Abstract
The concept of the hypothesis-driven or observational-based expansion of the therapeutic application of drugs is very seductive. This is due to a number of factors, such as lower cost of development, higher probability of success, near-term clinical potential, patient and societal benefit, and also the ability to apply the approach to rare, orphan, and underresearched diseases. Another highly attractive aspect is that the “barrier to entry” is low, at least in comparison to a full drug discovery operation. The availability of high-performance computing, and databases of various forms have also enhanced the ability to pose reasonable and testable hypotheses for drug repurposing, rescue, and repositioning. In this article we discuss several factors that are currently underdeveloped, or could benefit from clearer definition in articles presenting such work. We propose a classification scheme—drug repositioning evidence level (DREL)—for all drug repositioning projects, according to the level of scientific evidence. DREL ranges from zero, which refers to predictions that lack any experimental support, to four, which refers to drugs approved for the new indication. We also present a set of simple concepts that can allow rapid and effective filtering of hypotheses, leading to a focus on those that are most likely to lead to practical safe applications of an existing drug. Some promising repurposing leads for malaria (DREL-1) and amoebic dysentery (DREL-2) are discussed.
Introduction
A wide and increasing number of approaches and strategies for drug repositioning have been proposed, ranging from text mining or in silico screening, via in vitro/ex vivo screening, study in animal disease models, and finally to observational studies from human trials. In contrast to first-in-class agents, where the need to validate both the drug safety and the causal role of the targeted mechanism, there is less cost and risk for repositioning studies, akin to follow-up drugs.1 The costs and speed of performing these types of studies range from very low and fast for computational studies to expensive and slow for postapproval observational studies. In this article, we use our personal experience to outline various issues that can have large impact on the success of drug repurposing, repositioning, and re-patenting, and map these onto a number of examples from the literature. Specifically, we address the general case where there is no a priori hypothesis for drug reuse, no assumption over target mechanism, and where a drug library is tested, somehow, against either a single or panel of screens. In most cases, we argue that drug repositioning is vastly more complicated than typically imagined and currently implemented. Via more rigorous experimental design, data preparation, and prior art curation, more effective and successful drug repositioning could be accomplished. We illustrate these points with reference to some recent examples of the screening of drug collections in the neglected diseases therapeutic area.
The basic workflow for many drug repositioning projects is to assemble a set of known drugs—either as physical samples or, in the case of computational methods, reliable 2D structures, and then “screen” these in a relevant system (either a physical assay, or in silico)—Figure 1. It is generally assumed that experimental bioassays are more reliable and predictive than computational assays; however, experimental conditions, number of concentrations tested, as well as number of replicates can influence the accuracy of experiment. In contrast to reliable experimental data, the far lower cost and lower barrier to entry have made computational approaches of high interest and effort in the research community.
Fig. 1.
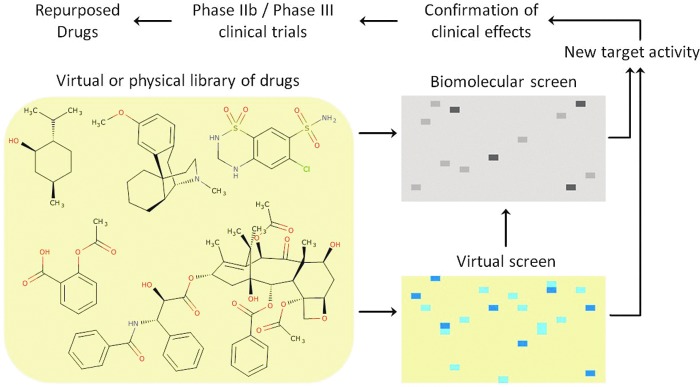
General purpose drug repositioning workflow. Color images are available online at www.liebertpub.com/adt
What are the Approved Drugs?
In its simplest definition, approved drugs are well-understood chemical and biological ingredients that regulatory agencies have approved for human use in the context of disease. In research, medicines are considered by reference to the pharmaceutical contained within, and no distinction is generally made between the term “drug” and the term “active pharmaceutical ingredient” (API). However, from a regulatory and drug development perspective, a drug is a particular combination of APIs with inactive ingredients that is formulated under defined and consistent conditions for human use. Here, we use “drug” when referring to API, unless clarity requires the distinction. For practical purposes, the set of realistic drugs that could be repositioned is far smaller than the total number of approved drugs—regardless of intellectual property or cost. For example, there are restrictions on handling and distribution of regulated substances, such as opiates and anabolic steroids. These restrictions also apply to radiochemicals. Some drugs are not sufficiently stable to survive long-term storage and handling; specifically, protein therapeutics often have poor stability in buffer/DMSO stock and do not cope well with repeated freeze–thaw cycles.
Some approved drugs would not be classed as “therapeutic” in most settings aimed at drug reuse—for example, magnesium chloride and the amino acid arginine are approved drugs, but would not fit many alternative definitions of a drug. Additionally, there are differences in the geographical approval of drugs, as many drugs are not approved in all territories. The concept of regulatory approval by the U.S. FDA or the European Medicines Agency (EMA) is often used as a de facto definition of approval, but opportunities exist in identifying drugs approved in some locations but not others. This factor may double the number of distinct APIs for consideration, but it is not likely to significantly increase the number of distinct mechanisms of action. Finally, several studies include well-known tool compounds, compounds in clinical trials, veterinary drugs, and so on. These factors conspire to make the construction and selection of a definitive and appropriate “approved drugs chemical library” for drug repositioning a complex task.
The Importance of Knowing the Pharmacologically Active Form
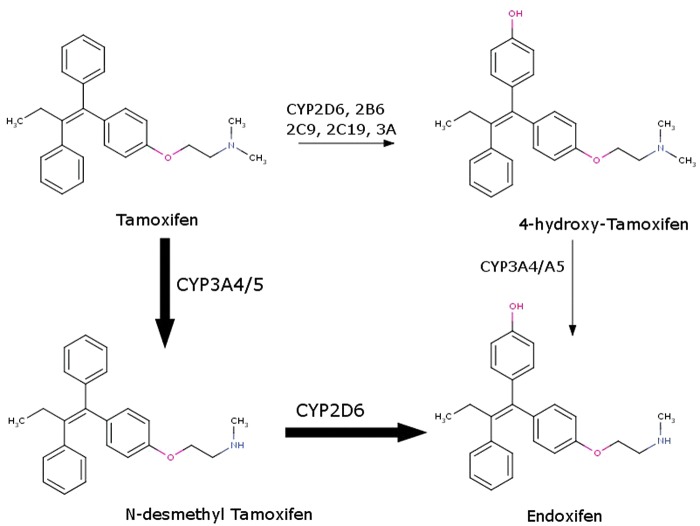
During in vitro or in silico evaluations, one needs to recall that many drugs are prodrugs either by design (e.g., most simple carboxylate esters) or are in vivo converted to active metabolites. Thus, the substances being screened can often differ from the chemicals responsible for the therapeutic effect. A classic example is tamoxifen (Fig. 2), which is converted to 4-hydroxy-tamoxifen and endoxifen (4-hydroxy-N-desmethyl tamoxifen) via cytochrome P450 metabolism.2 Indeed, the parent compound tamoxifen has low affinity and little/no efficacy at the molecular target—the estrogen alpha receptor.3 Biomedical literature abounds on reports of the activity of tamoxifen, yet in many cases it is unclear what compound was actually tested. Of course, the prodrug may well have independent and distinct pharmacological activity, but almost certainly the exposure of the prodrug form will be (by design) low in a human clinical setting, and lead to inconsistencies with results derived from in vitro bioassays.
Fig. 2.
Biotransformation pathways of tamoxifen toward active metabolites. Color images are available online at www.liebertpub.com/adt
Overall, about 10% of APIs are designed as typically not bioactive prodrugs, but many more drugs are converted into active metabolites, each with their own distinct target spectrum and ADMET properties. For example, the drug imipramine (which is a norepinephrine and serotonin pump inhibitor) represents, at steady state, only ∼31% of the initial dose; the rest is converted to the metabolites 2-hydroxy-imipramine (8%) and desipramine (39%), and the inactive metabolite 2-hydroxy-desipramine, 22% of the dose, respectively.4
Finally, there are several drugs that have been developed and approved as “prodrug” and “bioactive” form. For example, the antidepressant pairs amitriptyline & nortriptyline, and imipramine & desipramine, respectively, are individually marketed drugs, whereas terfenadine is now withdrawn, replaced by its less toxic active metabolite fexofenadine, which is marketed as an antihistamine. Any drug library should consider including annotations of these alternative forms, which should all be added to the screening deck for in vitro and in silico drug repositioning screening.
Although computational studies appear simpler, there is much ambiguity and complexity present when dealing with the proper assembly of a chemically correct representation of APIs for computational studies. Some stages of preparing compounds for computational studies are straightforward, for example, salt-stripping, assuming that the salt component is not responsible for any desired pharmaceutical activity. There is also a surprising amount of variation in drug structures reported in public and private databases, with some of these being simple errors, and others being more subtle differences. In much the same way as for the creation of a physical set of drugs for repositioning screening, these differences will complicate application and decrease success of computational studies.
Curation of Drug Structures
Although it seems a trivial task, the curation of a correct set of structures for approved drugs is difficult and arguably still not available at the required level of accuracy.5 Much of this challenge relates to the reporting of drug structures in the published and regulatory literature. For example, for several classes of structures, the stereochemistry is implicit—steroids and sugars in particular often do not have explicitly represented stereocenters in many databases, since to those in the community this is implicitly depicted in a well-understood, tacit manner. Although this information is essential for many (but not all) computational applications, it often leads to errors, especially when converting graphically correct representations of structures to 3D objects.6
Many drugs are dosed as racemic mixtures, a mixture of usually two distinct physical forms, differing solely in the configuration around chiral centers. For 2D-based computational methods, this stereochemical distinction remains ambiguous: for example, most fingerprint-based similarity methods do not adequately distinguish between the R- and S- enantiomers and identical similarity scores will be typically produced. However, for 3D-based methods such as docking and shape similarity, this stereochemical difference is crucial, and requires the explicit enumeration and consideration of all dosed stereoisomers. Additional factors that critically affect the success of docking are the treatment of different tautomeric forms, protonation states, and so on. Certain idiosyncratic examples further complicate this treatment: for example, midazolam is solubilized in an open-ring form (Fig. 3) under the acidic conditions required to solubilize the API in a syrup formulation, due to the acid-catalyzed ring opening of the 4,5-double bond of the diazepine ring. In man, midazolam is converted (10%) into its alpha-hydroxy (active) metabolite. Thalidomide is dosed as a racemate, but the two stereoisomers interconvert (epimerise) in vivo, making studies on a defined stereoisomer more difficult to interpolate into an in vivo setting. Together, these factors conspire to make 3D-based (in silico) approaches to drug repositioning quite challenging and irreproducible.
Fig. 3.
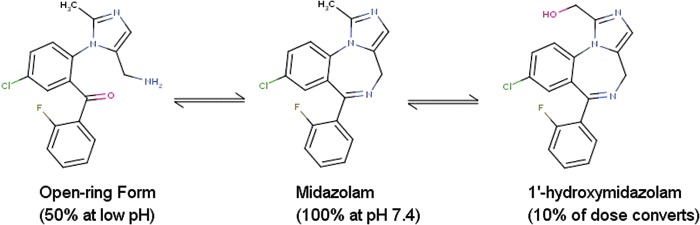
Multiple forms of midazolam with significant in vivo exposure. Color images are available online at www.liebertpub.com/adt
A further issue once these sets of compounds are used for drug repositioning is the consideration of the dosing properties of the drug—there are many differing dosage modalities, with three general classes covering the majority of delivery routes: oral, where the drug is administered by mouth and then absorbed through the intestinal tract (or sublingually); topical, where the drug is absorbed through various epithelial routes (including inhalation, ophthalmological, and intranasal); and finally injected, where the drug is injected either directly into the circulation, or into another body compartment. Some APIs can be reformulated to be delivered via different routes, which is a legitimate drug repurposing activity. However, physical chemistry and safety issues often restrict delivery options. The dosing route can also greatly restrict the options for effective drug repositioning. For example, injectables often need reconstitution just prior to use, and require trained staff for delivery and often an in-patient treatment setting, with associated high costs.
Known Safety Data
Safety is a very significant practical issue; ∼30–40% of FDA-approved drugs are approved with boxed warnings—meaning that they have known and important safety issues, but in the context of the approved indication, these are tolerable and are on the appropriate side of a risk–benefit analysis. The tolerable safety liabilities of a drug crucially depend on the target disease, with higher risk aversion in common chronic conditions such as obesity and diabetes, or patient groups such as in pediatric or pregnancy settings, and relatively less safety risk aversion in end-of-life diseases, such as terminal cancer.
The in vivo distribution of a drug can also greatly affect prospects for realistic drug repositioning. Certain compartments of the body are privileged, or unusual with respect to drug access—for example, the eye is physically isolated, and therefore drugs dosed directly to the eye often have high organ-specific exposure and low systemic availability. Another side of this effect is that many drugs do not efficiently enter (or are effluxed from) the central nervous system (CNS). If a drug does not have good CNS exposure, or if it penetrates the CNS but is pumped out by efflux transporters, it is unlikely that new indications and usage will be found for a neurological condition.
Dose Levels and Exposure
The dosing of a drug is arguably the most complex and unpredictable area of drug repositioning. An API will be approved in a clearly specified formulation at well-defined dosage strength levels; any use at dosage above these parameters will require substantial development costs, whereas the ideal for drug repositioning is to use an existing approved specific product. Matching the desired therapeutic profile to different disease and safety settings requires new trials, dosage forms, stability studies, and so on. For example, the phosphodiesterase V inhibitor, sildenafil, with dose strengths of 25, 50, and 100 mg, when marketed for erectile dysfunction as Viagra™, was reformulated to dose strengths of 5 and 20 mg, respectively, when marketed for pulmonary hypertension as Revatio™, respectively.
Mapping in vitro bioactivity levels to dosage is also complex; as a general rule, caution should be applied where in vitro concentrations are significantly (e.g., orders of magnitude) higher that previously reported concentrations for the same drug observed in clinical settings. In particular, sustained concentrations of an unbound drug in excess of 1 μM would be unusual, and so activity that is observed in vivo at concentrations higher than this require extra validation and study to be considered credible candidates for drug repositioning. There are many exceptions to this general rule though: Some drugs with long half-lives require several days/weeks to reach therapeutic concentrations (and consequently, to achieve efficacy); other idiosyncrasies, such as active transport or uptake into a particular compartment, enterohepatic recirculation, and so on, can add more levels of complexity for specific drugs. However, for proposed drug-reuse opportunities, comparison to the known human pharmacokinetic profile and properties should be considered essential as part of the analysis and publication processes. Overall, these factors require a high degree of consistent annotation of the drug library, and careful matching and prioritization against the desired product profile.
Categories for Assessment of Drug Repurposing
Despite major implications for healthcare providers, clinicians, and patients, there are multiple stages of development in the arena of drug repurposing that remain unrecognized. This may lead to heightened expectations, misunderstanding, and sometimes confusion regarding the availability of certain medicines; the degree of confidence in their safety and efficacy; as well as their overall usage. Sometimes, observations relevant at the in vitro level surface in mainstream mass media reports as almost available, just around-the-corner cures. One explanation for the lack of clarity in this area is that most scientists believe that the key effort in drug repurposing is the identification of a novel target, and presumably a novel mechanism of action, safety and efficacy notwithstanding. On the other hand, it appears easier to repurpose drugs from other indications toward cancer, because of the higher level of accepted systemic toxicity that anticancer agents have compared to other drugs. Most examples of successful drug repositioning involve exploiting the originally anticipated target, in a new disease, in new pathological setting, or simply in new dosage or new formulations. The data mining and computational approaches required for these “on-target” and “off-target” strategies are inherently rather different.
Without having performed a systematic evaluation of drug repurposing projects from publications, patent literature, and databases, we suggest that drug repurposing projects can be classified based on the quality of scientific evidence (Table 1). The assessment distinguishes five drug repositioning evidence level (DREL) stages given the amount and quality of evidence available (i.e., 0–4), similar to the classification scheme used for quantifying drug–drug interactions.7 In practical terms, this classification suggests that some drug repurposing claims are not substantiated, as no experimental evidence is provided, nor can it be inferred from the literature. The level of confidence increases as the evidence progresses from in vitro studies to animal and human studies, with higher weight being assigned to those data with direct clinical relevance. Examples of drug repurposing projects classified according to evidence are given in Table 2.
Table 1.
Classification of Drug Repurposing Claims According to Scientific Evidence
| Drug repositioning evidence level | Quality of scientific evidence |
|---|---|
| 0 | No evidence; includes in silico predictions without confirmation |
| 1 | In vitro studies with limited value for predicting in vivo/human situation |
| 2 | Animal studies with hypothetical relevance in man |
| 3 | Incomplete studies in man at the appropriate dose, e.g., proof of concept; very few cases or inference from medical records; some clinical effects observed |
| 4 | Well-documented clinical end points observed for the repurposed drug at doses within safety limits |
Table 2.
Examples of Drug Repurposing, Classified According to Evidence
| Drug repositioning evidence level | Active pharmaceutical ingredient | Comments |
|---|---|---|
| 0 | Many examples | Quite often, such articles are published in informatics/computational journals without experimental evidence |
| 1 | Benzbromarone | Showed in vitro activity as quorum sensing inhibitor; could not be confirmed in animal models9 |
| 2 | Astemizole | Showed effective activity as radiosensitizer when co-administered to mice with xenograft tumors9 |
| 3 | Ketorolac | Confirmed in vitro and in vivo activity as Rac1 and Cdc42 GTP-ase inhibitor*; undergoing clinical trial for ovarian cancer10 |
| 4 | Sildenafil | Revatio™ for pulmonary hypertension following initial launch as Viagra™ for male erectile dysfunction |
*Oprea TI, Sklar LA, Agola JO, et al. Novel activities of select NSAID R-enantiomers against Rac1 and Cdc42 GTPases. In Review.
By classifying drug repurposing and rescuing projects according to the five DREL levels, we anticipate that there will be less subjectivity when evaluating such projects. For example, while DREL-0 may appear controversial to some, it is in fact intended to distinguish facts from computations, until such time that experimental evidence progresses that project to DREL-1, in vitro evidence. Indeed, many articles from journals aimed at the computational and informatics community do not disclose experimental confirmation. By the same token, those compounds that work at very high concentrations in vitro (DREL-1) may achieve limited effects in vivo (DREL-2), or indeed under clinical conditions (DREL-3) due to toxicity. Such a classification scheme, when attached to individual projects, may assist the community to rapidly evaluate the level of development for each project, thus tempering heightened societal expectations for fast cures, when evidence does not warrant it.
For example, ketorolac is marketed as a racemic mixture. The S-isomer, known for its preferential cyclooxygenase subtype 1 inhibitory activity, is the basis for this compound's efficacy as nonsteroidal anti-inflammatory drug (NSAID).8 From a virtual screen9 following R-naproxen as initial lead for GTP-ase inhibition, in vitro and enantiopure R- and S- ketorolac evaluation showed that R-ketorolac is a Rac1 and Cdc42 GTP-ase inhibitor,* which is the basis for racemic ketorolac's clinical trial evaluation for ovarian, Fallopian tube, and primary peritoneal cancer.10
Examples of Drug Repositioning in Neglected Disease Research: The Importance of the Product Profile
We discuss two examples taken from the neglected disease therapeutic literature. Many neglected diseases have approved therapies, although with poor tolerability and other undesired pharmaceutical properties. This may be due to the lack of investment in optimization of the initial drug prototypes, in contrast to the process that occurs in the pharmaceutical sector when large revenues can be secured for next-generation, safer, more efficacious agents. This makes work in this field accessible to academic groups, and attractive to public or not-for-profit funding agencies. An early example of this was the identification of astemizole as a potential treatment for malaria, following a phenotypic screen.11
Case Study 1: Phenotypic Screening of Approved Drugs for Reuse as Malaria Therapies—Drel-1
Malaria is a major health burden for the world, and despite progress in drug discovery,12 there remains a need for novel, effective therapies. This need has been thrown into sharp focus by the emergence of resistance against the newest artemisinin class of drugs. Current agents for malaria disease treatment and prophylaxis are oral, and have low cost of use: these include aminoquinolines, biguanides, methanolquinolines, diaminopyrimidines, artemisinin, and derivatives, which are often prescribed in combination. New agents would need to be low cost and orally dosed, with few liabilities for drug–drug interactions. Moreover, the epidemiology of malaria has additional important constraints for drug development. For example, a malaria agent would need to be safe and well tolerated in not only pediatric, but also geriatric, and display consistent exposure and efficacy in populations with other comorbidities, such as tuberculosis or malnourishment. Additional factors would be good tolerance in pregnancy and understood exposure to babies while breastfeeding. Specialist dosage or monitoring (e.g., liver function tests) would not be realistic in areas where malaria is endemic.
Yuan et al.13 recently reported the screening of the NIH Chemical Genomics Centre Pharmaceutical collection14 in a cell-based assay for malaria, and discovered 32 highly active confirmed hits, and then characterized the properties of these hits with genetics and gene expression studies. The compounds (detailed in Table 1 of Yuan et al.13) are reported as the highly active set, and exclude already known, established antimalarial drugs, which all pass the selection criteria used for compounds in this table. This is an important control with respect to both setting relevant affinity levels but also allow direct comparison and studies of differentiation with respect to existing therapies. This is a list of potential leads for future antimalarial discovery.
In order to explore the likely best candidates for practical repositioning as a novel antimalarial therapy, we performed the following simple checks (these can be readily performed with the drug's prescribing information) on the list of 32, after confirming that the compound listed as suberoylanilide is suberoylanilide hydroxamic acid (SAHA) with the authors.
1. Whether the drugs were already approved for human use
2. The dosage/absorption route—oral dosage is preferred
3. Special clinical monitoring should be avoided
Of these 32, 1 is a chemical with little public annotation (alazanine triclofenate), 5 are currently approved for animal use only, and 1 compound, lestautinib, is in late-stage human trials, but not approved. Seventeen of the remaining 25 are dosed only parenterally/topically or by inhalation. This leaves eight that have the desired profile of being orally dosed and currently approved for human usage. Of these, six have restrictive use or appear to be poorly tolerated: for example, fumagilin is hospital-use only and requires careful monitoring. This leaves two candidates for consideration as realistic “drug-repurposing” opportunities—dextroamphetamine saccharate and orlistat.
Dextroamphetamine, a psychostimulant and nootropic agent, would clearly have significant practical, distribution, dosing, compliance, regulatory, and abuse issues.15 Orlistat is used as an oral over-the-counter (in some territories) and prescription drug to assist with weight loss. It reduces the absorption of fat from food intake, leading to a variety of significant gastrointestinal side effects that affect tolerability. In populations that are in malaria-endemic regions, a drug that could be administered to underweight malnourished patients which blocks fat absorption, as well of that of essential fat-soluble vitamins, is far from optimal. A larger issue though is that orlistat works in the lumen of the stomach and intestine, where the target human enzyme is secreted, and so for obesity treatment, orlistat does not need to be bioavailable, and indeed it has negligible systemic exposure. The presence of malaria in the circulatory system is therefore inconsistent with the known observed pattern of drug exposure of orlistat in humans.16
In summary, from the initial list of 32 hits, few realistic drug repositioning opportunities exist. While some of the hits could serve as prototypes for further development as proposed in the article, there appear to be no attractive drugs for immediate clinical studies.
Case 2: Phenotypic Screening of Approved Drugs for Application as Amoebic Dysentery therapies—Drel-2
Amoebiasis, caused by infection with the protozoa Entamoeba histolytica, is a significant cause of infant death, and currently has medicines with limited efficacy and low safety margin (e.g., metronidazole). As for malaria, new therapies would ideally be low cost and orally dosed, with a key difference being in this case that the pathogen exists largely in the gut and not in other body tissues or circulation. The pathology of amoebiasis adds challenges to drug discovery, specifically inflamed gut tissue, fluid loss, and often against a background of poor sanitation and nutrition. Morbidity needs to be focused on pediatric populations, with preference for good tolerability and a wide therapeutic index.
Debnath et al.17 reported the screening of a 910-compound screening library containing a mix of both drugs and other bioactives in a technically challenging phenotypic screen (E. histolytica is an anaerobe and sensitive to exposure to atmospheric oxygen). The primary screen identified 11 compounds as active, with the most potent, the approved anti-inflammatory auranofin, having an EC50 of 0.5 μM. Auranofin is an unusual drug, in that it functions as a delivery agent for the element gold, but has been used as an anti-inflammatory for over 25 years. The mechanism of action of auranofin is not well understood, but it is generally agreed that auranofin is rapidly metabolized to release elemental gold in vivo. The article explicitly discusses the exposure of auranofin from human studies, and uses this as support of application of auranofin as an opportunity for drug repositioning. This is a well-designed application of drug repositioning according to the workflow presented in Figure 1. However, deeper analysis of known drug properties for auranofin reveals several issues with its application to the proposed disease indication.
Auranofin18 is a specialist drug that may require physician supervision given the high incidence of toxicity risks, as it is one of the few drugs with a well-characterized human LD50. Specific contraindications are bowel lesions and damage, which unfortunately are parts of the clinical tableau of amoebic dysentery. The potency of auranofin (0.5 μM) in the assay is favorably compared to the steady-state plasma levels of gold (3.5 μM) observed in humans,17 and cited as evidence for its repositioning potential. Mean plasma gold levels of 0.62±0.195 mg/L (increasing to 0.68±0.452 mg/mL after 6 months) were measured19 after 3 months of 6 mg/day oral dosing of auranofin. This indeed corresponds to plasma gold levels between 3.137 and 3.452 μM/L, which compare well against the potency of auranofin (EC50=0.5 μM) against E. histolytica. The pharmacokinetic parameters for auranofin following oral administration are 25% oral bioavailability, 40% fraction unbound, and 408 hours half-life. In whole blood, auranofin gold is 60% distributed to plasma, 37% intracellularly, and 3% to cell membranes, respectively.20 The apparent volume of distribution of auranofin gold has not been reported; it penetrates in synovial fluid (1.7:1 gold concentration ratio compared to whole blood) and does not reach detectable skin levels following long-term administration.20
ClinicalTrials.gov lists an on-going phase 1 trial conducted by Quintiles to study the pharmacokinetics of 7-day oral dosing of 6 mg auranofin for amoebiasis.19 Given the above half-life and peak plasma concentration, we estimate that by day 7 of a 6 mg/day auranofin regimen, the total gold plasma concentration will reach 2.5 μM/L, or 1.002 μM/L unbound. While a 3-month dosing regimen is likely to render auranofin effective for chronic amoebic dysentery, we estimate that the 7-day, 6 mg/day oral regimen auranofin is not likely to achieve an effective amoebicidal concentration. While the estimated gold plasma level unbound is close to the EC50, we anticipate that the concentration needed to effectively block 100% of E. histolytica is higher, perhaps as much as 3 μM/L.17 The authors discuss auranofin gold blood levels,17 but do not state that it takes 3 months to achieve steady-state concentrations, even though the information is clearly stated in the auranofin package insert.18 Taking this information into account would have allowed better prioritization of the opportunity to reposition auranofin.
The price of auranofin in India is significantly cheaper compared to the United States: 115 INR (Indian rupees), or 1.84 USD, for ten 3 mg tablets,21 compared to 6.33 USD for ten 3 mg tablets.22 The 7-day oral regimen for auranofin does not appear to be cost-limiting. The cost for chronic administration, however, would exceed 2070 INR, or 33.12 USD, in India ($114 in the United States22), which represents the price for the one hundred eighty 3 mg tablets that are needed for a 3-month therapy. Since the annual median per capita income in India is 616 USD,23 we believe that chronic auranofin therapy for amoebic dysentery is cost-prohibitive for the Indian market, without governmental subsidy.
To summarize, a perfunctory examination of auranofin pharmacodynamics suggests that this drug is efficacious against E. histolytica.17 However, given the pharmacokinetic profile of auranofin, the prospect of achieving amoebicidal gold plasma levels is not likely after a 7-day 6 mg/day oral regimen. This rules out auranofin as a candidate for the chemotherapeutic management of acute amoebic dysentery. Whereas chronic administration for 3 months would achieve the steady-state gold blood levels required for amoebicidal activity, this prospect does not appear realistic in third-world countries such as India for economic reasons.
Conclusions
In this article, we have highlighted some issues that need to be considered by those wishing to successfully repurpose drugs for new clinical applications. Given a general outline of the drug repurposing workflow, from both computational and experimental perspectives, we identified areas that need special consideration. We suggested a robust classification scheme, DREL, that can be used to evaluate drug repositioning projects according to the level of scientific evidence, and discussed two repurposing projects, for malaria and amebiasis, using DREL classification.
Many drug repositioning projects stop at the in vitro level. This highlights the arduous path that compounds have to follow before meeting all qualifications for regulatory approval, which applies even for approved drugs. Such requirements will clearly be different for every disease, but we hope that concepts outlined here will facilitate a more realistic prioritization of repositioning opportunities.
Acknowledgment
This work was supported in part by NIH Grant 1U54CA189205-01 (T.I.O. and J.P.O.).
Disclosure Statement
No competing financial interests exist.
Oprea TI, Sklar LA, Agola JO, et al. Novel activities of select NSAID R-enantiomers against Rac1 and Cdc42 GTPases. In Review.
References
- 1.Cavalla D. Predictive methods in drug repositioning: gold mine or just a bigger haystack. Drug Discov Today. 2013;18:523–532 [DOI] [PubMed] [Google Scholar]
- 2.Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310:1062–1075 [DOI] [PubMed] [Google Scholar]
- 3.Furr BJA, Jordan VC. The pharmacology and clinical uses of tamoxifen. Pharmacol Ther. 1984;25:127–205 [DOI] [PubMed] [Google Scholar]
- 4.Brøsen K, Klysner R, Gram LF, Otton SV, Bech P, Bertilsson L. Steady-state concentrations of imipramine and its metabolites in relation to the sparteine/debrisoquine polymorphism. Eur J Clin Pharmacol. 1986;30:679–684 [DOI] [PubMed] [Google Scholar]
- 5.Williams AJ, Ekins S. A quality alert and call for improved curation of public chemistry databases. Drug Discov Today. 2011;16:747–750 [DOI] [PubMed] [Google Scholar]
- 6.Oprea TI, Olah M, Ostopovici L, Rad R, Mracec M. On the propagation of errors in the QSAR literature. In: Ford M, Livingstone D, Dearden J, Van de Waterbeemd H, eds. EuroQSAR 2002—Designing Drugs and Crop Protectants: Processes, Problems and Solutions. New York: Blackwell Publishing; 2003:314–315 [Google Scholar]
- 7.Jansman FGA, Reyners AKL, van Roon EN, et al. . Consensus-based evaluation of clinical significance and management of anticancer drug interactions. Clin Ther. 2011;33:305–314 [DOI] [PubMed] [Google Scholar]
- 8.Handley DA, Cervoni P, McCray JE, McCullough JR. Preclinical enantioselective pharmacology of (R)- and (S)- ketorolac. J Clin Pharmacol. 1998;38:25S–35S [DOI] [PubMed] [Google Scholar]
- 9.Oprea TI, Bauman JE, Bologa CG, et al. . Drug repurposing from an academic perspective. Drug Discov Today Therap Strategies. 2011;8:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ClinicalTrials.gov. Availability & effect of post-OP ketorolac on ovarian, fallopian tube or primary peritoneal cancer. http://clinicaltrials.gov/ct2/show/NCT01670799 (Last accessed November22, 2014)
- 11.Chong CR, Chen X, Shi L, Liu JO, Sullivan DJ. A clinical drug library screen identifies astemizole as an antimalarial agent. Nat Chem Biol. 2006;2:415–416 [DOI] [PubMed] [Google Scholar]
- 12.Medicines for Malaria Venture. Interactive R&D portfolio. www.mmv.org/research-development/rd-portfolio (Last accessed November22, 2014)
- 13.Yuan J, Cheng KC-C, Johnson RL, et al. . Chemical genomic profiling for antimalarial therapies, response signatures and molecular targets. Science. 2011;333:724–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang R, Southall N, Wang Y, et al. . The NCGC pharmaceutical collection: a comprehensive resource of clinically approved drugs enabling repurposing and chemical genomics. Sci Transl Med. 2011;3:80ps16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paladin Labs Inc. Prescribing Information: Dexedrine® (dextroamphetamine sulfate). www.paladin-labs.com/pdf_files/Dexedrine.pdf (Last accessed November22, 2014)
- 16.Highlights of Prescribing Information. www.gene.com/download/pdf/xenical_prescribing.pdf (Last accessed November22, 2014)
- 17.Debnath A, Parsonage D, Andrade RM, et al. . A high throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat Med. 2012;18:956–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dailymed. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=05c34ddf-a0f7-4267-83f5-d02be3defc37 combined with information from Product Monograph. Ridaura® www.xediton.com/RIDAURA_PRODUCT%20MONOGRAPH.pdf [the PDF contains an error regarding the units of measurement, it states picograms (pg) intead of micrograms (μg)]. (Last accessed November22, 2014)
- 19.ClinicalTrials.gov. Auranofin PK following oral dose administration. https://clinicaltrials.gov/ct2/show/NCT02089048 (Last accessed November22, 2014)
- 20.Chaffman M, Brogden RN, Heel RC, Speight TM, Avery GS. Auranofin. Drugs 1984;27:378–424 [DOI] [PubMed] [Google Scholar]
- 21.Thedu. Goldar (3 mg)—Auranofin—tablet. http://thedu.in/compare_prices/index.php/medicines-g/article/39214-goldar-3-mg-auranofin-tablet (Last accessed November22, 2014)
- 22.Unitedpharmacies. Ridaura: Auranofin. www.unitedpharmacies.com/Ridaura-Auranofin.html (Last accessed November22, 2014)
- 23.Business Standard. India's median per capita income lowest among BRICS: Gallup. www.business-standard.com/article/economy-policy/india-s-median-per-capita-income-lowest-among-brics-gallup-113121600968_1.html (Last accessed November22, 2014)