Abstract
The commonly used inhalation anesthetic, isoflurane, can permeate rapidly through the placental barrier and thus cause toxicity to the central nervous system of the developing fetus. In this study, we treated pregnant mice with clinically relevant concentration of isoflurane early on in development (days 3.5–6.5), and then found that the fetus growth was inhibited by isoflurane. We further used the mouse embryonic stem cell (mES cell) to be the early development model to investigate the mechanism of the embryotoxicity of isoflurane and found that isoflurane inhibited self-renewal of mES cells. In addition, neuronal differentiation from the mES cells treated with isoflurane was also inhibited. Overexpression of E-cadherin attenuated the effects of isoflurane on self-renewal and the subsequent neuronal differentiation. We also found that miR-9 can be upregulated by isoflurane. Overexpression of miR-9 inhibited the self-renewal and subsequent neuronal differentiation. E-cadherin was directly targeted by miR-9. Overexpression of E-cadherin can abolish the function of miR-9 or isoflurane on self-renewal and subsequent neuronal differentiation. These data suggested that isoflurane inhibits self-renewal and neuronal differentiation of mES cells, possibly by regulating the miR-9-E-cadherin signaling. The result of the current study may provide a novel idea for preventing the toxicity of inhalation anesthetics in the developing fetal brain in clinical practice when pregnant women accept nonobstetric surgery under inhalation general anesthesia.
Introduction
Nowadays, between 0.75% and 2% of pregnant women require nonobstetric surgery [1]. In the United States, about 75,000 pregnant women undergo nonobstetric surgery each year [2]. Isoflurane, a commonly used inhalation anesthetic that could readily cross the placental barrier, could decrease the self-renewal of neuron stem cells at clinically relevant concentrations and inhibit the survival, proliferation, and differentiation of human neural progenitor cells [3–5]. A previous study found that isoflurane significantly inhibited fetal growth in pregnant mice [6]. A recent study found that a rat exposed to isoflurane in utero during early gestation is behaviorally abnormal as an adult [7]. These studies suggest that isoflurane may have potential toxicity effects of isoflurane on embryonic development. Therefore, the embryotoxicity in embryonic development of the fetus of pregnant women who receive general anesthesia with isoflurane at the early stage of the pregnancy has become a major health issue for both the medical community and the public.
Embryonic stem (ES) cells are derived from the inner cell mass of blastocysts and are characterized by self-renewal and pluripotency [8]. E-cadherin is a critical molecule that regulates mouse embryonic stem cell (mES cell) self-renewal and pluripotent potential [9,10]. E-cadherin-mediated cell–cell contact is also critical for the generation of induced pluripotent stem cells [11]. A previous study showed that E-cadherin maintains the self-renewal and pluripotency of mES cells by enhancing the expression of Nanog and Oct4 through activating the Lif (leukemia inhibitory factor)-stat3 signaling [12]. The mES cells cultured on E-cadherin-coated plates show a higher proliferative capacity and lower dependence on leukemia inhibitory factor [13]. These observations suggest that E-cadherin plays an important role in the self-renewal of stem cells.
Mature microRNAs (miRNAs) are single-stranded RNA molecules, 20–23 nucleotides (nt) in length, that control gene expression post-transcriptionally in many cellular processes. These molecules typically reduce the stability of mRNAs [14]. MiR-9 is expressed in mES cells committed to differentiation to neurons and not at earlier stages [15]. E-cadherin is highly expressed during early embryonic development and downregulated upon neuronal differentiation [16]. However, the relationship between the miR-9 and E-cadherin in mES cells is still unknown.
In the current study, we found that anesthesia with 1.4% isoflurane for 2 h daily for 3 days reduced fetal growth and development. To explore the underlying mechanism, we next treated mES cells with isoflurane to examine the potential effects of isoflurane on the self-renewal of mES cells. Moreover, we also investigated the subsequent neuronal differentiation of these isoflurane-treated mES cells. In a preliminary bioinformatics analysis using TargetScan, miRanda, and miRBase [17–19], we predicted that miR-9 could bind to 3′ untranslated region (UTR) of E-cadherin. In subsequent experiments, we found that isoflurane could inhibit self-renewal of mES cells. The neural differentiation of these isoflurane-treated mES cells is inhibited. MiR-9 inhibited the expression of E-cadherin by targeting the mRNA 3′UTR. Isoflurane repressed self-renewal of mES cells by the miR-9-E-cadherin pathway and led to inhibition of the neural differentiation of isoflurane-treated mES cells.
In conclusion, isoflurane inhibited self-renewal and subsequent neuronal differentiation of mES cells, possibly by upregulating miR-9, and subsequent downregulation of E-cadherin. The miR-9-E-cadherin pathway may be a target for preventing the toxicity of inhalation anesthetics in the developing fetal brain in clinical practice when pregnant women accept nonobstetric surgery under inhalation general anesthesia.
Materials and Methods
Animals
All animal experiments were approved by the Animal Care Committee of the Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine. Two-month-old C57BL6/J mice (SLAC laboratory animal) with one-quarter males and three-quarters females were housed in clean cages and the room temperature was maintained at 24°C±1°C, with a 12-h light–12-h dark cycle and free rodent diet and water. The male and female mice were mixed at the ratio of 1:3 to mate overnight, and mating was confirmed by the presence of a vaginal plug the following morning. The date of plug positivity was designated as day 0.5 (E0.5), and pregnant mice were treated with 1.4% isoflurane for 2 h daily for 3 days from day 3.5 (E3.5) to day 6.5 (E6.5). At day 18 (E18), pregnant mice were executed by cervical vertebra luxation and the fetal mice were harvested by cesarean section. The fetuses were then weighed and observed.
Cell culture
E14 ES cells, purchased from the Institute of Biochemistry and Cell Biology in Shanghai, China (SCSP-204), were seeded in a plate precoated by 0.1% gelatin in Dulbecco's modified Eagle's medium (DMEM; Gibco) containing 15% ES-fetal bovine serum (FBS; Gibco), glutamine (1:100; Invitrogen), nonessential amino acid (1:100; Invitrogen), 2-mercaptoethanol (1:550; Invitrogen), and Lif (1:10,000; Invitrogen) at a density of 2×105 cells per well in six-well plates and cultured at 37°C in a humidified atmosphere containing 5% CO2. NIH3T3 cells were cultured in DMEM containing 10% FBS.
Cell treatment
The isoflurane group was exposed to 2% isoflurane, 5% CO2 plus 21% O2, and the control group conditions included 5% CO2 plus 21% O2 for 2, 4, and 6 h, as previously described [20]. We chose this concentration of isoflurane treatment directly because it is clinically relevant and has been shown to induce apoptotic cell death, Aβ accumulation, neuroinflammation in H4 human neuroglioma cells, and neurons in Xie's studies [20,21]. A Drager Vamos gas analyzer (Drager) was used to monitor the concentration of CO2, O2, and isoflurane. In some experiments, cells were transfected with a pre-miR-9 (50 nM), miR-9 inhibitor (100 nM), or both at 12 h before isoflurane exposure. For conditional expression of E-cadherin in mES cells (ptight-tet-on/rtTA-E-cadherin), E-cadherin expression was turned on by DOX exposure (5 μg/mL) at 12 h before isoflurane treatment.
Neural differentiation of mES cells
Cultured E14 mES cells were dissociated to single cells with 0.05% trypsin 2, 4, and 6 h after being treated with isoflurane for 3 min at 37°C, and then neutralized with DMEM containing 10% FBS. After being counted, mES cells were washed with DMEM/F12 and resuspended in a Petri dish at a density of 25,000–50,000/mL with neural differentiation medium (NDM): 50% DMEM/F12 (Invitrogen), 50% neural basal (Invitrogen), N2 supplement (1:100; Invitrogen), l-glutamax (1:100; Invitrogen), CD lipid concentrate (1:100; Invitrogen), 3.7 μM N-acetylcysteine (Sigma-Aldrich), and 0.1 mM 2-mercaptoethanol (Amresco). The medium was changed every 2 days. At day 12, embryoid bodies (EBs) were digested with Accutase (Innovative Cell Technologies) for 3 min, and washed twice with DMEM/F12, then plated onto polyornithine (Sigma-Aldrich)/laminin (Invitrogen)-coated coverslips for staining at day 14 or plated onto a laminin (Invitrogen)-coated six-well plate for RNA analysis at day 14 with NDM medium supplemented with B27 (Invitrogen).
Construction of luciferase reporter gene
Fragments of the 3′UTR of E-cadherin were amplified from the DNA of mES cells by polymerase chain reaction (PCR), with the following primers: forward: 5′-GGCGAG CTCGGACCACTATGCATGCTGC-3′, with the sacI restriction site; reverse: 5′-GGCCTCGAGGTCTCACCGCCT GTGTACC-3′ with an xbaI restriction site. The product was inserted into the luciferase reporter vector, pGL3cm (Promega).
Construction of luciferase reporter of mutant UTR
E-cadherin 3′UTR mutation was generated by replacing the miRNA-binding site sequence with miRNA seed sequences and insertion of the mutant sequence into luciferase reporter vector (pGL3cm).
Establishment of inducible E-cadherin overexpression in mES cell lines
The mES cells were dissociated with 0.05% trypsin and transfected at a density of 5,000/mL with rtTA lentivirus (106 transducing units/mL) supplemented with 8 μg/mL polybrene. Forty-eight hours later, cells were selected using G418 (50 μg/mL). A stably transfected cell line was selected and infected with pTight-E-cadherin overexpression lentivirus for 48 h in 8 μg/mL polybrene before selection with puromycin (5 μg/mL). The medium was changed every day for 7 days until the identification of the single-cell clone under a microscope. Clones were picked up and dissociated with trypsin and plated onto gelatin-coated 24-well plates. The expression of E-cadherin was examined using a western blot after adding Dox.
Reverse transcription PCR and real-time quantity PCR for miRNA
Total RNA was extracted using Trizol reagent (Sigma-Aldrich) and reverse transcribed using M-MLV reverse transcriptase (Promega) using primers published in a previous study [12]. The real-time quantitative PCR included 40 cycles of amplification. Expression of target genes (2ΔΔCt) was normalized against endogenous glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Real-time quantity PCR (qRT-PCR) was carried out using an Mx3000P system (Stratagene).
Reverse transcription PCR and real-time quantity PCR for miRNA
miRNA was reverse transcribed to cDNA using a stem–loop reverse transcription primer (RiboBio Co). Quantitative RT-PCR was carried out using the Mx3000P system (Stratagene). Small nuclear RNA U6 was used as an internal control. Target gene expression (2ΔΔCt) was normalized against endogenous U6 RNA. The miRNA qRT-PCR primers were synthesized by RiboBio.
Transfection of pre-miRNA or miRNA inhibitor
mES cells were transfected with a chemically synthesized pre-miRNA or miRNA inhibitor (Biolend) with Lipofectamine 2000.
Luciferase assay
NIH3T3 cells (1×105) were transfected with 200 ng UTR or mutant UTR reporter, 10 ng Renilla vector, and 50 nM chemically synthesized pre-miR-9 (Biolend) with Lipofectamine 2000. Cells lysates were harvested 24 h after transfection for the dual-luciferase assay (Promega).
Western blotting analysis
Cell lysate was resuspended using 5× loading lysis buffer (250 mM Tris–HCl (pH6.8) 5% DTT, 10% sodium dodecyl sulfate (SDS), 0.5% bromophenol blue 0.025 g, 50% glycerine). The membrane was then incubated with a primary antibody against E-cadherin (BD Biosciences) or GAPDH (Cell Signaling Technology). Signals were visualized by enhanced chemiluminescence (ECL; Thermo).
Alkaline phosphatase detection staining
Alkaline phosphatase (AP) is a universal pluripotent marker for all types of pluripotent stem cells, including ES cells, embryonic germ cells, and induced pluripotent stem cells. The pluripotent status of stem cells can be characterized by a high level of AP expression. Detection of AP-positive activity can show the self-renewal of mES cells.
AP detection staining was done by using the FastRed alkaline phosphatase kit (Sigma-Aldrich) to detect AP activity, according to the manufacturer's protocol. The cells were fixed for 2 min with phosphate-buffered saline (PBS) containing 4% paraformaldehyde (PFA) at room temperature. After washing with PBS, the reagent of the kit was added into the dish to react for 15 min.
Immunostaining
For immunostaining, cells were fixed for 20 min with PBS containing 4% PFA at room temperature. After extensive washing with PBS, cells were treated with PBS containing 0.2% Triton X-100 for 8 min at room temperature. After treatment by Triton X-100, PBS containing 10% FBS (Gibco) was added into the cells for 1 h. Then, the antibodies can be added. The primary antibodies included anti-Oct4 (Cell Signaling Technology), anti-Nanog (Santa Cruz Biotechnology), anti-SSEA1 (Santa Cruz Biotechnology), and anti-Tuj1 (Abcam).
Statistical analyses
Results are presented as mean±standard deviation from three independent experiments. We used GraphPad Prism 5 software (GraphPad Software, Inc.) for all statistical analyses. Statistical significance was determined using Student's t-test or one-way analysis of variance (ANOVA) to compare differences with the control group, followed by the Bonferroni correction where appropriate. *P<0.05, **P<0.01, ***P<0.001.
Results
Anesthesia with 1.4% isoflurane for 2 h daily for 3 days reduced fetal growth and development
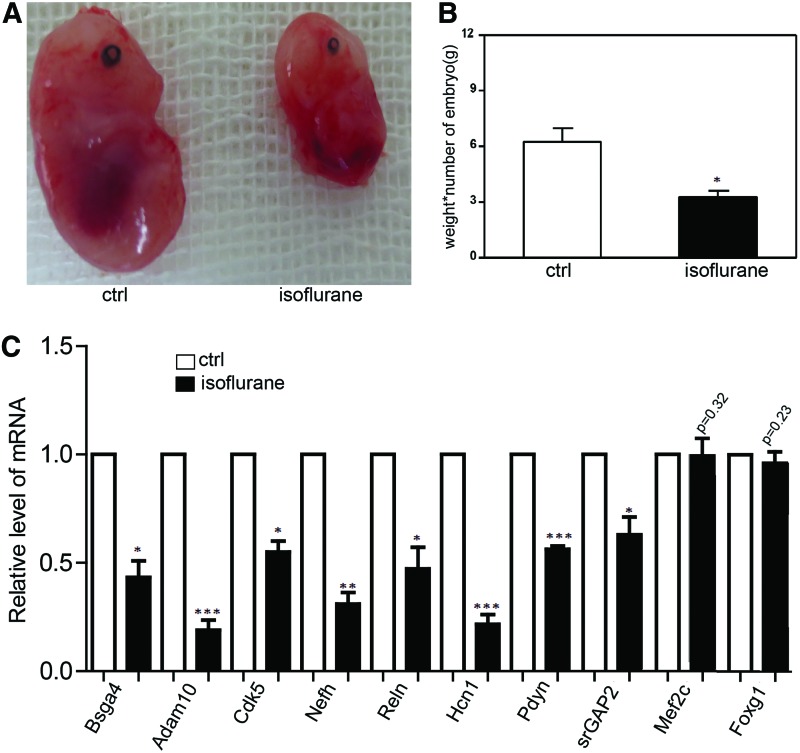
Previous studies suggested that inhaled anesthetics might have a negative impact on fetal growth and development at discrete times during pregnancy. This study was designed to test the impact of isoflurane at a time in the beginning of organogenesis during pregnancy. We set up the model, anesthesia with 1.4% isoflurane for 2 h daily for 3 days to pregnant mice to simulate pregnant women undergoing long-time nonobstetric surgery, as the previous study [6]. Interestingly, we found that anesthesia with 1.4% isoflurane for 2 h daily for 3 days reduced fetal growth and development (Fig. 1A). Isoflurane reduced fetal growth compared with fetal weights×number of fetuses between the control group and isoflurane group (Fig. 1B). We also found that the placental growth was reduced by isoflurane. The size and weight of the placenta were also smaller and lower than the control (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/scd). The placenta weights×number were also lower than the control group (Supplementary Fig. S1B). We detected the expression of brain-related genes of the mouse and found that the level of many genes was downregulated in the fetal mice, which were from the pregnant mice treated with isoflurane (Fig. 1C).
FIG. 1.
Isoflurane reduced fetal growth and development. (A) Anesthesia with 1.4% isoflurane for 2 h daily for 3 days reduced fetal growth and development. Ctrl means the fetal mouse from a pregnant mouse without treatment. Isoflurane means fetal mouse from a pregnant mouse treated with 1.4% isoflurane. (B) Isoflurane reduced fetal growth compared with fetal weights×number of fetuses between the control group and isoflurane group. Data shown are mean±SD (n=20). *P<0.05. (C) Detection of gene expression in the mouse brain by qRT-PCR. Data shown are mean±SD (n=6). *P<0.05, **P<0.01, ***P<0.001. SD, standard deviation; qRT-PCR, reverse transcription-real time PCR. Color images available online at www.liebertpub.com/scd
Isoflurane represses the neural differentiation by influencing the self-renewal of mES cells
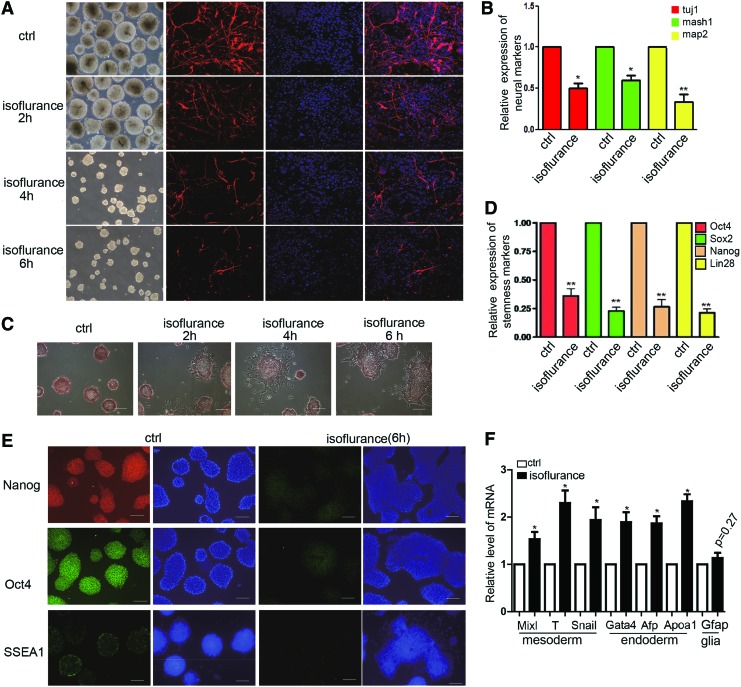
To study the effects of isoflurane on embryonic development, we performed neural differentiation from mES cells as the model to mimic the neural development in vivo. To find the effect of isoflurane on neural differentiation, we exposed the mES cells to 2% concentration of isoflurane for 2, 4, and 6 h. Then, we used these treated mES cells to perform neural differentiation and found that the size of EBs derived from isoflurane-treated mES cells was significantly smaller than the control group at day 10 (Fig. 2A). Furthermore, we found that the amounts of tuj1-positive cells derived from the mES cells treated with isoflurane were much lower than in the control group on day 14 (Fig. 2A). Then, we detected the mRNA level of neural markers, tuj1, mash1, and map2, between the control group and 6-h group after treatment with isoflurane and found the downregulation of tuj1, mash1, and map2 in the isoflurane-treated group (Fig. 2B). These phenomena of the effect of isoflurane on neural differentiation from mES cells made us hypothesize that isoflurane may damage the self-renewal of mES cells. We thus cultured the mES cells that have been treated for 2, 4, and 6 h with isoflurane, respectively, for another 48 h. AP detection staining showed that capability of self-renewal could be repressed by isoflurane with the treatment time extension (Fig. 2C). The expression level of stemness markers, Oct4, Sox2, Nanog, and Lin28, was also downregulated (Fig. 2D). Furthermore, we used immunostaining to detect the stemness markers, Nanog, Oct4, and SSEA1, and found the downregulation of these genes (Fig. 2E). We further detected the expression of markers of the mesoderm and endoderm and found that the level of these markers was higher than the control group on day 14 of neural differentiation (Fig. 2F). We also found that more cells, which had been treated with isoflurane for 6 h, underwent the apoptosis than the control group on day 14 of neural differentiation (Supplementary Fig. S2A, B). These results showed that isoflurane made the mES cells instable by repressing self-renewal and influencing the neural differentiation.
FIG. 2.
Isoflurane damages neuronal differentiation by repressing the self-renewal of mES cells. (A) Immunostaining of tuj1 showed the gradual serious inhibition of neural differentiation from the mES cells treated with isoflurane for 2, 4, and 6 h. Ctrl means control groups with no isoflurane. The scale bar represents 25 μm. (B) Detection of the mRNA level of neural markers, tuj1, mash1, and map2, in the group treated with isoflurane compared with the control group by qRT-PCR. Ctrl means mES cells treated without isoflurane. Isoflurane means cell treated with isoflurane for 6 h. Data shown are mean±SD (n=3). *P<0.05, **P<0.01. (C) AP detection staining showed the inhibition of mES cell self-renewal by isoflurane at 2, 4, and 6 h. Ctrl means control groups treated without isoflurane. The scale bar represents 100 μm. (D) Detection of the mRNA level of stemness makers, Oct4, Sox2, Nanog, and Lin28, in mES cells treated with isoflurane for 6 h and the control group. Ctrl means mES cells treated without isoflurane. Data shown are mean±SD (n=3). **P<0.01. (E) Immunostaining of stemness markers, Oct4, Nanog, and SSEA1, in the mES cells treated with isoflurane for 6 h and the control group. Ctrl means mES cells treated without isoflurane. The scale bar represents 100 μm. (F) Detection of the mRNA level of the markers of the mesoderm, endoderm, and glia on day 7 of neural induction. Data shown are mean±SD (n=3). *P<0.05. mES cells, mouse embryonic stem cell; AP, alkaline phosphatase. Color images available online at www.liebertpub.com/scd
Isoflurane represses the self-renewal of mES cells by upregulating the level of miR-9
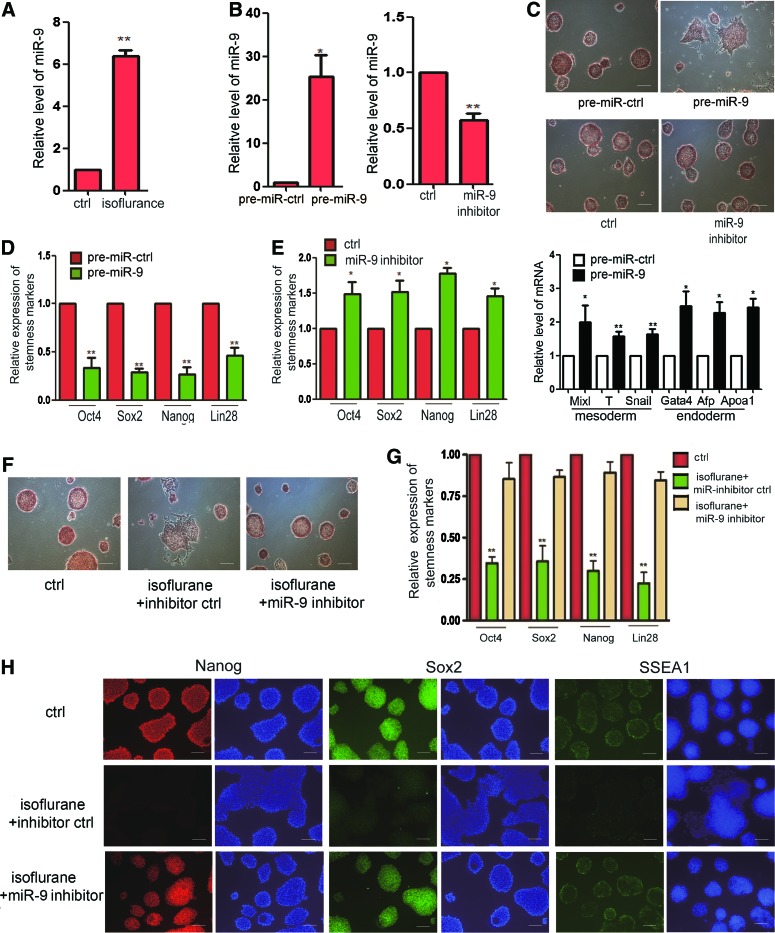
To find the mechanism of the effect of isoflurane on mES cells, we treated the mES cells with 2% isoflurane for 6 h and then detected that miR-9 was upregulated (Fig. 3A). Then, we wanted to know whether miR-9 regulates the self-renewal of mES cells. We thus transfected the pre-miR-9 or miR-9 inhibitor into the mES cells (Fig. 3B). We found that mES cells transfected with miR-9 did not maintain the capacity of self-renewal detected by AP detection staining and began to differentiate in advance (Fig. 3C), but the miR-9 inhibitor did not influence the self-renewal of mES cells (Fig. 3C). We transfected the miR-9 inhibitor into mES cells and cultured the cells for another 72 h. We found that the cells began to differentiate. We detected that the expression of Mixl, T, and Snail, which are the markers of the mesoderm, and the Gata1, Afp, Apoa1, which are the markers of the endoderm, in the miR-9-transfected group is higher than the control group (Fig. 3C). We also performed the real-time quantity PCR (qRT-PCR) to detect the stemness markers of mES cells, which were transfected with pre-miR-9 and miR-9 inhibitor. We found that mES cells transfected with miR-9 downregulated the level of stemness markers, Oct4, Sox2, Nanog, and Lin28, and the miR-9 inhibitor upregulated the expression of these stemness markers (Fig. 3D). We also used immunostaining to confirm the regulation of miR-9 on self-renewal of mES cells (Fig. 3E). Next, we performed the rescue experiment. We found that the miR-9 inhibitor abrogated the influence of isoflurane on mES cell self-renewal and recovered the clone maintaining and stemness marker expression (Fig. 3F, G, H). These results showed that isoflurane influences the mES cell self-renewal by regulating the level of miR-9 in mES cells.
FIG. 3.
Isoflurane represses the self-renewal of mES cells by upregulating the level of miR-9. (A) Level of miRNA-9 detected by qRT-PCR in mES cells treated with isoflurane for 6 h. Ctrl means mES cells with no isoflurane. Data shown are mean±SD (n=3). **P<0.01. (B) Level of miRNA-9 in mES cells transfected with pre-miR-9 or miR-9 inhibitor and control groups. Ctrl means mES cells transfected with miRNA control or miRNA inhibitor control. miR-9 means mES cells transfected with pre-miR-9. Data shown are mean±SD (n=3). *P<0.05, **P<0.01. (C) Representative pictures of AP detection staining of mES cells transfected with pre-miR-9 or miR-9 inhibitor and control groups. (D) Stemness markers, Oct4, Sox2, Nanog, and Lin28, in mES cells transfected by pre-miR-9 and miRNA control groups detected by qRT-PCR. Data shown are mean±SD (n=3). **P<0.01. (E) Stemness markers in miR-9 inhibitor and miRNA inhibitor control groups detected by qRT-PCR. Data shown are mean±SD (n=3). *P<0.05. (F) AP detection staining of mES cells treated with isoflurane or isoflurane plus miR-9 inhibitor compared with control mES cells. Ctrl means the control group treated with no isoflurane and miRNA control. (G) Detection of stemness markers, Oct4, Sox2, Nanog, and Lin28, in mES cells by qRT-PCR in (F). Data shown are mean±SD (n=3). **P<0.01. (H) Immunostaining of stemness markers, Nanog, Sox2, and SSEA1, of mES cells in (F). The scale bar represents 100 μm. miRNA, microRNA. Color images available online at www.liebertpub.com/scd
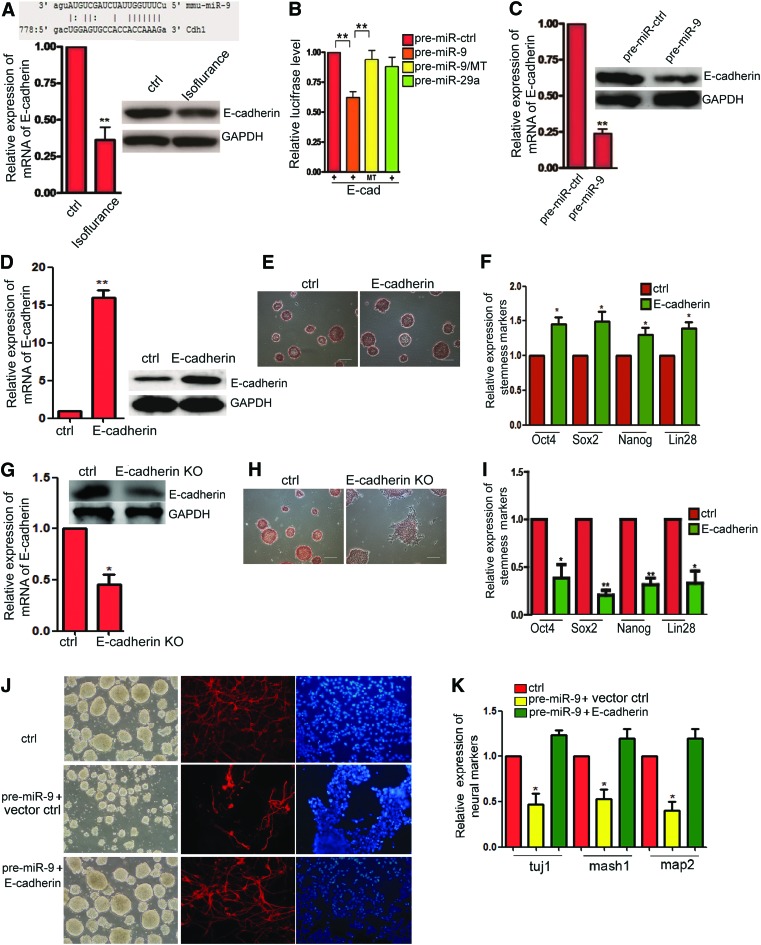
E-cadherin is the target of miR-9 and can be regulated by isoflurane to regulate the mES cell self-renewal
MiRNAs perform their function by targeting the downstream mRNA and inhibiting the translation of protein. So, we wanted to find out the target gene of miR-9. Using the TargetScan, miRanda, and miRBase, we predicted that E-cadherin might be targeted by miR-9 (Fig. 4A). Then, we detected that the expression of E-cadherin was downregulated in the mES cells treated for 6 h with isoflurane in both mRNA and protein levels (Fig. 4A). Then, we performed the luciferase assay to confirm that. We engineered luciferase reporters that had the wild-type 3′UTRs of E-cadherin or the mutant UTRs to perform the luciferase assay to study the relationship between E-cadherin and miR-9. In 3T3 cells, the luciferase reporters were cotransfected with the miR-9 precursor (pre-miR-9), which was processed into mature miRNAs. A pre-miR control with no homology to the genome was used to control the nonspecific effects of expression; the pre-miR control did not affect the reporter activities. miR-29a was another negative control, which had no predicted target site on E-cadherin 3′UTR. The pre-miR-9 significantly reduced the luciferase activities of the wild-type E-cadherin compared with the two negative controls. In contrast, mutant reporters were not repressed by pre-miR-9 (Fig. 4B). A mutation in the miR-9-binding seed region in E-cadherin 3′UTR abrogated the suppressive effect of miR-9 (Fig. 3B). Furthermore, we detected that overexpression of miR-9 downregulated the expression of E-cadherin in both mRNA and protein levels (Fig. 4C). We overexpressed the E-cadherin in mES cells (Fig. 4D) and found that mES cells still maintained the clones and the ability of self-renewal by AP detection staining (Fig. 4E). We also found that the stemness markers were upregulated slightly in E-cadherin-overexpressed mES cells (Fig. 4F). We also found that downregulation of E-cadherin caused the repression of self-renewal of mES cells (Fig. 4G–I). We further found that mES cells transfected with pre-miR-9 had lower expression of neuronal markers, tuj1, mash1, and map2, and the capacity of neural differentiation was also inhibited. Additionally, expression of markers of the mesoderm and endoderm is much higher in the miR-9-transfected group than the control, which is similar to the isoflurane-treated group in Fig. 2. Overexpression of E-cadherin restored the lower expression of neuronal markers and neural differentiation, which was inhibited in mES cells tranfected with pre-miR-9 (Fig. 4J, K).
FIG. 4.
Isoflurane represses self-renewal of mES cells to inhibit neurogenesis by increasing miR-9 and subsequent repressing of E-cadherin (E-cad). (A) Above picture: prediction of the E-cadherin 3′UTR-binding site. Bottom panel means mRNA and protein levels of E-cadherin in mES cells treated with isoflurane detected by qRT-PCR and western blot. Ctrl means mES cells treated with no isoflurane. Data shown are mean±SD (n=3). **P<0.01. (B) Luciferase reporter assays were performed with vectors containing DNA fragments corresponding to the putative wild-type or mutant target sites for miR-9 in the 3′UTRs of the E-cadherin mRNAs. Firefly luciferase activity was normalized to Renilla luciferase activity. E-cad means luciferase reporter gene vector with wild-type E-cad 3′UTR. MT means mutant luciferase reporter gene vector with the mutant miRNA-233-binding site. Pre-miR-29a was used as a negative miRNA control in the assays. Data shown are mean±SD (n=3). **P<0.01. (C) Pre-miR-9 decreases the expression of E-cadherin in mRNA and protein levels. Ctrl means miRNA control. Data shown are mean±SD (n=3). **P<0.01. (D) Detection of mRNA and protein levels of E-cadherin by qRT-PCR and western blot in mES cells. Ctrl means overexpression vector control. Data shown are mean±SD (n=3). **P<0.01. (E) AP detection staining of mES cells that overexpressed E-cadherin and control group. Ctrl means overexpression vector control. (F) Detection of mRNA of stemness markers, Oct4, Sox2, Nanog, and SSEA1, in mES cells. Ctrl means shRNA vector control. Data shown are mean±SD (n=3). *P<0.05. (G) Knockdown of E-cadherin in mES cells detected by qRT-PCR and western blot. Data shown are mean±SD (n=3). *P<0.05. (H) AP detection staining of mES cells, knockdown of E-cadherin and control groups. E-cadherin KO means knockdown of E-cadherin in mES cells. (I) Detection of stemness markers, Oct4, Sox2, Nanog, and SSEA1, of mES cells by qRT-PCR. Data shown are mean±SD (n=3). *P<0.05, **P<0.01. (J) Immunostaining of tuj1. Ctrl means control groups with no isoflurane. The scale bar represents 25 μm. (K) Detection of the mRNA level of neural makers, tuj1, mash1, and map2. Bottom panel is the detection of the expression level of markers of the mesoderm and endoderm. Data shown are mean±SD (n=3). *P<0.05. Color images available online at www.liebertpub.com/scd
Isoflurane influences neural differentiation in mES cells through regulating the miR-9-E-cadherin pathway
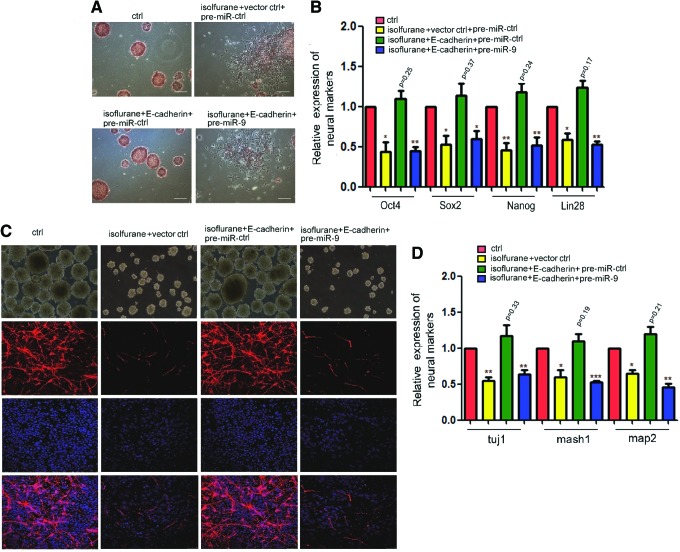
To find out whether the isoflurane influence on mES cell self-renewal could be mediated by E-cadherin, we performed the rescue experiment and found that overexpression of E-cadherin abrogated the effect of isoflurane on mES cell self-renewal by AP detection staining (Supplementary Fig. S3A). Overexpression of E-cadherin also abrogated the effect of isoflurane on stemness markers by qRT-PCR (Supplementary Fig. S3B) and immunostaining (Supplementary Fig. S3C). Then, we performed the AP detection staining to show that overexpressing E-cadherin rescues the inhibition of mES cell self-renewal caused by isoflurane. Overexpression of miR-9 abrogated the function of E-cadherin (Fig. 5A). Overexpressing E-cadherin also attenuated the decrease of stemness genes of mES cells by isoflurane. Pre-miR-9 abrogated the effect of overexpressing E-cadherin in isoflurane-treated cells (Fig. 5B). Next, we established the inducible E-cadherin overexpression plasmid, which expressed E-cadherin only after adding Dox. We overexpressed E-cadherin in mES cells by adding Dox 6 h before being exposed to isoflurane, and then induced them to neural differentiation without Dox and found that the inhibition of self-renewal of mES cells caused by isoflurane was attenuated. In our study, we found that the ability of neural differentiation of cells, which cotransfected with pre-miR-9 6 h before being exposed to isoflurane, could abrogate the effect of only overexpressing E-cadherin (Fig. 5C, D). These results confirmed that isoflurane repressed self-renewal of mES cells by the miR-9-E-cadherin pathway and the neural differentiation of isoflurane-treated mES cells was inhibited.
FIG. 5.
Isoflurane inhibits neuronal differentiation of mES cells through miR-9/E-cadherin signaling. (A) AP detection staining of mES cells. Overexpression of E-cadherin restored isoflurane or pre-miR-9 induced low self-renewal capacity of mES cells. (B) Detection of mRNA of stemness markers, Oct4, Sox2, Nanog, and Lin 28, by qRT-PCR in mES cells treated the same as in (A). *P<0.05, **P<0.01. (C) Immunostaining of neural marker, tuj1, in neural differentiation from mES cells treated the same as in (A). The scale bar represents 25 μm. (D) Detection of the mRNA level in mES cells treated the same as in (C) by qRT-PCR. Error bars represent the SD of three independent experiments. *P<0.05, **P<0.01, ***P<0.001, one-way ANOVA. ANOVA, analysis of variance. Color images available online at www.liebertpub.com/scd
Discussion
Many pregnant women are exposed to inhalation anesthetics for nonobstetric surgery [1]. Inhalation anesthetics readily cross the placental barrier; however, the embryotoxicity is still largely unknown. In this study, we found that anesthesia with 1.4% isoflurane (the clinical concentration) for 2 h daily for 3 days inhibited fetal growth and development of fetus during pregnancy. This finding showed a very important clinical significance because many pregnant women undergo nonobstetric surgery each year and isoflurane is a commonly used inhalation anesthetic in clinical practice and has the ability to readily cross the placental barrier [2]. Previous studies showed that isoflurane decreased self-renewal of cultured rat neural stem cells and human neural progenitor cells [3,4]. To know the underlying mechanism, we treated mES cells with isoflurane to examine the potential repression of isoflurane on self-renewal. We found that isoflurane decreased the expression of stemness genes (Nanog, Oct4, Sox2, and Lin28) and repressed the self-renewal of mES cells and promoted it to differentiate in advance at a clinically relevant concentration.
E-cadherin is highly expressed during early embryonic development and is essential for maintaining clonal formation and self-renewal of mES cells in early embryonic development [12]. E-cadherin also is required for STAT3 phosphorylation, resulting in the expression of stemness genes, such as Nanog and Oct4, and ultimately facilitating maintenance of pluripotency in mES cells [13]. In our experiments, isoflurane downregulated E-cadherin expression in both mRNA and protein levels in mES cells. Downregulation of E-cadherin reduced the mES cell self-renewal, leading to the downregulation of stemness genes. Interestingly, overexpressing E-cadherin attenuated isoflurane-induced decrease of self-renewal and the expression of stemness genes in mES cells. These findings suggest that isoflurane could inhibit the self-renewal of mES cells by downregulating E-cadherin expression. We next found that isoflurane increased miR-9 in mES cells. A bioinformatics analysis using TargetScan, miRanda, and miRBase suggested that miR-9 could bind to the 3′UTR of E-cadherin [17–19]. Pre-miR-9 produced similar functions to isoflurane. The miR-9 inhibitor attenuated the function of isoflurane or E-cadherin downregulation on self-renewal in mES cells. These results indicated that inhibition of isoflurane on the self-renewal of mES cells is mediated by upregulating miR-9. Self-renewal of mES cells, which transfected with pre-miR-9 before being exposed to isoflurane, abrogated the effect of overexpressing E-cadherin or transfecting with the miR-9 inhibitor. These results suggested that isoflurane decreased the self-renewal of mES cells through miR-9-E-cadherin signaling, which may be a potential target therapy to prevent the embryotoxicity of isoflurane.
Inhalation anesthetics affecting the developing fetal brain are a major health issue in clinical practice when pregnant women accept nonobstetric surgery under general anesthesia. Most of the studies of influence of isoflurane on offspring were focused on the neurotoxicity in learning and memory impairment and cognitive dysfunction [1–6]. A previous study found inhalation anesthetic exposure in utero during early gestation produced behaviorally abnormal adults and it impairs the developing brain upon administration to pregnant rodents during middle gestation [7,22]. Moreover, under normal conditions, mES cells express a low level of miR-9. E-cadherin, in contrast, is highly expressed [23,24]. Upon differentiation to neurons, miR-9 increases, while E-cadherin decreases [24,25]. A previous study showed that miR-9 plays an important role in neural progenitor differentiation [15]. Additionally, miR-9 is upregulated during the later stage of neuron differentiation, but is still lower in the early stage of differentiation, or even lowest in ES cells [26]. For these reasons above, we performed the subsequent neuronal differentiation of these isoflurane-treated mES cells to observe the subsequent development. In this study, we found that isoflurane repressed self-renewal of mES cells by the miR-9-E-cadherin pathway and led to inhibition of the neural differentiation of isoflurane-treated mES cells. There was cell apoptosis during neural differentiation and the expression of mesoderm and endoderm markers was higher than the control group. Because the cells of the mesoderm and endoderm cannot be sustained in the induction system of neural differentiation, we thought that the increase of apoptosis cells might be due to the increase of cells differentiating to the mesoderm and endoderm. We also hypothesized that isoflurane damaged the self-renewal of mES cells and initiated the differentiation toward the mesoderm and endoderm, leading to the weak induction of neural differentiation.
In this study, pregnant mice were treated with isoflurane at a time in the beginning of organogenesis to simulate pregnant women undergoing long-time nonobstetric surgery. We found that exposure to isoflurane at an earlier gestational period could produce more severe damage to the whole body development. Additionally, we also found that the placental growth was reduced by isoflurane. The placenta is the primary interface between the fetus and mother and plays an important role in maintaining fetal development and growth. The major substrates required for fetal growth include oxygen, glucose, amino acids, and fatty acids and their transport processes depend on morphological characteristics of the placental blood flow [27]. The reduction of the weight and size of the placenta suggests the lower placental blood flow. Therefore, we thought that the reduction of fetal growth may not only be relevant to the effect of isoflurane on the placental growth but may also result from the effect of isoflurane on the self-renewal of ES cells.
As the mechanism of development of different organs is not the same, the mechanisms of influence by isoflurane on organs are various. Moreover, there are also different differentiation induction systems for different somatic cells in vitro; the whole body develops into different parts of different germ layers, and different organs even different somatic cells need to be investigated in a different way in future. Since there is a limit, we only chose the nervous system as our target; the mechanism of the effect of isoflurane on other organs and the placenta needs further studies.
In summary, isoflurane induced reduction of fetal growth and development of the fetus during pregnancy in vivo. It may result from the effect of isoflurane on self-renewal of mES cells. The miR-9-E-cadherin signaling may be a potential target therapy to prevent the embryotoxicity of isoflurane.
Supplementary Material
Acknowledgments
This research was supported by the National Natural Science Foundation of China (81300934, 81272038, 81301655). Research funds were from the Shanghai Municipal Commission of Health and Family Planning (grant no. 201440356).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ní Mhuireachtaigh R. and O'Gorman DA. (2006). Anesthesia in pregnant patients for nonobstetric surgery. J Clin Anesth 18:60–66 [DOI] [PubMed] [Google Scholar]
- 2.Reitman E. and Flood P. (2011). Anaesthetic considerations for non-obstetric surgery during pregnancy. Br J Anaesth 107:i72–i78 [DOI] [PubMed] [Google Scholar]
- 3.Culley DJ, Boyd JD, Palanisamy A, Xie Z, Kojima K, Vacanti CA, Tanzi RE. and Crosby G. (2011). Isoflurane decreases self-renewal capacity of rat cultured neural stem cells. Anesthesiology 115:754–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao X, Yang Z, Liang G, Wu Z, Peng Y, Joseph DJ, Inan S. and Wei H. (2013). Dual effects of isoflurane on proliferation, differentiation, and survival in human neuroprogenitor cells. Anesthesiology 118:537–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronca AE, Abel RA. and Alberts JR. (2007). Maternal anesthesia via isoflurane or ether differentially affects pre-and postnatal behavior in rat offspring. Dev Psychobiol 49:675–684 [DOI] [PubMed] [Google Scholar]
- 6.Thaete LG, Levin SI. and Dudley AT. (2013). Impact of anaesthetics and analgesics on fetal growth in the mouse. Lab Anim 47:175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palanisamy A, Baxter MG, Keel PK, Xie Z, Crosby G. and Culley DJ. (2011). Rats exposed to isofurane in utero during early gestation are behaviorally abnormal as adults. Anesthesiology 114:521–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marthiens V, Gavard J, Lambert M. and Mège RM. (2002). Cadherin-based cell adhesion in neuromuscular development. Biol Cell 94:315–326 [DOI] [PubMed] [Google Scholar]
- 9.Larue L, Antos C, Butz S, Huber O, Delmas V, Dominis M. and Kemler R. (1996). A role for cadherins in tissue formation. Development 122:3185–3194 [DOI] [PubMed] [Google Scholar]
- 10.Uda Y, Poh YC, Chowdhury F, Wu DC, Tanaka TS, Sato M. and Wang N. (2011). Force via integrins but not E-cadherin decreases Oct3/4 expression in embryonic stem cells. Biochem Biophys Res Commun 415:396–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T, Yuan D, Wei B, Jiang J, Kang J, Ling K, Gu Y, Li J, Xiao L. and Pei G. (2010). E-cadherin-mediated cell-cell contact is critical for induced pluripotent stem cell generation. Stem Cells 28:1315–1325 [DOI] [PubMed] [Google Scholar]
- 12.del Valle I, Rudloff S, Carles A, Li Y, Liszewska E, Vogt R. and Kemler R. (2013). E-cadherin is required for the proper activation of the Lifr/Gp130 signaling pathway in mouse embryonic stem cells. Development 140:1684–1692 [DOI] [PubMed] [Google Scholar]
- 13.Nagaoka M, Koshimizu U, Yuasa S, Hattori F, Chen H, Tanaka T, Okabe M, Fukuda K. and Akaike T. (2006). E-cadherin-coated plates maintain pluripotent ES cells without colony formation. PLoS One 20:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wienholds E. and Plasterk RH. (2005). MicroRNA function in animal development. FEBS Lett 579:5911–5922 [DOI] [PubMed] [Google Scholar]
- 15.Bonev B, Pisco A. and Papalopulu N. (2011). MicroRNA-9 reveals regional diversity of neural progenitors along the anterior-posterior axis. Dev Cell 20:19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawkins K, Mohamet L, Ritson S, Merry CL. and Ward CM. (2012). E-cadherin and, in its absence, N-cadherin promotes Nanog expression in mouse embryonic stem cells via STAT3 phosphorylation. Stem Cells 30:1842–1851 [DOI] [PubMed] [Google Scholar]
- 17.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP. and Burge CB. (2003). Prediction of mammalian microRNA targets. Cell 115:787–798 [DOI] [PubMed] [Google Scholar]
- 18.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A. and Enright AJ. (2006). miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res 34:D140–D144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, Burge CB. and Bartel DP. (2005). The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science 310:1817–1821 [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Zhang J, Yang L, Dong Y, Zhang Y. and Xie Z. (2013). Isoflurane and sevoflurane increase interleukin-6 levels through the nuclear factor-kappa B pathway in neuroglioma cells. Br J Anaesth 110 (Suppl. 1):i82–i91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE. and Xie Z. (2012). Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol 71:687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng H, Dong Y, Xu Z, Crosby G, Culley DJ, Zhang Y, and Xie Z. (2013). Sevoflurane anesthesia in pregnant mice induces neurotoxicity in fetal and offspring mice. Anesthesiology 118:516–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coolen M, Thieffry D, Drivenes Ø, Becker TS. and Bally-Cuif L. (2012). miR-9 controls the timing of neurogenesis through the direct inhibition of antagonistic factors. Dev Cell 22:1052–1064 [DOI] [PubMed] [Google Scholar]
- 24.Wu W, Tian Y, Wan H, Ma J, Song Y, Wang Y. and Zhang L. (2013). Expression of β-catenin and E- and N-cadherin in human brainstem gliomas and clinicopathological correlations. Int J Neurosci 123:318–323 [DOI] [PubMed] [Google Scholar]
- 25.Tan SL, Ohtsuka T, González A. and Kageyama R. (2012). MicroRNA9 regulates neural stem cell differentiation by controlling Hes1 expression dynamics in the developing brain. Genes Cells 17:952–961 [DOI] [PubMed] [Google Scholar]
- 26.Delaloy Celine, Liu Lei, Lee Jin-A, Su Hua, Shen Fanxia, Yang Guo-Yuan, Young William L, Ivey Kathy N. and Gao Fen-Biao. (2010). MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell Stem Cell 6:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S, Regnault TR, Barker PL, Botting KJ, McMillen IC, McMillan CM, Roberts CT. and Morrison J. (2015). Placental adaptations in growth restriction. Nutrients 7:360–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.