecting between competing defense strategies, such as CME vs. CMI responses, in accordance with prior theories positing that mPFC mediates behavioral strategy selection by altering how stimuli are mapped onto responses in other brain regions (Miller and Cohen 2001). To further investigate the roles of dlPAG and mPFC in defensive responding, we analyzed their spike activity on a shorter time scale (see below).
Pip-Evoked Responses
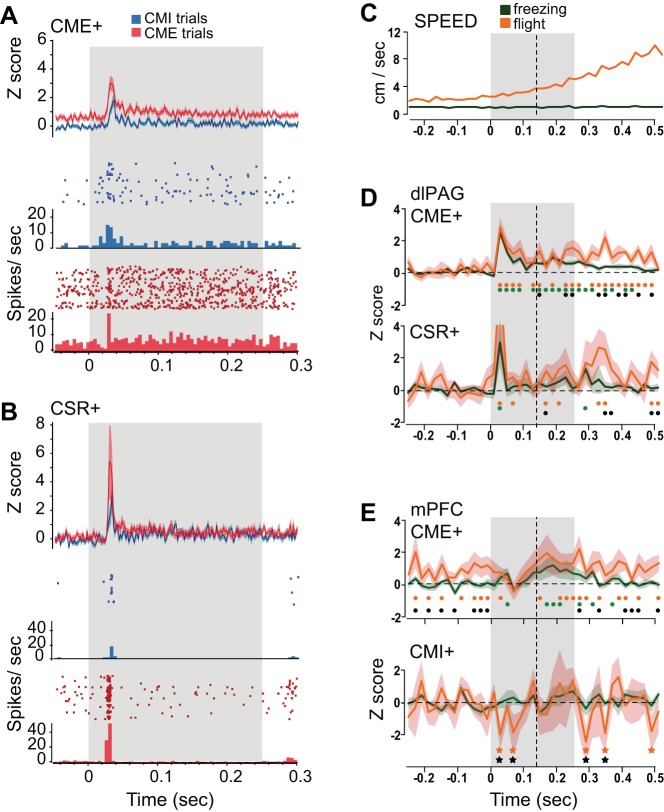
Short-latency responses to CS pips were analyzed on a fast time scale to assess whether neurons exhibited sensory-evoked responses to the auditory pips. About half of the dlPAG cells (34 of 74, or 46%) exhibited excitatory responses that were time locked to pip onset, with a mean onset latency of 32.7 ± 1.7 ms (no dlPAG cells showed evidence of being inhibited by pip onset). None of the pip-responsive cells in dlPAG belonged to the MOV+ category, supporting the interpretation that motor rather than sensory influences were the primary determinant of firing rate changes for MOV+ cells. Pip responses were observed for 58% (23/40) of CME+ cells (Fig. 5A) and 50% (10/20) of CSR+ cells (Fig. 5B) in dlPAG; in addition, one of five NR cells was pip responsive. Pip responsiveness was observed in 36% (12/33) of PAG cells recorded contralateral and 51% (21/41) of cells recorded ipsilateral to the shocked eyelid, which was not a significant difference (χ21,74 = 2.2, P = 0.14). Unlike dlPAG neurons, none of the individual mPFC neurons exhibited significant short-latency CS-evoked responses (either excitatory or inhibitory) that were time locked to the CS onset. However, pip-evoked inhibition was observed for population-averaged responses of CMI+ cells in mPFC, as discussed further below.
Fig. 5.
Pip-evoked responses of dlPAG and mPFC neurons. A: short-latency pip-evoked responses of CME+ neurons in dlPAG, Top: population-averaged firing rates (n = 40); bottom: rasters and PSTHs during CMI and CME trials for 1 example of a pip-responsive CME+ cell (bin size = 2 ms; gray shaded region indicates time window of the 250-ms pip presentation). B: same as A except data are shown for the population of CSR+ cells in dlPAG (n = 20, top) and 1 example of a pip-responsive CSR+ cell (bottom). C: mean movement speed calculated in 20-ms bins within a time window surrounding all freezing and flight pips (see materials and methods for definitions) during which neurons were recorded; gray shading indicates the 250-ms pip, and dashed line marks the time at which the mean speed first exceeds the pre-pip baseline during flight trials (Z test, P < 0.05 of individual time points against all samples from the 500-ms period before pip onset). D: population-averaged responses of CME+ and CSR+ cells in dlPAG during freezing vs. flight pips (20-ms bins); colored dots (green for freezing and orange for flight) mark time points at which the population Z score exceeded 0 (P < 0.05 against the mean pre-pip firing rate for freezing pips), whereas black dots mark time points at which the Z score for flight pips exceeded that for freezing pips (P < 0.05). E: same as D except data are shown for CME+ and CMI+ cells in mPFC; bottom: colored stars mark time points at which the population Z score fell significantly below 0 (P < 0.05 against the mean pre-pip firing rate for freezing pips).
To further dissociate sensory, behavioral, and motivational influences on CS-evoked firing of recorded neurons, individual CS pips from post-shock trials were parsed for membership in two categories, freezing or flight, based on the animal's behavior during each pip (pre-shock trials were not included, to eliminate the confounding influence of recent shock on behavior and neural activity). Freezing pips were defined as those during which the animal remained motionless before and after the pip, whereas flight pips were defined as those for which the animal's movement speed was significantly greater during the post- than pre-pip period (note that pips not belonging to either category were discarded from the analysis).
During flight pips, averaged movement speed became significantly elevated above the pre-pip baseline at a motor response latency of ∼140 ms after pip onset (dashed vertical lines in Fig. 5, C–E). Both freezing and flight pips elicited phasic spiking responses (concentrated in the 20–40-ms bin) from CME+ and CSR+ cells in dlPAG (Fig. 5D). For CME+ cells, the initial phasic response was similar in magnitude for both freezing and flight pips. The phasic response was followed by a sustained elevation of firing, which was significantly larger during flight than freezing pips at time points beyond (but not before) the 140-ms motor response latency (black dots in Fig. 5D, top). A pair of binomial tests confirmed that this result, whereby the flight pip response exceeded the freezing pip response only at time points after (but not before) onset of movement, was extremely unlikely to arise by chance (P < 0.00001).
Firing rates of CME+ cells in mPFC were also larger for flight pips than freezing pips but at different time points (black dots in Fig. 5E, top). When flight pips were compared against freezing pips, CME+ cells in mPFC showed higher firing rates during flight pips for the 13 time bins (260 ms) before pip onset (P < 0.0001, based on a binomial test for 7/13 successes at 5% success rate), as well as for the 12 time bins (240 ms) after pip offset (P < 0.001, based on 6/12 successes), but not during the pip period itself (0/13 successes). This lack of elevated firing rate during the pip period of flight pips appeared to be caused by a phasic inhibition of mPFC CME+ cells at ∼80 ms after the onset of flight pips, which reduced their firing rate to a level similar to that seen during freezing pips (Fig. 5E).
One possible explanation for the elevated firing rates of mPFC CME+ cells before the onset of flight pips could be that CME+ cells in mPFC were sensitive to movement speed because the rats' average movement speed was slightly elevated before the onset of flight pips compared with freezing pips (Fig. 5C). However, movement speed was even more elevated after the offset than before the onset of flight pips (Fig. 5C), yet CME+ cells in mPFC exhibited similar firing rates both before onset and after offset flight pips (Fig. 5E, top). Moreover, movement speed was lower during the CX period of CME than CMI trials (see Fig. 2B), but baseline firing rates of CME+ cells did not decrease along with movement speed during the CX period of flight trials (Fig. 3D, left). These results imply that movement speed was not a primary influence on the firing rates of CME+ cells in mPFC.
In contrast with CME+ cells, population-averaged responses of CMI+ cells in mPFC were smaller for flight pips than freezing pips, mainly at time points clustered after pip onset and offset (black stars in Fig. 5E, bottom). Binomial tests did not reveal a significant overall reduction in firing rate during the flight pip period (P = 0.14, 2/13 successes) but did indicate a significantly reduced firing rate during the time period spanning 250 ms before onset to 250 ms after offset of flight pips when compared against freezing pips (P = 0.04, 5/38 successes). By definition, CMI+ cells increased their firing rates during the CS of CMI trials, so it would be desirable to test whether these cells increased their firing rates during a subset of pips that evoked a decrease in movement speed (in the same way that flight pips evoked an increase in movement speed). Unfortunately, it was not possible to perform such an analysis because CMI behavior tended to be expressed tonically over a time scale of seconds, and thus movement cessation was not phasically evoked by pips in the same manner as CME behavior. Because very few pips were followed at short latency by a significant decrease in movement speed, it was not possible to analyze neural activity during pips that evoked movement cessation.
Shock-Evoked Responses
Shock-evoked responses of dlPAG and mPFC neurons were analyzed on a short time scale by plotting PSTHs triggered by individual shock pulses. In dlPAG, more than half of the recorded cells (41 of 74) were excited by shock pulses (see example in Fig. 6A, left) with a mean onset latency of 15.4 ± 1.1 ms, whereas about a quarter of the cells (17 of 74) were inhibited by shock pulses (see example in Fig. 6A, right). Shock responsiveness in dlPAG was not contingent on the type classification of a cell (Fisher's exact test, P = 0.66), but was contingent on whether a cell was recorded in dPAG vs. lPAG (Fisher's exact test, P = 0.02). Binomial tests revealed that this was because cells inhibited by shock were found exclusively in dPAG and not in lPAG (P = 0.02), whereas cells excited by shock were evenly distributed between dPAG and lPAG (P = 0.43), as were cells that were NR to shocks (P = 0.60). A 3 × 2 χ2 test revealed that shock responsiveness was also asymmetric across hemispheres (χ21,74 = 7.16, P = 0.028); while excitatory responses to shock were similar among cells recorded in the dlPAG ipsilateral (58%) vs. contralateral (54%) to the shocked eyelid, inhibitory responses were more prevalent in the ipsilateral (33%) than contralateral (15%) hemisphere and non-shock-responsive cells were rarer in the ipsilateral (9%) than contralateral (32%) hemisphere.
Fig. 6.
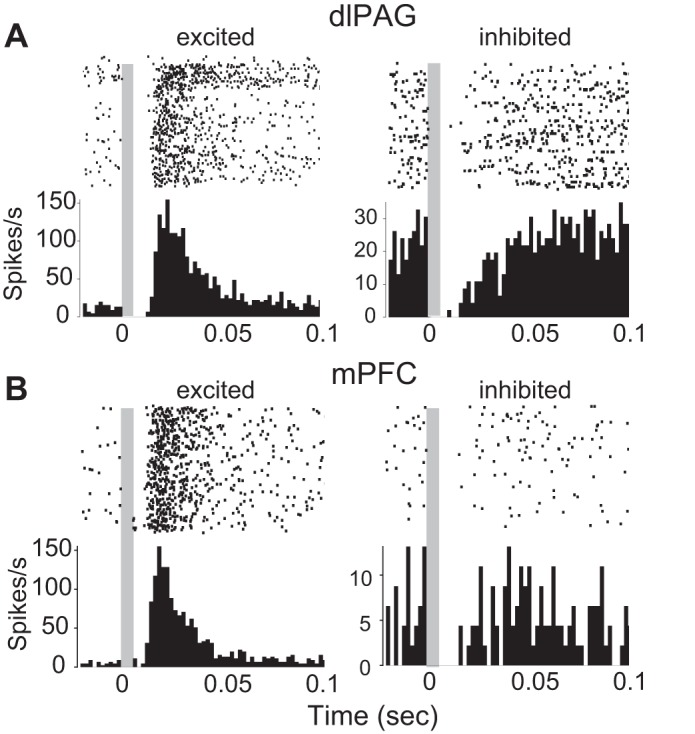
Shock-evoked responses of dlPAG and mPFC neurons. A: spike rasters and PSTHs for 2 example cells recorded in dlPAG that were excited (left) and inhibited (right) by shock pulses; t = 0 marks onset of the 2-ms shock pulse, and gray shading indicates the ∼6-ms time window during which spike recording was occluded by stimulus artifact (see materials and methods). B: same as A except data are shown for 2 example cells recorded in mPFC.
In mPFC, about a third of the cells (24 of 71) were excited after the onset of shock pulses (see example in Fig. 6B, left), whereas only 4% were inhibited by shock (3 of 71 cells, all CMI+ cells recorded ipsilateral to the shocked eyelid; see example in Fig. 6B, right), and the remaining cells (44 of 71) were NR to shocks. A χ2 test indicated that the proportion of cells excited by shock differed significantly among mPFC subregions (χ22,71 = 8.8, P = 0.01). Approximately half of the cells in ACC (15 of 31) were excited by shock with an onset latency of 16.1 ± 1.5 ms, and half of the cells in IL (5 of 11) were excited by shock with an onset of 13.2 ± 2.3 ms. By contrast, only 14% (4 of 29) of cells in PL were excited by shock, with a longer mean onset latency of 28.8 ± 9.3 ms. A 3 × 2 χ2 test revealed that shock responsiveness of mPFC neurons was not contingent on whether they were recorded ipsilateral vs. contralateral from the shocked eyelid (χ21,71 = 3.83, P = 0.15).
Longer-lasting effects of the shock US were analyzed by averaging behavior and neural activity during CS-US pairing trials throughout a 160-s time window beginning with the onset of the CX period (that is, 20 s before CS onset), and ending 2 min after offset of the shock US (Fig. 7). Rats exhibited turning away from the shocked eyelid during the CS and US, but turning ceased within a few seconds following US offset (Fig. 7A, top). By contrast, movement speed remained elevated above the CX baseline for nearly 2 min following shock offset (Fig. 7A, bottom). Visual observation indicated that, during this time period, animals exhibited bouts of freezing (which became longer in duration with increasing latency from shock offset) punctuated by rapid scurrying among different locations (which became less frequent with increasing latency from shock offset), as if the shock made them uncertain of which location in the environment was the safest place to freeze. In dlPAG, firing rates of CME+, CSR+, and MOV+ cells remained elevated above baseline throughout much of this post-shock period of motor activity (Fig. 7B). CSR+ cell firing rates fell below the CX baseline near the end of the motor activity period.
Fig. 7.
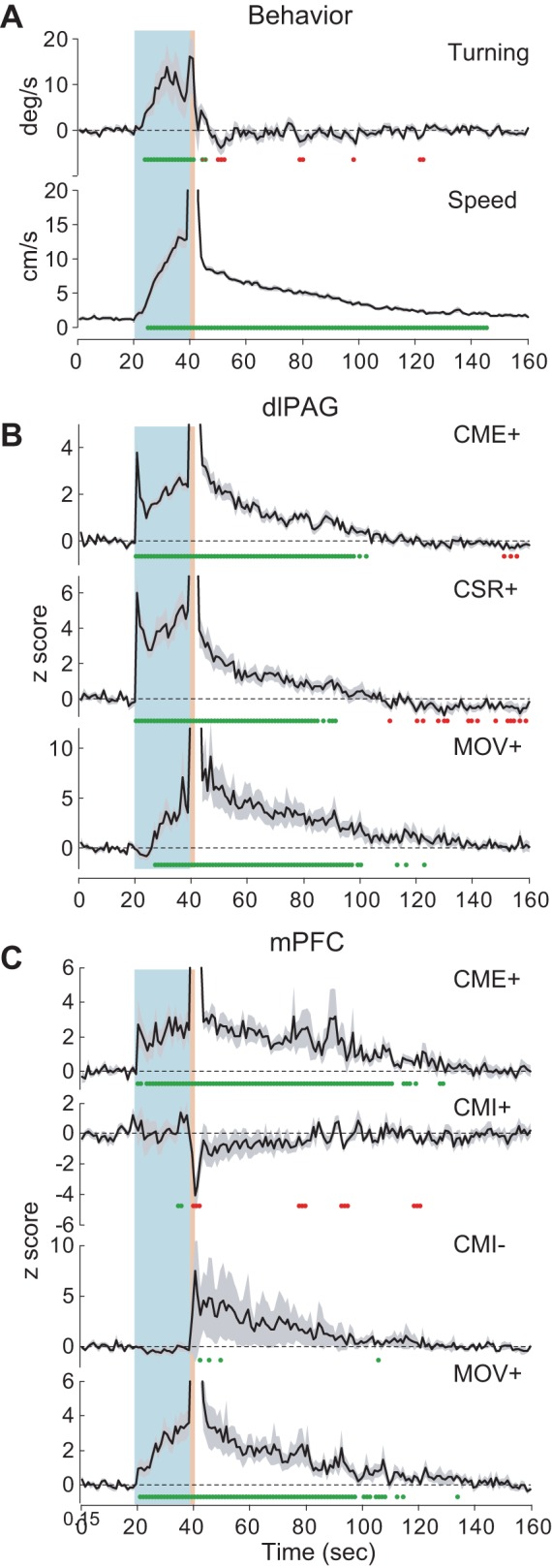
Post-shock responses of dlPAG and mPFC neurons. A: mean turning velocity (top) and movement speed (bottom) averaged over all CS-US pairings (CMI and CME trials combined) during which neurons were recorded; blue and red shaded regions indicate the CS and US periods, respectively, whereas green dots indicate time points at which the plotted value is significantly (P < 0.05) above or below the mean from the CX baseline period. B: population-averaged firing rates during CS-US pairings for CME+, CSR+, and MOV+ cells recorded in dlPAG. C: population-averaged firing rates during CS-US pairings for CME+, CMI+, CMI-, and MOV+ cells recorded in mPFC.
In mPFC, firing rates of CME+, CMI-, and MOV+ cells were elevated above baseline throughout much of the post-shock period of motor activity (Fig. 7C). Conversely, the population average firing rate for CMI+ cells decreased during the post-shock period. Post-shock firing rate changes for CMI+ and CMI- cells did not reach the P < 0.05 significance level at most time points, probably because the sample size was smaller than for other cell types.
DISCUSSION
Here we recorded neural activity from mPFC and dlPAG while rats expressed two distinct defensive behaviors, CMI vs. CME responses, elicited by the same fear-conditioned CS. During each experimental session, shock delivery served as a catalyst for inducing a transition in the rat's defensive response strategy; the CS elicited only CMI responses during pre-shock trials but then began to elicit CME responses during post-shock trials. This behavioral transition may be interpreted as a rightward shift along the “predatory imminence continuum,” whereby an animal's defensive response strategy changes as a function of its proximity to danger (Bolles 1970; Fanselow and Lester 1988). CMI responses may be classified as a “post-encounter” defensive behavior that is expressed when danger is present but not proximal, whereas CME responses may be classified as a “circa-strike” (or in this case, “circa-shock”) defensive behavior that is expressed when danger is in close proximity. Hence, a CS that has not recently been paired with shock may signal a distal threat that elicits CMI responses, whereas, after recently being paired with shock, the same CS may evoke a greater sense of proximity to danger and thus begin to trigger CME responses (Tarpley et al. 2010).
Our single-unit recording experiments indicated that about 40% of mPFC neurons were strategy-selective cells that responded differentially to the CS, depending on whether it elicited CMI or CME responses from the rat. This result is consistent with prior evidence that mPFC participates in regulating the selection of defensive-response strategies, as a function of proximity to danger (Mobbs et al. 2007, 2009). However, a limitation of our experimental design was that the rat's behavioral strategy was confounded with other variables such as recency of shock delivery and perhaps also the degree of fear during the CS (because the rat's expectation of the shock may have been greater during post-shock trials than pre-shock trials). As discussed below, even when these confounding factors are considered, our findings suggest that some mPFC neurons may indeed participate in regulating the animal's selection of defensive actions as a function of predatory imminence.
Stimulus-Selective Cells
Stimulus-selective neurons (those that responded to the CS regardless of whether it elicited CMI or CME behavior) constituted 27% (20/74) of the neurons recorded in dlPAG, all of which were CSR+ cells excited by the CS, but <6% (4/71) of the neurons recorded in mPFC. Interestingly, all of the stimulus-selective mPFC neurons (3 CSR+ cells and 1 CSR- cell) were located in the PL subregion, so that 15% (4/29) of PL neurons were stimulus selective. Because the CS predicted the same threat (the eyelid shock US) during both CMI and CME trials, it was not possible to dissociate whether stimulus-selective neurons were tuned for the auditory properties of the CS or its motivational valence properties. Clearly, if any recorded neuron functioned as a low-threshold “fear cell” that fired whenever the rat's anticipation of shock exceeded the minimum required for eliciting CMI behavior, then that neuron should be expected to behave as a stimulus-selective cell in our experiment (because the low threshold for shock anticipation of the cell would presumably be exceeded during both CMI and CME trials). By this reasoning, the paucity of stimulus-selective neurons in mPFC (especially in the ACC and IL subregions) implies a paucity of cells that simply responded whenever the rat's anticipation of shock exceeded the minimum threshold for eliciting CMI behaviors. One possible explanation for this lack of low-threshold fear cells in mPFC could be that prefrontal activity did not simply signal the rat's anticipation of shock during the CS but more specifically regulated the selection of appropriate defensive response strategies as function of predatory imminence. As discussed below, this interpretation is further supported by our observations that strategy-selective neurons were more abundant than stimulus-selective neurons in mPFC.
Strategy-Selective Cells
Strategy-selective cells were prevalent in both dlPAG, where they constituted more than half (40/74) of the recorded neurons, and mPFC, where they constituted 39% (28/71) of the recorded neurons. However, the distributions and firing properties of strategy-selective cells differed in dlPAG vs. mPFC.
CME+ neurons.
All of the strategy-selective neurons in dlPAG (except for 1) were CME+ cells that increased their firing rates during the CS when it elicited CME but not CMI responses. By contrast, only half (14/28) of the strategy-selective neurons in mPFC were CME+ cells (the rest were mostly CMI- or CMI+ cells, discussed further below). Prefrontal CME+ cells could have been involved in regulating the rat's behavioral strategy to express CME rather than CMI responses during the CS. Alternatively, CME+ cells might instead have been “high-threshold fear cells” that were insensitive to the rat's behavior during the CS but only responded to the CS when it evoked a strong anticipation of shock (during post-shock trials) but not when it evoked a weaker anticipation of shock (during pre-shock trials). It is difficult to dissociate between these possibilities based solely on the selectivity of the neurons for CME trials. However, because CME+ neurons were the only cell type (other than MOV+ cells) that was abundant in both dlPAG and mPFC, it was possible to compare the time course of CME+ cell activity across the two structures. Following CS pips that elicited short-latency movement responses, the firing rates of CME+ cells in dlPAG increased after the onset of the pip, at about the same latency as the movement response evoked by the pip. It is thus possible that CME+ neurons in dlPAG were involved in motor activation to drive the CME response, in accordance with prior evidence that the dorsal PAG orchestrates active escape and avoidance behaviors (Bandler and DePaulis 1988; De Oca et al. 1998; Fanselow 1991). By contrast, firing rates of CME+ cells in mPFC were already significantly elevated before the onset of CS pips that evoked short-latency movement responses and did not increase further after the onset of the pip. Hence, it was the tonic firing rates of mPFC CME+ cells (both before and after the pip), rather than their phasic responses to the pip, that appeared to differ depending whether the pip evoked a movement response. This suggests that CME+ cells in mPFC did not drive CME responses on their own (because their firing rates did not change dynamically along with motor behavior). Instead, the tonic activity of CME+ cells in mPFC may have influenced how downstream structures, such as amygdala and PAG, mapped sensory inputs encoding the CS onto motor circuits that controlled competing defensive behaviors, thereby biasing the rat's defensive-response strategy toward the selection of CME responses and away from CMI responses during states of high predatory imminence. This interpretation accords well with theories proposing that mPFC participates in behavioral strategy selection by altering how sensory stimuli are mapped onto motor responses in downstream structures (Miller and Cohen 2001).
Population-averaged responses (Fig. 4) show that CME+ cells exhibited sustained firing throughout the CS during CME but not CMI trials (which is why they were statistically classified as CME+ cells), but they also exhibited brief (<1 s) transient responses to the first pip at the onset of the CS period during both CME and CMI trials. This was true in both dlPAG (Fig. 4A) and mPFC (Fig. 4B). One possible explanation for this could be that CME+ cells were in competition with other neurons (such as CMI+ cells) for control of the rat's behavior; if so, then both CME+ and CMI+ cells might have responded to the initial onset of the CS during all trials but only continued to show sustained firing during trials where they “won” the competition for behavioral control (which, in the case of CME+ cells, would of course occur only during CME trials). Another possible interpretation could be that CME+ cells were involved in conveying a temporal difference (TD) prediction error for aversive events (McNally et al. 2011). By definition, a TD prediction error signal is generated at moments when the rat's expectation of the US increases. Because of this, a TD error signal responds transiently to a sudden increase in expectation of the US but responds in a sustained manner when expectation of the US increases gradually over time. It is thus possible that, during pre-shock CMI trials, the rat's expectation of the US increased suddenly at the CS onset (thus generating a brief TD prediction error signal), but then expectation remained constant and did not continue to increase so that the TD error signal ended and did not persist throughout the CS. By contrast, during post-shock CME trials, the rat's expectation of the US might have continued to ramp up throughout the CS period, resulting in a sustained TD error signal as the rat's expectation of the US grew steadily from one moment to the next. Midbrain dopamine (DA) neurons (which are thought to signal TD errors for rewarding rather than aversive events) have similarly been observed to exhibit either transient or sustained responses to an appetitive CS, depending on experimental conditions (Fiorillo et al. 2003). Like DA neurons, dlPAG neurons are located in the midbrain and have been implicated in signaling prediction errors during aversive conditioning (Johansen et al. 2011). Hence, CME+ neurons in dlPAG (and possibly mPFC as well) could play some role in signaling aversive prediction errors.
CMI+ neurons.
Prior studies have reported that 25–30% of neurons in the PL subregion of mPFC increase their firing rates during conditioned freezing responses (Burgos-Robles et al. 2009; Sotres-Bayon et al. 2012; but for conflicting results see Chang et al. 2010). Accordingly, we observed that 35% (10/29) of PL neurons belonged to one of the three cell types (CMI+, CSR+, and MOV-) that increased their firing rates during the CS when it elicited movement-suppression behavior (that is, during CMI trials). Neurons belonging to these three cell types would likely have been classified as freezing cells in prior studies, where freezing was the only measure of conditioned fear. In the present study, a comparison between CMI vs. CME trials made it possible to further discern that 10% (3/29) of PL neurons were CMI+ cells that were excited by the CS only during CMI (but not CME) trials; another 10% (3/29) of PL neurons were stimulus-selective CSR+ cells excited by the CS during both CMI and CME trials (see above), and one PL neuron was a MOV- cell that always fired during low movement speed, regardless of whether the CS was present or absent. Interestingly, only half (3/6) of the CMI+ cells we recorded in mPFC were located in PL subregion, and these were all classified as principal cells, whereas CMI+ cells recorded outside of PL (2 in ACC, 1 in IL) were all classified as interneurons. It is difficult to draw firm conclusions from such a small sample of cells, but these findings are consistent with prior evidence that output relayed by PL projection neurons could promote freezing (Burgos-Robles et al. 2009; Corcoran and Quirk 2007; Laurent and Westbrook 2009; Senn et al. 2014), whereas inhibitory interneurons might conversely suppress ACC and IL activity during freezing.
CMI+ cells did not increase their firing rates during the CS period of CME trials, so it seems unlikely that their firing rates were directly correlated with the rat's anticipation of shock (if anything, the rat's anticipation of shock should have been greater during CME than CMI trials). Thus, if CMI+ cells signaled anticipation of shock, they must have done so nonmonotonically, firing only when the shock was weakly but not strongly anticipated. A simpler interpretation is that CMI+ firing rates were monotonically correlated with expression of CMI responses, rather than nonmonotonically correlated with fear or anticipation of shock.
CMI- neurons.
Another type of strategy-selective cell we observed was CMI- neurons, which were inhibited by the CS during CMI (but not CME) trials and constituted 8% (6/71) of the neurons recorded in mPFC. Courtin at al. (2014) have reported that prefrontal cells with similar firing properties (which suppress their activity during freezing behavior) are predominantly interneurons, but only one of the CMI- cells we recorded here (located in ACC) was classified as an interneuron, whereas the rest were classified as principal cells (all located in IL or PL). As discussed above for CMI+ cells, the fact that CMI- cell-firing rates were suppressed during CMI but not CME trials implies that the suppression was correlated with the expression of CMI behaviors, rather than with fear or anticipation of shock (because shock anticipation during CME trials should have been equal or greater than during CMI trials). Hence, like CMI+ cells, CMI- cells might project to downstream targets in amygdala or PAG, where they could participate in disinhibiting behavioral circuits that promote the expression of CMI responses (or alternatively, in inhibiting circuits that promote CME responses).
Shock-Evoked Responses
Many neurons in both mPFC and dlPAG exhibited short latency responses to shock pulses (Fig. 6). In mPFC, a disproportionate number of shock-responsive neurons were recorded from the ACC subregion, which participates in processing nociceptive sensory information in rodents (Johansen et al. 2001). In dlPAG, more than half of the neurons were shock responsive, in accordance with the fact that PAG is a major relay center that conveys ascending nociceptive information from the spinal cord to higher structures (Willis and Westlund 1997) and also exerts descending modulatory influences on nociceptive processing in the spinal cord (Basbaum and Fields 1984). We did not find evidence that shock responsiveness was more prevalent among some cell types than others; instead, shock responsiveness was similarly prevalent among all cell types in both mPFC and dlPAG. Hence, it appears that there was ample opportunity for the shock stimulus to widely and directly influence neural processing in both structures. Several cell types shifted their baseline firing rates for up to 2 min after shock delivery, in conjunction with changes in the rats' tonic motor activity (Fig. 7). Hence, shock delivery may have affected the activity of multiple cell types in mPFC and dlPAG, in ways that might have promoted the transition in behavioral strategy from CMI to CME responses following recent shock delivery.
Summary and Conclusions
Our findings suggest that strategy-selective mPFC neurons may participate in regulating which defensive response strategy a rat chooses to perform in the presence of a fear-conditioned CS, in accordance with prior theories positing that mPFC controls behavioral strategies by modulating how downstream structures map sensory stimuli onto specific motor responses (Miller and Cohen 2001). One such downstream structures could be the amygdala, which stores memories of the association between the CS and US during fear conditioning (Davis 1992; Herry and Johansen 2014; Johansen et al. 2011; Maren 2003) and may also participate in selecting appropriate defensive responses to the CS because different output pathways from the amygdala appear to mediate distinct modes of defensive responding (Amorapanth et al. 2000; Gozzi et al. 2010; Killcross et al. 1997). PAG is another downstream target of mPFC neurons that could mediate the selection of appropriate defensive responses to a threat. The ACC and PL subregions of mPFC project to the dorsal columns of PAG, whereas the PL and IL subregions project to the ventral PAG (Floyd et al. 2000). It has been proposed that the dorsal and ventral columns of PAG may compete with one another to drive CME vs. CMI defenses, respectively (Bandler and DePaulis 1988; De Oca et al. 1998; Fanselow 1991; Vianna et al. 2001; Walker et al. 1997). Hence, mPFC is well positioned to mediate the outcome of competition between PAG columns and thereby regulate which defensive behavior is expressed to a threatening stimulus. Recent evidence also suggests that inhibitory outputs from mPFC to the ventral striatum might mediate avoidance behavior (Lee et al. 2014), so the ventral striatum is another target where mPFC might exert an influence over the mapping from threatening sensory stimuli onto specific behavioral defense responses. Further study is warranted to more deeply investigate how defensive action selection might be mediated by interactions between strategy-selective mPFC neurons, such as those recorded here, and downstream regions.
GRANTS
This work was supported by NIH R01 MH073700 awarded to H. T. Blair and by NIH training grant 5 T32 NS058280-03 to L. R. Halladay.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.R.H. and H.T.B. conception and design of research; L.R.H. and H.T.B. analyzed data; L.R.H. and H.T.B. interpreted results of experiments; L.R.H. and H.T.B. prepared figures; L.R.H. and H.T.B. edited and revised manuscript; L.R.H. and H.T.B. approved final version of manuscript; L.R.H. and H.T.B. performed experiments; L.R.H. and H.T.B. drafted manuscript.
ACKNOWLEDGMENTS
We thank Michael Fanselow and Jason Tarpley for helpful discussions.
REFERENCES
- Abeles M. Quantification, smoothing, and confidence limits for single-units' histograms. J Neurosci Methods 5: 317–325, 1982. [DOI] [PubMed] [Google Scholar]
- Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat Neurosci 3: 74–79, 2000. [DOI] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex 11: 441–451, 2001. [DOI] [PubMed] [Google Scholar]
- Bandler R, DePaulis A. Elicitation of intraspecific defense reactions in the rat from midbrain periaqueductal gray by microinjection of kainic acid, without neurotoxic effects. Neurosci Lett 88: 291–296, 1988. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 7: 309–338, 1984. [DOI] [PubMed] [Google Scholar]
- Bolles RC. Species-specific defense reactions and avoidance learning. Psychol Rev 77: 32–48, 1970. [Google Scholar]
- Bravo-Rivera C, Roman-Ortiz C, Brignoni-Perez E, Sotres-Bayon F, Quirk GJ. Neural structures mediating expression and extinction of platform-mediated avoidance. J Neurosci 34: 9736–9742, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron 53: 871–880, 2007. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci 29: 8474–8472, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Berke JD, Maren S. Single-unit activity in the medial prefrontal cortex during immediate and delayed extinction of fear in rats. PLoS One 5: e11971, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Maren S. Strain difference in the effect of infralimbic cortex lesions on fear extinction in rats. Behav Neurosci 124: 391–397, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate fears. J Neurosci 27: 840–844, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TC, Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 505: 92–96, 2014. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear, and anxiety. Annu Rev Neurosci 15: 353–375, 1992. [DOI] [PubMed] [Google Scholar]
- De Oca BM, DeCola JP, Maren S, Fanselow MS. Distinct regions of the periaqueductal gray are involved in the acquisition and expression of defensive responses. J Neurosci 18: 3426–3432, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron 82: 966–980, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. The midbrain periaqueductal gray as a coordinator of action in response to fear and anxiety. In: The Midbrain Periaqueductal Gray Matter: Functional, Anatomical and Neurochemical Organization, edited by Depaulis A, Bandler R. New York, NY: Plenum, 1991, pp. 151–175. [Google Scholar]
- Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. In: Evolution and Learning, edited by Bolles RC, Beecher MD. Hillsdale, NJ: Earlbaum, 1988, p. 185–211. [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science 299: 1898–1902, 2003. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J Comp Neurol 422: 556–578, 2000. [DOI] [PubMed] [Google Scholar]
- Garcia R, Chang CH, Maren S. Electrolytic lesions of the medial prefrontal cortex do not interfere with long-term memory of extinction of conditioned fear. Learn Mem 13: 14–17, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Balderston NL, Helmstetter FJ. Prefrontal cortical regulation of fear learning. Trends Neurosci 37: 455–464, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Jain A, Giovannelli A, Bertollini C, Crestan V, Schwartz AJ, Tsetsenis T, Ragozzino D, Gross CT, Bifone A. A neural switch for active and passive fear. Neuron 67: 656–666, 2010. [DOI] [PubMed] [Google Scholar]
- Halladay LR, Blair HT. The role of mu-opioid receptor signaling in the dorsolateral periaqueductal gray on conditional and unconditional responding to threatening and aversive stimuli. Neuroscience 216: 82–93, 2012. [DOI] [PubMed] [Google Scholar]
- Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci 17: 1644–1654, 2014. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature 454: 600–606, 2008. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci USA 98: 8077–8082, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell 147: 509–524, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature 388: 377–380, 1997. [DOI] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem 16: 486–493, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn Mem 16: 520–529, 2009. [DOI] [PubMed] [Google Scholar]
- Lee AT, Vogt D, Rubenstein JL, Sohal VS. A class of GABAergic neurons in the prefrontal cortex sends long-range projections to the nucleus accumbens and elicits acute avoidance behavior. J Neurosci 34: 11519–11525, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. The amygdala, synaptic plasticity, and fear memory. Ann NY Acad Sci 985: 106–113, 2003. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 14: 417–428, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty VB, Grace AA. Selective activation of medial prefrontal-to-accumbens projection neurons by amygdala stimulation and Pavlovian conditioned stimuli. Cereb Cortex 18: 1961–1972, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP, Johansen JP, Blair HT. Placing prediction into the fear circuit. Trends Neurosci 34: 283–292, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420: 70–74, 2002. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci 118: 389–394, 2004. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202, 2001. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science 317: 1079–1083, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Petrovic P, Dolan RJ, Frith CD. From threat to fear: the neural organization of defensive fear systems in humans. J Neurosci 29: 12236–12243, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Steriotaxic Coordinates. San Diego, CA: Academic, 1997. [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 20: 6225–6231, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modulate the intrinsic excitability of infralimbic neurons. J Neurosci 28: 4028–4036, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Grundemann J, Fadok JP, Muller C, Letzkus JJ, Luthi A. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81: 428–437, 2014. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12: 154–167, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36: 529–538, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol 20: 231–235, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Diaz-Mataix L, Bush DE, LeDoux JE. Dissociable roles for the ventromedial prefrontal cortex and amygdala in fear extinction: NR2B contribution. Cereb Cortex 19: 474–482, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76: 804–812, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpley JW, Shlifer IG, Halladay LR, Blair HT. Conditioned turning behavior: a Pavlovian fear response expressed during the post-encounter period following aversive stimulation. Neuroscience 169: 1689–1704, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna DM, Landeira-Fernandez J, Brandao ML. Dorsolateral and ventral regions of the periaqueductal gray matter are involved in distinct types of fear. Neurosci Biobehav Rev 25: 711–719, 2001. [DOI] [PubMed] [Google Scholar]
- Walker DL, Cassella JV, Lee Y, De Lima TC, Davis M. Opposing roles of the amygdala and dorsolateral periaqueductal gray in fear-potentiated startle. Neurosci Biobehav Rev 21: 743–753, 1997. [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol 14: 2–31, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]