Abstract
High-frequency stimulation is known to entrain spike activity downstream and upstream of several clinical deep brain stimulation (DBS) targets, including the cerebellar-receiving area of thalamus (VPLo), subthalamic nucleus (STN), and globus pallidus (GP). Less understood are the fidelity of entrainment to each stimulus pulse, whether entrainment patterns are stationary over time, and how responses differ among DBS targets. In this study, three rhesus macaques were implanted with a single DBS lead in VPLo, STN, or GP. Single-unit spike activity was recorded in the resting state in motor cortex during VPLo DBS, in GP during STN DBS, and in STN and pallidal-receiving area of motor thalamus (VLo) during GP DBS. VPLo DBS induced time-locked spike activity in 25% (n = 15/61) of motor cortex cells, with entrained cells following 7.5 ± 7.4% of delivered pulses. STN DBS entrained spike activity in 26% (n = 8/27) of GP cells, which yielded time-locked spike activity for 8.7 ± 8.4% of stimulus pulses. GP DBS entrained 67% (n = 14/21) of STN cells and 32% (n = 19/59) of VLo cells, which showed a higher fraction of pulses effectively inhibiting spike activity (82.0 ± 9.6% and 86.1 ± 16.6%, respectively). Latency of phase-locked spike activity increased over time in motor cortex (58%, VPLo DBS) and to a lesser extent in GP (25%, STN DBS). In contrast, the initial inhibitory phase observed in VLo and STN during GP DBS remained stable following stimulation onset. Together, these data suggest that circuit-level entrainment is low-pass filtered during high-frequency stimulation, most notably for glutamatergic pathways. Moreover, phase entrainment is not stationary or consistent at the circuit level for all DBS targets.
Keywords: deep brain stimulation, mechanisms, entrainment, peri-stimulus time histogram, subthalamic nucleus, globus pallidus, thalamus, motor cortex
high-frequency stimulation of subcortical structures, known as deep brain stimulation (DBS), has emerged in the past decades as a highly effective surgical therapy for medication-refractory movement disorders and a promising therapy for numerous other neurological and neuropsychiatric disorders (Wichmann and DeLong 2006). Despite the clinical success, refinement of DBS remains hampered by the lack of clear mechanistic understanding of how DBS affects brain areas distal to the stimulated electrode(s) and how to modulate this activity in a consistent way to appropriately ameliorate specific symptoms.
Computational modeling studies have suggested that DBS preferentially activates axonal efferents, afferents, and fibers of passage near the active electrode(s) (Johnson and McIntyre 2008; McIntyre et al. 2004; Miocinovic et al. 2006) because axons, compared with cell bodies, have a lower threshold for generating action potentials with typical DBS waveforms (McIntyre and Grill 2000; Ranck 1975). Accordingly, electrophysiological studies have shown that high-frequency stimulation can generate phase-locked antidromic spike activity (i.e., spike activity that occurs at a specific phase of the interpulse interval) in regions that project to or through the region undergoing stimulation (Li et al. 2007). Phase-locked spike activity has also been observed distal to the stimulated target with interpulse interval periods exhibiting increased or decreased probability of spiking that is consistent with activation of axonal efferents yielding putatively monosynaptic (Agnesi et al. 2013; Anderson et al. 2003; Hashimoto et al. 2003; Moran et al. 2011; Santaniello et al. 2015) and multisynaptic (Kita et al. 2005; Montgomery 2006) responses. Although axonal conduction fidelity can be robust for the high-stimulation frequencies that are typical of DBS therapy (∼80–185 Hz) (Chomiak and Hu 2007; Windels et al. 2003), several studies have shown axonal conduction and synaptic conduction failure (Chomiak and Hu 2007; Iremonger et al. 2006; Jensen and Durand 2009; Rosenbaum et al. 2014; Zheng et al. 2011) with high-frequency stimulation.
We hypothesized that 1) the fidelity of spike entrainment to high-frequency stimulation depends on the circuit-level connections to or from current clinical targets of DBS and that 2) the phase-locked spike activity in upstream and downstream cell populations is not stationary over time, with excitatory pathways exhibiting less stationarity than inhibitory pathways. To evaluate the fidelity of frequency entrainment to DBS, we introduce a simple but intuitive measurement called the effective pulse fraction (EPF) representing the number of single-unit spikes, within a phase of an interpulse interval, that are induced or suppressed by stimuli divided by the number of stimulus pulses used to entrain the cell. We show that the EPF metric can be applied to nuclei with predominantly glutamatergic (excitatory, eEPF) or GABAergic (inhibitory, iEPF) projections to or from the stimulated nucleus. To further characterize entrainment fidelity, we also evaluated the consistency of the delays in the phase-locked spike activity following onset of DBS. The eEPF and the temporal fidelity measures were calculated for 1) motor cortex spike activity during cerebellar-receiving area of thalamus (VPLo) DBS and 2) globus pallidus (GP) spike activity during subthalamic nucleus (STN) DBS. Similarly, the iEPF and temporal fidelity measures were calculated for 3) pallidal-receiving area of motor thalamus (VLo) and STN spike activity during GP DBS.
MATERIALS AND METHODS
Animals.
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Minnesota and complied with United States Public Health Service policy on the humane care and use of laboratory animals. Three adult rhesus monkey (Macaca mulatta; one male, monkey K, 11.0 kg, 12 yr old; and two females, monkey R, 4.9 kg, 9 yr old, and monkey F, 8 kg, 25 yr old) were used in this study. Monkey K received an intraputaminal infusion of 3-nitropropionic acid (Agnesi et al. 2013), whereas monkeys R and F were rendered moderately parkinsonian with the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, as described previously (Hashimoto et al. 2003; Johnson et al. 2012). The animals were housed with environmental enrichment, provided with water ad libitum, and given a range of food options including fresh fruit and vegetables. All efforts were made to provide good care and alleviate any discomfort, including administration of analgesics before and after surgery.
Surgery.
In an aseptic procedure under isoflurane anesthesia, animals were instrumented with cranial chambers (Crist Instruments). Monkeys K and R were implanted with a coronal chamber that facilitated microelectrode mapping of the ventral posterior lateral pars oralis nucleus (VPLo, cerebellar-receiving area of motor thalamus) and primary motor cortex (M1) (monkey K), as well as mapping of the GP, STN, and thalamus (monkey R). Monkey F was instrumented with one sagittal chamber to access the STN and one coronal chamber allowing access to the GP. In monkey K, microelectrode mapping was performed to identify the VPLo using a combination of unit-spike responses to passive joint manipulation and microstimulation-evoked movements at thresholds <30 μA (Vitek et al. 1996). Sensorimotor regions of the GP and STN were identified by unit-spike responses to passive and active movements. For each monkey, the recording track that yielded a long run of spike activity responding to movement was used for implanting a scaled-down version of the human DBS lead (NuMed, 0.75-mm diameter with 0.5-mm contact height and 0.5-mm spacing between electrode contacts; 4 annular contacts in monkeys R and F and 8 annular contacts in monkey K) (Fig. 1).
Fig. 1.
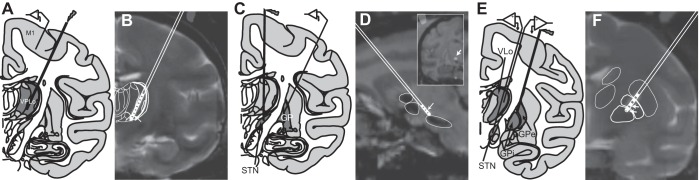
Experimental design used to investigate the fidelity of deep brain stimulation (DBS) entrainment. A: microelectrode recordings were performed in the motor cortex, while DBS was delivered in the cerebellar-receiving area of the thalamus (VPLo). B: overlap of preoperative MRI and postoperative CT (obtained using the stereotactic navigation software Monkey Cicerone) showing location of the DBS lead in monkey K. C: microelectrode recordings were performed in the globus pallidus (GP), while DBS was delivered in the subthalamic nucleus (STN). D: overlap of MRI/CT showing location of the DBS electrode in monkey F. Note the sagittal trajectory of the DBS lead implant in monkey F. Inset: MRI with CT overlay in the coronal plane at the level of the active electrode contact. E: microelectrode recordings were performed in the pallidal-receiving area of the thalamus (VLo) and in the STN during pallidal DBS. GPi, inhibitory GP; GPe, excitatory GP. F: MRI/CT overlap showing DBS lead positioning in monkey R.
DBS.
In monkey K the contact used was the most ventral contact within VPLo (contact 1, −0.3 mA, 130 Hz, 90-μs pulse width). In monkey R the two contacts below and above the lamina separating the external and internal segments of GP were used, as this location was found to be the most effective setting at reducing bradykinesia and rigidity (contact 0 or 1, −1 V or −1.5 V, 135 Hz, 90-μs pulse width) (Johnson et al. 2012). This stimulation location was likely to affect both excitatory GP (GPe) and inhibitory GOP (GPi) efferent pathways directly, especially because DBS targeted to the GPi volume likely stimulates GPe efferents projecting through GPi (Johnson and McIntyre 2008). In monkey F, bipolar stimulation through the middle two contacts was most effective at reducing bradykinesia and rigidity (contact 1 negative, contact 2 positive, 2.8 V, 135 Hz, 60-μs pulse width). DBS was delivered for intervals of 1 min (VPLo and STN DBS) or 30 s (GP DBS) with at least 3-min intervals of no stimulation between stimulation bouts (∼5–10 per recording session). Primates were awake and sitting at rest in a primate chair during the recordings.
Extracellular recordings.
Microelectrode recordings were performed using tungsten microwires (impedance 0.5–1.0 MΩ at 1 kHz; FHC) and collected using an SNR system (Alpha Omega) before, during, and after delivery of high-frequency stimulation. Stimulation artifacts were removed using a previously described template subtraction procedure (Agnesi et al. 2013; Hashimoto et al. 2002) reducing the period of recording obscured by stimulation artifacts to a small blanked period (average ∼0.5–1.0 ms). To prevent bias of the data, similarly blanked regions were introduced in the pre- and post-DBS recording epochs using virtual stimulation time stamps at the same stimulation pulse frequency. Template-subtracted spike recordings were thresholded and sorted in Offline Sorter (Plexon) to identify spike activity. Time stamps of spike activity, stimulation pulses, and virtual stimulation pulses were used to generate peri-stimulus time histograms (PSTHs, 0.1-ms bins) to characterize the degree of entrainment in spike activity to the stimulation pulse train. For cortical and pallidal recordings, neuronal activity was considered entrained to DBS if the PSTH during stimulation showed an increase in spike activity for at least two consecutive bins that exceeded the mean plus 6 SDs of the DBS-OFF baseline PSTH rate. The choice of a conservatively high significance threshold ensured the absence of false positives such that the analysis was only performed on cells that were strongly entrained to DBS. The use of a lower threshold would have included less significant excitatory phases of entrainment, and thus the overall entrainment fidelity would be even lower than that found in this study (see below). For thalamic and STN recordings, spike activity was considered entrained to GP DBS if the PSTH displayed an inhibitory phase lower, for at least five consecutive bins, than the mean − 3 SDs of the DBS-OFF baseline PSTH rate. The choice of significance threshold was chosen to include cell responses with a fairly strong phase-locked inhibition. Significance thresholds that were >3 SDs below the mean extended into negative spike probabilities for the majority of VLo and STN recordings and thus were not practical to implement in this study.
eEPF.
To quantify how faithfully nuclei with known glutamatergic afferents from DBS targets [i.e., GPe/GPi from STN (Sato et al. 2000a) or M1 from VPLo (Darian-Smith et al. 1990)] were able to follow high-frequency stimulation, we calculated the fraction of stimulus pulses that effectively produced a spike within an entrained phase of the interpulse interval. This pulse fraction was normalized by subtracting the equivalent measure calculated from the DBS-OFF baseline data. More specifically, for an interpulse interval phase (i.e., defined by 2 or more bins) with a statistically significant increase in spike activity, the eEPF was defined by the difference between the number of actual stimulus pulses followed by a spike (pfs) minus the number of virtual stimulus pulses followed by a spike in the baseline period (pfsb), all divided by the number of delivered stimulus pulses minus the pfsb measure.
| (1) |
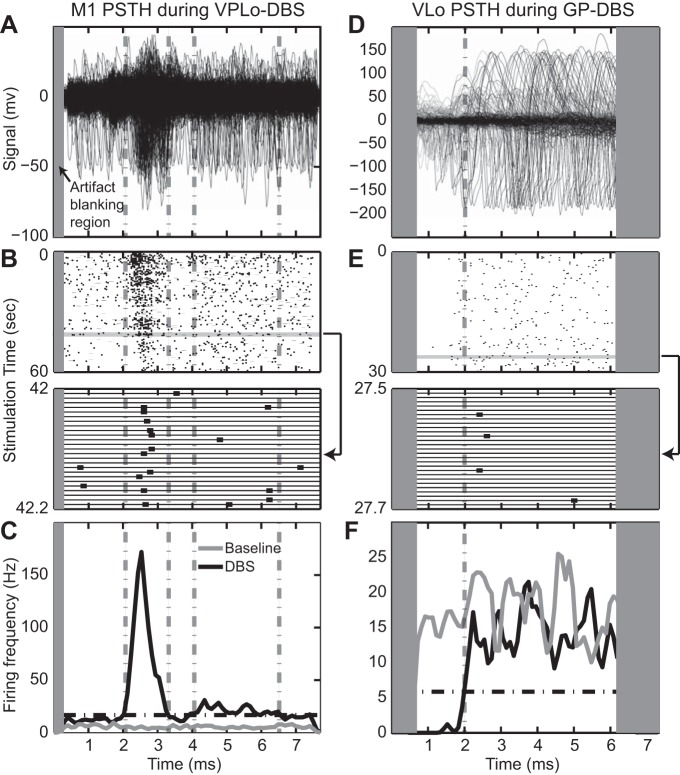
The eEPF numerator thus represents the number of additional spikes introduced by stimulation, whereas the denominator represents the number of pulses “available” to produce an additional spike within a particular entrained phase of the PSTH. An example PSTH is shown in Fig. 2, A–C in which a cell in M1 exhibited a higher probability of spiking between 2.5 and 3.0 ms from the onset of each VPLo DBS stimulus pulse. This entrained phase (from 2.5 to 3.0 ms) defined the period with which to calculate the eEPF.
Fig. 2.
Examples of phase-locked spike activity to high-frequency stimulation. Overlap of stimulus-triggered spike activity in an M1 cell during VPLo DBS (A) and VLo cell during GP DBS (D). Time zero on the x-axis coincides with delivery of each stimulus pulse. Gray rectangle represents the region of the interpulse interval blanked by the stimulation artifact template subtraction algorithm. B and E: corresponding raster for the entire interpulse interval (top) and for a 0.2-s subsection. Bottom: 2 cells are present in the recording; only the larger responded to DBS and was used to generate the spike rasters and peri-stimulus time histogram (PSTH) plots. C and F: PSTH of the entire recording, showing significance level (black dot-dash line) and phases of significant excitation or inhibition (gray dot-dash lines). Note that the PSTH bin width was 0.1 ms, and the spike frequency in the example PSTHs reflects this bin width.
The eEPF was calculated as a single value for the 60 s of stimulation and in nonoverlapping 1-s windows. The eEPFs for the first and second 30 s after onset of stimulation were compared for each entrained phase using a Wilcoxon rank sum test (P = 0.05, Bonferroni correction for multiple comparisons, n = 19 in motor cortex, n = 13 in GP) to evaluate the fraction of phases whose spike entrainment fidelity to the DBS pulse train changed between the initial and next 30-s period following DBS onset. The choice of recording duration stemmed from both the retrospectively available recording durations in a number of the data sets used in this study as well as general approximations of DBS wash-in and wash-out times for alleviating the majority of tremor (Perera et al. 2015), rigidity (Levin et al. 2009), and bradykinesia (Cooper et al. 2014).
iEPF.
To quantify the fidelity of neuronal entrainment to GP DBS resulting from putative activation of GABAergic efferents innervating VLo (Parent et al. 2001) and STN (Sato et al. 2000a), we measured the fraction of pulses that effectively suppressed spike activity within an interpulse interval phase that probabilistically would have otherwise occurred with no stimulation present. An example of this probabilistic sparsity of spike activity with a 2-ms duration following each stimulus pulse is shown in Fig. 2, D–F. PSTH bins deemed to exhibit a significant decrease in phase entrainment were those with spike rates over five or more bins that were below the mean minus 3 SDs of the baseline PSTH for the DBS-OFF condition (dot-dash line). For interpulse interval phases exhibiting statistically significant inhibition, the iEPF was defined by the normalized difference between the number of stimulus pulses followed by a spike (pfs) and the number of stimulus pulses that were predicted to result in a phase-locked spike based on the baseline DBS-OFF condition (pfsb):
| (2) |
This metric calculates the effectiveness of phasic suppression of ongoing spike activity and does not necessarily reflect the effectiveness of GABAergic projections in hyperpolarizing membrane potentials of the recorded neurons. The iEPF was calculated as a single value for 30 s of stimulation in nonoverlapping 1-s windows. The iEPF of the initial 15 s and the last 15 s following stimulation onset were compared for each entrained cell recording using a Wilcoxon rank sum test (P = 0.05, Bonferroni corrected for multiple comparisons, n = 11 in STN, n = 16 in VLo).
PSTH stationarity.
To evaluate the temporal fidelity of the excitatory and/or inhibitory phases of the PSTH responses, we measured the temporal delay between spikes and the immediately preceding DBS pulse. Only spikes occurring in the significantly entrained phase of the PSTH (M1 and GP recordings) or belonging to a 2-ms-wide phase centered around the sample where the PSTH returned above significance level (STN and VLo recordings) were used for this analysis. The delays measured during the first half of a stimulation trial and the second half of a stimulation trial were compared using a Wilcoxon rank sum test (Bonferroni correction, based on the number of entrained cells) to establish whether the phase entrainment remained stationary or varied over the duration of stimulation.
RESULTS
Entrainment of circuit-level spike activity during high-frequency stimulation.
In each subject, high-frequency electrical stimulation entrained neuronal spike activity in a subset of neurons in regions connected with the stimulated nucleus. This entrainment consisted of phase(s) within the PSTH that reflected a statistically significant increase or decrease in the probability with which a given cell spiked (Fig. 2). For instances in which stimulation was delivered to nuclei with known glutamatergic efferents (i.e., VPLo DBS and STN DBS), PSTHs of cells recorded downstream of those targets showed significant interpulse interval phases of excitation in 15/61 motor cortex cells (25%) for a total of 19 excitatory phases (4 cells displayed 2 excitatory phases) during VPLo DBS and in 8/27 GP cells (26%) for a total of 13 excitation phases (3 cells showed 2 excitatory phases, 1 cell showed 3 excitatory phases) during STN DBS (See Table 1). When more than one entrained phase was detected in a PSTH for a given cell, all significant phases were used in the eEPF analysis. Two cells in motor cortex and one cell in GP exhibited a broad increase in firing probability during the entire interpulse interval and were not included in the overall eEPF calculation.
Table 1.
Summary of recordings
| DBS Target | Recording Location | Entrained Cells | EPF of Entrained Cells: Average % (range) |
|---|---|---|---|
| VPLo | M1 | 15/61 | 7.5% (0.5–25.5%) |
| STN | GP | 8/27 | 8.7% (0.4–39.9%) |
| GPe/GPi | STN | 14/21 | 82.2% (69.1–96.2%) |
| GPe/GPi | VLo | 19/59 | 86.1% (48.1–99.5%) |
DBS, deep brain stimulation; EPF, effective pulse fraction; VPLo, cerebellar-receiving area of thalamus; STN, subthalamic nucleus; GPe, excitatory globus pallidus; GPi, inhibitory globus pallidus; VLo, pallidal-receiving area of motor thalamus.
On the other hand, unit-spike recordings in nuclei that are innervated by GABAergic projections from the GP showed interpulse interval periods of significant inhibition in 14/21 (67%) of STN cells and 19/59 (32%) of VLo cells. Inhibition was categorized as general (widespread reduction in spiking probability during the entire interpulse interval), initial (reduction of spiking immediately after delivery of each stimulus pulse followed by a return to baseline spiking probability; see Fig. 2, D–F), or localized (reduction observed within a limited range in the interpulse interval that did not start immediately after stimulus pulse delivery). The majority of the cells in the STN (11/14, 79%) and VLo (16/19, 84%) exhibited responses consistent with the initial inhibition pattern. Generalized inhibition was observed in two STN cells, whereas localized inhibition was observed in one STN cell and three thalamic cells. Because of their paucity within the recorded population, cells displaying general or localized inhibition were not analyzed further.
Excitatory spike-pulse fraction during high-frequency stimulation.
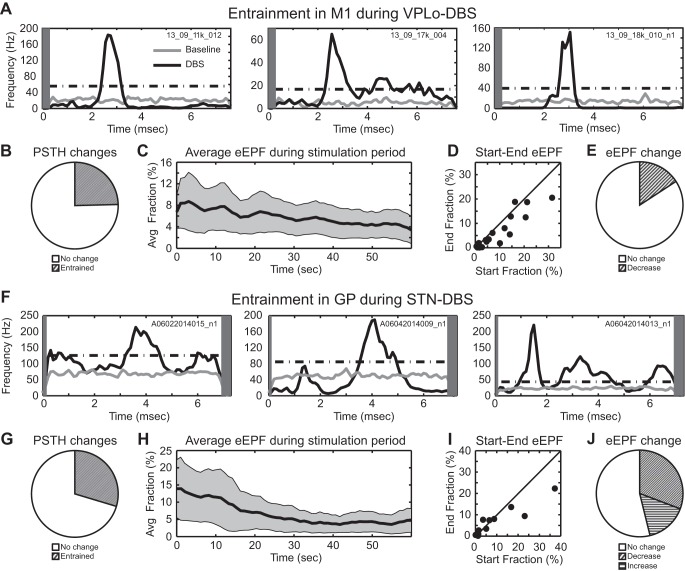
The entrainment patterns of cells in M1 during VPLo DBS never followed a one-to-one pattern of each stimulus pulse eliciting a unit-spike response in M1 at a fixed interpulse phase delay. Examples of PSTHs displaying stimulus entrainment are shown for three cells in M1 during VPLo DBS (Fig. 3A). Responses were generally narrow phases of entrainment crossing above significance threshold between 1.1 and 5.1 ms (mean 2.4 ms, median 2.3 ms) and returning below threshold between 1.3 and 6.5 ms (mean 3.2, median 3.1 ms) for an average phase duration of 0.9 ± 0.91 ms. On average, the eEPF of the 19 entrained phases was 7.5 ± 7.4%, which exhibited a slight downward trend over the minute-long stimulation period (Fig. 3C). When the eEPF of each entrained phase was compared between the initial and final 30 s of stimulation, the majority of entrained phases did not exhibit a statistically significant change in their eEPF (Fig. 3, D and E). However, in a minority of cases (3/19 entrained phases, 16%), a statistically significant reduction in the eEPF value between the beginning and end of the minute-long stimulation period was observed.
Fig. 3.
Excitatory entrainment during VPLo DBS and STN DBS. Example PSTHs of M1 cells during VPLo DBS (A) and GP cells during STN DBS (F). Gray rectangle represents the region of the interpulse interval that was blanked during stimulus artifact template subtraction. Fraction of M1 cells (B) and GP cells (E) showing significant entrainment to DBS. Average excitatory effective pulse fraction (eEPF) in M1 (C) and GP (H) as a function of time (mean ± 2 SE). D and I: comparison of the eEPF measured in the initial and in the final 30 s of each 1-min stimulation trial. E and J: fraction of excitatory interpulse interval phases whose eEPF significantly changed during DBS.
Similar results were found in the GP during STN DBS, as shown in three pallidal cells (Fig. 3F). In this case, entrainment was characterized by excitatory phases of entrainment in the PSTH starting between 1.0 and 5.9 ms (mean 3.1, median 3.4 ms) and ending between 1.7 and 6.9 ms (mean 3.9 median 4.1 ms) for an average entrained phase duration of 0.76 ± 0.48 ms. On average, the eEPF of the 12 entrained phases was 8.7 ± 8.3%, which was characterized by a downward trend in the eEPF of the minute-long stimulation period (Fig. 3H). When the eEPF of each entrained phase was compared between the initial and final 30 s of stimulation, four entrained phases exhibited a significant decrease in eEPF and two entrained phases exhibited a significant increase in eEPF (Fig. 3J). Roughly 50% of the recorded population had no statistically significant change in the eEPF over the stimulation period.
Inhibitory spike-pulse fraction during high-frequency stimulation.
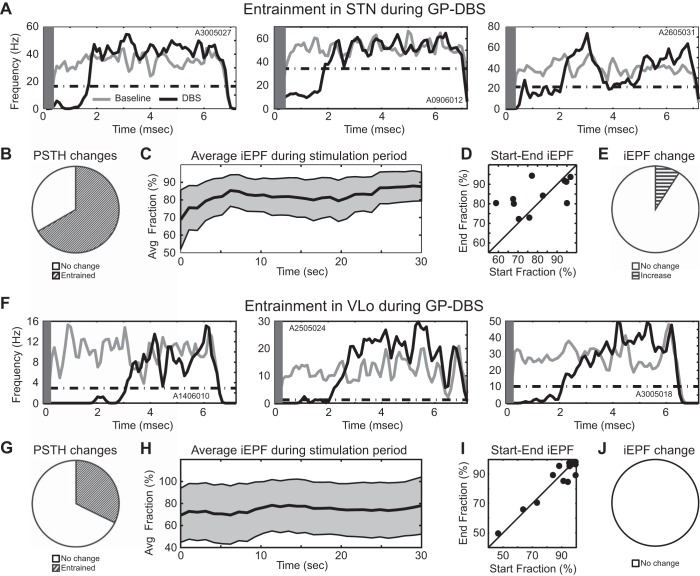
An iEPF measure was calculated as a decrease in the likelihood that a cell would initiate a spike within a phase of the interpulse interval relative to a baseline period without stimulation. Examples of three entrained cells within the STN are shown in the context of GP DBS (Fig. 4A). The initial phase of inhibition lasted between 1.5 and 3.5 ms following stimulus pulse onset for a mean inhibitory phase duration lasting until 1.9 ± 0.6 ms after stimulus pulse delivery. On average, the iEPF was 82.2 ± 9.6% and was relatively stable over time when considering the entire recorded STN cell population (Fig. 4C). The iEPF at the beginning compared with the end of the 30-s-long stimulation period showed that only one STN cell had a statistically significant temporal change in its iEPF (Fig. 4, D and E); however, the scatter was fairly high (Fig. 4D).
Fig. 4.
Inhibitory entrainment during GP DBS. Example PSTHs of STN cells (A) and VLo cells (F) during GP DBS. Gray rectangle represents the region of the interpulse interval that was blanked during stimulus artifact template subtraction. Fraction of STN cells (B) and VLo cells (E) showing significant entrainment to DBS. Average inhibitory EPF (iEPF) in STN (C) and VLo (H) as a function of time (mean ± 2 SE). D and I: comparison of the iEPF measured in the initial and in the final 15 s of each 30-s stimulation trial. E and J: fraction of inhibitory interpulse interval phases whose iEPF significantly changed during DBS.
Similar findings were observed in VLo during GP DBS with an initial inhibitory phase within the PSTH as shown for three VLo cells (Fig. 4F). The initial inhibitory phase lasted between 0.7 and 3.2 ms for a mean inhibition phase duration lasting until 1.7 ± 0.7 ms after stimulus pulse delivery. On average, the iEPF was 86.1 ± 16.6% and remained relatively constant across the 30-s-long stimulation period (Fig. 4H). No VLo cell exhibited a significant change in its iEPF between the beginning and end of the stimulation period (Fig. 4, I and J).
Stationarity of the phase-locking period over time.
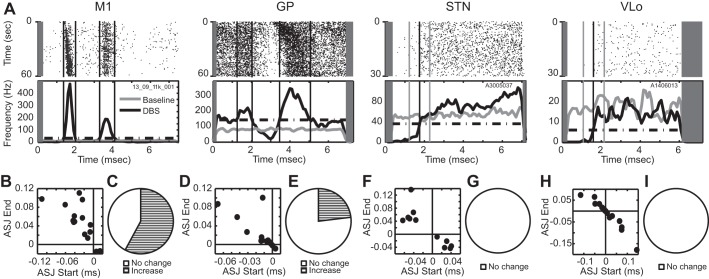
The phase of the stimulus-induced entrainment was not necessarily stationary over the duration of stimulation, in that the precise delay between the stimulus pulses and the phase-locked spike activity was found to vary over time, most notably for the excitatory responses in M1 and GP during VPLo DBS and STN DBS, respectively. The measured delays at the beginning compared with those at the end of stimulation showed that a number of M1 and GP neurons exhibited an increase in the time delay of the phase-locked spike activity (Fig. 5A). An average signed jitter was calculated for each cell by averaging the difference between the mean phase-locked spike delay and each phase-locked spike delay measured in the first half of the stimulation trial (x-axis) or in the second half of the stimulation trial (y-axis) (Fig. 5, B–I). For entrained cells in M1 and GP, the majority of the data points in these plots were in the top left quadrant, indicating a tendency toward increasing delays in phase-locked spike activity over time. This included statistically significant delays in phase-locked spike activity in M1 (11/19, 58%) and phase-locked spike activity in GP (3/13, 23%) between the first and last half of the minute-long stimulation trial (Fig. 5, C and E). The phase-locked inhibitory responses observed in STN and VLo during GP DBS were stationary over the 30-s recording period (Fig. 5, G and I).
Fig. 5.
Temporal fidelity of phase entrainment to DBS. A: examples of entrainment in the 4 recorded nuclei with raster plots (top) and corresponding PSTHs (bottom). Gray rectangle represents the region of the interpulse period blanked during template subtraction. Average signed jitter (ASJ) at the beginning and end of stimulation for all significant excitatory phases in M1 (VPLo DBS) (B), GP (STN DBS) (D), STN (GP DBS) (F), and VLo (GP DBS) (H). Fraction of excitatory phases in M1 (C), GP (E), STN (G), and VLo (I), in which the phase-locked spike delay statistically changed over the stimulation period.
DISCUSSION
This study quantified and compared frequency and phase entrainment of neuronal spike activity in four nuclei downstream (and in some cases also upstream) of three clinical DBS targets. The data indicate that high-frequency stimulation in VPLo and STN was effectively low-pass filtered in the entrained spike activity of M1 and GP, respectively. Moreover, entrainment in M1 during VPLo stimulation, and to a lesser extent in GP during STN stimulation, was not fully stationary over time. In contrast, high-frequency stimulation in the GP was robustly effective in delivering phasic inhibitory entrainment of spike activity in VLo and STN.
Low-pass entrainment filter to high-frequency stimulation.
The fidelity with which high-frequency stimulation is able to elicit phase-locked spike activity in a nucleus downstream (or upstream) of the stimulated target provides a measure to assess the broader network-level effects of DBS. In this study, we found that, despite the extensive projections existing between VPLo and primary motor cortex (Holsapple et al. 1991; Strick 1975, 1985) and between STN and GP (Sato et al. 2000a, 2000b), only a minor fraction of stimulus pulses in a high-frequency pulse train (7.5% in M1 and 8.5% in GP) was classified as effective (i.e., able to elicit a spike within a phase-locked window of the interpulse interval). Our study also found that entrainment phase (delays in excitatory phases ranged from 1.1–5.1 ms), entrainment type (single, double, or even triple excitatory phases), and entrainment fidelity (eEPF ranging between 0.5–25.0% in M1 and 0.4–40.0% in GP) were variable among the cells entrained with excitatory phase responses. During GP DBS, a higher fraction of GP DBS pulses was estimated to be effective in suppressing a probabilistically expected spike in STN and VLo (iEPFs were 82% and 86%, respectively). At the same time, there was a fairly large variability in the length of the stimulus-evoked inhibition (0.7 to 3.2 ms in STN and 1.5 to 3.5 ms in GP) and in the iEPF (69–96% in STN and 48–99% in GP).
Although the data in our study do not necessarily identify a specific mechanism for these findings, several factors are likely involved. For one, it is possible that, because of the stochastic nature of axons (Segev and Schneidman 1999), not all axons projecting from the stimulated area are activated with each and every stimulus pulse. Interestingly, the phase-locked excitatory responses to stimulation that we observed with delays <2 ms and with relatively low jitter, which could be attributable to antidromic activation, also showed an inability to entrain one to one with high-frequency stimulation. This finding is consistent with a study in brain slices showing that high-frequency stimulation of the motor thalamus could only entrain antidromic activity within corticothalamic axons at firing rates <50 Hz (Iremonger et al. 2006). Similar findings have been reported for other axonal pathways (Jensen and Durand 2009; Rosenbaum et al. 2014; Zheng et al. 2011) although some fiber tracts, particularly heavily myelinated tracts, can follow high-frequency stimulation pulse trains (Chomiak and Hu 2007).
Previous studies have noted a range of mechanistic rationales for axonal conduction failure, including depolarization block (Beurrier et al. 2001), an accumulation of intracellular calcium that activates calcium-dependent potassium channels thereby hyperpolarizing the membrane (Debanne 2004), a limited depolarizing after-potential stemming from a lack of a peri-axonal space between the axolemma and myelin sheath (Richardson et al. 2000), accumulation of extracellular potassium within the periaxonal space (Bellinger et al. 2008), and difficulties in propagating action potentials at high frequencies through axonal branches (Debanne 2004; Grill et al. 2008). In theory, in the case of M1 activity during VPLo DBS, sustained excitation could also be limited by increased inhibition via disynaptic activation of inhibitory interneurons, which may sustain higher firing frequencies (Martina and Jonas 1997) and are less susceptible to conduction failure compared with glutamatergic neurons (Meeks and Mennerick 2004). However, as Iremonger et al. (2006) noted, cortical neurons are still unable to follow the high-frequency pattern of stimulation even in the presence of GABA blockade. In addition, at high frequencies of stimulation, synaptic transmission is likely to undergo at least partial vesicle depletion (Anderson et al. 2006), which was suggested to shape the cortical response to high-frequency thalamic stimulation in slices using a voltage-sensitive dye (Urbano et al. 2002). Such depletion of available neurotransmitter will increase the transmission failure rate and account for the inability of recorded cells to follow the imposed high-frequency pattern, especially over time as the vesicle depletion presumably becomes more pronounced.
Imperfect fidelity of phase entrainment.
Wash-in and wash-out effects of DBS for treating essential tremor and Parkinson's disease are known to follow different time scales depending on the motor sign and the stimulation target (Cooper et al. 2014; Hristova et al. 2000; Johnson et al. 2008; Temperli et al. 2003). Although the data reported in our study only account for the first minute following stimulation onset, even in this case, we did observe phase-locked spike activity stemming from VPLo DBS and STN DBS that was not consistently stationary over time. These results are similar to those observed in GP during GP DBS (Erez et al. 2009; McCairn and Turner 2009) and in the GP during STN DBS (Moran et al. 2011). Several mechanisms are likely to be involved. Fast recurrent activation of synaptic terminals can result in depletion of readily available neurotransmitter vesicles. In this case, once a limiting frequency is exceeded, excitatory postsynaptic potentials begin to decrease in amplitude (Abbott et al. 1997; Tsodyks and Markram 1997), leading to a slower buildup of the poststimulus response (Zucker and Regehr 2002). It is also possible that other nonsynaptic mechanisms might be involved, such as channel dynamics (Johnson and McIntyre 2008) or precession-like interactions between multiple oscillators (Montgomery and Gale 2008). Another potential reason for the phase shift of time-locked responses could be a decrease of the speed of axonal conduction. It has been shown that, during repetitive stimulation, conduction velocity of lobster axons was decreased by about 30% (Grossman et al. 1979), whereas synaptic delay was extended by 1–2 ms in crayfish motor neurons (Hatt and Smith 1976).
DBS is also known to induce synaptic plasticity, even on short time scales of stimulation. In brain slices, it was shown that STN DBS could induce short- and long-term potentiation as well as long-term depression (Shen et al. 2003). Although presynaptic long-term depression could also be responsible for slower buildup of poststimulus responses together with vesicle depletion, the phase shift in phase-locked spike activity was previously reported to be short term and able to recover during roughly 40-s intervals between stimulation blocks (McCairn and Turner 2009), suggesting that this form of phase shift, observed in our study as well, may be short lived and thus more likely related to neurotransmitter depletion or axonal conduction delays.
Differences between glutamatergic and GABAergic transmission.
Our study also found significant differences in the entrainment responses that depended on the type of downstream projection likely to be modulated with high-frequency stimulation. Putative monosynaptic glutamatergic transmission was characterized by phase-locked increases in spike activity that were delayed >2 ms, whereas inhibitory transmission generated phase-locked decreases in spike activity almost immediately after each stimulus pulse. It is important to consider, however, that the stimulus artifact obscured spike activity within the first 0.5–1.0 ms after each stimulus pulse.
A second discrepancy between transmission modes consisted of the degree of effective pulses. Whereas eEPF measures were in the 8% range, iEPF measures were around 80–90%. Methodological differences in the calculation of the eEPF and iEPF may explain the large discrepancy observed, but the choice of 6 SDs for excitatory phases and 3 SDs for inhibitory phases is unlikely to be the origin of these differences. The lower significance thresholds of the iEPF calculation ultimately included cells with lower degrees of entrainment. It is also possible that GABAergic projections compared with glutamatergic projections could possess higher axonal and synaptic fidelity. Additionally, intrinsic functional topology of the motor network could also be responsible for the difference. Single pallidal spikes can exert a brief but strong inhibitory effect on thalamocortical relay cells in vivo in singing zebra finches that depend on the neural activity present in the thalamocortical circuit (Goldberg et al. 2012). For glutamatergic projections, repetitive stimulation has been reported to reduce the amplitude of presynaptic potential in invertebrate and mammalian axons (Geiger and Jonas 2000; Grossman et al. 1979; Wang and Kaczmarek 1998) because of sodium channel inactivation, amplified by nanomolar ranges of tetrodotoxin (Brody and Yue 2000). Also inactivation of sodium channels by high external concentration of potassium increases the proportion of conduction failures during repetitive stimulation (Meeks and Mennerick 2004). GABA-containing axons instead are less affected by the aforementioned manipulation of sodium currents (He et al. 2002; Meeks and Mennerick 2004; Prakriya and Mennerick 2000), possibly making them less prone to axonal failure. Moreover, inhibitory synapses are known to be generally located closer to the soma and thus possibly more effective than excitatory synapses in modulating postsynaptic activity, especially in the context of partial axonal conduction failure because of high-frequency stimulation.
The third difference between recording locations consisted of the fidelity of phase entrainment to the high-frequency stimulation. Although the interpulse interval times of the phase-locked spike activity were found to increase in a subset of excitatory phases (11/19 in motor cortex and 3/12 in GP), the times when phase-locked inhibition of spike activity returned to its baseline level were found to be stable over the recording period for VLo and STN during GP DBS. Again the methodological differences, together with the fact that stimulation in the GP was delivered only for 30 s compared with 60 s in VPLo and STN stimulation, suggest some caution in the interpretation of this data.
Limitations and other considerations.
The findings of this study motivate the investigation of phase and frequency entrainment fidelity over longer durations of stimulation coupled with quantitative measures of behavioral therapy, which will facilitate testing the potential emergence of long-term potentiation and depression that may require longer stimulus periods than used in this study (Shen et al. 2003). Additionally, the number of entrained cells reported in this study was maintained at a conservative level (that is, the thresholds for phase-locked activity were high) to study phase and frequency entrainment in only those cells with the strongest entrainment. It is also important to note that the difference in EPF calculation (eEPF and iEPF) consisted of different denominators. The denominator used in the eEPF measure represents the number of delivered pulses that are theoretically capable of altering the behavior of the recorded cell (i.e., the number of pulses that would not be expected to be followed by a spike in the excitatory phase of the PSTH for the eEPF measure). For the iEPF measure, the extracellular recordings were not able to directly measure inhibitory postsynaptic potentials downstream of the stimulated target, thus making it infeasible to know how effective each stimulus pulse was in generating a hyperpolarizing current. Instead, we considered the number of spikes in a given PSTH phase that were effectively inhibited by the stimulation.
Conclusion.
Computational models of neuronal activity during DBS have proven to be a useful tool to investigate the physiological mechanisms underlying therapy with DBS. However, to date there have been several assumptions made in terms of the fidelity of transmission of high-frequency stimulation on downstream nuclei. Clinical studies have indicated that alleviation of motor signs of DBS have wash-in and wash-out latencies that depend on stimulation target and the motor sign under investigation. Here, we show that phase-locked spike activities in nuclei that are directly connected with three different clinical targets of DBS show, with different degrees, characteristic low-pass filtered responses to high-frequency stimulation. This pattern depended on the type of projection and notably for glutamatergic projections resulted in a temporal variation in the PSTH following stimulation onset.
GRANTS
This work was supported by grants from the National Institutes of Health (NS-081118, NS-037019, and NS077657) and the Michael J. Fox Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: F.A., K.B.B., J.L.V., and M.D.J. conception and design of research; F.A., A.M., and M.D.J. performed experiments; F.A., A.M., and M.D.J. analyzed data; F.A., A.M., K.B.B., J.L.V., and M.D.J. interpreted results of experiments; F.A. and M.D.J. prepared figures; F.A. and M.D.J. drafted manuscript; F.A., A.M., K.B.B., J.L.V., and M.D.J. edited and revised manuscript; F.A., A.M., K.B.B., J.L.V., and M.D.J. approved final version of manuscript.
REFERENCES
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science 275: 220–224, 1997. [DOI] [PubMed] [Google Scholar]
- Agnesi F, Connolly AT, Baker KB, Vitek JL, Johnson MD. Deep brain stimulation imposes complex informational lesions. PLoS One 8: e74462, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M, Postupna N, Ruffo M. Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol 89: 1150–1160, 2003. [DOI] [PubMed] [Google Scholar]
- Anderson TR, Hu B, Iremonger K, Kiss ZH. Selective attenuation of afferent synaptic transmission as a mechanism of thalamic deep brain stimulation-induced tremor arrest. J Neurosci 26: 841–850, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger SC, Miyazawa G, Steinmetz PN. Submyelin potassium accumulation may functionally block subsets of local axons during deep brain stimulation: a modeling study. J Neural Eng 5: 263–274, 2008. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Bioulac B, Audin J, Hammond C. High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol 85: 1351–1356, 2001. [DOI] [PubMed] [Google Scholar]
- Brody DL, Yue DT. Release-independent short-term synaptic depression in cultured hippocampal neurons. J Neurosci 20: 2480–2494, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomiak T, Hu B. Axonal and somatic filtering of antidromically evoked cortical excitation by simulated deep brain stimulation in rat brain. J Physiol 579: 403–412, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SE, Driesslein KG, Noecker AM, McIntyre CC, Machado AM, Butson CR. Anatomical targets associated with abrupt versus gradual washout of subthalamic deep brain stimulation effects on bradykinesia. PLoS One 9: e99663, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith C, Darian-Smith I, Cheema SS. Thalamic projections to sensorimotor cortex in the macaque monkey: use of multiple retrograde fluorescent tracers. J Comp Neurol 299: 17–46, 1990. [DOI] [PubMed] [Google Scholar]
- Debanne D. Information processing in the axon. Nat Rev Neurosci 5: 304–316, 2004. [DOI] [PubMed] [Google Scholar]
- Erez Y, Czitron H, McCairn K, Belelovsky K, Bar-Gad I. Short-term depression of synaptic transmission during stimulation in the globus pallidus of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates. J Neurosci 29: 7797–7802, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Jonas P. Dynamic control of presynaptic Ca(2+) inflow by fast-inactivating K(+) channels in hippocampal mossy fiber boutons. Neuron 28: 927–939, 2000. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Farries MA, Fee Ms. Integration of cortical and pallidal inputs in the basal ganglia-recipient thalamus of singing birds. J Neurophysiol 108: 1403–1429, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill WM, Cantrell MB, Robertson MS. Antidromic propagation of action potentials in branched axons: implications for the mechanisms of action of deep brain stimulation. J Comput Neurosci 24: 81–93, 2008. [DOI] [PubMed] [Google Scholar]
- Grossman Y, Parnas I, Spira ME. Differential conduction block in branches of a bifurcating axon. J Physiol 295: 283–305, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Elder C, Vitek J. A template subtraction method for stimulus artifact removal in high-frequency deep brain stimulation. J Neurosci Methods 113: 181186, 2002. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder C, Okun M, Patrick S, Vitek J. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci 23: 1916–1923, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatt H, Smith DO. Synaptic depression related to presynaptic axon conduction block. J Physiol 259: 367–393, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zorumski CF, Mennerick S. Contribution of presynaptic Na(+) channel inactivation to paired-pulse synaptic depression in cultured hippocampal neurons. J Neurophysiol 87: 925–936, 2002. [DOI] [PubMed] [Google Scholar]
- Holsapple JW, Preston JB, Strick PL. The origin of thalamic inputs to the “hand” representation in the primary motor cortex. J Neurosci 11: 2644–2654, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristova A, Lyons K, Tröster AI, Pahwa R, Wilkinson SB, Koller WC. Effect and time course of deep brain stimulation of the globus pallidus and subthalamus on motor features of Parkinson's disease. Clin Neuropharmacol 23: 208–11, 2000. [DOI] [PubMed] [Google Scholar]
- Iremonger KJ, Anderson TR, Hu B, Kiss ZH. Cellular mechanisms preventing sustained activation of cortex during subcortical high-frequency stimulation. J Neurophysiol 96: 613–621, 2006. [DOI] [PubMed] [Google Scholar]
- Jensen AL, Durand DM. High frequency stimulation can block axonal conduction. Exp Neurol 220: 57–70, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, McIntyre CC. Quantifying the neural elements activated and inhibited by globus pallidus deep brain stimulation. J Neurophysiol 100: 2549–2563, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Miocinovic S, McIntyre C, Vitek J. Mechanisms and targets of deep brain stimulation in movement disorders. Neurotherapeutics 5: 294–308, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Zhang J, Ghosh D, McIntyre CC, Vitek JL. Neural targets for relieving parkinsonian rigidity and bradykinesia with pallidal deep brain stimulation. J Neurophysiol 108: 567–577, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Tachibana Y, Nambu A, Chiken S. Balance of monosynaptic excitatory and disynaptic inhibitory responses of the globus pallidus induced after stimulation of the subthalamic nucleus in the monkey. J Neurosci 25: 8611–8619, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J, Krafczyc S, Valkovic P, Eggert T, Claasen J, Botzel K. Objective measurement of muscle rigidity in parkinsonian patients treated with subthalamic stimulation. Mov Disord 24: 57–63, 2009. [DOI] [PubMed] [Google Scholar]
- Li S, Arbuthnott GW, Jutras MJ, Goldberg JA, Jaeger D. Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. J Neurophysiol 98: 3525–3537, 2007. [DOI] [PubMed] [Google Scholar]
- Martina M, Jonas P. Functional differences in Na+ channel gating between fast-spiking interneurones and principal neurones of rat hippocampus. J Physiol 505: 593–603, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCairn K, Turner R. Deep brain stimulation of the globus pallidus internus in the parkinsonian primate: local entrainment and suppression of low-frequency oscillations. J Neurophysiol 101: 1941–1960, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre C, Grill W. Selective microstimulation of central nervous system neurons. Ann Biomed Eng 28: 219233, 2000. [DOI] [PubMed] [Google Scholar]
- McIntyre C, Grill W, Sherman D, Thakor N. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol 91: 1457–1469, 2004. [DOI] [PubMed] [Google Scholar]
- Meeks JP, Mennerick S. Selective effects of potassium elevations on glutamate signaling and action potential conduction in hippocampus. J Neurosci 24: 197–206, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miocinovic S, Parent M, Butson C, Hahn P, Russo G, Vitek J, McIntyre C. Computational analysis of subthalamic nucleus and lenticular fasciculus activation during therapeutic deep brain stimulation. J Neurophysiol 96: 1569–1580, 2006. [DOI] [PubMed] [Google Scholar]
- Montgomery EB. Effects of GPi stimulation on human thalamic neuronal activity. Clin Neurophysiol 117: 26912702, 2006. [DOI] [PubMed] [Google Scholar]
- Montgomery EB, Gale JT. Mechanisms of action of deep brain stimulation (DBS). Neurosci Biobehav Rev 32: 388–407, 2008. [DOI] [PubMed] [Google Scholar]
- Moran A, Stein E, Tischler H, Belelovsky K, Bar-Gad I. Dynamic stereotypic responses of Basal Ganglia neurons to subthalamic nucleus high-frequency stimulation in the parkinsonian primate. Front Syst Neurosci 5: 21, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent M, Lévesque M, Parent A. Two types of projection neurons in the internal pallidum of primates: single-axon tracing and three-dimensional reconstruction. J Comp Neurol 439: 162–175, 2001. [DOI] [PubMed] [Google Scholar]
- Perera T, Yohanandan SAC, Vogel AP, McKay CM, Jones M, Peppard R, McDermott HJ. Deep brain stimulation wash-in and wash-out times for tremor and speech. Brain Stim 8: 359, 2015. [Google Scholar]
- Prakriya M, Mennerick S. Selective depression of low-release probability excitatory synapses by sodium channel blockers. Neuron 26: 671–682, 2000. [DOI] [PubMed] [Google Scholar]
- Ranck J. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98: 417440, 1975. [DOI] [PubMed] [Google Scholar]
- Richardson AG, McIntyre CC, Grill WM. Modelling the effects of electric fields on nerve fibres: influence of the myelin sheath. Med Biol Eng Comput 38: 438–446, 2000. [DOI] [PubMed] [Google Scholar]
- Rosenbaum R, Zimnik A, Zheng F, Turner RS, Alzheimer C, Doiron B, Rubin JE. Axonal and synaptic failure suppress the transfer of firing rate oscillations, synchrony and information during high frequency deep brain stimulation. Neurobiol Dis 62: 86–99, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaniello S, McCarthy MM, Montgomery EB, Gale JT, Kopell N, Sarma SV. Therapeutic mechanisms of high-frequency stimulation in Parkinson's disease and neural restoration via loop-based reinforcement. Proc Natl Acad Sci USA 112: E586–E595, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Lavallée P, Lévesque M, Parent A. Single-axon tracing study of neurons of the external segment of the globus pallidus in primate. J Comp Neurol 417: 17–31, 2000a. [PubMed] [Google Scholar]
- Sato F, Parent M, Levesque M, Parent A. Axonal branching pattern of neurons of the subthalamic nucleus in primates. J Comp Neurol 424: 142–152, 2000b. [DOI] [PubMed] [Google Scholar]
- Segev I, Schneidman E. Axons as computing devices: basic insights gained from models. J Physiol (Paris) 93: 263–270, 1999. [DOI] [PubMed] [Google Scholar]
- Shen KZZ, Zhu ZTT, Munhall A, Johnson SW. Synaptic plasticity in rat subthalamic nucleus induced by high-frequency stimulation. Synapse 50: 314–319, 2003. [DOI] [PubMed] [Google Scholar]
- Strick PL. Multiple sources of thalamic input to the primate motor cortex. Brain Res 88: 372–377, 1975. [DOI] [PubMed] [Google Scholar]
- Strick P. How do the basal ganglia and cerebellum gain access to the cortical motor areas? Behav Brain Res 18: 107123, 1985. [DOI] [PubMed] [Google Scholar]
- Temperli P, Ghika J, Villemure J, Burkhard P, Bogousslavsky J, Vingerhoets F. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology 60: 78–81, 2003. [DOI] [PubMed] [Google Scholar]
- Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci USA 94: 719–723, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Leznik E, Llinás RR. Cortical activation patterns evoked by afferent axons stimuli at different frequencies: an in vitro voltage-sensitive dye imaging study. Thalamus Relat Syst 1: 371–378, 2002. [Google Scholar]
- Vitek JL, Ashe J, DeLong MR, Kaneoke Y. Microstimulation of primate motor thalamus: somatotopic organization and differential distribution of evoked motor responses among subnuclei. J Neurophysiol 75: 2486–2495, 1996. [DOI] [PubMed] [Google Scholar]
- Wang LY, Kaczmarek LK. High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature 394: 384–388, 1998. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong M. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron 52: 197–204, 2006. [DOI] [PubMed] [Google Scholar]
- Windels F, Bruet N, Poupard A, Feuerstein C, Bertrand A, Savasta M. Influence of the frequency parameter on extracellular glutamate and γ-aminobutyric acid in substantia nigra and globus pallidus during electrical stimulation of subthalamic nucleus in rats. J Neurosci Res 72: 259–267, 2003. [DOI] [PubMed] [Google Scholar]
- Zheng F, Lammert K, Nixdorf-Bergweiler BE, Steigerwald F, Volkmann J, Alzheimer C. Axonal failure during high frequency stimulation of rat subthalamic nucleus. J Physiol 589: 2781–2793, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol 64: 355–405, 2002. [DOI] [PubMed] [Google Scholar]