Abstract
Steviol glycosides are natural constituents of Stevia rebaudiana (Bert.) Bert. (Asteraceae) that have recently gained worldwide approval as nonnutritive sweeteners by the Joint Food and Agriculture Organization/World Organization Expert Committee on Food Additives. Cheminformatic tools suggested that the aglycone steviol and several of its phase I metabolites were predicted as potential anticonvulsant agents effective in the seizure animal model maximal electroshock seizure (MES) test. Thus, aqueous infusion from S. rebaudiana was tested in the MES test (mice, intraperitoneal administration), confirming dose-dependent anticonvulsant effect. Afterward, isolated stevioside and rebaudioside A were tested in the MES test, with positive results. Though drug repositioning most often focuses on known therapeutics, this article illustrates the possibilities of this strategy to find new functionalities and therapeutic indications for food constituents and natural products.
Introduction
Drug repositioning refers to finding new therapeutic indications for already-known drugs.1 Such strategy has recently raised much interest within the international drug development community, including a number of public programs to promote repositioning launched by national health authorities in developed countries such as the United States and United Kingdom.2,3 Since already-known drugs have already undergone safety assessment and pharmacokinetic characterization, repositioning often allows acceding clinical trials early in the drug discovery process, speeding up the development of novel therapeutics. Though drug repositioning often focuses on previously known therapeutics, it also presents great potential to identify novel functionalities and therapeutic applications of food constituents.
We have recently identified, through cheminformatic tools, the anticonvulsant activity of artificial sweeteners acesulfame, cyclamate, and saccharin.4 These positive results led us to wonder whether natural sweeteners also have anticonvulsant effects. Natural products (NP) remain one of the most important sources of lead structures in the drug discovery field. About 30% of the small-molecule new approved drugs introduced to the market between 1981 and 2002 were NP or NP derivatives (without considering peptides or proteins obtained from organisms and cell lines by biotechnological means).5 Furthermore, NPs present high chemical diversity and a large overlap in chemical space with approved drugs.6,7
Stevia, a member of the Asteraceae family, is a plant native to South America; extracts of the leaves of the stevia plant have a long traditional use to sweeten food and beverages in Argentina, and have also been used for several years in Japan and China and some European countries.8,9 Very recently, stevia was approved for use as a sweetener by the Joint Food and Agriculture Organization World Organization Expert Committee on Food Additives.10
Here we report the application of ligand-based computational models to predict whether stevia constituents and/or their phase I metabolites might have anticonvulsant properties. Afterward, an aqueous infusion of stevia leaves and isolated steviosides was tested in seizure models (mice, intraperitoneally [ip]) to confirm our predictions.
Materials and Methods
Prediction of Stevia Constituents' Anticonvulsant Activity
Two independent and previously reported ligand-based computational models were used to predict the potential anticonvulsant effect of stevia constituents and their phase I metabolites (Fig. 1) in the maximal electroshock seizure (MES) test.
Fig. 1.
Chemical structures of the constituents of stevia (and some phase I metabolites) screened with our computational models.
The first model applied to screen the stevia constituents was a four-feature linear classificatory model based on conformation-independent topological molecular descriptors and information indices.11 The molecular descriptors included in this model were computed with Dragon Software for molecular descriptors calculation v. 4. 0 (Milano Chemometrics, 2003). The model consists of four molecular descriptors:
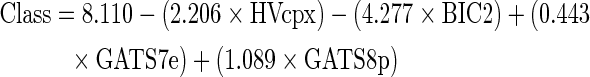 |
where a positive value of the model's response (Çlass) is linked to anticonvulsant effect in the MES test, while a negative value is associated to lack of anticonvulsant effect. HVcpx denotes the graph vertex complexity index, BIC2 stands for the bond information content (neighborhood symmetry of second order), GATS7e symbolizes Geary autocorrelation lag 7, weighted by atomic Sanderson electronegativities, and GATS8p denotes Geary autocorrelation lag 8, weighted by atomic polarizabilities.
HVcpx and BIC2 belong to a group of topological indices known as information indices, which are obtained by combining graph theory and Shannon's information theory.12 Information indices provide information on the local or global complexity of the molecule. For example, HVcpx reflects the average vertex complexity; the vertex complexity  is computed from the topological distance matrix as follows13:
is computed from the topological distance matrix as follows13:
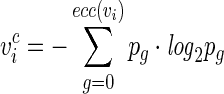 |
The previous sum runs from zero to the vertex eccentricity ecc(vi); pg is the probability of randomly selecting a distance equal to g from the considered vertex/atom. A completely symmetric vertex is associated to a low vertex complexity. Thus, the inclusion of these information indices in the model may reflect the relatively simpler molecular topology of the anticonvulsants used to train the model compared to compounds from other therapeutic categories included as training instances in the inactive class.
Regarding Geary autocorrelations, they describe the distribution of a given atomic property in the molecule. These indices will assume larger values when the considered molecule includes atoms with differences in the atomic property under consideration (electronegativity, polarizability) at the topological distance examined (the lag). The model might be reflecting that (1) anticonvulsants, compared to the inactive compounds included in the training set, tend to possess rather compact structures, and thus anticonvulsants present less pairs of atoms separated by long interatomic distances, and (2) anticonvulsants tend to present, in their molecular structure, one or two polar regions, with the other part of the molecule being rather hydrophobic, in contrast with drugs from other therapeutic categories presenting a more uniform charge distribution.
The second model is a Sybyl pharmacophore obtained through the active analog approach that proposes the geometrical arrangement of hydrophobic and polar moieties required to elicit the protective effects in the MES test.14 Such model has been derived from the superposition of the rigid anticonvulsants phenytoin and carbamazepine, and was later refined through the alignment of 15 additional drugs active in the MES test with the common capability of blocking voltage-gated sodium channels. The common pattern emerging from such alignment includes a hydrophobic chain (atoms 5–7; Fig. 2) and a polar moiety (atoms 1–3; Fig. 2). The hydrophobic chain is usually (though not necessarily) of aromatic nature. Whereas some of the anticonvulsant compounds used to infer the pharmacophore show two hydrophobic regions, a single hydrophobic tail is present in other compounds, this being the minimum structural requisite.
Fig. 2.
Sybyl pharmacophore. Atoms 1–3 represent a polar moiety; atoms 5–7 represent a hydrophobic tail.
Pharmacophore superposition was conducted with Hyperchem v. 8.0 (Hypercube, 2007). To that purpose, Hyperchem's Conformational Search module was used with the PM3 optimization method and an RMS gradient of 0.05 kcal/(Å·mol). At least 250 optimizations were performed; optimizations were conducted until convergence (i.e., until no new low-energy conformers are found). The lowest energy conformer of each drug was then superposed onto the pharmacophore, and the energy difference (conformational strain energy) between the lowest energy conformer and the conformer that approach the most to the pharmacophore conformation was calculated for each molecule. In other words, an induced fit onto the pharmacophore was performed using the lowest energy conformer, and the energy difference between the bioactive conformation and the minimum energy conformer was computed. RMS for the superposition of the minimum energy conformer and the pharmacophore (RMS 1) and the induced conformer and the pharmacophore (RMS 2) were also calculated. It is worth highlighting that these two models have previously been jointly applied to screen NP libraries, with good results.15
Plant Material and Preparation of Extracts
Authenticated stevia [Stevia rebaudiana (Bert.) Bert.] plants from ecological crops were field-grown in La Capilla, Partido Florencio Varela, Buenos Aires Province, Argentina (34° 55′ 43″ S–58° 16′ 29″ W). A voucher specimen was deposited in Exact Sciences Faculty, Carlos Spegazzini Museum, UNLP, Argentina (LPE 1163). The leaves were harvested in early summer (Nov–Dec 2012) from fully grown shoots, air-dried at room temperature, and ground. Powdered drug (20 g) was extracted by infusion and lyophilized (yield: 34.98%). For further enrichment of the diterpene glycosides, the extract (20 mL) was shaken with 20 mL n-butanol, and the n-butanol phase was separated, evaporated to 3 mL, and used for TLC. An amount of 20 μL was spotted on precoated silicagel 60 F254 plates (0.25 mm thick; E. Merck, Darmstadt) and developed with chloroform–methanol–water (65:25:4). After development, chromatograms were air-dried, sprayed with Liebermann-Bouchard reagent, and heated for 5–10 min at 110°C (evaluation: vis and UV366). The chromatographic profile of the stevia folia extract shows nine gray zones, distributed over the whole Rf range. Four of them are found in the Rf range 0.1–0.3. According to bibliographical data, the zones at Rf 0.2 and 0.3 correspond to rebaudioside A and stevioside, respectively.16
Anticonvulsant Preclinical Screening
The evaluation of the anticonvulsant activity was performed by following the anticonvulsant drug development program of the National Institutes of Health. Adult male Swiss mice (18–23 g) were used as experimental animals. Animals of the same age and weight have been selected in order to minimize biological variability. The animals were maintained on a 12-hour light/dark cycle and allowed free access to food and water, except during the time they were removed from their cages for testing. Mice received a daily administration of saline (0.1 mL ip) for 1 week. This procedure allows the animals to habituate to manipulation, avoiding downregulation of GABA receptors induced by acute handling, which could affect the susceptibility to convulsions.17
The dry aqueous infusion from S. rebaudiana was properly dissolved in water in all the cases, without solubility problems. A maximal volume of 5 mL/kg of the freshly made solutions was injected ip to groups of two to four mice, at doses of 30 and 100 mg/kg. MES were elicited in mice by delivering a 60 Hz/50 mA electrical stimuli for 0.2 s via ear clip electrodes, at 0.5 and 4 hours after the drug injection, using an UGO Basile equipment. A drop of saline was applied on each ear before placing the electrodes to ensure adequate electrical contact. In these conditions, maximal seizures are produced in virtually all normal mice. The maximal seizure typically consists of a short period of tonic flexion followed by a longer period of tonic extension of the hind limbs and a final clonic episode. Blockade of the hind limbs tonic extension due to the drug treatment is taken as the end point. The tonic component is considered abolished if the hind leg tonic extension does not exceed a 90° angle with the trunk. The rotorod test was used to determine possible neurotoxic effects of the test drugs. Control animals are manipulated as described above except that they receive vehicle (saline) instead of the drug solution.
Quantitative studies were conducted at 4 hours, which was the time at which maximal anticonvulsant effect was observed, as shown in the Results and Discussion section. The ED50 was determined by treating groups of six albino mice. The method of Litchfield and Wilcoxon was used to compute the ED50 value.18
The isolated steviosides were tested as described in the previous paragraphs. Rebaudioside A was acquired from Sigma Aldrich. Stevioside was a gentle gift from Prof. Kolb Kozlobsky, Faculty of Exact Sciences, National University of Misiones. It was obtained through preparative chromatography, and selected fractions were collected and purified. The compounds in each sample were identified by isocratic HPLC, comparing their retention times with those of the reference compounds, according the Kolb et al.'s method.19
Results and Discussion
Our models predicted that steviol and all its phase I metabolites tested would have anticonvulsant activity. Table 1 shows the output of the linear classificatory model for these compounds; the values of the molecular descriptors for stevia components and its derivatives are also presented. Table 2 shows the strain energy and RMS values correspondent to the superposition with the pharmacophore. Steviosides were predicted as non-anticonvulsants in the MES test. Superposition of steviol on the pharmacophore model is presented in Figure 3. The conformational strain energy for steviol and its metabolites was, in all cases, below 5 kcal/mol, which indicates that steviol and its metabolites can probably assume a conformation similar to those of the active rigid analogs from which our pharmacophore was inferred.20
Table 1.
Model Parameters and Output for the Stevia Components and Metabolites for the Classificatory Linear Model
| Mol ID | HVcpx | BIC2 | GATS7e | GATS8p | Class | Prediction |
|---|---|---|---|---|---|---|
| 15-Hydroxisteviol | 2.82 | 0.67 | 1.20 | 0.64 | 0.27 | Anticonvulsant |
| 15-Oxosteviol | 2.82 | 0.65 | 1.29 | 0.64 | 0.39 | Anticonvulsant |
| α-Epoxisteviol | 2.83 | 0.62 | 1.39 | 0.62 | 0.53 | Anticonvulsant |
| Dulcosido A | 4.00 | 0.64 | 0.96 | 1.01 | −1.93 | Inactive |
| Isoesteviol | 2.81 | 0.58 | 1.29 | 0.60 | 0.66 | Anticonvulsant |
| Rebaudioside A | 4.05 | 0.61 | 0.98 | 1.03 | −1.89 | Inactive |
| Rebaudioside B | 3.73 | 0.64 | 1.04 | 0.97 | −1.36 | Inactive |
| Rebaudioside C | 4.05 | 0.62 | 0.98 | 1.02 | −1.92 | Inactive |
| Steviol | 2.81 | 0.62 | 1.29 | 0.60 | 0.48 | Anticonvulsant |
| Steviolbioside | 3.65 | 0.68 | 0.97 | 0.96 | −1.37 | Inactive |
| Stevioside | 4.01 | 0.64 | 0.96 | 1.02 | −1.91 | Inactive |
Table 2.
Strain Energy and RMS Values from the Superposition onto the Pharmacophore
| Mol ID | Energy lowest (kcal/mol) | Energy of the induced conformer (kcal/mol) | ΔE (kcal/mol) | RMS 1 | RMS 2 |
|---|---|---|---|---|---|
| 15-Hydroxysteviol | −5414.86 | −5411.67 | 3.19 | 0.8767 | 0.6030 |
| 15-Oxosteviol | −5297.74 | −5295.02 | 2.72 | 0.6135 | 0.4433 |
| Steviol | −5314.60 | −5312.23 | 2.37 | 0.8491 | 0.5533 |
| Isosteviol | −5333.51 | −5333.31 | 0.20 | 0.4257 | 0.4060 |
| α-Epoxisteviol | −5396.37 | −5394.87 | 1.50 | 0.6327 | 0.6046 |
Fig. 3.

Superposition of steviol on the pharmacophore model.
On the basis of these results, we decided to evaluate the pharmacological effect of an aqueous infusion of stevia leaves in the MES test. Initial results confirmed the anticonvulsant activity of the infusion, which later proved to be dose dependent (Tables 3 and 4), with an estimated ED50 of 70.3 mg/kg (47.4–104.3) (mice, ip, 4 hours). Neither of the tested doses presented neurotoxicity in the rotorod test. Quantitative studies with stevia aqueous extract were conducted at 4 hours. The ED50 was determined by treating groups of six albino mice. Different doses were used for each drug at the time of peak effect. The method of Litchfield and Wilcoxon was used to compute the ED50 value.
Table 3.
Anticonvulsant Effect of the Aqueous Infusion from Stevia Leaves at Doses of 30 and 100 mg/kg
aNumber of protected animals/number of tested animals.
bNumber of animals presenting motor impairment/number of tested animals.
MES, maximal electroshock seizure.
Table 4.
Dose-Dependent Anticonvulsant Effect of Stevia Infusion in the MES Test
aNumber of protected animals/number of tested animals.
bNumber of dead animals/number of tested animals.
Having confirmed the anticonvulsant effect of the aqueous extract from stevia leaves, we decided to test the isolated stevioside and rebaudioside A, using a similar protocol to the previously described for the aqueous infusion. Both drugs showed protective effect in the preclinical testing model. The results are presented in Table 5.
Table 5.
Anticonvulsant Effect of Isolated Steviosides
| MES testa | Neurotoxicityb | ||||
|---|---|---|---|---|---|
| Drug | Doses (mg/kg) | 0.5 h | 4 h | 0.5 h | 4 h |
| Rebaudioside A | 30 | 0/2 | 0/2 | 0/2 | 0/2 |
| 100 | 2/4 | 2/4 | 0/4 | 0/4 | |
| Stevioside | 30 | 0/2 | 3/4 | 0/2 | 0/4 |
| 100 | 3/4 | 1/4 | 0/4 | 0/4 | |
aNumber of protected animals/number of tested animals.
bNumber of animals presenting motor impairment/number of tested animals.
Noteworthy, the isolated steviosides presented anticonvulsant properties in mice, though our models suggested that they do not have intrinsic activity. This may reflect a misprediction of the model or, alternatively, the model might be correct and the steviosides may have no intrinsic anticonvulsant activity, in which case the anticonvulsant effect could be attributed to the aglycone and/or its metabolization products (which are predicted as anticonvulsants). This appears to be in agreement with the fact that, at 30 mg/kg (the lower doses tested), stevioside only shows anticonvulsant effects at 4 hours (presumably, after metabolization to steviol has taken place). At higher doses (100 mg/kg), the effect at 0.5 hours is higher than that at 4 hours (it should be taken into consideration that biotransformation frequently follows first-order kinetics; therefore, the higher the dose the sooner that the metabolization product might reach effective blood levels). Further studies (e.g., binding studies) are needed to confirm whether the steviosides or their metabolites (as predicted by our models) are the species responsible for the observed effect in the MES test. Our results demonstrate not only the anticonvulsant activity of stevia constituents in mice, but also the potential of computer-aided drug repositioning to discover new functionalities of food and NP constituents.
Conclusions
S. rebaudiana aqueous extract and isolated natural sweeteners stevioside and rebaudioside A present protective effects in the acute seizure animal model MES (mice, ip). In the case of the aqueous infusion, such effect was proven to be dose dependent. These observations complement recent previous reports from our group that indicate that artificial sweeteners also have anti-MES effects. These facts lend weight to the hypothesis of a structural link between sweet taste receptor and any of the molecular targets of antiepileptic drugs. Our results also confirm the potential of computer-aided approaches to discover new drugs among the NP biodiversity and new therapeutic indications for food additives or constituents. Further studies are required to verify whether the results reported here are significant for application in human therapeutics, taking into consideration that important differences in steviosides' metabolisms in rodents and human have been reported.8
Abbreviations Used
- NP
natural products
- MES
maximal electroshock seizure
Acknowledgments
M.E. Di Ianni is a CONICET fellowship holder. A. Talevi is a member of the Scientific Research Career at CONICET. L.E. Bruno-Blanch, M.A. Rosella, M.E. Del Valle, and A.V. Enrique are researchers at Facultad de Ciencias Exactas, Universidad Nacional de La Plata.
The authors would like to thank UNLP (Incentivos X-597), CONICET (PIP 11220090100603), ANPCyT (PICTs 2010-2531 and 2010-1774), and SECYT (Grant 11-X582) for providing funds to develop the research. We would specially like to thank Prof. Kolb for his gentle gift of isolated steviosides.
Disclosure Statement
The authors declare no conflict of interest related to the content of this article.
References
- 1.Sleigh SH, Barton CL. Repurposing strategies for therapeutics. Pharm Med. 2010;24:151–159 [Google Scholar]
- 2.Allison M. NCATS launches drug repurposing program. Nat Biotechnol. 2012;30:571–572 [DOI] [PubMed] [Google Scholar]
- 3.Marusina K, Welsch DJ, Rose L, Brock D, Bahr N. The CTSA Pharmaceutical Assets Portal—a public-private partnership model for drug repositioning. Drug Discov Today Ther Strateg. 2011;8:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talevi A, Enrique AV, Bruno-Blanch LE. Anticonvulsant activity of artificial sweeteners: a structural link between sweet-taste receptor T1R3 and brain glutamate receptors. Bioorg Med Chem Lett. 2012;22:4072–4074 [DOI] [PubMed] [Google Scholar]
- 5.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;66:1022–1037 [DOI] [PubMed] [Google Scholar]
- 6.Gu J, Gui Y, Chen L, Yuan G, Lu HZ, Xu X. Use of natural products as chemical library for drug discovery and network pharmacology. PLoS One. 2013;8:e62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanagita K, Nonaka K, Nakata M. Genetic study of the craniofacial growth in male rats by factor scores. J Craniofac Genet Dev Biol. 1992;12:13–21 [PubMed] [Google Scholar]
- 8.Geuns JM. Stevioside. Phytochemistry. 2003;64:913–921 [DOI] [PubMed] [Google Scholar]
- 9.Mortensen A. Sweeteners permitted in the European Union, safety aspects. Scand J Food Nutr. 2006;50:104–116 [Google Scholar]
- 10.Shankar P, Ahuja S, Sriram K. Non-nutritive sweeteners: review and update. Nutrition. 2013;29:1293–1299 [DOI] [PubMed] [Google Scholar]
- 11.Talevi A, Bellera CL, Castro EA, Bruno-Blanch LE. A successful virtual screening application: prediction of anticonvulsant activity in MES test of widely used pharmaceutical and food preservatives methylparaben and propylparaben. J Comput Aided Mol Des. 2007;21:527–538 [DOI] [PubMed] [Google Scholar]
- 12.Raychaudhury C, Ray SK, Ghosh JJ, Roy AB, Basak SC. Discrimination of isomeric structures using information theoretic topological indices. J Comput Chem. 1984;5:581–588 [Google Scholar]
- 13.Todeschini R, Consonni V. Handbook of Molecular Descriptors. Weinheim, Germany: Wiley-VCH; 2000 [Google Scholar]
- 14.Tasso SM, Moon S, Bruno-Blanch LE, Estiu GL. Characterization of the anticonvulsant profile of valpromide derivatives. Bioorg Med Chem. 2004;12:3857–3869 [DOI] [PubMed] [Google Scholar]
- 15.Gavernet L, Talevi A, Castro EA, Bruno-Blanch LE. A combined virtual screening 2D and 3D QSAR methodology for the selection of new anticonvulsant candidates from a natural product library. QSAR Combinatorial Sci. 2008;27:1120–1129 [Google Scholar]
- 16.Wagner H. Drugs containing sweet-tasting terpene glycosides. In: Sabine B, ed. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed. Berlin: Springer-Verlag; 1996:329–332 [Google Scholar]
- 17.Skilbeck KJ, Johnston GA, Hinton T. Stress and GABA receptors. J Neurochem. 2010;112:1115–1130 [DOI] [PubMed] [Google Scholar]
- 18.Litchfield JT, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–113 [PubMed] [Google Scholar]
- 19.Kolb N, Herrera JL, Ferreyra DJ, Uliana RF. Analysis of sweet diterpene glycosides from Stevia rebaudiana: improved HPLC method. J Agric Food Chem. 2001;49:538–4541 [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Pang YP. Normal-mode-analysis-monitored energy minimization procedure for generating small-molecule bound conformations. PLoS One. 2007;2:e1025. [DOI] [PMC free article] [PubMed] [Google Scholar]




